Role of Radiomics to Predict Malignant Transformation of Sinonasal Inverted Papilloma: A Systematic Review
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design and Reporting Guidelines
2.2. Search Strategy
2.3. Inclusion Criteria
- Study design:
- Cohort studies.
- Original research (>20 patients).
- Participants:
- Patients with sinonasal inverted papilloma.
- Intervention:
- Radiomic signature development.
- Outcomes:
- Ability to predict malignant transformation.
2.4. Study Selection, Data Extraction, and Critical Appraisal
2.5. Statistical Analysis
2.6. Systematic Review Registration
3. Results
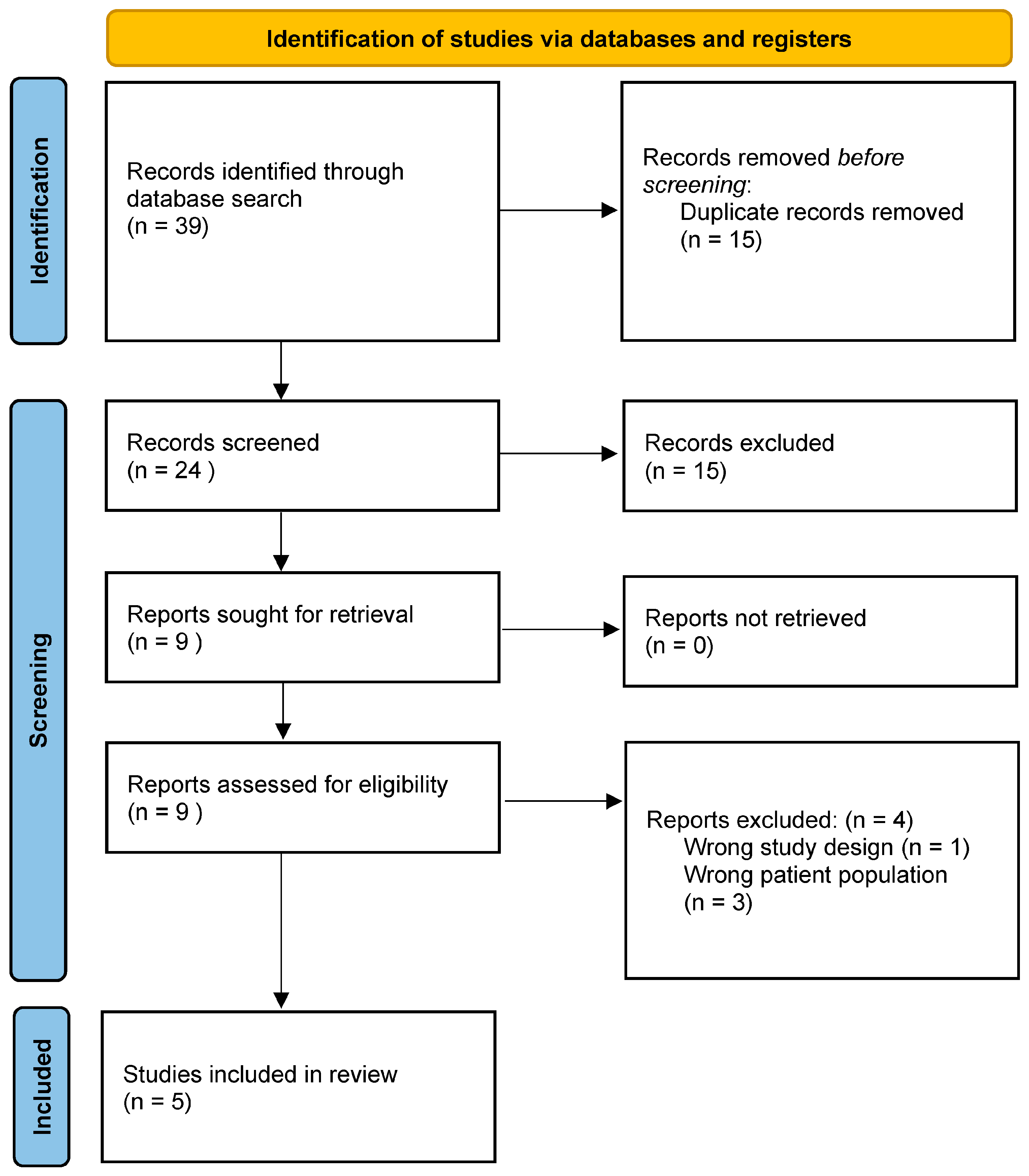
3.1. Search Results
3.2. Methodological Characteristics and Quality of Studies
3.3. Participant Characteristics
3.4. Acquisition Parameters
3.5. Development of Signatures
3.6. Performance of Signatures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Viitasalo, S.; Ilmarinen, T.; Aaltonen, L.; Hagström, J.; Hytönen, M.; Hammarén-Malmi, S.; Pietarinen, P.; Järvenpää, P.; Kinnari, T.; Geneid, A.; et al. Sinonasal inverted papilloma—Malignant transformation and non-sinonasal malignancies. Laryngoscope 2023, 133, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Mendenhall, W.M.; Hinerman, R.W.; Malyapa, R.S.; Werning, J.W.; Amdur, R.J.; Villaret, D.B.; Mendenhall, N.P. Inverted papilloma of the nasal cavity and paranasal sinuses. Am. J. Clin. Oncol. 2007, 30, 560–563. [Google Scholar] [CrossRef]
- Melroy, C.T.; Senior, B.A. Benign sinonasal neoplasms: A focus on inverting papilloma. Otolaryngol. Clin. North Am. 2006, 39, 601–617. [Google Scholar] [CrossRef] [PubMed]
- Mirza, S.; Bradley, P.J.; Acharya, A.; Stacey, M.; Jones, N.S. Sinonasal inverted papillomas: Recurrence, and synchronous and metachronous malignancy. J. Laryngol. Otol. 2007, 121, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.H.; Tong, C.C.L.; Penta, M.; Patel, V.S.; Palmer, J.N.; Adappa, N.D.; Nayak, J.V.; Hwang, P.H.; Patel, Z.M. Imaging predictors for malignant transformation of inverted papilloma. Laryngoscope 2019, 129, 777–782. [Google Scholar] [CrossRef]
- Ramkumar, S.; Ranjbar, S.; Ning, S.; Lal, D.; Zwart, C.; Wood, C.; Weindling, S.; Wu, T.; Mitchell, J.; Li, J.; et al. MRI-Based Texture Analysis to Differentiate Sinonasal Squamous Cell Carcinoma from Inverted Papilloma. AJNR Am. J. Neuroradiol. 2017, 38, 1019–1025. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Veritas Health Innovation. Covidence Systematic Review Software; Veritas Health Innovation: Melbourne, Australia, 2014. [Google Scholar]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M.; QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.T.H.M.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef]
- Liu, G.S.; Yang, A.; Kim, D.; Hojel, A.; Voevodsky, D.; Wang, J.; Tong, C.C.; Ungerer, H.; Palmer, J.N.; Kohanski, M.A.; et al. Deep learning classification of inverted papilloma malignant transformation using 3D convolutional neural networks and magnetic resonance imaging. Int. Forum Allergy Rhinol. 2022, 12, 1025–1033. [Google Scholar] [CrossRef]
- Xia, Z.; Lin, N.; Chen, W.; Qi, M.; Sha, Y. Multiparametric MRI-based radiomics nomogram for predicting malignant transformation of sinonasal inverted papilloma. Clin. Radiol. 2024, 79, e408–e416. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Yu, Q.; Li, Q.; Peng, J.; Lv, F.; Gong, B.; Zhang, X. MRI radiomics-based machine learning model integrated with clinic-radiological features for preoperative differentiation of sinonasal inverted papilloma and malignant sinonasal tumors. Front. Oncol. 2022, 12, 1003639. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Liu, Y.; Tao, J.; Li, Z.; Qu, X.; Guo, J.; Xian, J. Preoperative Prediction of Malignant Transformation of Sinonasal Inverted Papilloma Using MR Radiomics. Front. Oncol. 2022, 12, 870544. [Google Scholar] [CrossRef]
- Barry, N.; Kendrick, J.; Molin, K.; Li, S.; Rowshanfarzad, P.; Hassan, G.M.; Dowling, J.; Parizel, P.M.; Hofman, M.S.; Ebert, M.A. Evaluating the impact of the Radiomics Quality Score: A systematic review and meta-analysis. Eur. Radiol. 2025, 35, 1701–1713. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.-L.; Ren, J.; Jin, Z.-Y.; Liu, X.-Y.; He, Y.-L.; Li, Y.; Xue, H.-D. A systematic review and meta-analysis of CT and MRI radiomics in ovarian cancer: Methodological issues and clinical utility. Insights Into Imaging 2023, 14, 117. [Google Scholar] [CrossRef]
- Stanzione, A.; Gambardella, M.; Cuocolo, R.; Ponsiglione, A.; Romeo, V.; Imbriaco, M. Prostate MRI radiomics: A systematic review and radiomic quality score assessment. Eur. J. Radiol. 2020, 129, 109095. [Google Scholar] [CrossRef]
- Du, L.; Yuan, Q.; Han, Q. A new biomarker combining multimodal MRI radiomics and clinical indicators for differentiating inverted papilloma from nasal polyp invaded the olfactory nerve possibly. Front. Neurol. 2023, 14, 1151455. [Google Scholar] [CrossRef]
- He, S.; Zhao, Y.; Shi, L.; Yang, X.; Wang, X.; Luo, Y.; Wang, M.; Zhang, X.; Li, X.; Yu, D.; et al. Utilizing radiomics for differential diagnosis of inverted papilloma and chronic rhinosinusitis with polyps based on unenhanced CT scans. Sci. Rep. 2024, 14, 19299. [Google Scholar] [CrossRef]
- McKee, S.P.; Liang, X.; Yao, W.C.; Anderson, B.; Ahmad, J.G.; Allen, D.Z.; Hasan, S.; Chua, A.J.; Mokashi, C.; Islam, S.; et al. Predicting sinonasal inverted papilloma attachment using machine learning: Current lessons and future directions. Am. J. Otolaryngol. 2025, 46, 104549. [Google Scholar] [CrossRef]
- Miao, S.; Cheng, Y.; Li, Y.; Chen, X.; Chen, F.; Zha, D.; Xue, T. Prediction of recurrence-free survival and risk factors of sinonasal inverted papilloma after surgery by machine learning models. Eur. J. Med. Res. 2024, 29, 528. [Google Scholar] [CrossRef]
- Harding-Theobald, E.; Louissaint, J.; Maraj, B.; Cuaresma, E.; Townsend, W.; Mendiratta-Lala, M.; Singal, A.G.; Su, G.L.; Lok, A.S.; Parikh, N.D. Systematic review: Radiomics for the diagnosis and prognosis of hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2021, 54, 890–901. [Google Scholar] [CrossRef]
- Liu, F.; Ning, Z.; Liu, Y.; Liu, D.; Tian, J.; Luo, H.; An, W.; Huang, Y.; Zou, J.; Liu, C.; et al. Development and validation of a radiomics signature for clinically significant portal hypertension in cirrhosis (CHESS1701): A prospective multicenter study. eBioMedicine 2018, 36, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Primakov, S.; Beuque, M.; Woodruff, H.; Halilaj, I.; Wu, G.; Refaee, T.; Granzier, R.; Widaatalla, Y.; Hustinx, R.; et al. Radiomics for precision medicine: Current challenges, future prospects, and the proposal of a new framework. Methods 2021, 188, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Zhou, J.; Zhang, H.; Qiu, L.; Tan, H.; Hu, Y.; Shi, H. Relationship between KRAS mutations and dual time point 18F-FDG PET/CT imaging in colorectal liver metastases. Abdom. Radiol. 2019, 44, 2059–2066. [Google Scholar] [CrossRef] [PubMed]
- Raunig, D.L.; McShane, L.M.; Pennello, G.; Gatsonis, C.; Carson, P.L.; Voyvodic, J.T.; Wahl, R.L.; Kurland, B.F.; Schwarz, A.J.; Gönen, M.; et al. Quantitative imaging biomarkers: A review of statistical methods for technical performance assessment. Stat. Methods Med. Res. 2015, 24, 27–67. [Google Scholar] [CrossRef]
- Kessler, L.G.; Barnhart, H.X.; Buckler, A.J.; Choudhury, K.R.; Kondratovich, M.V.; Toledano, A.; Guimaraes, A.R.; Filice, R.; Zhang, Z.; Sullivan, D.C.; et al. The emerging science of quantitative imaging biomarkers terminology and definitions for scientific studies and regulatory submissions. Stat. Methods Med. Res. 2015, 24, 9–26. [Google Scholar] [CrossRef]
- Balagurunathan, Y.; Kumar, V.; Gu, Y.; Kim, J.; Wang, H.; Liu, Y.; Goldgof, D.B.; Hall, L.O.; Korn, R.; Zhao, B.; et al. Test-retest reproducibility analysis of lung CT image features. J. Digit. Imaging 2014, 27, 805–823. [Google Scholar] [CrossRef]
- Berenguer, R.; del Rosario Pastor-Juan, M.; Canales-Vazquez, J.; Castro-García, M.; Villas, M.V.; Masilla Legorburo, F.; Sabater, S. Radiomics of CT Features May Be Nonreproducible and Redundant: Influence of CT Acquisition Parameters. Radiology 2018, 288, 407–415. [Google Scholar] [CrossRef]
- Pfaehler, E.; Zhovannik, I.; Wei, L.; Boellaard, R.; Dekker, A.; Monshouwer, R.; El Naqa, I.; Bussink, J.; Gillies, R.; Wee, L.; et al. A systematic review and quality of reporting checklist for repeatability and reproducibility of radiomic features. Phys. Imaging Radiat. Oncol. 2021, 20, 69–75. [Google Scholar] [CrossRef]
- Park, J.E.; Park, S.Y.; Kim, H.J.; Kim, H.S. Reproducibility and Generalizability in Radiomics Modeling: Possible Strategies in Radiologic and Statistical Perspectives. Korean J. Radiol. 2019, 20, 1124–1137. [Google Scholar] [CrossRef]
- Kumar, V.; Gu, Y.; Basu, S.; Berglund, A.; Eschrich, S.A.; Schabath, M.B.; Forster, K.; Aerts, H.J.W.L.; Dekker, A.; Fenstermacher, D.; et al. Radiomics: The process and the challenges. Magn. Reson. Imaging 2012, 30, 1234–1248. [Google Scholar] [CrossRef] [PubMed]
| Study | Country | Journal | Study Design | Primary Outcome |
|---|---|---|---|---|
| Liu 2022 [11] | USA | International Forum of Allergy & Rhinology | Retrospective, Multicentre | Classification of IP malignant transformation using 3D CNNs |
| Xia 2024 [12] | China | Clinical Radiology | Retrospective, Single centre | Prediction of malignant transformation in IP based on MR radiomics and clinical risk factors. |
| Gu 2022 [13] | China | Frontiers in Oncology | Retrospective, Single centre | Differentiation of SNIP and MST using MR radiomics models |
| Yan 2022 [14] | China | Frontiers in Oncology | Retrospective, Single centre | Preoperative prediction of the malignant transformation of IP using MR radiomics |
| Ramkumar 2017 [6] | USA | American Journal of Neuroradiology | Retrospective, Single centre | MRI-Based texture analysis to differentiate IP from IP-SCC |
| Study | No. Patients | Age | Dataset Splitting | M:F | |
|---|---|---|---|---|---|
| Liu 2022 [11] | 64 IP | 26 IP-SCC | Mean age: IP: 59.7, IP-SCC: 62.9 | 72 (training), 18 (test) | IP: 45:19 IP-SCC: 18:8 |
| Xia 2024 [12] | 143 IP | 75 IP-SCC | Mean age: Training: 56.10, Test: 59.18 | 153 (training), 65 (test) | Training: 110:43 Test: 48:17 |
| Gu 2022 [13] | 106 SNIP | 141 MST | Mean age: IP: 58.1, SCC: 54.2 | 135 (training), 58 (test 1), 54 (test 2) | Training: 93:42 Test 1: 40:18 Test 2: 35:19 |
| Yan 2022 [14] | 144 IP | 92 IP-SCC | Not specified | 157 (training), 79 (test) | IP: 97:47 IP-SCC: 72:20 |
| Ramkumar 2017 [6] | 22 IP | 24 IP-SCC | Mean age: IP: 58.1, SCC: 54.2 | 33 (training), 13 (test) | IP: 17:5 IP-SCC: 19:5 |
| Study | Phase | Model | Field Strength | FOV (cm2) | TE/TR (ms) | ST (mm) | SI (mm) | Matrix | Contrast |
|---|---|---|---|---|---|---|---|---|---|
| Liu 2022 [11] | T1WI CE-T1WI T2WI | - | 1.5–3.0 T | - | - | - | - | - | Gadolinium enhancement |
| Xia 2024 [12] | T2WI DWI CE-T1WI | Magnetom Verio | 3 T | 18–22 × 18–22 | 384–4700/9–99 | 6 mm 3 mm 5 mm | 0.6 mm 1.2 mm 0.6 mm | 640 × 592 160 × 160 640 × 592 | 0.1 mmol/kg Magnevist at 2 mL/s. |
| Gu 2022 [13] | T1WI FSE T2WI CE-T1WI | Magnetom Essenza Magnetom Skyra GE Signa HDxt | 1.5–3.0 T | 22–24 × 22–24 | 400–4260/10–93 | 4–5 mm | 1 mm | 256 × 204 288 × 224 320 × 224 | 0.1 mL/kg Gd⁃DTPA and rate of 2.5 mL/s |
| Yan 2022 [14] | T1WI FSE T2WI FSE CE-T1WI T1WI TSE T2WI TSE | Ingenia GE Signa-HDxt | 3 T | 21–22 × 19–22 | 400–4720/6–90 | 4–5 mm | 0.3–0.5 mm | 264–512 × 201–256 | 0.1 mL/kg Gd-DTPA |
| Ramkumar 2017 [6] | T1WI FSE T2WI FSE CE-T1WI FSE | - | 1.5–3.0 T | ≤20 | - | ≤5 mm | - | 256 × 192 | Gadolinium enhancement |
| Study | Segmentation Software | Radiomics Software | Performance of Signature (Training) | Performance of Signature (Test) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUC | Accuracy | Sens | Spec | 95% CI | AUC | Accuracy | Sens | Spec | 95% CI | ||||
| Liu 2022 [11] | - | Training performed in PyTorch 1.8 | - | - | - | - | - | 0.80 | 77.9% | 0.667 | 0.815 | 0.68–0.9 | |
| Xia 2024 [12] | ITK-SNAP (v3.8.0) 3D Slicer (v4.11) | PyRadiomics v3.0.1 | 0.987 | - | - | - | 0.975–1.00 | 0.989 | - | - | - | 0.973–1.00 | |
| Gu 2022 [13] | ITK-SNAP (v3.6.0) | PyRadiomics v3.0.1 | 0.901 | 81.5% | 0.831 | 0.879 | 0.837–0.946 | Test set 1 | 0.878 | 0.788 | 0.788 | 0.800 | 0.765–0.949 |
| Test set 2 | 0.914 | 0.935 | 0.833 | 0.696 | 0.806–0.973 | ||||||||
| Yan 2022 [14] | ITK-SNAP (v3.6.0) | PyRadiomics (v3.0.1) | 0.954 | 87.3% | 0.857 | 0.883 | 0.926–0.982 | 0.940 | 0.873 | 0.793 | 0.920 | 0.888–0.992 | |
| Ramkumar 2017 [6] | - | R statistical and computing software, Python 2.7+custom code | - | 90.9% | 0.941 | 0.875 | - | - | 84.6% | 0.857 | 0.833 | - | |
| Study | Pre-Processing | Feature Selection Process | Selected Features | Nomogram/CNN Models |
|---|---|---|---|---|
| Liu 2022 [11] | Image volumes were resampled to a size of 128 × 128 × 64 voxels and normalised | Manual segmentation of 16 × 16-pixel regions of interest Feature extraction with CNNs | 446 images of distinct MRI sequences | 3D CNNs trained using backpropagation and Adam-optimized stochastic gradient descent: All-Net, Small-All-Net, and Elastic-All-Net |
| Xia 2024 [12] | Resampling and signal intensity normalisation Z-score standardisation | LASSO Minimum redundancy maximum relevance Pearson’s or Spearman’s correlation Statistical testing | CE wavelet-LLH GLSZM zone entropy, T2 wavelet-HLH glcm Idmn, ADC original first order skewness, CE wavelet-LLL first order interquartile range, T2 wavelet-LHH first order kurtosis, CE original shape maximum 2D diameter row, ADC wavelet-LHL GLSZM grey level non-uniformity, CE wavelet-LHL first order maximum, CE wavelet-LLH GLDM large dependence high grey level emphasis, CE original shape flatness, T2 original first order 10 percentile, ADC original first order 10 percentile | Clinical and radiomics model: (1.188 × epistaxis) + (2.503 × T2 equal signal) + (0.675 × extranasal invasion) + (0.978 × loss of CCP) + (2.899 × rad-score)—1.458 |
| Gu 2022 [13] | Z-score standardisation Images resampled to a consistent voxel spacing of 1 × 1 × 1 mm | Spearman correlation coefficients 10-fold cross-validation Recursive feature elimination | 1130 radiomics features were extracted: wavelet-LLH_firstorder_10Percentile, wavelet-LHL_firstorder_Mean, wavelet-LHL_glszm_SmallAreaLowGrayLevelEmphasis, wavelet-LHH_glszm_SmallAreaEmphasis, wavelet-HHH_glcm_Idm, wavelet-LLL_glcm_MCC, log-sigma-1-0-mm-3D_firstorder_10Percentile, log-sigma-1-0-mm-3D_firstorder_90Percentile, log-sigma-2-0-mm-3D_glcm_ClusterShade, log-sigma-2-0-mm-3D_glszm_SmallAreaLowGrayLevelEmphasis, log-sigma-3-0-mm-3D_firstorder_Median, log-sigma-3-0-mm-3D_firstorder_RootMeanSquared, log-sigma-3-0-mm-3D_glcm_ClusterShade, log-sigma-3-0-mm-3D_glcm_Correlation, log-sigma-3-0-mm-3D_glcm_MaximumProbability, log-sigma-3-0-mm-3D_glszm_LargeAreaLowGrayLevelEmphasis, log-sigma-3-0-mm-3D_glszm_SmallAreaEmphasis, log-sigma-3-0-mm-3D_ngtdm_Complexity | Combination of radiomics features and clinic-radiological features |
| Yan 2022 [14] | B-Spline interpolation Z-score standardisation | Multivariable logistic regression Support vector machine Minimum redundancy Maximum relevance | A total of 3948 radiomic features: squareroot_glszm_SizeZoneNonUniformity, wavelet. HLL_glrlm_RunEntropy, square_ngtdm_Coarseness, gradient_glszm_SizeZoneNonUniformityNormalized, original_shape_SurfaceVolumeRatio, logarithm_glcm_ClusterProminenc, logarithm_firstorder_Minimum, logarithm_glszm_SizeZoneNonUniformity, logarithm_glcm_Contrast, squareroot_ngtdm_Complexity, logarithm_glszm_SizeZoneNonUniformity, logarithm_glcm_DifferenceVariance, square_firstorder_Minimum, logarithm_glszm_LargeAreaLowGrayLevelEmphasis, logarithm_glszm_GrayLevelNonUniformityNormalized | MR radiomic features and morphological features |
| Ramkumar 2017 [6] | Resampling and/or zero-padding and normalisation | Manual ROI extraction Sequential forward-feature selection 16 × 16 square ROIs | 231 texture features were calculated for each case (77 texture features per MR imaging contrast × 3 contrasts) 13 GLCM features 12 LBP features 10 DOST features 18 LoGHist features 8 GFB features | Texture analysis integrated into machine-learning models |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waters, C.; Deshwal, A.; Cuddihy, T.O.; Jones, H.; Temperley, H.C.; Kaye-Coyle, H.; O’Sullivan, N.J.; Mac Curtain, B.M.; Kelly, M.E.; Young, O. Role of Radiomics to Predict Malignant Transformation of Sinonasal Inverted Papilloma: A Systematic Review. Cancers 2025, 17, 2175. https://doi.org/10.3390/cancers17132175
Waters C, Deshwal A, Cuddihy TO, Jones H, Temperley HC, Kaye-Coyle H, O’Sullivan NJ, Mac Curtain BM, Kelly ME, Young O. Role of Radiomics to Predict Malignant Transformation of Sinonasal Inverted Papilloma: A Systematic Review. Cancers. 2025; 17(13):2175. https://doi.org/10.3390/cancers17132175
Chicago/Turabian StyleWaters, Caitlin, Avinash Deshwal, Tom O. Cuddihy, Holly Jones, Hugo C. Temperley, Hannah Kaye-Coyle, Niall J. O’Sullivan, Benjamin M. Mac Curtain, Michael E. Kelly, and Orla Young. 2025. "Role of Radiomics to Predict Malignant Transformation of Sinonasal Inverted Papilloma: A Systematic Review" Cancers 17, no. 13: 2175. https://doi.org/10.3390/cancers17132175
APA StyleWaters, C., Deshwal, A., Cuddihy, T. O., Jones, H., Temperley, H. C., Kaye-Coyle, H., O’Sullivan, N. J., Mac Curtain, B. M., Kelly, M. E., & Young, O. (2025). Role of Radiomics to Predict Malignant Transformation of Sinonasal Inverted Papilloma: A Systematic Review. Cancers, 17(13), 2175. https://doi.org/10.3390/cancers17132175






