Clinical Significance and Prognostic Value of TLR4 and AGER in Inflammatory Breast Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics
2.2. Subject and Data Collection
2.3. TLR4 and AGER Immunofluorescence
2.4. TLR4 and AGER Gene Expression Analysis
2.5. Statistical Analysis
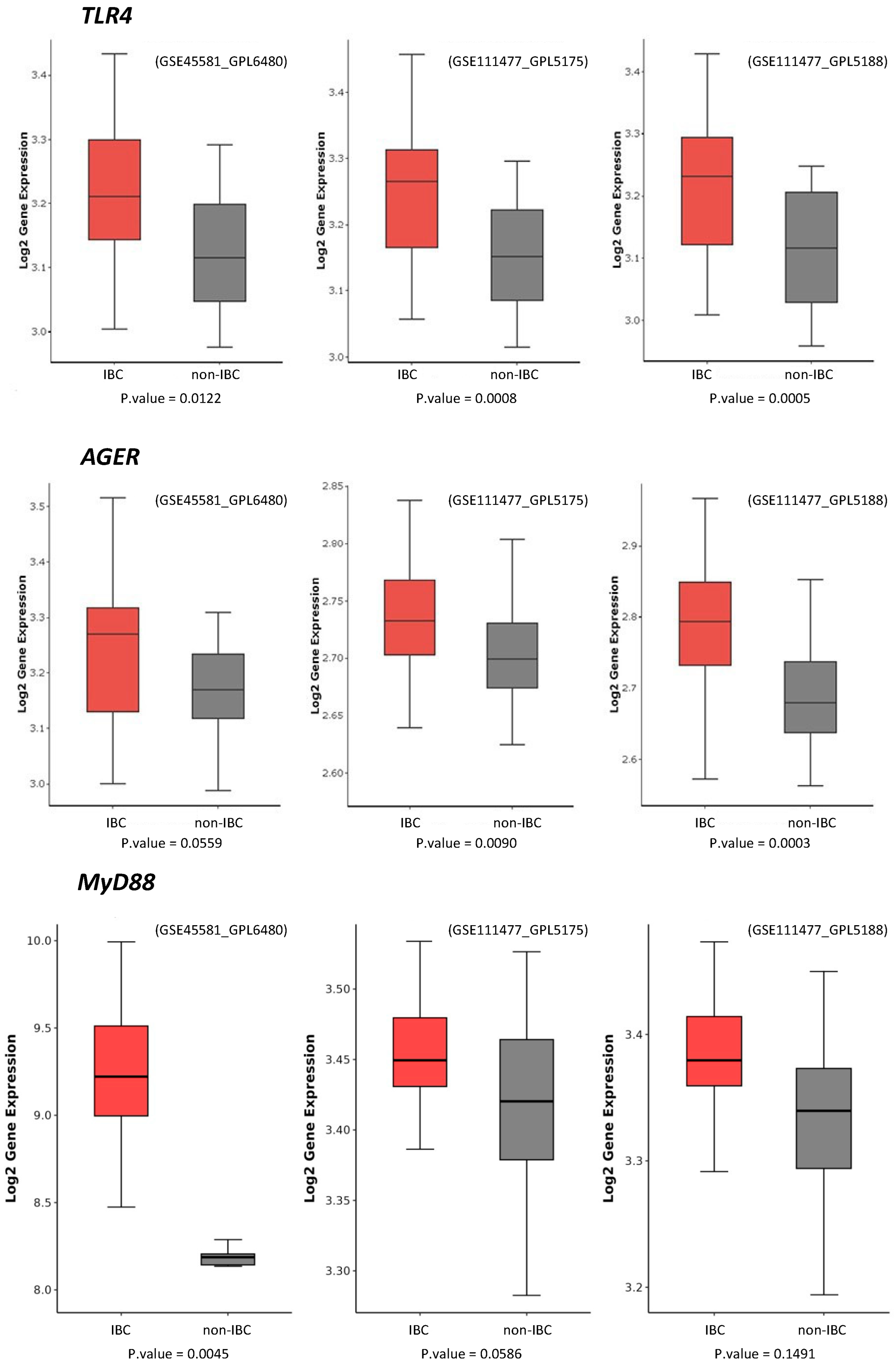
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGER | Advanced Glycation End Products Receptor |
| BC | Breast Cancer |
| BMI | Body Mass Index |
| IBC | Inflammatory Breast Carcinoma |
| TLR4 | Toll-like Receptor Type 4 |
| ER | Estrogen Receptor |
| PR | Progesterone Receptor |
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.d.O.; Lima, F.C.d.S.d.; Martins, L.F.L.; Oliveira, J.F.P.; Almeida, L.M.d.; Cancela, M.d.C. Estimated Cancer Incidence in Brazil, 2023-2025. Rev. Bras. Cancerol. 2023, 69, e-213700. [Google Scholar] [CrossRef]
- Instituto Nacional de Câncer José Alencar Gomes da Silva (INCA). Estimativa 2023: Incidencia de Cancer No Brasil; INCA: Rio de Janeiro, Brasil, 2023; ISBN 9786588517093. [Google Scholar]
- Menta, A.; Fouad, T.M.; Lucci, A.; Le-Petross, H.; Stauder, M.C.; Woodward, W.A.; Ueno, N.T.; Lim, B. Inflammatory Breast Cancer: What to Know About This Unique, Aggressive Breast Cancer. Surg. Clin. N. Am. 2018, 98, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Hance, K.W.; Anderson, W.F.; Devesa, S.S.; Young, H.A.; Levine, P.H. Trends in Inflammatory Breast Carcinoma Incidence and Survival: The Surveillance, Epidemiology, and End Results Program at the National Cancer Institute. J. Natl. Cancer Inst. 2005, 97, 966–975. [Google Scholar] [CrossRef]
- Hirko, K.A.; Chen, W.Y.; Willett, W.C.; Rosner, B.A.; Hankinson, S.E.; Beck, A.H.; Tamimi, R.M.; Eliassen, A.H. Alcohol Consumption and Risk of Breast Cancer by Molecular Subtype: Prospective Analysis of the Nurses’ Health Study after 26 Years of Follow-Up. Int. J. Cancer 2016, 138, 1094–1101. [Google Scholar] [CrossRef]
- Jagsi, R.; Mason, G.; Overmoyer, B.A.; Woodward, W.A.; Badve, S.; Schneider, R.J.; Lang, J.E.; Alpaugh, M.; Williams, K.P.; Vaught, D.; et al. Inflammatory Breast Cancer Defined: Proposed Common Diagnostic Criteria to Guide Treatment and Research. Breast Cancer Res. Treat. 2022, 192, 235–243. [Google Scholar] [CrossRef]
- Schairer, C.; Hablas, A.; Eldein, I.A.B.S.; Gaafar, R.; Rais, H.; Mezlini, A.; Ayed, F.B.; Ayoub, W.B.; Benider, A.; Tahri, A.; et al. Risk Factors for Inflammatory and Non-Inflammatory Breast Cancer in North Africa. Breast Cancer Res. Treat. 2020, 184, 543–558. [Google Scholar] [CrossRef]
- De Heredia, F.P.; Gómez-Martínez, S.; Marcos, A. Chronic and Degenerative Diseases: Obesity, Inflammation and the Immune System. Proc. Nutr. Soc. 2012, 71, 332–338. [Google Scholar] [CrossRef]
- El-Shinawi, M.; Mohamed, H.T.; Abdel-Fattah, H.H.; Ibrahim, S.A.A.; El-Halawany, M.S.; Nouh, M.A.; Schneider, R.J.; Mohamed, M.M. Inflammatory and Non-Inflammatory Breast Cancer: A Potential Role for Detection of Multiple Viral DNAs in Disease Progression. Ann. Surg. Oncol. 2016, 23, 494–502. [Google Scholar] [CrossRef]
- Silvera, D.; Arju, R.; Darvishian, F.; Levine, P.H.; Zolfaghari, L.; Goldberg, J.; Hochman, T.; Formenti, S.C.; Schneider, R.J. Essential Role for EIF4GI Overexpression in the Pathogenesis of Inflammatory Breast Cancer. Nat. Cell Biol. 2009, 11, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Olejnik, J.; Hume, A.J.; Mühlberger, E. Toll-like Receptor 4 in Acute Viral Infection: Too Much of a Good Thing. PLoS Pathog. 2018, 14, e1007390. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Recognition of Microorganisms and Activation of the Immune Response. Nature 2007, 449, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Palanissami, G.; Paul, S.F.D. RAGE and Its Ligands: Molecular Interplay Between Glycation, Inflammation, and Hallmarks of Cancer—A Review. Horm. Cancer 2018, 9, 295–325. [Google Scholar] [CrossRef]
- Lv, W.; Chen, N.; Lin, Y.; Ma, H.; Ruan, Y.; Li, Z.; Li, X.; Pan, X.; Tian, X. Macrophage Migration Inhibitory Factor Promotes Breast Cancer Metastasis via Activation of HMGB1/TLR4/NF Kappa B Axis. Cancer Lett. 2016, 375, 245–255. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, A.; Song, I.H.; Park, I.A.; Yu, J.H.; Ahn, J.H.; Gong, G. Cytoplasmic Expression of High Mobility Group B1 (HMGB1) Is Associated with Tumor-infiltrating Lymphocytes (TILs) in Breast Cancer. Pathol. Int. 2016, 66, 202–209. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, L.; Liu, S. Targeting HMGB1: An Available Therapeutic Strategy for Breast Cancer Therapy. Int. J. Biol. Sci. 2022, 18, 3421–3434. [Google Scholar] [CrossRef]
- Sims, G.P.; Rowe, D.C.; Rietdijk, S.T.; Herbst, R.; Coyle, A.J. HMGB1 and RAGE in Inflammation and Cancer. Annu. Rev. Immunol. 2010, 28, 367–388. [Google Scholar] [CrossRef]
- Jialal, I.; Kaur, H.; Devaraj, S. Toll-like Receptor Status in Obesity and Metabolic Syndrome: A Translational Perspective. J. Clin. Endocrinol. Metab. 2014, 99, 39–48. [Google Scholar] [CrossRef]
- Wu, K.; Zhang, H.; Fu, Y.; Zhu, Y.; Kong, L.; Chen, L.; Zhao, F.; Yu, L.; Chen, X. TLR4/MyD88 Signaling Determines the Metastatic Potential of Breast Cancer Cells. Mol. Med. Rep. 2018, 18, 3411–3420. [Google Scholar] [CrossRef]
- Ahmed, A.; Redmond, H.P.; Wang, J.H. Links between Toll-like Receptor 4 and Breast Cancer. Oncoimmunology 2013, 2, e22945. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yin, J.; Shen, W.; Gao, R.; Liu, Y.; Chen, Y.; Li, X.; Liu, C.; Xiang, R.; Luo, N. TLR4 Promotes Breast Cancer Metastasis via Akt/GSK3β/β-Catenin Pathway upon LPS Stimulation. Anat. Rec. 2017, 300, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Nankali, M.; Karimi, J.; Goodarzi, M.T.; Saidijam, M.; Khodadadi, I.; Razavi, A.N.E.; Rahimi, F. Increased Expression of the Receptor for Advanced Glycation End-Products (RAGE) Is Associated with Advanced Breast Cancer Stage. Oncol. Res. Treat. 2016, 39, 622–628. [Google Scholar] [CrossRef]
- Pan, H.; He, L.; Wang, B.; Niu, W. The Relationship between RAGE Gene Four Common Polymorphisms and Breast Cancer Risk in Northeastern Han Chinese. Sci. Rep. 2014, 4, 4355. [Google Scholar] [CrossRef]
- Rodríguez del Águila, M.M.; González-Ramírez, A.R. Sample Size Calculation. Allergol. Immunopathol. 2014, 42, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Woodward, W.A.; Krishnamurthy, S.; Yamauchi, H.; El-Zein, R.; Ogura, D.; Kitadai, E.; Niwa, S.I.; Cristofanilli, M.; Vermeulen, P.; Dirix, L.; et al. Genomic and Expression Analysis of Microdissected Inflammatory Breast Cancer. Breast Cancer Res. Treat. 2013, 138, 761–772. [Google Scholar] [CrossRef]
- Lerebours, F.; Vacher, S.; Guinebretiere, J.M.; Rondeau, S.; Caly, M.; Gentien, D.; Van Laere, S.; Bertucci, F.; de la Grange, P.; Bièche, L.; et al. Hemoglobin Overexpression and Splice Signature as New Features of Inflammatory Breast Cancer? J. Adv. Res. 2021, 28, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Sean, D.; Meltzer, P.S. GEOquery: A Bridge between the Gene Expression Omnibus (GEO) and BioConductor. Bioinformatics 2007, 23, 1846–1847. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma Powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Schwender, H.; Müller, T. Computing thousands of test statistics simultaneously in R. Stat. Comput. Graph. Am. Stat. Assoc. 2007, 18, 5–10. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: Cham, Switzerland, 2016. [Google Scholar][Green Version]
- Amornsupak, K.; Thongchot, S.; Thinyakul, C.; Box, C.; Hedayat, S.; Thuwajit, P.; Eccles, S.A.; Thuwajit, C. HMGB1 Mediates Invasion and PD-L1 Expression through RAGE-PI3K/AKT Signaling Pathway in MDA-MB-231 Breast Cancer Cells. BMC Cancer 2022, 22, 578. [Google Scholar] [CrossRef]
- Sharaf, H.; Matou-Nasri, S.; Wang, Q.; Rabhan, Z.; Al-Eidi, H.; Al Abdulrahman, A.; Ahmed, N. Advanced Glycation Endproducts Increase Proliferation, Migration and Invasion of the Breast Cancer Cell Line MDA-MB-231. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2015, 1852, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Zhang, J.; Wu, X.; Yan, X.; Qin, X.; Wang, Y. Prognostic and Clinicopathological Significance of TLR4 Expression in Patients with Breast Cancer: A Meta-Analysis. Front. Oncol. 2024, 14, 1344130. [Google Scholar] [CrossRef]
- Schairer, C.; Laurent, C.A.; Moy, L.M.; Gierach, G.L.; Caporaso, N.E.; Pfeiffer, R.M.; Kushi, L.H. Obesity and Related Conditions and Risk of Inflammatory Breast Cancer: A Nested Case–Control Study. Breast Cancer Res. Treat. 2020, 183, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, M.; Guo, C.; Koka, H.; Zhu, B.; Deng, J.; Hu, N.; Zhou, B.; Garcia-Closas, M.; Lu, N.; Yang, X.R. Impact of Breast Cancer Risk Factors on Clinically Relevant Prognostic Biomarkers for Primary Breast Cancer. Breast Cancer Res. Treat. 2021, 189, 483–495. [Google Scholar] [CrossRef]
- Atoum, M.F.; Alzoughool, F.; Al-Hourani, H. Linkage Between Obesity Leptin and Breast Cancer. Breast Cancer 2020, 14, 117822341989845. [Google Scholar] [CrossRef]
- Wang, C.-H.; Wang, P.-J.; Hsieh, Y.-C.; Lo, S.; Lee, Y.-C.; Chen, Y.-C.; Tsai, C.-H.; Chiu, W.-C.; Chu-Sung Hu, S.; Lu, C.-W.; et al. Resistin Facilitates Breast Cancer Progression via TLR4-Mediated Induction of Mesenchymal Phenotypes and Stemness Properties. Oncogene 2018, 37, 589–600. [Google Scholar] [CrossRef]
- Muoio, M.G.; Talia, M.; Lappano, R.; Sims, A.H.; Vella, V.; Cirillo, F.; Manzella, L.; Giuliano, M.; Maggiolini, M.; Belfiore, A.; et al. Activation of the S100A7/RAGE Pathway by IGF-1 Contributes to Angiogenesis in Breast Cancer. Cancers 2021, 13, 621. [Google Scholar] [CrossRef]
- Bustreo, S.; Osella-Abate, S.; Cassoni, P.; Donadio, M.; Airoldi, M.; Pedani, F.; Papotti, M.; Sapino, A.; Castellano, I. Optimal Ki67 Cut-off for Luminal Breast Cancer Prognostic Evaluation: A Large Case Series Study with a Long-Term Follow-Up. Breast Cancer Res. Treat. 2016, 157, 363–371. [Google Scholar] [CrossRef]
- Inwald, E.C.; Klinkhammer-Schalke, M.; Hofstädter, F.; Zeman, F.; Koller, M.; Gerstenhauer, M.; Ortmann, O. Ki-67 Is a Prognostic Parameter in Breast Cancer Patients: Results of a Large Population-Based Cohort of a Cancer Registry. Breast Cancer Res. Treat. 2013, 139, 539–552. [Google Scholar] [CrossRef]
- Li, J.; Gonzalez-Angulo, A.M.; Allen, P.K.; Yu, T.K.; Woodward, W.A.; Ueno, N.T.; Lucci, A.; Krishnamurthy, S.; Gong, Y.; Bondy, M.L.; et al. Triple-Negative Subtype Predicts Poor Overall Survival and High Locoregional Relapse in Inflammatory Breast Cancer. Oncologist 2011, 16, 1675–1683. [Google Scholar] [CrossRef] [PubMed]
- Méndez, O.; Pérez, J.; Soberino, J.; Racca, F.; Cortés, J.; Villanueva, J. Clinical Implications of Extracellular HMGA1 in Breast Cancer. Int. J. Mol. Sci. 2019, 20, 5950. [Google Scholar] [CrossRef]
- Muoio, M.G.; Pellegrino, M.; Rapicavoli, V.; Talia, M.; Scavo, G.; Sergi, V.; Vella, V.; Pettinato, S.; Galasso, M.G.; Lappano, R.; et al. RAGE Inhibition Blunts Insulin-Induced Oncogenic Signals in Breast Cancer. Breast Cancer Research 2023, 25, 84. [Google Scholar] [CrossRef] [PubMed]
- Magna, M.; Hwang, G.H.; McIntosh, A.; Drews-Elger, K.; Takabatake, M.; Ikeda, A.; Mera, B.J.; Kwak, T.; Miller, P.; Lippman, M.E.; et al. RAGE Inhibitor TTP488 (Azeliragon) Suppresses Metastasis in Triple-Negative Breast Cancer. NPJ Breast Cancer 2023, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.; Woodward, W.A.; Wang, X.; Reuben, J.M.; Ueno, N.T. Inflammatory Breast Cancer Biology: The Tumour Microenvironment Is Key. Nat. Rev. Cancer 2018, 18, 485–499. [Google Scholar] [CrossRef]
- Rajput, S.; Volk-Draper, L.D.; Ran, S. TLR4 Is a Novel Determinant of the Response to Paclitaxel in Breast Cancer. Mol. Cancer Ther. 2013, 12, 1676–1687. [Google Scholar] [CrossRef]
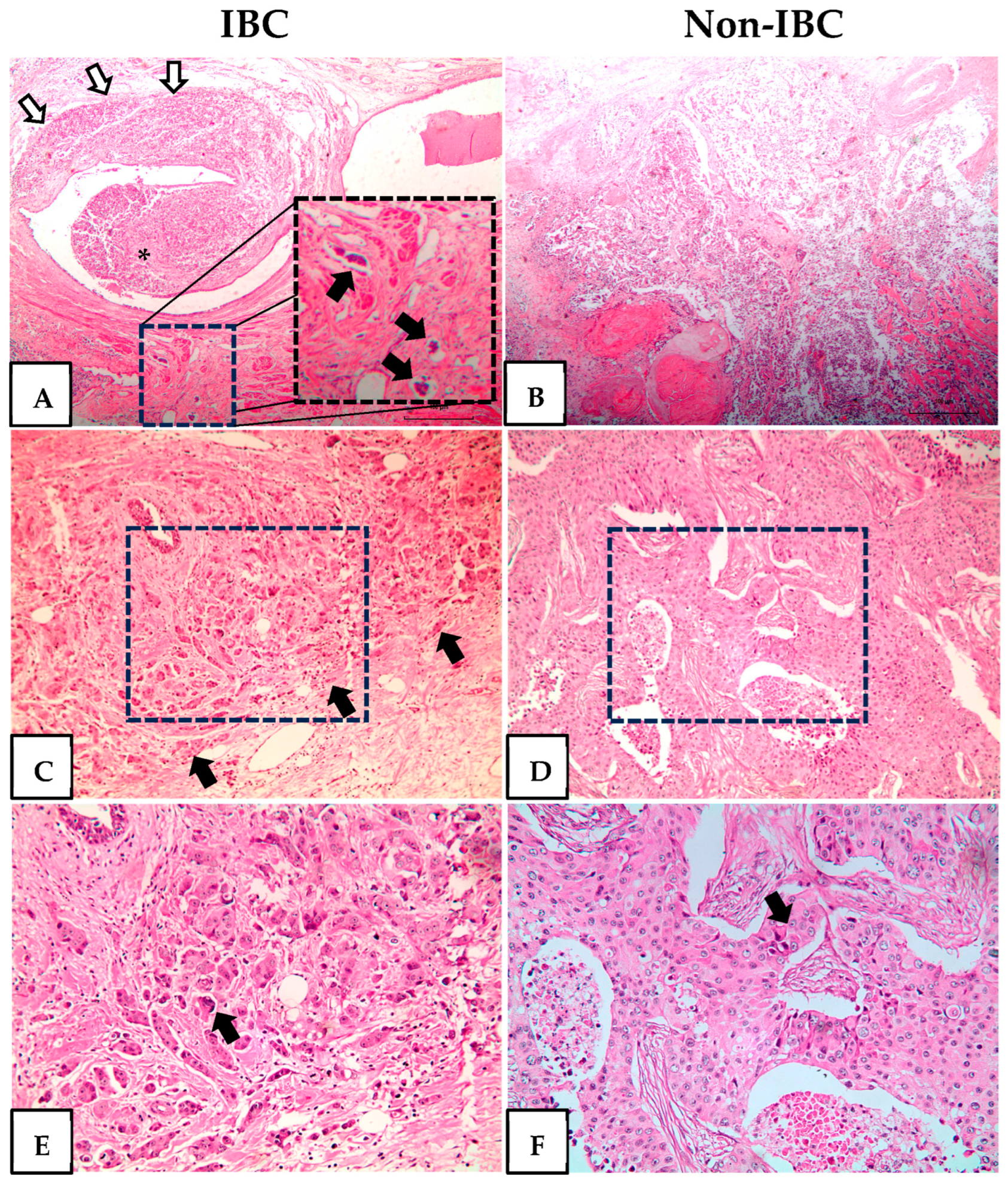
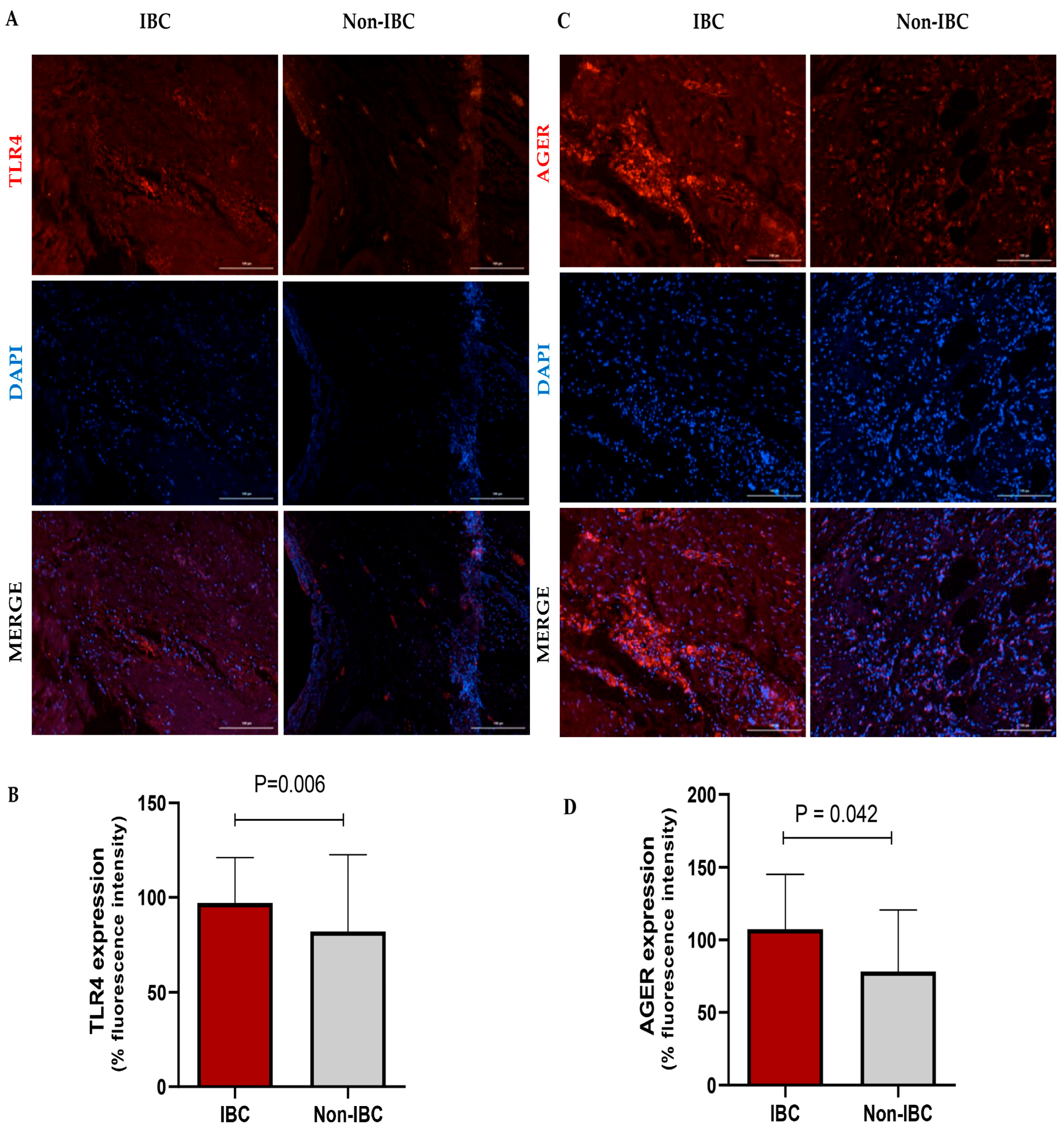
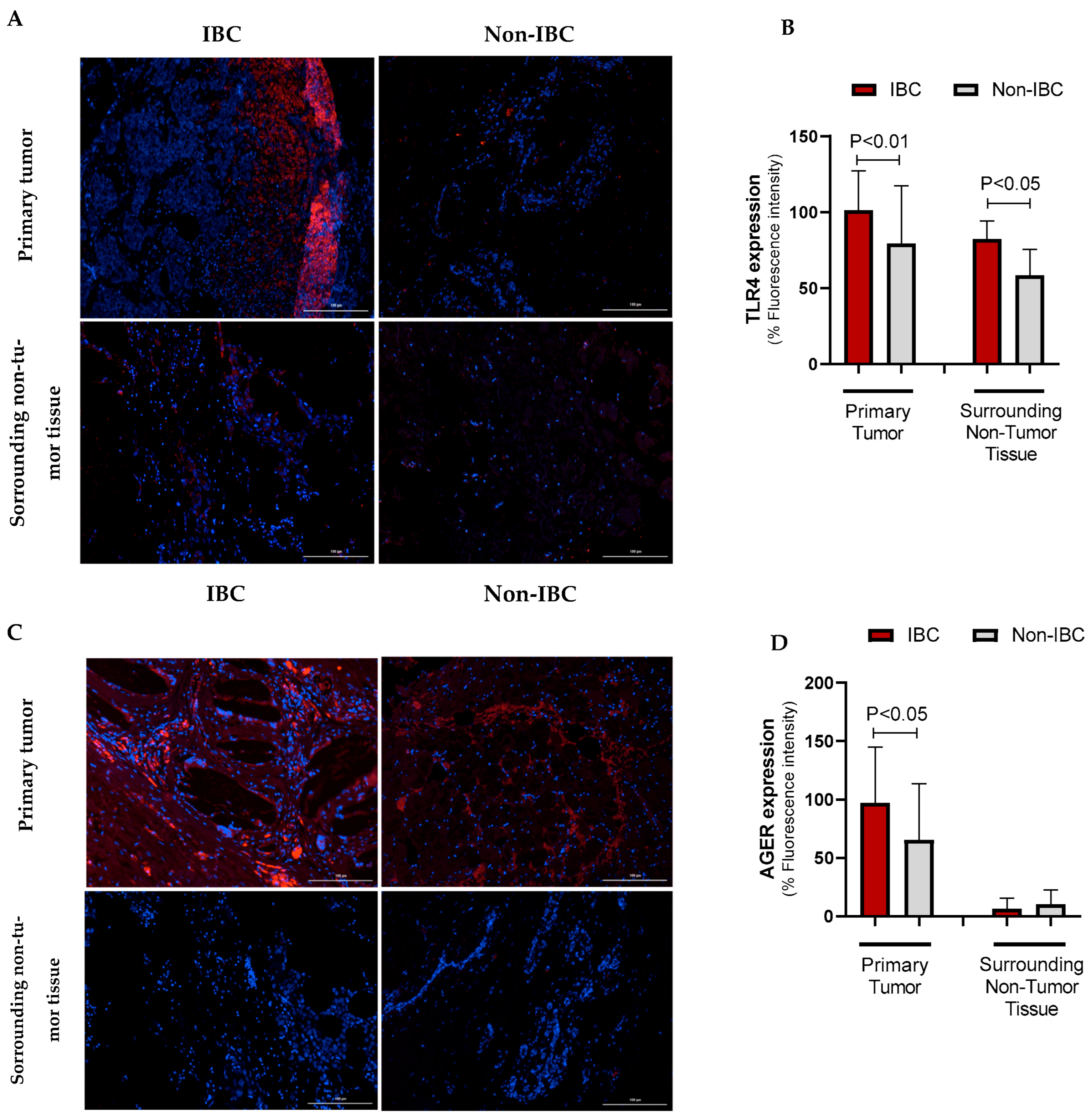
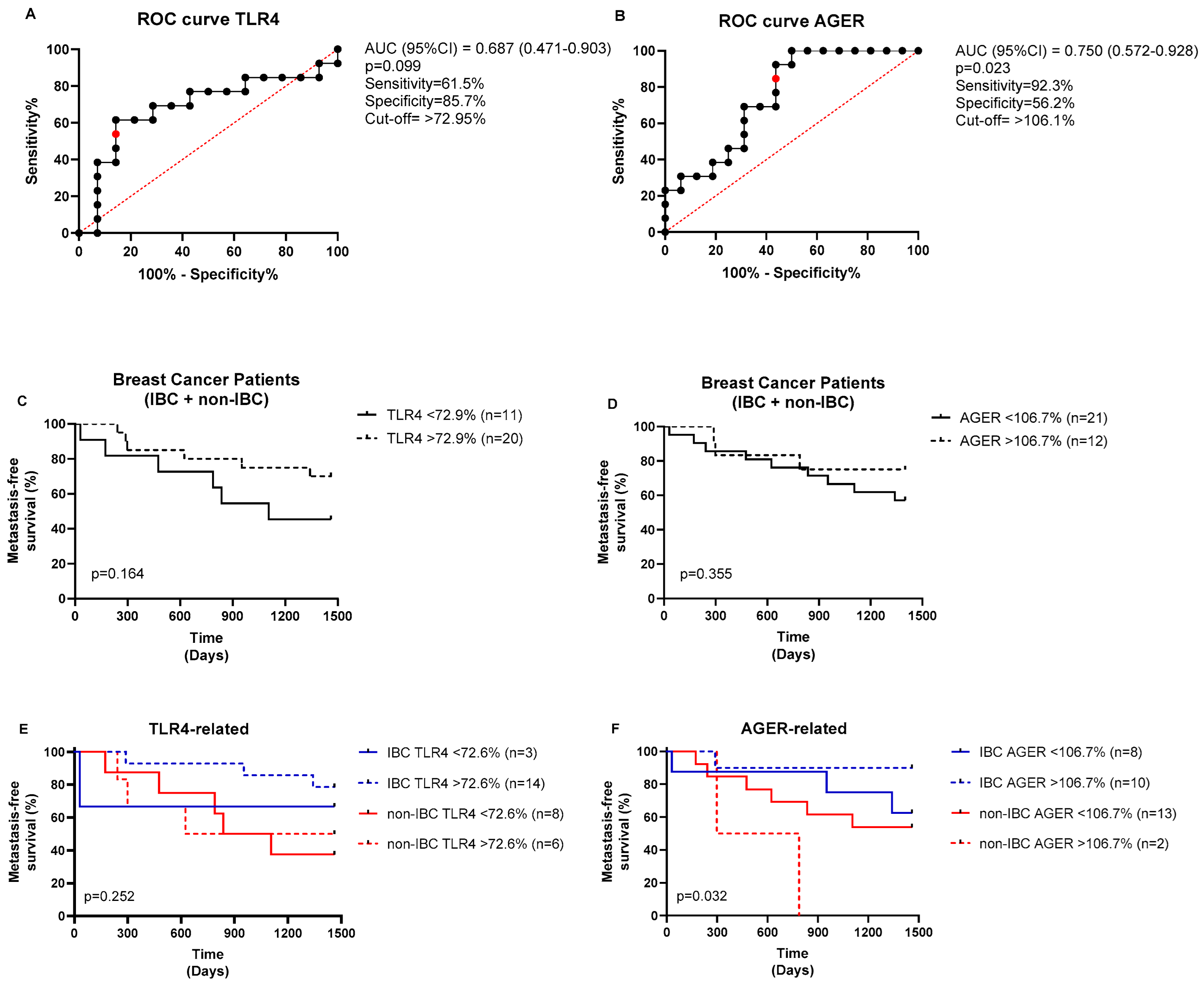
| Parameter | IBC (n = 27) No. (%) | Non-IBC (n = 24) No. (%) | p-Value |
|---|---|---|---|
| Median age in years (range) | 55 (36–83) | 57 (32–91) | 0.996 |
| Family history of cancer | 0.781 | ||
| No | 14 (51.9%) | 11 (45.8%) | |
| Yes | 13 (48.1%) | 13 (54.2%) | |
| Obesity | 0.304 | ||
| No (BMI < 30) | 18 (72.0%) | 18 (85.7%) | |
| Yes (BMI ≥ 30) | 7 (28.0%) | 3 (14.3%) | |
| Lymph node (LN) | 0.444 | ||
| Negative (N0) | 3 (11.1%) | 5 (21.7%) | |
| Positive (N1/N2/N3) | 24 (88.9%) | 18 (78.3%) | |
| Metastasis | 0.577 | ||
| M0 | 14 (51.9%) | 10 (41.7%) | |
| M1 | 13 (48.1%) | 14 (58.3%) | |
| Hormone receptors | |||
| Negative ER | 8 (29.6%) | 7 (29.2%) | 0.999 |
| Positive ER | 19 (70.4%) | 17 (70.8%) | |
| Negative PR | 10 (37.0%) | 8 (33.3%) | 0.999 |
| Positive PR | 17 (63.0%) | 16 (66.7%) | |
| HER2/neu (HER2) | |||
| Negative HER2 | 22 (81.5%) | 19 (79.2%) | 0.999 |
| Positive HER2 | 5 (18.5%) | 5 (20.8%) | |
| Ki-67 | |||
| Ki-67 < 20% | 5 (18.5%) | 7 (30.4%) | 0.507 |
| Ki-67 ≥ 20% | 22 (81.5%) | 16 (69.6%) | |
| TNBC | 0.731 | ||
| No | 21 (77.8%) | 20 (83.3%) | |
| Yes | 6 (22.2%) | 4 (16.7%) |
| Variable | TLR4 Expression (%) | AGER Expression (%) | ||||
|---|---|---|---|---|---|---|
| IBC (n = 17) Mean ± SD | Non-IBC (n = 15) Mean ± SD | p-Value | IBC (n = 18) Mean ± SD | Non-IBC (n = 16) Mean ± SD | p-Value | |
| Age | 0.438 | 0.826 | ||||
| <45 a | 99.7 ± 39.8 | 84.8 ± 16.9 | 83.7 ± 28.7 | 65.1 ± 36.4 | ||
| ≥45 a | 86.7 ± 21.9 | 95.7 ± 58.1 | 112.2 ± 38.4 | 86.4 ± 45.1 | ||
| Family history of cancer | 0.229 | 0.227 | ||||
| No | 85.4 ± 25.5 | 77.5 ± 30.2 | 97.3 ± 35.6 | 84.3 ± 48.0 | ||
| Yes | 94.7 ± 26.9 | 119.0 ± 61.1 | 117.0 ± 40.9 | 68.5 ± 31.4 | ||
| Obesity | 0.107 | 0.054 | ||||
| No (BMI < 30 Kg/m2) | 80.9 ± 16.8 | 98.4 ± 48.3 | 101.5 ± 34.4 | 86.6 ± 40.9 | ||
| Yes (BMI ≥ 30 Kg/m2) | 112.9 ± 23.3 a | 74.0 ± 8.5 | 133.8 ± 33.0 | 8.2 ± 0.0 | ||
| LN status | 0.072 | 0.814 | ||||
| Negative | 74.3 ± 22.3 | 97.4 ± 50.7 | 141.7 ± 0.0 | 97.7 ± 0.0 | ||
| Positive | 97.0 ± 20.2 | 69.9 ± 16.1 | 105.4 ± 38.0 | 75.7 ± 44.6 | ||
| Metastasis | 0.656 | 0.386 | ||||
| M0 | 93.5 ± 32.8 | 97.2 ± 48.9 | 107.4 ± 35.0 | 96.6 ± 8.6 | ||
| M1 | 77.9 ± 12.6 | 96.4 ± 48.3 | 107.5 ± 40.7 | 74.2 ± 45.9 | ||
| Hormone receptor | 0.538 | 0.309 | ||||
| Negative ER | 81.9 ± 23.6 | 72.2 ± 10.4 | 108.4 ± 49.4 | 51.3 ± 36.7 | ||
| Positive ER | 90.0 ± 25.5 | 101.0 ± 53.4 | 110.6 ± 38.0 | 90.7 ± 39.9 | ||
| Negative PR | 84.0 ± 19.8 | 82.9 ± 18.7 | 0.844 | 98.6 ± 44.7 | 76.1 ± 57.4 | 0.705 |
| Positive PR | 91.1 ± 27.1 | 95.6 ± 54.7 | 114.1 ± 37.6 | 80.21 ± 29.2 | ||
| HER2 | 0.110 | 0.890 | ||||
| Negative | 85.3 ± 21.8 | 96.6 ± 49.5 | 111.4 ± 39.6 | 84.4 ± 44.4 | ||
| Positive | 117.1 ± 37.0 | 70.3 ± 12.6 | 92.6 ± 0.0 | 60.3 ± 32.4 | ||
| Ki-67 | 0.002 * | 0.049 * | ||||
| Ki67 < 20% | 86.7 ± 35.1 | 138.2 ± 51.7 | 95.0 ± 33.2 | 96.7 ± 47.4 | ||
| Ki67 ≥ 20% | 89.7 ± 22.5 | 67.9 ± 14.3 b | 121.1 ± 36.5 | 64.2 ± 33.5 | ||
| TNBC | 0.923 | 0.045 * | ||||
| No | 92.9 ± 23.8 | 93.3 ± 48.4 | 115.5 ± 35.3 | 87.7 ± 40.3 | ||
| Yes | 71.0 ± 25.2 | 74.9 ± 9.8 | 136.8 ± 6.8 | 33.6 ± 35.9 | ||
| Variable | p-Value | HR | CI95% | |
|---|---|---|---|---|
| Age (<50) | 0.151 | 0.115 | 0.006 | 2.207 |
| Radical mastectomy | 0.022 * | 0.002 | 0.006 | 0.392 |
| Group (IBC vs non-IBC) | 0.062 | 12.803 | 0.787 | 20.833 |
| Histologic grade (III) | 0.101 | 0.164 | 0.019 | 1.421 |
| Clinical Stage | 0.582 | 1.879 | 0.199 | 17.764 |
| T | 0.406 | 1.675 | 0.496 | 5.656 |
| N | 0.377 | 0.632 | 0.229 | 1.747 |
| M | 0.306 | 5.948 | 0.195 | 181.135 |
| Immunohistochemistry profile | 0.071 | 3.578 | 0.896 | 14.286 |
| BMI | 0.101 | 2.123 | 0.864 | 5.218 |
| TLR4 (high) | 0.043 * | 1.029 | 1.010 | 1.064 |
| AGER (high) | 0.598 | 0.992 | 0.965 | 1.021 |
| Best response to neoadjuvant chemotherapy | 0.341 | 4.839 | 0.188 | 124.617 |
| Neoadjvant chemotherapy | 0.052 | 0.045 | 0.002 | 1029 |
| Adjuvant chemotherapy | 0.652 | 0.346 | 0.003 | 34.970 |
| Hormone therapy | 0.025 * | 0.034 | 0.002 | 0.650 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paiva, L.D.A.S.; Teles, A.C.F.; Souza, J.d.S.; Oliveira, P.R.A.; Alves, B.E.S.; Coelho, M.T.B.; Cajado, A.G.; Maia, I.F.V.C.; Silva, P.G.B.; Cavalcante, D.I.M.; et al. Clinical Significance and Prognostic Value of TLR4 and AGER in Inflammatory Breast Cancer. Cancers 2025, 17, 2182. https://doi.org/10.3390/cancers17132182
Paiva LDAS, Teles ACF, Souza JdS, Oliveira PRA, Alves BES, Coelho MTB, Cajado AG, Maia IFVC, Silva PGB, Cavalcante DIM, et al. Clinical Significance and Prognostic Value of TLR4 and AGER in Inflammatory Breast Cancer. Cancers. 2025; 17(13):2182. https://doi.org/10.3390/cancers17132182
Chicago/Turabian StylePaiva, Luiza Darla Aguiar Silva, Ana Carolina Filgueiras Teles, Jeferson dos Santos Souza, Pedro Ruan Amorim Oliveira, Bianca Elen Souza Alves, Mariana Timbaúba Benício Coelho, Aurilene Gomes Cajado, Isabelle Fátima Vieira Camelo Maia, Paulo Goberlânio Barros Silva, Diane Isabelle Magno Cavalcante, and et al. 2025. "Clinical Significance and Prognostic Value of TLR4 and AGER in Inflammatory Breast Cancer" Cancers 17, no. 13: 2182. https://doi.org/10.3390/cancers17132182
APA StylePaiva, L. D. A. S., Teles, A. C. F., Souza, J. d. S., Oliveira, P. R. A., Alves, B. E. S., Coelho, M. T. B., Cajado, A. G., Maia, I. F. V. C., Silva, P. G. B., Cavalcante, D. I. M., Cunha, M. d. P. S. S., Arruda, L. M., Lima-Júnior, R. C. P., Rogatto, S. R., & Wong, D. V. T. (2025). Clinical Significance and Prognostic Value of TLR4 and AGER in Inflammatory Breast Cancer. Cancers, 17(13), 2182. https://doi.org/10.3390/cancers17132182










