Changes in the Characteristics of Kidney Cancer Detection During the COVID-19 Pandemic
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Setting
2.2. Impact of COVID-19 Pandemic Regulations in Hungary
2.3. Study Design
2.4. Data Analysis
3. Results
3.1. Distribution and Baseline Characteristics of Patients with Kidney Cancer Before and During the COVID-19 Pandemic
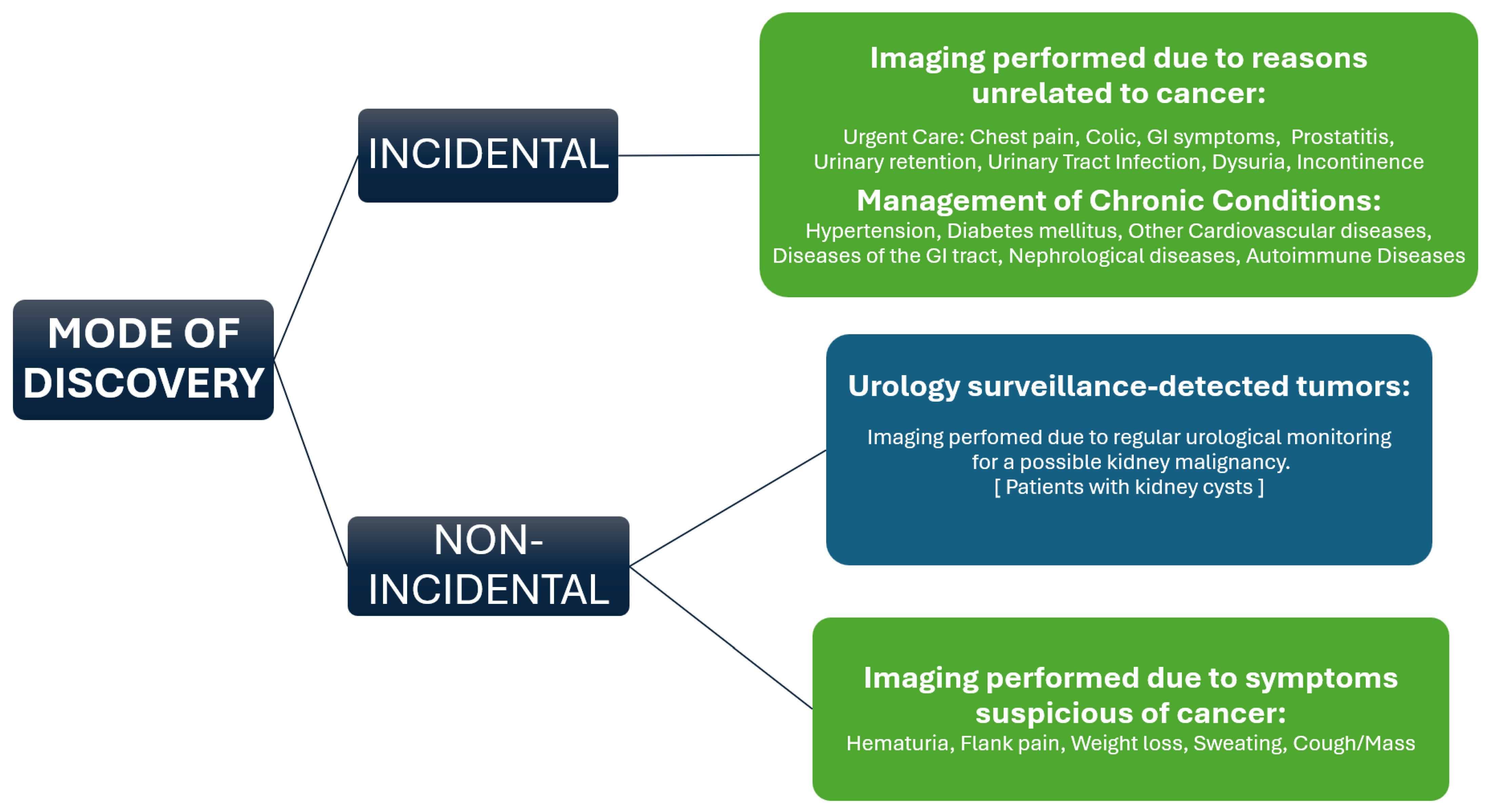
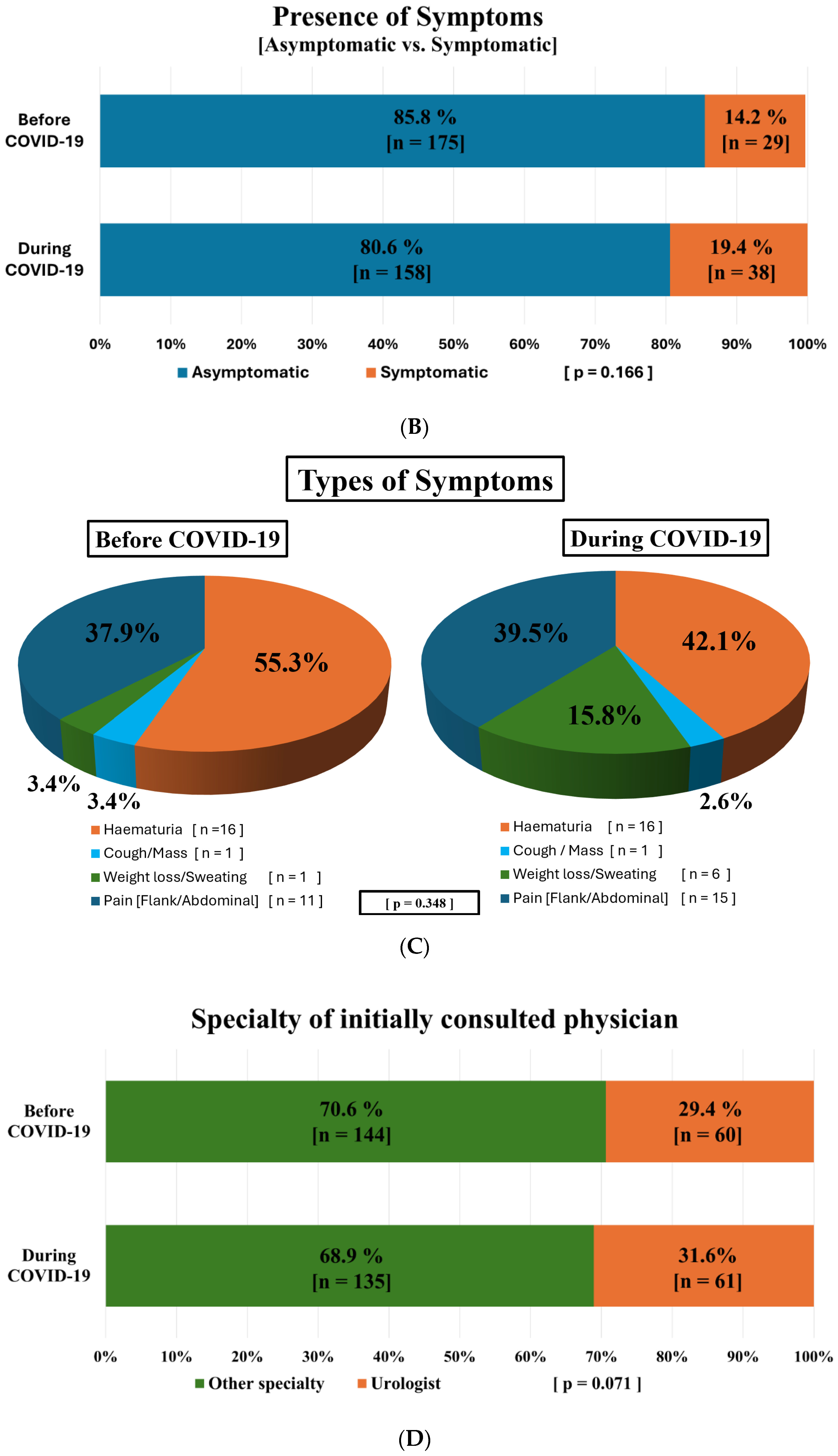
3.2. Modes of Tumor Detection and Influencing Factors Before and During the COVID-19 Pandemic
3.3. Predictive Factors for Advanced Tumor Stage and Incidental Discovery Before and During the COVID-19 Pandemic
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CCI | Charlson Comorbidity Index |
| CEUS | Contrast Enhances Ultrasound |
| EU | European Union |
| KC | Kidney Cancer |
| RCC | Renal Cell Carcinoma |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
| UK | United Kingdom |
| UP UC | University of Pécs Clinical Center Urology Clinic |
| US | Ultrasound |
References
- Capitanio, U.; Bensalah, K.; Bex, A.; Boorjian, S.A.; Bray, F.; Coleman, J.; Gore, J.L.; Sun, M.; Wood, C.; Russo, P. Epidemiology of Renal Cell Carcinoma. Eur. Urol. 2019, 75, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Kenessey, I.; Nagy, P.; Polgár, C. The Hungarian situation of cancer epidemiology in the second decade of the 21st century. Magy. Onkol. 2022, 66, 175–184. [Google Scholar] [PubMed]
- Hollingsworth, J.M.; Miller, D.C.; Daignault, S.; Hollenbeck, B.K. Rising Incidence of Small Renal Masses: A Need to Reassess Treatment Effect. JNCI J. Natl. Cancer Inst. 2006, 98, 1331–1334. [Google Scholar] [CrossRef] [PubMed]
- Lughezzani, G.; Paciotti, M.; Fasulo, V.; Casale, P.; Saita, A. Gender-specific risk factors for renal cell carcinoma. Curr. Opin. Urol. 2019, 29, 272–278. [Google Scholar] [CrossRef]
- Mancini, M.; Righetto, M.; Baggio, G. Gender-Related Approach to Kidney Cancer Management: Moving Forward. Int. J. Mol. Sci. 2020, 21, 3378. [Google Scholar] [CrossRef]
- Znaor, A.; Lortet-Tieulent, J.; Laversanne, M.; Jemal, A.; Bray, F. International Variations and Trends in Renal Cell Carcinoma Incidence and Mortality. Eur. Urol. 2015, 67, 519–530. [Google Scholar] [CrossRef]
- Hu, S.L.; Weiss, R.H. Management of the Incidental Kidney Mass in the Nephrology Clinic. Clin. J. Am. Soc. Nephrol. 2018, 13, 1407–1409. [Google Scholar] [CrossRef]
- Vasudev, N.S.; Wilson, M.; Stewart, G.D.; Adeyoju, A.; Cartledge, J.; Kimuli, M.; Datta, S.; Hanbury, D.; Hrouda, D.; Oades, G.; et al. Challenges of early renal cancer detection: Symptom patterns and incidental diagnosis rate in a multicentre prospective UK cohort of patients presenting with suspected renal cancer. BMJ Open 2020, 10, e035938. [Google Scholar] [CrossRef]
- Bahadoram, S.; Davoodi, M.; Hassanzadeh, S.; Bahadoram, M.; Barahman, M.; Mafakher, L. Renal cell carcinoma: An overview of the epidemiology, diagnosis, and treatment. G. Ital. Nefrol. 2022, 39, 2022. [Google Scholar]
- Kościelecka, K.E.; Kuć, A.J.; Kubik, D.M.; Męcik-Kronenberg, T.; Ceglarz, D. Impact of the Covid-19 pandemic on the availability of medical care among oncological patients. Wiad. Lek. 2021, 74, 1542–1551. [Google Scholar] [CrossRef]
- Barranco, R.; Messina, C.; Bonsignore, A.; Cattrini, C.; Ventura, F. Medical Liability in Cancer Care During COVID-19 Pandemic: Heroes or Guilty? Front. Public Health 2020, 8, 602988. [Google Scholar] [CrossRef] [PubMed]
- Riera, R.; Bagattini, Â.M.; Pacheco, R.L.; Pachito, D.V.; Roitberg, F.; Ilbawi, A. Delays and Disruptions in Cancer Health Care Due to COVID-19 Pandemic: Systematic Review. JCO Glob. Oncol. 2021, 7, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Bekele, B.B.; Alhaffar, B.A.; Wasnik, R.N.; Sándor, J. The Effect of the COVID-19 Pandemic on the Social Inequalities of Health Care Use in Hungary: A Nationally Representative Cross-Sectional Study. Int. J. Environ. Res. Public Health 2022, 19, 2258. [Google Scholar] [CrossRef]
- Naidich, J.J.; Boltyenkov, A.; Wang, J.J.; Chusid, J.; Hughes, D.; Sanelli, P.C. Impact of the Coronavirus Disease 2019 (COVID-19) Pandemic on Imaging Case Volumes. J. Am. Coll. Radiol. 2020, 17, 865–872. [Google Scholar] [CrossRef]
- Kuusk, T.; Cullen, D.; Neves, J.B.; Campain, N.; Barod, R.; Boleti, E.; El-Sheihk, S.; Grant, L.; Kelly, J.; Marchetti, M.; et al. Impact of the first surge of the COVID-19 pandemic on a tertiary referral centre for kidney cancer. BJU Int. 2021, 128, 752–758. [Google Scholar] [CrossRef]
- Yildirim, H.; Bins, A.D.; Hurk, C.v.D.; van Moorselaar, R.J.A.; van Oijen, M.G.H.; Bex, A.; Zondervan, P.J.; Aben, K.K.H. The impact of the COVID-19 pandemic on renal cancer care. World J. Urol. 2024, 42, 1–7. [Google Scholar] [CrossRef]
- Janes, W.I.; Fagan, M.G.; Andrews, J.M.; Harvey, D.R.; Warden, G.M.; Johnston, P.H.; Organ, M.K. Impacts of the COVID-19 pandemic on diagnosis of renal cell carcinoma and disease stage at presentation. Can. Urol. Assoc. J. 2023, 18, E113–E119. [Google Scholar] [CrossRef]
- Elleisy, M.; Dräger, D.L.; Zettl, H.; Hakenberg, O.W. COVID-19 Pandemic Impact on Uro-Oncological Disease Outcomes at a German Referral Center. Urol. Int. 2024, 109, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gansler, T.; Fedewa, S.A.; Flanders, W.D.; Pollack, L.A.; Siegel, D.A.; Jemal, A. Prevalence of Cigarette Smoking among Patients with Different Histologic Types of Kidney Cancer. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1406–1412. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Collins, P.M.; Madden, A.; O’connell, C.; Omer, S.A.; Inder, M.S.; Casey, R.G.; Flynn, R.J.; Thomas, A.Z.; Smyth, L.G.; Manecksha, R.P. Urological service provision during the COVID-19 period: The experience from an Irish tertiary centre. Ir. J. Med Sci. (1971-) 2021, 190, 455–460. [Google Scholar] [CrossRef]
- Oderda, M.; Roupret, M.; Marra, G.; Merseburger, A.S.; Oderda, G.; Falcone, M.; Ceruti, C.; Shariat, S.F.; Gontero, P. The Impact of COVID-19 Outbreak on Uro-oncological Practice Across Europe: Which Burden of Activity Are We Facing Ahead? Eur. Urol. 2020, 78, 124–126. [Google Scholar] [CrossRef]
- Naspro, R.; Da Pozzo, L.F. Urology in the time of corona. Nat. Rev. Urol. 2020, 17, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Kelemenic-Drazin, R.; Budisavljevic, A.; Plavetic, N.D.; Fucak, I.K.; Silovski, T.; Dobric, V.T.; Nalbani, M.; Curic, Z.; Boric-Mikez, Z.; Ladenhauser, T.; et al. Impact of the coronavirus disease pandemic on cancer care in Croatia: A multicentre cross-sectional study. Ecancermedicalscience 2021, 15, 1263. [Google Scholar] [CrossRef] [PubMed]
- Scelo, G.; Larose, T.L. Epidemiology and Risk Factors for Kidney Cancer. J. Clin. Oncol. 2018, 36, 3574–3581. [Google Scholar] [CrossRef] [PubMed]
- Scelo, G.; Li, P.; Chanudet, E.; Muller, D.C. Variability of Sex Disparities in Cancer Incidence over 30 Years: The Striking Case of Kidney Cancer. Eur. Urol. Focus 2018, 4, 586–590. [Google Scholar] [CrossRef]
- Yu, C.-P.; Ho, J.-Y.; Huang, Y.-T.; Cha, T.-L.; Sun, G.-H.; Yu, D.-S.; Chang, F.-W.; Chen, S.-P.; Hsu, R.-J.; Wang, X.W. Estrogen Inhibits Renal Cell Carcinoma Cell Progression through Estrogen Receptor-β Activation. PLoS ONE 2013, 8, e56667. [Google Scholar] [CrossRef]
- Hunt, J.D.; van der Hel, O.L.; McMillan, G.P.; Boffetta, P.; Brennan, P. Renal cell carcinoma in relation to cigarette smoking: Meta-analysis of 24 studies. Int. J. Cancer 2004, 114, 101–108. [Google Scholar] [CrossRef]
- Aguilar-Palacio, I.; Obón-Azuara, B.; Castel-Feced, S.; Malo, S.; Teresa, J.; Rabanaque, M.J. Gender health care inequalities in health crisis: When uncertainty can lead to inequality. Arch. Public Health 2024, 82, 1–10. [Google Scholar] [CrossRef]
- Reisch, T.; Heiler, G.; Hurt, J.; Klimek, P.; Hanbury, A.; Thurner, S. Behavioral gender differences are reinforced during the COVID-19 crisis. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Bruce, S.F.; Huysman, B.; Bharucha, J.; Massad, L.S.; Mullen, M.M.; Hagemann, A.R.; Fuh, K.C.; McCourt, C.K.; Thaker, P.H.; Khabele, D.; et al. Impact of the COVID-19 pandemic on referral to and delivery of gynecologic oncology care. Gynecol. Oncol. Rep. 2022, 39, 100928. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Sendino, Á.; Guallar-Castillón, P.; Banegas, J.R.; Rodríguez-Artalejo, F. Gender differences in the utilization of health-care services among the older adult population of Spain. BMC Public Health 2006, 6, 155. [Google Scholar] [CrossRef] [PubMed]
- Macintyre, S.; Hunt, K.; Sweeting, H. Gender differences in health: Are things really as simple as they seem? Soc. Sci. Med. 1996, 42, 617–624. [Google Scholar] [CrossRef]
- Jayant, K.; Rao, R.S.; Nene, B.M.; Dale, P.S. Improved stage at diagnosis of cervical cancer with increased cancer awareness in a rural Indian population. Int. J. Cancer 1995, 63, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.-P.; Yoo, H.S.; Lee, J.-S. The impact of the pandemic declaration on public awareness and behavior: Focusing on COVID-19 google searches. Technol. Forecast. Soc. Chang. 2021, 166, 120592. [Google Scholar] [CrossRef]
- Young, A.M.; Ashbury, F.D.; Schapira, L.; Scotté, F.; I Ripamonti, C.; Olver, I.N. Uncertainty upon uncertainty: Supportive Care for Cancer and COVID-19. Support. Care Cancer 2020, 28, 4001–4004. [Google Scholar] [CrossRef]
- Kupcova, I.; Danisovic, L.; Klein, M.; Harsanyi, S. Effects of the COVID-19 pandemic on mental health, anxiety, and depression. BMC Psychol. 2023, 11, 1–7. [Google Scholar] [CrossRef]
- Moore, C.A.; Ruisch, B.C.; Samayoa, J.A.G.; Boggs, S.T.; Ladanyi, J.T.; Fazio, R.H. Contracting COVID-19: A longitudinal investigation of the impact of beliefs and knowledge. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Gareev, I.; Gallyametdinov, A.; Beylerli, O.; Valitov, E.; Alyshov, A.; Pavlov, V.; Izmailov, A.; Zhao, S. The opportunities and challenges of telemedicine during COVID-19 pandemic. Front. Biosci. 2021, 13, 291–298. [Google Scholar] [CrossRef]
- Novara, G.; Checcucci, E.; Crestani, A.; Abrate, A.; Esperto, F.; Pavan, N.; De Nunzio, C.; Galfano, A.; Giannarini, G.; Gregori, A.; et al. Telehealth in Urology: A Systematic Review of the Literature. How Much Can Telemedicine Be Useful During and After the COVID-19 Pandemic? Eur. Urol. 2020, 78, 786–811. [Google Scholar] [CrossRef]
- Jayson, M.; Sanders, H. Increased incidence of serendipitously discovered renal cell carcinoma. Urology 1998, 51, 203–205. [Google Scholar] [CrossRef]
- Turner, R.M.; Morgan, T.M.; Jacobs, B.L. Epidemiology of the Small Renal Mass and the Treatment Disconnect Phenomenon. Urol. Clin. N. Am. 2017, 44, 147–154. [Google Scholar] [CrossRef]
- DI Trapani, E.; Dell’Oglio, P.; Larcher, A.; Nini, A.; Muttin, F.; Cianflone, F.; Dehò, F.; Matloob, R.; DI Trapani, D.; Freschi, M.; et al. Pathological High-risk Renal Cell Carcinoma: Trends in Clinical Characteristics over 25 Years. Anticancer Res. 2018, 38, 4123–4130. [Google Scholar] [CrossRef]
- Dinmohamed, A.G.; Visser, O.; Verhoeven, R.H.A.; Louwman, M.W.J.; van Nederveen, F.H.; Willems, S.M.; Merkx, M.A.W.; Lemmens, V.E.P.P.; Nagtegaal, I.D.; Siesling, S. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol. 2020, 21, 750–751. [Google Scholar] [CrossRef]
- Peacock, H.M.; De Gendt, C.; Silversmit, G.; Nuyts, S.; Casselman, J.; Machiels, J.-P.; Giusti, F.; van Gool, B.; Poorten, V.V.; Van Eycken, L. Stage shift and relative survival for head and neck cancer during the 2020 COVID-19 pandemic: A population-based study of temporal trends. Front. Oncol. 2023, 13, 1253968. [Google Scholar] [CrossRef] [PubMed]
- Mangone, L.; Marinelli, F.; Bonfante, G.; Bisceglia, I.; Morabito, F.; Masini, C.; Bergamaschi, F.A.M.; Pinto, C. The Impact of COVID-19 on New Kidney Cancer Diagnosis: Stage and Treatment in Northern Italy. Int. J. Environ. Res. Public Health 2023, 20, 4755. [Google Scholar] [CrossRef] [PubMed]
- Christakoudi, S.; Kakourou, A.; Markozannes, G.; Tzoulaki, I.; Weiderpass, E.; Brennan, P.; Gunter, M.; Dahm, C.C.; Overvad, K.; Olsen, A.; et al. Blood pressure and risk of cancer in the European Prospective Investigation into Cancer and Nutrition. Int. J. Cancer 2019, 146, 2680–2693. [Google Scholar] [CrossRef]
- Chow, W.-H.; Gridley, G.; Fraumeni, J.F.J.; Järvholm, B. Obesity, Hypertension, and the Risk of Kidney Cancer in Men. N. Engl. J. Med. 2000, 343, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Andras, I.; Territo, A.; Telecan, T.; Medan, P.; Perciuleac, I.; Berindean, A.; Stanca, D.V.; Buzoianu, M.; Coman, I.; Crisan, N. Role of the Laparoscopic Approach for Complex Urologic Surgery in the Era of Robotics. J. Clin. Med. 2021, 10, 1812. [Google Scholar] [CrossRef]
- Pinsky, P.F.; Dunn, B.; Gierada, D.; Nath, P.H.; Munden, R.; Berland, L.; Kramer, B.S. Incidental renal tumours on low-dose CT lung cancer screening exams. J. Med. Screen. 2016, 24, 104–109. [Google Scholar] [CrossRef]
- Rossi, S.H.; Klatte, T.; Usher-Smith, J.A.; Fife, K.; Welsh, S.J.; Dabestani, S.; Bex, A.; Nicol, D.; Nathan, P.; Stewart, G.D.; et al. A Decision Analysis Evaluating Screening for Kidney Cancer Using Focused Renal Ultrasound. Eur. Urol. Focus 2021, 7, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Rouprêt, M. Smoking Status Is Not Sufficient to Accurately Target Patients Who Would Benefit from Screening for Bladder and Kidney Cancer. Eur. Urol. Focus 2015, 1, 52–53. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.H.; Klatte, T.; Usher-Smith, J.; Stewart, G.D. Epidemiology and screening for renal cancer. World J. Urol. 2018, 36, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- Hartmann-Boyce, J.; Highton, P.; Rees, K.; Onakpoya, I.; Suklan, J.; Curtis, F.; O’MAhoney, L.; Morris, E.; Kudlek, L.; Morgan, J.; et al. The impact of the COVID-19 pandemic and associated disruptions in health-care provision on clinical outcomes in people with diabetes: A systematic review. Lancet Diabetes Endocrinol. 2024, 12, 132–148. [Google Scholar] [CrossRef]


| Before COVID-19 | During COVID-19 | Chi-Square Test’s p Value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Sex | 0.023 | ||||
| Male Female Total | 139 | 68.1 | 112 | 57.1 | |
| 65 | 31.9 | 84 | 42.9 | ||
| 204 | 100.0 | 196 | 100.0 | ||
| Age | 0.307 | ||||
| 0–49 50–59 60–69 ≥70 Total | 30 | 14.7 | 27 | 13.8 | |
| 54 | 26.5 | 57 | 29.1 | ||
| 62 | 30.4 | 71 | 36.2 | ||
| 58 | 28.4 | 41 | 20.9 | ||
| 204 | 100.0 | 196 | 100.0 | ||
| Place of residence | 0.087 | ||||
| County seat City Other location Total | 54 | 26.5 | 54 | 27.6 | |
| 60 | 29.4 | 75 | 38.3 | ||
| 90 | 44.1 | 67 | 34.1 | ||
| 204 | 100.0 | 196 | 100.0 | ||
| Distance from UP UC [km] | 0.048 | ||||
| ≤40 >40 Total | 84 | 41.2 | 100 | 51.0 | |
| 120 | 58.8 | 96 | 49.0 | ||
| 204 | 100.0 | 196 | 100.0 | ||
| Smoking | 0.555 | ||||
| No Yes Total | 145 | 71.1 | 134 | 68.4 | |
| 59 | 28.9 | 62 | 31.6 | ||
| 204 | 100 | 196 | 100 | ||
| Charlson Comorbidity Index | 0.048 | ||||
| ≤4 ≥5 Total | 86 | 42.2 | 102 | 52.0 | |
| 118 | 57.8 | 94 | 48.0 | ||
| 204 | 100 | 196 | 100 | ||
| Stage | 0.632 | ||||
| I II III IV Total | 151 | 74.0 | 135 | 68.9 | |
| 9 | 4.4 | 12 | 6.1 | ||
| 32 | 15.7 | 38 | 19.4 | ||
| 12 | 5.9 | 11 | 5.6 | ||
| 204 | 100 | 196 | 100 | ||
| Stage | 0.417 | ||||
| I–II II–III Total | 160 | 78.4 | 147 | 75.0 | |
| 44 | 21.6 | 49 | 25.0 | ||
| 204 | 100 | 196 | 100 | ||
| Type of initial imaging test | 0.088 | ||||
| US * CT MRI CEUS ** Plain chest X-ray Total | 157 | 77.0 | 126 | 64.4 | |
| 36 | 17.6 | 52 | 26.5 | ||
| 7 | 3.4 | 13 | 6.6 | ||
| 3 | 1.5 | 4 | 2.0 | ||
| 1 | 0.5 | 1 | 0.5 | ||
| 204 | 100 | 196 | 100 | ||
| (A) | ||||||
| STAGE | Before COVID-19 | During COVID-19 | p | |||
| Incidental | Incidental | |||||
| No | Yes | No | Yes | 0.001 | ||
| Early (I–II) | n [%] | 21 [58.3%] | 139 [82.7%] | 35 [64.8%] | 112 [78.9%] | |
| Advanced (III–IV) | n [%] | 15 [41.7%] | 29 [17.3%] | 19 [35.2%] | 30 [21.1%] | |
| Total | n [%] | 36 [100.0%] | 168 [100.0%] | 54 [100.0%] | 142 [100.0%] | |
| (B) | ||||||
| Initially Consulted Physician | Before COVID-19 | During COVID-19 | p | |||
| Incidental | Incidental | |||||
| No | Yes | No | Yes | 0.010 | ||
| Other specialty | n [%] | 19 [52.8%] | 125 [74.4%] | 29 [53.7%] | 106 [74.6%] | |
| Urologist | n [%] | 17 [47.2%] | 43 [25,6%] | 25 [46.3%] | 36 [25.4%] | |
| Total | n [%] | 36 [100%] | 168 [100%] | 54 [100%] | 142 [100%] | |
| (A) | ||||
| Before COVID-19 OR/CI 95% | During COVID-19 OR/CI 95% | Z-Value/p-Value | ||
| Incidental discovery | ‘Other’ specialist as initially contacted physician | 2.6 [1.240–5.454] | 2.54 [1.318–4.897] | 0.046/0.963 |
| (B) | ||||
| Before COVID-19 OR/CI 95% | During COVID-19 OR/CI 95% | Z-Value/p-Value | ||
| Advanced-stage | Non-incidental discovery | 3.42 [1.579–7.424] | 2.03 [1.018–4.035] | 0.987/0.324 |
| Presence of symptoms | 4.51 [1.972–10.321] | 2.76 [1.302–5.829] | 0.862/0.386 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rumi, L.; Szántó, Á.; Bányai, D.; Szabó, É.; Zemplényi, A.; Bellyei, S.; Mátyus, E.; Hubai, D.; Girán, J.; Kiss, I.; et al. Changes in the Characteristics of Kidney Cancer Detection During the COVID-19 Pandemic. Cancers 2025, 17, 2150. https://doi.org/10.3390/cancers17132150
Rumi L, Szántó Á, Bányai D, Szabó É, Zemplényi A, Bellyei S, Mátyus E, Hubai D, Girán J, Kiss I, et al. Changes in the Characteristics of Kidney Cancer Detection During the COVID-19 Pandemic. Cancers. 2025; 17(13):2150. https://doi.org/10.3390/cancers17132150
Chicago/Turabian StyleRumi, László, Árpád Szántó, Dániel Bányai, Éva Szabó, Antal Zemplényi, Szabolcs Bellyei, Emese Mátyus, Dóra Hubai, János Girán, István Kiss, and et al. 2025. "Changes in the Characteristics of Kidney Cancer Detection During the COVID-19 Pandemic" Cancers 17, no. 13: 2150. https://doi.org/10.3390/cancers17132150
APA StyleRumi, L., Szántó, Á., Bányai, D., Szabó, É., Zemplényi, A., Bellyei, S., Mátyus, E., Hubai, D., Girán, J., Kiss, I., Pozsgai, É., & Boronkai, Á. (2025). Changes in the Characteristics of Kidney Cancer Detection During the COVID-19 Pandemic. Cancers, 17(13), 2150. https://doi.org/10.3390/cancers17132150







