Exploring the Potential of a Deep Learning Model for Early CT Detection of High-Grade Metastatic Epidural Spinal Cord Compression and Its Impact on Treatment Delays
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval and Study Design
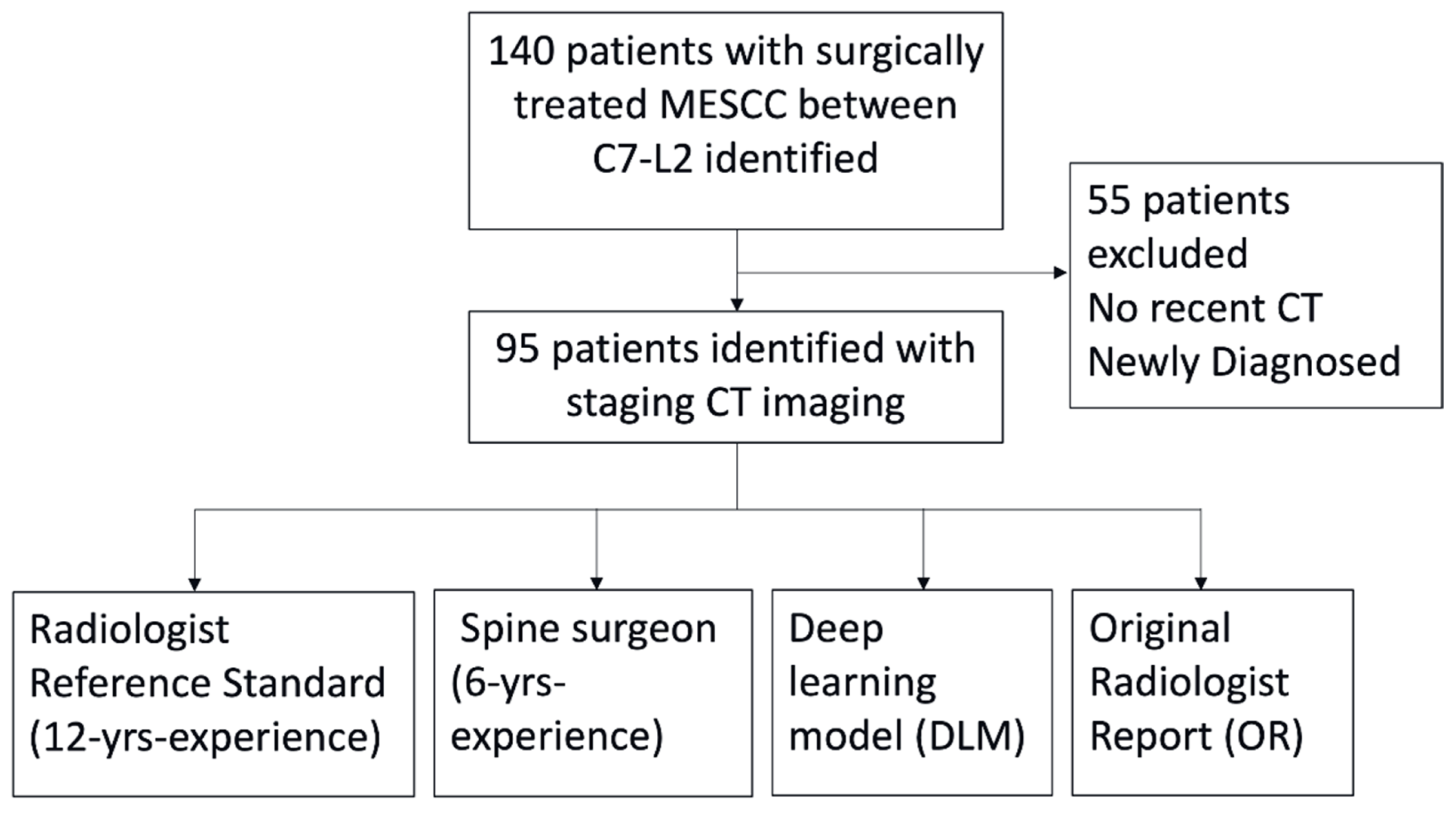
2.2. Patient Selection and Inclusion Criteria
2.3. Imaging Evaluation
2.4. Clinical Data Collection
2.5. Deep Learning Model Development
2.6. Statistical Analysis
3. Results
3.1. Patient Demographics
3.2. Imaging Evaluation for MESCC
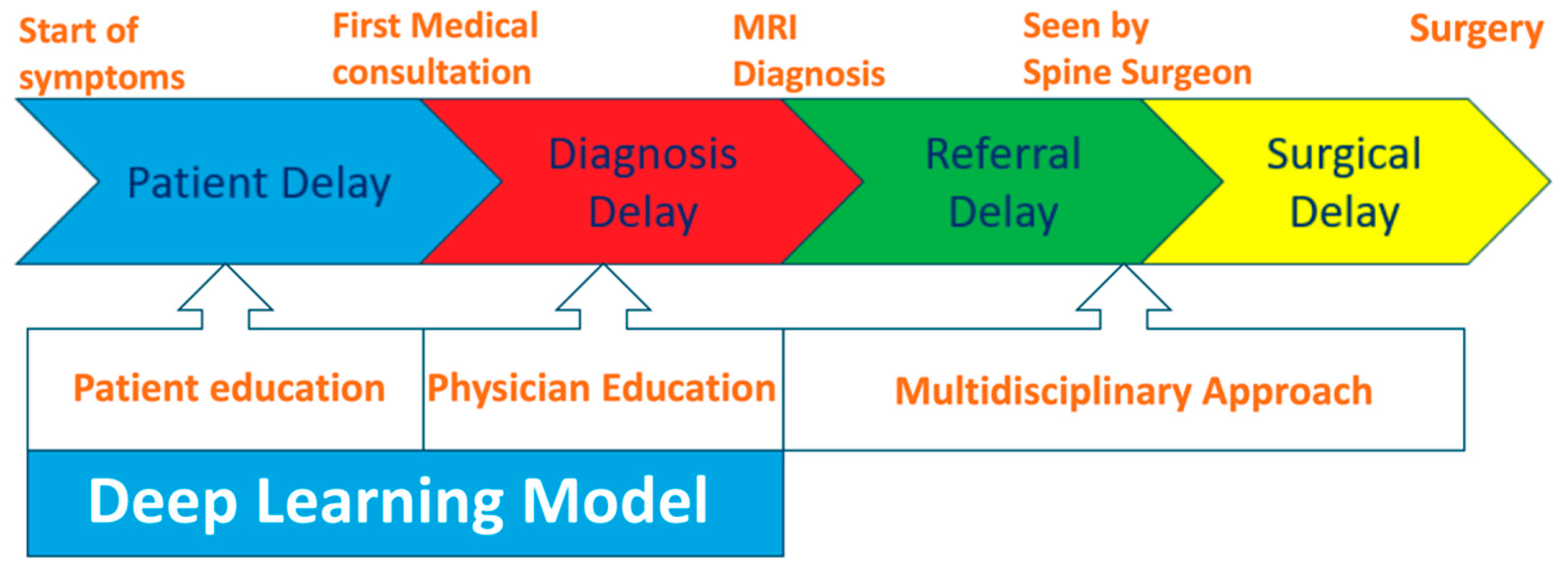
3.3. Delays in MESCC Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CI | Confidence interval |
| CT | Computed tomography |
| DICOM | Digital imaging and communications in medicine |
| DL | Deep learning |
| DLM | Deep learning model |
| ECOG | Eastern Cooperative Oncology Group |
| MESCC | Metastatic epidural spinal cord compression |
| MRI | Magnetic resonance imaging |
| OR | Original radiologist |
| PACS | Picture archiving and communication systems |
| SD | Standard deviation |
| SORG | Skeletal Oncology Research Group |
References
- Cole, J.S.; Patchell, R.A. Metastatic epidural spinal cord compression. Lancet Neurol. 2008, 7, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Bach, F.; Larsen, B.H.; Rohde, K.; Børgesen, S.E.; Gjerris, F.; Bøge-Rasmussen, T.; Agerlin, N.; Rasmusson, B.; Stjernholm, P.; Sørensen, P.S. Metastatic spinal cord compression. Occurrence, symptoms, clinical presentations and prognosis in 398 patients with spinal cord compression. Acta Neurochir. 1990, 107, 37–43. [Google Scholar] [CrossRef]
- Loblaw, D.A.; Laperriere, N.J.; Mackillop, W.J. A population-based study of malignant spinal cord compression in Ontario. Clin. Oncol. 2003, 15, 211–217. [Google Scholar] [CrossRef]
- van Tol, F.R.; Choi, D.; Verkooijen, H.M.; Oner, F.C.; Verlaan, J.J. Delayed presentation to a spine surgeon is the strongest predictor of poor postoperative outcome in patients surgically treated for symptomatic spinal metastases. Spine J. Off. J. N. Am. Spine Soc. 2019, 19, 1540–1547. [Google Scholar] [CrossRef]
- van Tol, F.R.; Suijkerbuijk, K.P.M.; Choi, D.; Verkooijen, H.M.; Oner, F.C.; Verlaan, J.J. The importance of timely treatment for quality of life and survival in patients with symptomatic spinal metastases. Eur. Spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 2020, 29, 3170–3178. [Google Scholar] [CrossRef] [PubMed]
- Patchell, R.A.; Tibbs, P.A.; Regine, W.F.; Payne, R.; Saris, S.; Kryscio, R.J.; Mohiuddin, M.; Young, B. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: A randomised trial. Lancet 2005, 366, 643–648. [Google Scholar] [CrossRef]
- Rades, D.; Huttenlocher, S.; Bajrovic, A.; Karstens, J.H.; Adamietz, I.A.; Kazic, N.; Rudat, V.; Schild, S.E. Surgery followed by radiotherapy versus radiotherapy alone for metastatic spinal cord compression from unfavorable tumors. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, e861–e868. [Google Scholar] [CrossRef]
- Chen, B.; Xiao, S.; Tong, X.; Xu, S.; Lin, X. Comparison of the Therapeutic Efficacy of Surgery with or without Adjuvant Radiotherapy versus Radiotherapy Alone for Metastatic Spinal Cord Compression: A Meta-Analysis. World Neurosurg. 2015, 83, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Weber-Levine, C.; Jiang, K.; Al-Mistarehi, A.-H.; Welland, J.; Hersh, A.M.; Horowitz, M.A.; Davidar, A.D.; Sattari, S.A.; Redmond, K.J.; Lee, S.H.; et al. The role of combination surgery and radiotherapy in patients with metastatic spinal cord compression: What are the remaining grey areas? A systematic review. Clin. Neurol. Neurosurg. 2025, 248, 108632. [Google Scholar] [CrossRef]
- Amelink, J.J.G.J.; Bindels, B.J.J.; Kasperts, N.; MacDonald, S.M.; Tobert, D.G.; Verlaan, J.-J. Radiotherapy and surgery: Can this combination be further optimized for patients with metastatic spine disease? Oncologist 2025, 30, oyae359. [Google Scholar] [CrossRef]
- Rades, D.; Küchler, J.; Graumüller, L.; Abusamha, A.; Schild, S.E.; Gliemroth, J. Radiotherapy with or without Decompressive Surgery for Metastatic Spinal Cord Compression: A Retrospective Matched-Pair Study Including Data from Prospectively Evaluated Patients. Cancers 2022, 14, 1260. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Kwon, J.W.; Lee, J.; Hyun, S.J.; Kim, K.J.; Jahng, T.A.; Kim, H.J. Direct decompressive surgery followed by radiotherapy versus radiotherapy alone for metastatic epidural spinal cord compression: A meta-analysis. Spine 2014, 39, E587–E592. [Google Scholar] [CrossRef]
- Di Perna, G.; Cofano, F.; Mantovani, C.; Badellino, S.; Marengo, N.; Ajello, M.; Comite, L.M.; Palmieri, G.; Tartara, F.; Zenga, F.; et al. Separation surgery for metastatic epidural spinal cord compression: A qualitative review. J. Bone Oncol. 2020, 25, 100320. [Google Scholar] [CrossRef] [PubMed]
- Barzilai, O.; Boriani, S.; Fisher, C.G.; Sahgal, A.; Verlaan, J.J.; Gokaslan, Z.L.; Lazary, A.; Bettegowda, C.; Rhines, L.D.; Laufer, I. Essential Concepts for the Management of Metastatic Spine Disease: What the Surgeon Should Know and Practice. Glob. Spine J. 2019, 9 (Suppl. S1), 98s–107s. [Google Scholar] [CrossRef]
- Tan, J.H.J.; Hallinan, J.; Lee, R.; Chan, Y.H.; Tan, T.H.; Ang, S.W.; Tan, L.T.I.; Tan, J.H.I.; Sin, Q.S.; Hey, D.H.W.; et al. Trends in surgical management of spinal metastases in a Singaporean tertiary referral center: A 17-year retrospective review. Front. Oncol. 2023, 13, 1297553. [Google Scholar] [CrossRef] [PubMed]
- Helweg-Larsen, S.; Sørensen, P.S.; Kreiner, S. Prognostic factors in metastatic spinal cord compression: A prospective study using multivariate analysis of variables influencing survival and gait function in 153 patients. Int. J. Radiat. Oncol. Biol. Phys. 2000, 46, 1163–1169. [Google Scholar] [CrossRef]
- Leviov, M.; Dale, J.; Stein, M.; Ben-Shahar, M.; Ben-Arush, M.; Milstein, D.; Goldsher, D.; Kuten, A. The management of metastatic spinal cord compression: A radiotherapeutic success ceiling. Int. J. Radiat. Oncol. Biol. Phys. 1993, 27, 231–234. [Google Scholar] [CrossRef]
- Maranzano, E.; Latini, P. Effectiveness of radiation therapy without surgery in metastatic spinal cord compression: Final results from a prospective trial. Int. J. Radiat. Oncol. Biol. Phys. 1995, 32, 959–967. [Google Scholar] [CrossRef]
- Loblaw, D.A.; Perry, J.; Chambers, A.; Laperriere, N.J. Systematic review of the diagnosis and management of malignant extradural spinal cord compression: The Cancer Care Ontario Practice Guidelines Initiative’s Neuro-Oncology Disease Site Group. J. Clin. Oncol. 2005, 23, 2028–2037. [Google Scholar] [CrossRef]
- Quraishi, N.A.; Rajagopal, T.S.; Manoharan, S.R.; Elsayed, S.; Edwards, K.L.; Boszczyk, B.M. Effect of timing of surgery on neurological outcome and survival in metastatic spinal cord compression. Eur. Spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 2013, 22, 1383–1388. [Google Scholar] [CrossRef]
- Bilsky, M.H.; Laufer, I.; Fourney, D.R.; Groff, M.; Schmidt, M.H.; Varga, P.P.; Vrionis, F.D.; Yamada, Y.; Gerszten, P.C.; Kuklo, T.R. Reliability analysis of the epidural spinal cord compression scale. J. Neurosurg. Spine 2010, 13, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Kuah, T.; Vellayappan, B.A.; Makmur, A.; Nair, S.; Song, J.; Tan, J.H.; Kumar, N.; Quek, S.T.; Hallinan, J. State-of-the-Art Imaging Techniques in Metastatic Spinal Cord Compression. Cancers 2022, 14, 3289. [Google Scholar] [CrossRef] [PubMed]
- Hallinan, J.T.P.D.; Ge, S.; Zhu, L.; Zhang, W.; Lim, Y.T.; Thian, Y.L.; Jagmohan, P.; Kuah, T.; Lim, D.S.W.; Low, X.Z.; et al. Diagnostic Accuracy of CT for Metastatic Epidural Spinal Cord Compression. Cancers 2022, 14, 4231. [Google Scholar] [CrossRef]
- Husain, Z.A.; Sahgal, A.; De Salles, A.; Funaro, M.; Glover, J.; Hayashi, M.; Hiraoka, M.; Levivier, M.; Ma, L.; Martínez-Alvarez, R.; et al. Stereotactic body radiotherapy for de novo spinal metastases: Systematic review. J. Neurosurg. Spine 2017, 27, 295–302. [Google Scholar] [CrossRef]
- Pezaro, C.; Omlin, A.; Perez-Lopez, R.; Mukherji, D.; Attard, G.; Bianchini, D.; Lorente, D.; Parker, C.; Dearnaley, D.; de Bono, J.S.; et al. Progressive computed tomography (CT) appearances preceding malignant spinal cord compression (MSCC) in men with castration-resistant prostate cancer. Clin. Radiol. 2015, 70, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.; Zhu, L.; Zhang, W.; Kuah, T.; Lim, D.S.W.; Low, X.Z.; Thian, Y.L.; Teo, E.C.; Tan, J.H.; Kumar, N.; et al. Application of Artificial Intelligence Methods for Imaging of Spinal Metastasis. Cancers 2022, 14, 4025. [Google Scholar] [CrossRef]
- Motohashi, M.; Funauchi, Y.; Adachi, T.; Fujioka, T.; Otaka, N.; Kamiko, Y.; Okada, T.; Tateishi, U.; Okawa, A.; Yoshii, T.; et al. A New Deep Learning Algorithm for Detecting Spinal Metastases on Computed Tomography Images. Spine 2024, 49, 390–397. [Google Scholar] [CrossRef]
- Ather, S.; Windson, R.; Singh, S.; Chowdhury, R.; Jamaluddin, A.; Fairbank, J. A novel deep-learning architecture to detect spinal metastases, vertebral fractures and cord compression using information extracted from radiological reports. Clin. Radiol. 2022, 77, e7–e8. [Google Scholar] [CrossRef]
- Hallinan, J.; Zhu, L.; Zhang, W.; Lim, D.S.W.; Baskar, S.; Low, X.Z.; Yeong, K.Y.; Teo, E.C.; Kumarakulasinghe, N.B.; Yap, Q.V.; et al. Deep Learning Model for Classifying Metastatic Epidural Spinal Cord Compression on MRI. Front. Oncol. 2022, 12, 849447. [Google Scholar] [CrossRef]
- Wang, D.; Sun, Y.; Tang, X.; Liu, C.; Liu, R. Deep learning-based magnetic resonance imaging of the spine in the diagnosis and physiological evaluation of spinal metastases. J. Bone Oncol. 2023, 40, 100483. [Google Scholar] [CrossRef]
- Lang, N.; Zhang, Y.; Zhang, E.; Zhang, J.; Chow, D.; Chang, P.; Yu, H.J.; Yuan, H.; Su, M.Y. Differentiation of spinal metastases originated from lung and other cancers using radiomics and deep learning based on DCE-MRI. Magn. Reson. Imaging 2019, 64, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Cao, G.; Hua, Y.; Hu, J.; Zheng, Y.; Wu, F.; Xu, S.; Rong, T.; Liu, B. Identification of Origin for Spinal Metastases from MR Images: Comparison Between Radiomics and Deep Learning Methods. World Neurosurg. 2023, 175, e823–e831. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fang, Z.; Lang, N.; Yuan, H.; Su, M.-Y.; Baldi, P. A multi-resolution approach for spinal metastasis detection using deep Siamese neural networks. Comput. Biol. Med. 2017, 84, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Hallinan, J.; Zhu, L.; Zhang, W.; Kuah, T.; Lim, D.S.W.; Low, X.Z.; Cheng, A.J.L.; Eide, S.E.; Ong, H.Y.; Muhamat Nor, F.E.; et al. Deep Learning Model for Grading Metastatic Epidural Spinal Cord Compression on Staging CT. Cancers 2022, 14, 3219. [Google Scholar] [CrossRef]
- Duan, S.; Weijie, D.; Yichun, H.; Yali, Z.; Zengsuonan, R.; Guanmei, C.; Fangfang, W.; Tianhua, R.; Liu, B. Accurate Differentiation of Spinal Tuberculosis and Spinal Metastases Using MR-Based Deep Learning Algorithms. Infect. Drug Resist. 2023, 16, 4325–4334. [Google Scholar] [CrossRef]
- Xiong, X.; Wang, J.; Hu, S.; Dai, Y.; Zhang, Y.; Hu, C. Differentiating Between Multiple Myeloma and Metastasis Subtypes of Lumbar Vertebra Lesions Using Machine Learning–Based Radiomics. Front. Oncol. 2021, 11, 601699. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, X.; Zhang, Z.; Jiang, Y. Deep Learning on MRI Images for Diagnosis of Lung Cancer Spinal Bone Metastasis. Contrast Media Mol. Imaging 2021, 2021, 5294379. [Google Scholar] [CrossRef]
- Hallinan, J.; Zhu, L.; Tan, H.W.N.; Hui, S.J.; Lim, X.; Ong, B.W.L.; Ong, H.Y.; Eide, S.E.; Cheng, A.J.L.; Ge, S.; et al. A deep learning-based technique for the diagnosis of epidural spinal cord compression on thoracolumbar CT. Eur. Spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 2023, 32, 3815–3824. [Google Scholar] [CrossRef]
- Haim, O.; Agur, A.; Gabay, S.; Azolai, L.; Shutan, I.; Chitayat, M.; Katirai, M.; Sadon, S.; Artzi, M.; Lidar, Z. Differentiating spinal pathologies by deep learning approach. Spine J. 2024, 24, 297–303. [Google Scholar] [CrossRef]
- Liu, K.; Qin, S.; Ning, J.; Xin, P.; Wang, Q.; Chen, Y.; Zhao, W.; Zhang, E.; Lang, N. Prediction of Primary Tumor Sites in Spinal Metastases Using a ResNet-50 Convolutional Neural Network Based on MRI. Cancers 2023, 15, 2974. [Google Scholar] [CrossRef]
- Roth, H.R.; Yao, J.; Lu, L.; Stieger, J.; Burns, J.E.; Summers, R.M. Detection of Sclerotic Spine Metastases via Random Aggregation of Deep Convolutional Neural Network Classifications. In Recent Advances in Computational Methods and Clinical Applications for Spine Imaging; Yao, J., Glocker, B., Klinder, T., Li, S., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 3–12. [Google Scholar]
- Noguchi, S.; Nishio, M.; Sakamoto, R.; Yakami, M.; Fujimoto, K.; Emoto, Y.; Kubo, T.; Iizuka, Y.; Nakagomi, K.; Miyasa, K.; et al. Deep learning–based algorithm improved radiologists’ performance in bone metastases detection on CT. Eur. Radiol. 2022, 32, 7976–7987. [Google Scholar] [CrossRef] [PubMed]
- Hallinan, J.; Zhu, L.; Zhang, W.; Ge, S.; Muhamat Nor, F.E.; Ong, H.Y.; Eide, S.E.; Cheng, A.J.L.; Kuah, T.; Lim, D.S.W.; et al. Deep learning assessment compared to radiologist reporting for metastatic spinal cord compression on CT. Front. Oncol. 2023, 13, 1151073. [Google Scholar] [CrossRef]
- van Tol, F.R.; Versteeg, A.L.; Verkooijen, H.M.; Öner, F.C.; Verlaan, J.J. Time to Surgical Treatment for Metastatic Spinal Disease: Identification of Delay Intervals. Glob. Spine J. 2023, 13, 316–323. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Hoskin, P.J.; Grover, A.; Bhana, R. Metastatic spinal cord compression: Radiotherapy outcome and dose fractionation. Radiother. Oncol. 2003, 68, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Levack, P.; Graham, J.; Collie, D.; Grant, R.; Kidd, J.; Kunkler, I.; Gibson, A.; Hurman, D.; McMillan, N.; Rampling, R.; et al. Don’t wait for a sensory level--listen to the symptoms: A prospective audit of the delays in diagnosis of malignant cord compression. Clin. Oncol. 2002, 14, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Husband, D.J. Malignant spinal cord compression: Prospective study of delays in referral and treatment. BMJ 1998, 317, 18–21. [Google Scholar] [CrossRef]
- Guzik, G. Analysis of factors delaying the surgical treatment of patients with neurological deficits in the course of spinal metastatic disease. BMC Palliat. Care 2018, 17, 44. [Google Scholar] [CrossRef] [PubMed]
- Rades, D.; Abrahm, J.L. The role of radiotherapy for metastatic epidural spinal cord compression. Nat. Rev. Clin. Oncol. 2010, 7, 590–598. [Google Scholar] [CrossRef]
- Rades, D.; Hansen, O.; Jensen, L.H.; Dziggel, L.; Staackmann, C.; Doemer, C.; Cacicedo, J.; Conde-Moreno, A.J.; Segedin, B.; Ciervide-Jurio, R.; et al. Radiotherapy for metastatic spinal cord compression with increased radiation doses (RAMSES-01): A prospective multicenter study. BMC Cancer 2019, 19, 1163. [Google Scholar] [CrossRef]
- Crocker, M.; Anthantharanjit, R.; Jones, T.L.; Shoeb, M.; Joshi, Y.; Papadopoulos, M.C.; Bell, B.A.; Rich, P. An extended role for CT in the emergency diagnosis of malignant spinal cord compression. Clin. Radiol. 2011, 66, 922–927. [Google Scholar] [CrossRef]
- Kim, L.; Narayanan, D.; Liu, J.; Pattanayak, P.; Turkbey, E.; Shen, T.C.; Linehan, W.M.; Pinto, P.A.; Summers, R.M. Radiologic reporting of MRI-proven thoracolumbar epidural metastases on body CT: 12-Year single-institution experience. Clin. Imaging 2023, 102, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.S.W.; Makmur, A.; Zhu, L.; Zhang, W.; Cheng, A.J.L.; Sia, D.S.Y.; Eide, S.E.; Ong, H.Y.; Jagmohan, P.; Tan, W.C.; et al. Improved Productivity Using Deep Learning-assisted Reporting for Lumbar Spine MRI. Radiology 2022, 305, 160–166. [Google Scholar] [CrossRef]
- Bennani, S.; Regnard, N.E.; Ventre, J.; Lassalle, L.; Nguyen, T.; Ducarouge, A.; Dargent, L.; Guillo, E.; Gouhier, E.; Zaimi, S.H.; et al. Using AI to Improve Radiologist Performance in Detection of Abnormalities on Chest Radiographs. Radiology 2023, 309, e230860. [Google Scholar] [CrossRef] [PubMed]
- Seah, J.C.Y.; Tang, C.H.M.; Buchlak, Q.D.; Holt, X.G.; Wardman, J.B.; Aimoldin, A.; Esmaili, N.; Ahmad, H.; Pham, H.; Lambert, J.F.; et al. Effect of a comprehensive deep-learning model on the accuracy of chest x-ray interpretation by radiologists: A retrospective, multireader multicase study. Lancet Digit. Health 2021, 3, e496–e506. [Google Scholar] [CrossRef] [PubMed]
- Miki, S.; Hayashi, N.; Masutani, Y.; Nomura, Y.; Yoshikawa, T.; Hanaoka, S.; Nemoto, M.; Ohtomo, K. Computer-Assisted Detection of Cerebral Aneurysms in MR Angiography in a Routine Image-Reading Environment: Effects on Diagnosis by Radiologists. Am. J. Neuroradiol. 2016, 37, 1038. [Google Scholar] [CrossRef]
- Najjar, R. Redefining Radiology: A Review of Artificial Intelligence Integration in Medical Imaging. Diagnostics 2023, 13, 2760. [Google Scholar] [CrossRef]
- Eadie, L.H.; Taylor, P.; Gibson, A.P. A systematic review of computer-assisted diagnosis in diagnostic cancer imaging. Eur. J. Radiol. 2012, 81, e70–e76. [Google Scholar] [CrossRef]
- Aggarwal, R.; Sounderajah, V.; Martin, G.; Ting, D.S.W.; Karthikesalingam, A.; King, D.; Ashrafian, H.; Darzi, A. Diagnostic accuracy of deep learning in medical imaging: A systematic review and meta-analysis. Npj Digit. Med. 2021, 4, 65. [Google Scholar] [CrossRef]
- Maghami, M.; Sattari, S.A.; Tahmasbi, M.; Panahi, P.; Mozafari, J.; Shirbandi, K. Diagnostic test accuracy of machine learning algorithms for the detection intracranial hemorrhage: A systematic review and meta-analysis study. Biomed. Eng. Online 2023, 22, 114. [Google Scholar] [CrossRef]
- Arbabshirani, M.R.; Fornwalt, B.K.; Mongelluzzo, G.J.; Suever, J.D.; Geise, B.D.; Patel, A.A.; Moore, G.J. Advanced machine learning in action: Identification of intracranial hemorrhage on computed tomography scans of the head with clinical workflow integration. NPJ Digit. Med. 2018, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, R.; Javaid, S. Deep Learning for Pneumonia Detection in Chest X-ray Images: A Comprehensive Survey. J. Imaging 2024, 10, 176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhao, G.; Liu, Y.; Huang, Y.; Long, J.; Li, N.; Yan, H.; Zhang, X.; Ma, J.; Zhang, Y. Clinic, CT radiomics, and deep learning combined model for the prediction of invasive pulmonary aspergillosis. BMC Med. Imaging 2024, 24, 264. [Google Scholar] [CrossRef] [PubMed]
- Ganaie, M.A.; Hu, M.; Malik, A.K.; Tanveer, M.; Suganthan, P.N. Ensemble deep learning: A review. Eng. Appl. Artif. Intell. 2022, 115, 105151. [Google Scholar] [CrossRef]
- Ooi, B.C.; Tan, K.-L.; Wang, S.; Wang, W.; Cai, Q.; Chen, G.; Gao, J.; Luo, Z.; Tung, A.K.H.; Wang, Y.; et al. SINGA: A Distributed Deep Learning Platform. In Proceedings of the 23rd ACM International Conference on Multimedia, Brisbane, Australia, 26–30 October 2015; Association for Computing Machinery: New York, NY, USA, 2015; pp. 685–688. [Google Scholar]
- Luo, Z.; Yeung, S.H.; Zhang, M.; Zheng, K.; Zhu, L.; Chen, G.; Fan, F.; Lin, Q.; Ngiam, K.Y.; Ooi, B.C. MLCask: Efficient Management of Component Evolution in Collaborative Data Analytics Pipelines. In Proceedings of the 2021 IEEE 37th International Conference on Data Engineering (ICDE), Chania, Greece, 19–22 April 2021. [Google Scholar]

| Characteristic | Included Patients (n = 95) |
| Age (years), mean ± SD (range) | 63 ± 9 (40–86) |
| Sex, n (%) | |
| Male | 47 (49.5%) |
| Female | 48 (50.5%) |
| Ethnicity, n (%) | |
| Chinese | 73 (76.8%) |
| Malay | 16 (16.8%) |
| Indian | 6 (6.4%) |
| Preoperative ECOG score, n (%) | |
| 0–2 | 75 (78.9%) |
| 3–4 | 20 (21.1%) |
| Charlson Comorbidity Index, mean ± SD (range) | 8 ± 2 (0–12) |
| Tumor subtype (SORG classification), n (%) | |
| Slow growth | 26 (27.4%) |
| Moderate growth | 34 (35.8%) |
| Rapid growth | 35 (36.8%) |
| Diagnosis, n (%) | |
| Known cancer | 70 (73.7%) |
| New diagnosis | 25 (26.3%) |
| Preoperative neurological status (Frankel), n (%) | |
| A | 5 (5.3%) |
| B or C | 50 (52.6%) |
| D or E | 40 (42.1%) |
| Readers * | Kappa (95% CI) | p-Value | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|
| Spine surgeon (J.H.J.T.) | 0.947 (0.893–1.000) | <0.001 | 97.62 (91.66–99.71) | 81.82 (48.22–97.72) |
| Deep learning model | 0.891 (0.816–0.967) | <0.001 | 100 (95.7–100) | 18.18 (2.3–51.8) |
| Original radiologist report | 0.125 (0.046–0.204) | <0.001 | 38.1 (27.71–49.34) | 100 (71.5–100) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hallinan, J.T.P.D.; Wu, J.; Liu, C.; Tran, H.A.; Lim, N.T.R.; Makmur, A.; Ong, W.; Wang, S.; Teo, E.C.; Chan, Y.H.; et al. Exploring the Potential of a Deep Learning Model for Early CT Detection of High-Grade Metastatic Epidural Spinal Cord Compression and Its Impact on Treatment Delays. Cancers 2025, 17, 2180. https://doi.org/10.3390/cancers17132180
Hallinan JTPD, Wu J, Liu C, Tran HA, Lim NTR, Makmur A, Ong W, Wang S, Teo EC, Chan YH, et al. Exploring the Potential of a Deep Learning Model for Early CT Detection of High-Grade Metastatic Epidural Spinal Cord Compression and Its Impact on Treatment Delays. Cancers. 2025; 17(13):2180. https://doi.org/10.3390/cancers17132180
Chicago/Turabian StyleHallinan, James Thomas Patrick Decourcy, Junran Wu, Changshuo Liu, Hien Anh Tran, Noah Tian Run Lim, Andrew Makmur, Wilson Ong, Shilin Wang, Ee Chin Teo, Yiong Huak Chan, and et al. 2025. "Exploring the Potential of a Deep Learning Model for Early CT Detection of High-Grade Metastatic Epidural Spinal Cord Compression and Its Impact on Treatment Delays" Cancers 17, no. 13: 2180. https://doi.org/10.3390/cancers17132180
APA StyleHallinan, J. T. P. D., Wu, J., Liu, C., Tran, H. A., Lim, N. T. R., Makmur, A., Ong, W., Wang, S., Teo, E. C., Chan, Y. H., Hey, H. W. D., Lau, L.-L., Thambiah, J., Wong, H.-K., Liu, G., Kumar, N., Ooi, B. C., & Tan, J. H. J. (2025). Exploring the Potential of a Deep Learning Model for Early CT Detection of High-Grade Metastatic Epidural Spinal Cord Compression and Its Impact on Treatment Delays. Cancers, 17(13), 2180. https://doi.org/10.3390/cancers17132180







