Advances and Challenges in Prostate Cancer Diagnosis: A Comprehensive Review
Simple Summary
Abstract
1. Introduction
1.1. Structure and Function of the Prostate Gland
1.2. Epidemiology of Prostate Cancer
1.3. Etiology of Prostate Cancer
1.4. Pathomorphological Classification of Prostate Cancer
2. Initial Diagnosis of Prostate Cancer
2.1. Rectal Palpation
2.2. Prostate-Specific Antigen
2.3. Screening and Early Detection
2.4. Genomic Classifiers in Risk Stratification
2.5. Imaging Studies
2.5.1. Transrectal Ultrasonography
2.5.2. Computed Tomography
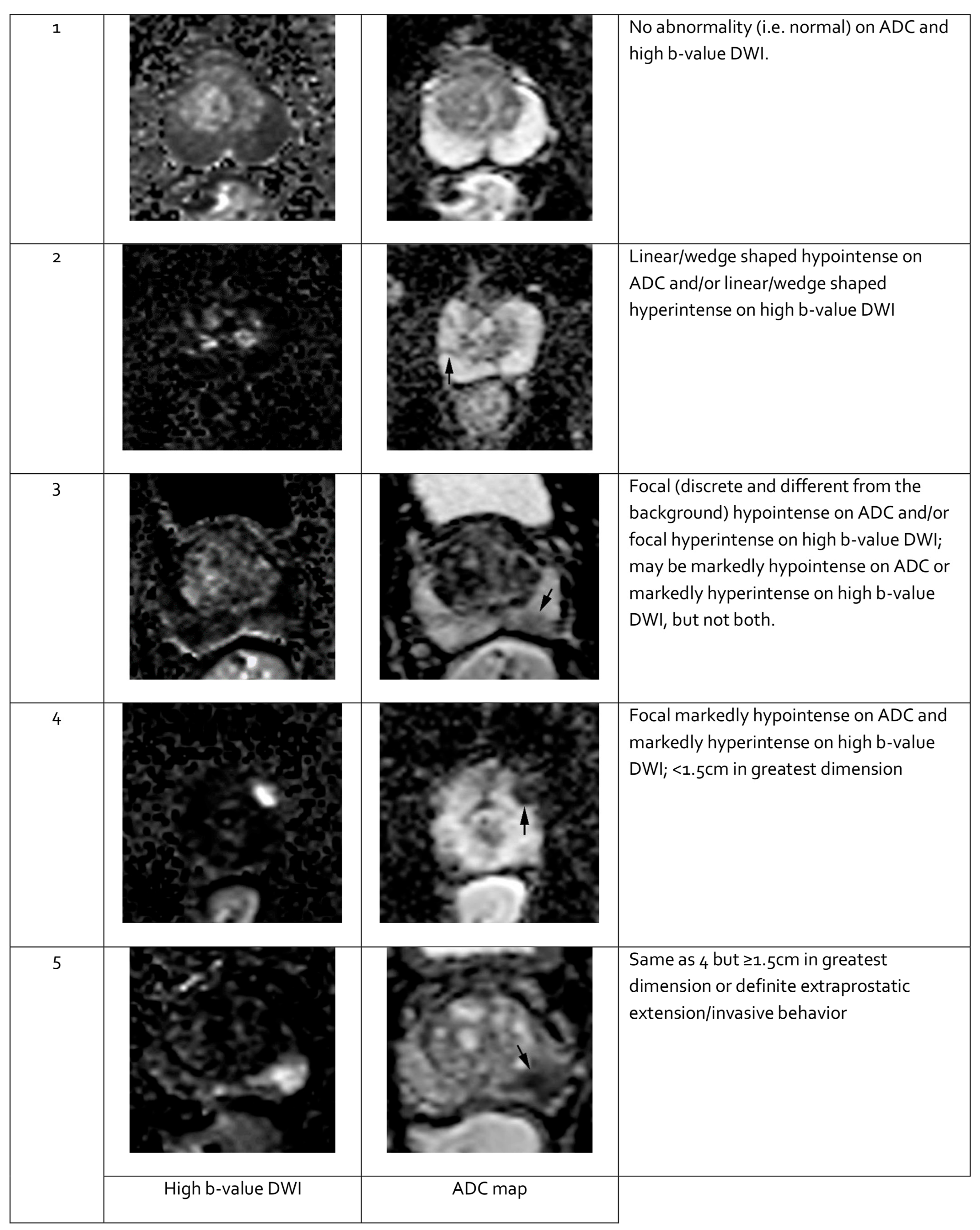
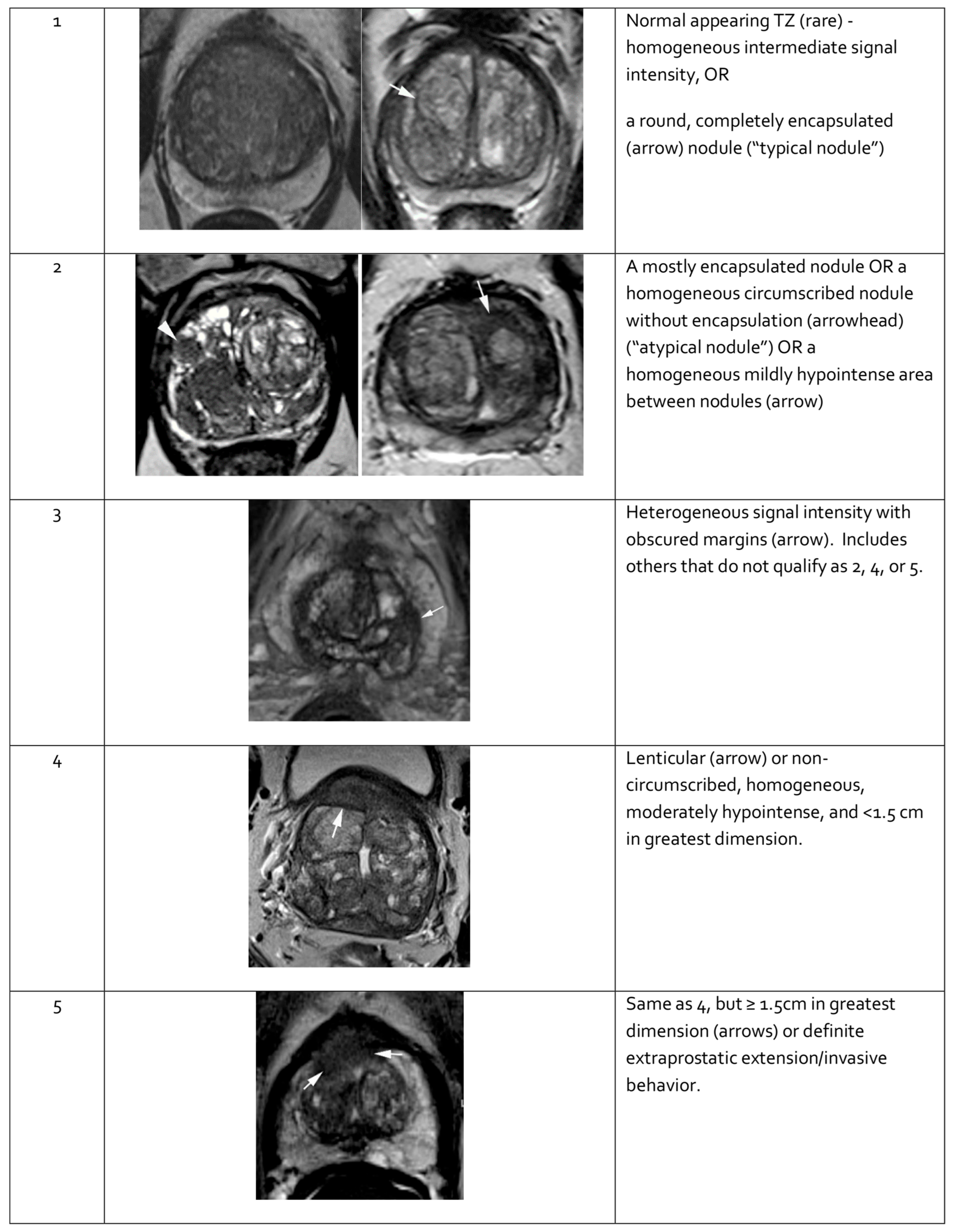
2.5.3. Multiparametric Magnetic Resonance Imaging
2.5.4. Prostate-Specific Membrane Antigen–Positron Emission Tomography–Computed Tomography
3. Prostate Biopsy
3.1. Historical Background
3.2. Systematic Biopsy
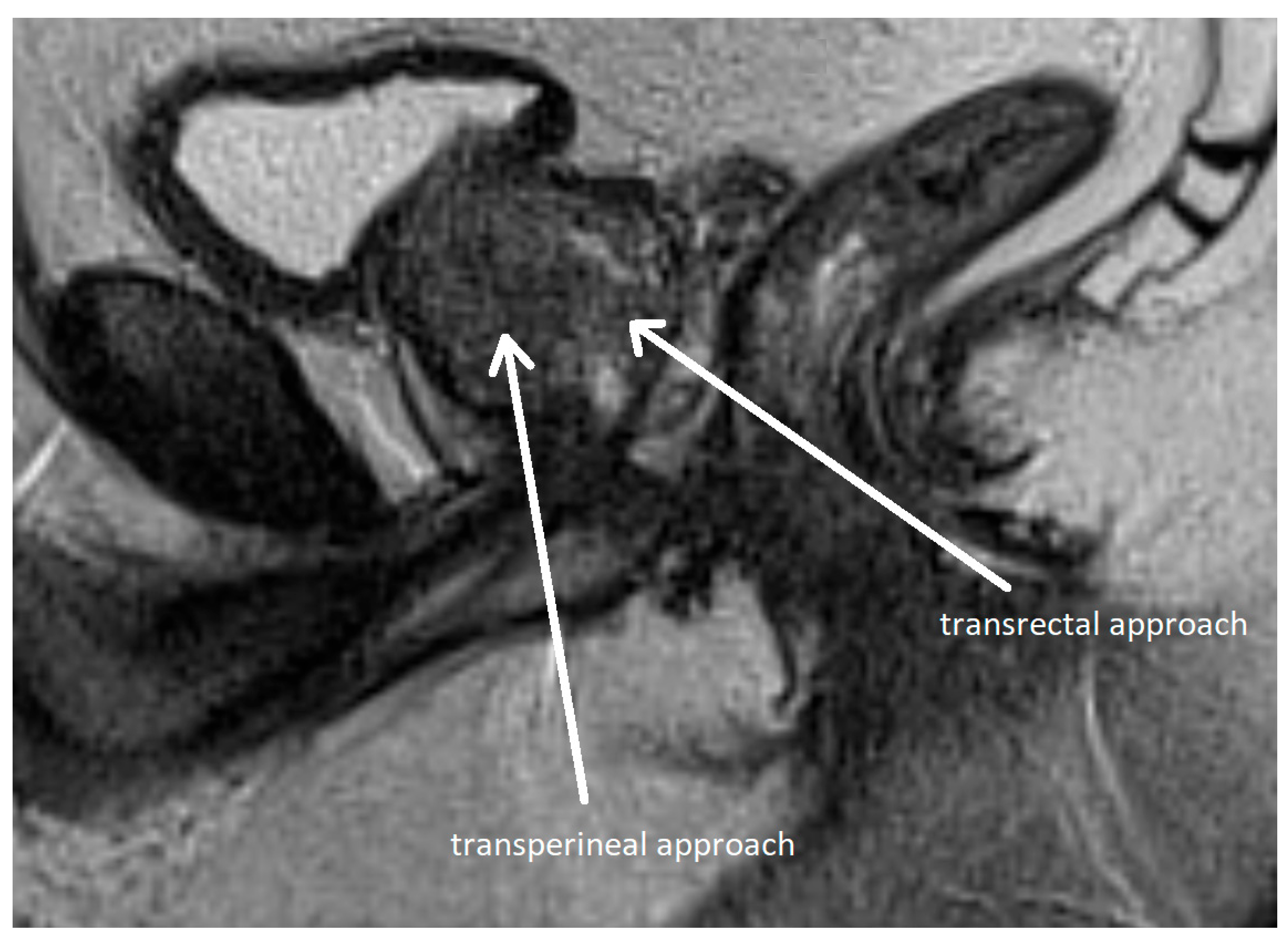
3.3. Fusion Biopsy
- Cognitive biopsy—after reviewing mpMRI images and the radiologic report, the operator mentally maps the lesion’s location and attempts to target the area under ultrasound guidance;
- Direct mpMRI-guided biopsy (in-bore)—tissue sampling is performed within the MRI scanner, with real-time visualization and targeting of the lesion;
- Fusion biopsy—dedicated software merges pre-acquired mpMRI images with real-time TRUS, allowing for a direct visualization of the marked lesions. The urologist then guides the biopsy needle to obtain tissue samples from these targets.
3.4. Limitations of Prostate Biopsy
4. Treatment of Prostate Cancer
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADC | apparent diffusion coefficient |
| ADT | androgen deprivation therapy |
| AS | active surveillance |
| BCR | biochemical recurrence |
| BPH | benign prostatic hyperplasia |
| C-index | concordance index |
| CAPRA-S | Cancer of the Prostate Risk Assessment Postsurgical Score |
| CCP | cell cycle progression |
| ComBx | combined fusion biopsy |
| CT | computed tomography |
| DCE | dynamic contrast enhancement |
| DRE | digital rectal examination |
| DWI | diffusion-weighted imaging |
| EAU | European Association of Urology |
| EBRT | external beam radiation therapy |
| EPE | extraprostatic extension |
| EU | European Union |
| fPSA | free prostate-specific antigen |
| f/tPSA | free-to-total prostate-specific antigen ratio |
| GPS | Genomic Prostate Score |
| GS | Gleason score |
| HPCa | hereditary prostate cancer |
| ISUP | International Society of Urological Pathology |
| LNI | lymph node invasion |
| LUTS | lower urinary tract symptoms |
| mpMRI | multiparametric magnetic resonance imaging |
| NCCN | National Comprehensive Cancer Network |
| PCa | prostate cancer |
| PI-RADS | Prostate Imaging—Reporting and Data System |
| PPV | positive predictive value |
| PROMIS | Prostate Magnetic Resonance Imaging Study |
| PSA | prostate-specific antigen |
| PSA-D | prostate-specific antigen density |
| PSA-DT | prostate-specific antigen doubling time |
| PSA-V | prostate-specific antigen velocity |
| PSMA-PET-CT | prostate-specific membrane antigen–positron emission tomography–computed tomography (PSMA-PET-CT) |
| SVI | seminal vesicle invasion |
| TNM | tumor–node–metastasis |
| tPSA | total prostate-specific antigen |
| TRUS | transrectal ultrasonography |
| TRUS-Bx | transrectal ultrasonography-guided biopsy |
| USA | United States of America |
References
- Wang, L.; Lu, B.; He, M.; Wang, Y.; Wang, Z.; Du, L. Prostate Cancer Incidence and Mortality: Global Status and Temporal Trends in 89 Countries From 2000 to 2019. Front. Public Health 2022, 10, 811044. [Google Scholar] [CrossRef] [PubMed]
- Raporty | Krajowy Rejestr Nowotworów. Available online: https://onkologia.org.pl/pl/raporty (accessed on 19 April 2025).
- Merriel, S.W.D.; Funston, G.; Hamilton, W. Prostate Cancer in Primary Care. Adv. Ther. 2018, 35, 1285–1294. [Google Scholar] [CrossRef]
- Gnanapragasam, V.J.; Greenberg, D.; Burnet, N. Urinary symptoms and prostate cancer—The misconception that may be preventing earlier presentation and better survival outcomes. BMC Med. 2022, 20, 264. [Google Scholar] [CrossRef] [PubMed]
- Aaron, L.; Franco, O.E.; Hayward, S.W. Review of Prostate Anatomy and Embryology and the Etiology of Benign Prostatic Hyperplasia. Urol. Clin. N. Am. 2016, 43, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Rebello, R.J.; Oing, C.; Knudsen, K.E.; Loeb, S.; Johnson, D.C.; Reiter, R.E.; Gillessen, S.; Van der Kwast, T.; Bristow, R.G. Prostate cancer. Nat. Rev. Dis. Prim. 2021, 7, 9. [Google Scholar] [CrossRef]
- Yacoub, J.H.; Oto, A. MR Imaging of Prostate Zonal Anatomy. Radiol. Clin. N. Am. 2018, 56, 197–209. [Google Scholar] [CrossRef]
- Stenman, U.-H.; Leinonen, J.; Zhang, W.-M.; Finne, P. Prostate-specific antigen. Semin. Cancer Biol. 1999, 9, 83–93. [Google Scholar] [CrossRef]
- Prostate Cancer—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/prost.html (accessed on 19 April 2025).
- Culp, M.B.; Soerjomataram, I.; Efstathiou, J.A.; Bray, F.; Jemal, A. Recent Global Patterns in Prostate Cancer Incidence and Mortality Rates. Eur. Urol. 2020, 77, 38–52. [Google Scholar] [CrossRef]
- CDC. U.S. Cancer Statistics Prostate Cancer Stat Bite; U.S. Department of Health and Human Services: Washington, DC, USA, 2021.
- What Are the Survival Rates for Prostate Cancer? | American Cancer Society. Available online: https://www.cancer.org/cancer/types/prostate-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 12 June 2025).
- OECD; European Commission. EU Country Cancer Profile: Poland 2025; EU Country Cancer Profiles; OECD Publishing: Paris, France, 2025. [Google Scholar]
- Barańska, K.; Miklewska, M.; Wnętrzak, I.; Wojciechowska, U.; Didkowska, J.A. Geographical disparities in survival rates for urological cancers in Poland from 2000 to 2015. Nowotwory 2023, 73, 347–353. [Google Scholar] [CrossRef]
- Witkowicz-Matolicz, A. Reversing the Rising Trend in Prostate Cancer Mortality in Poland | Cancerworld Magazine. Available online: https://cancerworld.net/reversing-the-rise-in-prostate-cancer-mortality-in-poland/ (accessed on 12 June 2025).
- Barber, L.; Gerke, T.; Markt, S.C.; Peisch, S.F.; Wilson, K.M.; Ahearn, T.; Giovannucci, E.; Parmigiani, G.; Mucci, L.A. Family History of Breast or Prostate Cancer and Prostate Cancer Risk. Clin. Cancer Res. 2018, 24, 5910–5917. [Google Scholar] [CrossRef]
- Vietri, M.T.; D’Elia, G.; Caliendo, G.; Resse, M.; Casamassimi, A.; Passariello, L.; Albanese, L.; Cioffi, M.; Molinari, A.M. Hereditary Prostate Cancer: Genes Related, Target Therapy and Prevention. Int. J. Mol. Sci. 2021, 22, 3753. [Google Scholar] [CrossRef] [PubMed]
- Gulati, R.; Cheng, H.H.; Lange, P.H.; Nelson, P.S.; Etzioni, R. Screening Men at Increased Risk for Prostate Cancer Diagnosis: Model Estimates of Benefits and Harms. Cancer Epidemiol. Biomark. Prev. 2017, 26, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Giri, V.N.; Hegarty, S.E.; Hyatt, C.; O’Leary, E.; Garcia, J.; Knudsen, K.E.; Kelly, W.K.; Gomella, L.G. Germline genetic testing for inherited prostate cancer in practice: Implications for genetic testing, precision therapy, and cascade testing. Prostate 2019, 79, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, T.; Frost, D.; Barrowdale, D.; Evans, D.G.; Bancroft, E.; Adlard, J.; Ahmed, M.; Barwell, J.; Brady, A.F.; Brewer, C.; et al. Prostate Cancer Risks for Male BRCA1 and BRCA2 Mutation Carriers: A Prospective Cohort Study. Eur. Urol. 2020, 77, 24–35. [Google Scholar] [CrossRef]
- Castro, E.; Goh, C.; Olmos, D.; Saunders, E.; Leongamornlert, D.; Tymrakiewicz, M.; Mahmud, N.; Dadaev, T.; Govindasami, K.; Guy, M.; et al. Germline BRCA Mutations Are Associated with Higher Risk of Nodal Involvement, Distant Metastasis, and Poor Survival Outcomes in Prostate Cancer. J. Clin. Oncol. 2013, 31, 1748–1757. [Google Scholar] [CrossRef]
- Mano, R.; Tamir, S.; Kedar, I.; Benjaminov, O.; Baniel, J.; Tabachnik, T.; Margel, D. Malignant Abnormalities in Male BRCA Mutation Carriers. JAMA Oncol. 2018, 4, 872–874. [Google Scholar] [CrossRef]
- Kamangar, F.; Dores, G.M.; Anderson, W.F. Patterns of Cancer Incidence, Mortality, and Prevalence Across Five Continents: Defining Priorities to Reduce Cancer Disparities in Different Geographic Regions of the World. J. Clin. Oncol. 2006, 24, 2137–2150. [Google Scholar] [CrossRef]
- Carlsson, S.; Assel, M.; Ulmert, D.; Gerdtsson, A.; Hugosson, J.; Vickers, A.; Lilja, H. Screening for Prostate Cancer Starting at Age 50–54 Years. A Population-based Cohort Study. Eur. Urol. 2017, 71, 46–52. [Google Scholar] [CrossRef]
- Albright, F.; Stephenson, R.A.; Agarwal, N.; Teerlink, C.C.; Lowrance, W.T.; Farnham, J.M.; Albright, L.A.C. Prostate cancer risk prediction based on complete prostate cancer family history. Prostate 2015, 75, 390–398. [Google Scholar] [CrossRef]
- Bell, K.J.L.; Del Mar, C.; Wright, G.; Dickinson, J.; Glasziou, P. Prevalence of incidental prostate cancer: A systematic review of autopsy studies. Int. J. Cancer 2015, 137, 1749–1757. [Google Scholar] [CrossRef]
- Braunhut, B.L.; Punnen, S.; Kryvenko, O.N. Updates on Grading and Staging of Prostate Cancer. Surg. Pathol. Clin. 2018, 11, 759–774. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 8th ed.; Union for International Cancer Control: Geneva, Switzerland, 2017; pp. 1–272. [Google Scholar]
- Kweldam, C.F.; van Leenders, G.J.; van der Kwast, T. Grading of prostate cancer: A work in progress. Histopathology 2019, 74, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Cornford, P.; Tilki, D.; van den Bergh, R.C.N.; Eberli, D.; De Meerleer, G.; De Santis, M.; Gillessen, S.; Henry, A.M.; van Leenders, G.J.L.H.; Oldenburg, J.; et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer–March 2025 Limited Update; EAU Guidelines Office: Arnhem, The Netherlands, 2025; ISBN 978-94-92671-29-5. [Google Scholar]
- Linkon, A.H.M.; Labib, M.M.; Hasan, T.; Hossain, M.; Jannat, M.-E. Deep learning in prostate cancer diagnosis and Gleason grading in histopathology images: An extensive study. Inform. Med. Unlocked 2021, 24, 100582. [Google Scholar] [CrossRef]
- Martin, R.M.; Donovan, J.L.; Turner, E.L.; Metcalfe, C.; Young, G.J.; Walsh, E.I.; Lane, J.A.; Noble, S.; Oliver, S.E.; Evans, S.; et al. Effect of a Low-Intensity PSA-Based Screening Intervention on Prostate Cancer Mortality. JAMA 2018, 319, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, S.; Assel, M.; Sjoberg, D.; Ulmert, D.; Hugosson, J.; Lilja, H.; Vickers, A. Influence of blood prostate specific antigen levels at age 60 on benefits and harms of prostate cancer screening: Population based cohort study. BMJ 2014, 348, g2296. [Google Scholar] [CrossRef]
- Vickers, A.J.; Ulmert, D.; Sjoberg, D.D.; Bennette, C.J.; Bjork, T.; Gerdtsson, A.; Manjer, J.; Nilsson, P.M.; Dahlin, A.; Bjartell, A.; et al. Strategy for detection of prostate cancer based on relation between prostate specific antigen at age 40–55 and long term risk of metastasis: Case-control study. BMJ 2013, 346, f2023. [Google Scholar] [CrossRef] [PubMed]
- Gelfond, J.; Choate, K.; Ankerst, D.P.; Hernandez, J.; Leach, R.J.; Thompson, I.M. Intermediate-Term Risk of Prostate Cancer is Directly Related to Baseline Prostate Specific Antigen: Implications for Reducing the Burden of Prostate Specific Antigen Screening. J. Urol. 2015, 194, 46–51. [Google Scholar] [CrossRef]
- Hugosson, J.; Roobol, M.J.; Månsson, M.; Tammela, T.L.J.; Zappa, M.; Nelen, V.; Kwiatkowski, M.; Lujan, M.; Carlsson, S.V.; Talala, K.M.; et al. A 16-yr Follow-up of the European Randomized study of Screening for Prostate Cancer. Eur. Urol. 2019, 76, 43–51. [Google Scholar] [CrossRef]
- Segundo, C.G.-S.; Gómez-Iturriaga, A.; Couñago, F. Are all prostate cancer patients “fit” for salvage radiotherapy? World J. Clin. Oncol. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Sekhoacha, M.; Riet, K.; Motloung, P.; Gumenku, L.; Adegoke, A.; Mashele, S. Prostate Cancer Review: Genetics, Diagnosis, Treatment Options, and Alternative Approaches. Molecules 2022, 27, 5730. [Google Scholar] [CrossRef]
- Eklund, M.; Jäderling, F.; Discacciati, A.; Bergman, M.; Annerstedt, M.; Aly, M.; Glaessgen, A.; Carlsson, S.; Grönberg, H.; Nordström, T. MRI-Targeted or Standard Biopsy in Prostate Cancer Screening. N. Engl. J. Med. 2021, 385, 908–920. [Google Scholar] [CrossRef] [PubMed]
- Kasivisvanathan, V.; Rannikko, A.S.; Borghi, M.; Panebianco, V.; Mynderse, L.A.; Vaarala, M.H.; Briganti, A.; Budäus, L.; Hellawell, G.; Hindley, R.G.; et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N. Engl. J. Med. 2018, 378, 1767–1777. [Google Scholar] [CrossRef] [PubMed]
- Ahdoot, M.; Wilbur, A.R.; Reese, S.E.; Lebastchi, A.H.; Mehralivand, S.; Gomella, P.T.; Bloom, J.; Gurram, S.; Siddiqui, M.; Pinsky, P.; et al. MRI-Targeted, Systematic, and Combined Biopsy for Prostate Cancer Diagnosis. N. Engl. J. Med. 2020, 382, 917–928. [Google Scholar] [CrossRef]
- Drost, F.-J.H.; Osses, D.F.; Nieboer, D.; Steyerberg, E.W.; Bangma, C.H.; Roobol, M.J.; Schoots, I.G. Prostate MRI, with or without MRI-targeted biopsy, and systematic biopsy for detecting prostate cancer. Cochrane Database Syst. Rev. 2019, 2019, CD012663. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.B.; Oberlin, D.T.; Razmaria, A.A.; Choy, B.; Zagaja, G.P.; Shalhav, A.L.; Meeks, J.J.; Yang, X.J.; Paner, G.P.; Eggener, S.E. Extraprostatic Extension Is Extremely Rare for Contemporary Gleason Score 6 Prostate Cancer. Eur. Urol. 2017, 72, 455–460. [Google Scholar] [CrossRef]
- Ross, H.M.; Kryvenko, O.N.; Cowan, J.E.; Simko, J.P.; Wheeler, T.M.; Epstein, J.I. Do Adenocarcinomas of the Prostate With Gleason Score (GS)≤6 Have the Potential to Metastasize to Lymph Nodes? Am. J. Surg. Pathol. 2012, 36, 1346–1352. [Google Scholar] [CrossRef]
- Naji, L.; Randhawa, H.; Sohani, Z.; Dennis, B.; Lautenbach, D.; Kavanagh, O.; Bawor, M.; Banfield, L.; Profetto, J. Digital Rectal Examination for Prostate Cancer Screening in Primary Care: A Systematic Review and Meta-Analysis. Ann. Fam. Med. 2018, 16, 149–154. [Google Scholar] [CrossRef]
- Magklara, A.; Cheung, C.C.; Asa, S.L.; Diamandis, E.P. Expression of prostate-specific antigen and human glandular kallikrein 2 in the thyroid gland. Clin. Chim. Acta 2000, 300, 171–180. [Google Scholar] [CrossRef]
- Balk, S.P.; Ko, Y.-J.; Bubley, G.J. Biology of Prostate-Specific Antigen. J. Clin. Oncol. 2003, 21, 383–391. [Google Scholar] [CrossRef]
- Pérez-Ibave, D.C.; Burciaga-Flores, C.H.; Elizondo-Riojas, M.-Á. Prostate-specific antigen (PSA) as a possible biomarker in non-prostatic cancer: A review. Cancer Epidemiol. 2018, 54, 48–55. [Google Scholar] [CrossRef]
- Wajdowicz, H.; Bartusik-Aebisher, D.; Aebisher, D. Prostate-specific antigen (PSA). In The Biochemical Guide to Proteins; StatPearls Publishing: Tampa, FL, USA, 2023; pp. 159–163. ISBN 9798886975352. [Google Scholar]
- Brosman, S.A.; Kim, E.D. Prostate-Specific Antigen Testing: Overview, Physiologic Characteristics of PSA, Other Prostate Cancer Markers. Available online: https://emedicine.medscape.com/article/457394-overview (accessed on 10 June 2025).
- David, M.K.; Leslie, S.W. Prostate-Specific Antigen; StatPearls: Petersburg, FL, USA, 2025. [Google Scholar]
- Oesterling, J.E. Serum Prostate-Specific Antigen in a Community-Based Population of Healthy Men. JAMA 1993, 270, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Newton, M.R.; Phillips, S.; Chang, S.S.; Clark, P.E.; Cookson, M.S.; Davis, R.; Fowke, J.H.; Herrell, S.D.; Baumgartner, R.; Chan, R.; et al. Smaller Prostate Size Predicts High Grade Prostate Cancer at Final Pathology. J. Urol. 2010, 184, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.; Talwar, M.; Singh, P. Low free to total PSA ratio is not a good discriminator of chronic prostatitis and prostate cancer: An Indian experience. Indian J. Cancer 2014, 51, 335–337. [Google Scholar] [CrossRef]
- Omri, N.; Kamil, M.; Alexander, K.; Alexander, K.; Edmond, S.; Ariel, Z.; David, K.; Gilad, A.E.; Azik, H. Association between PSA density and pathologically significant prostate cancer: The impact of prostate volume. Prostate 2020, 80, 1444–1449. [Google Scholar] [CrossRef]
- Yusim, I.; Krenawi, M.; Mazor, E.; Novack, V.; Mabjeesh, N.J. The use of prostate specific antigen density to predict clinically significant prostate cancer. Sci. Rep. 2020, 10, 20015. [Google Scholar] [CrossRef]
- Vickers, A.J.; Brewster, S.F. PSA Velocity and Doubling Time in Diagnosis and Prognosis of Prostate Cancer. Br. J. Med. Surg. Urol. 2012, 5, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Grossman, D.C.; Curry, S.J.; Owens, D.K.; Bibbins-Domingo, K.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Ebell, M.; Epling, J.W.; Kemper, A.R.; et al. Screening for Prostate Cancer. JAMA 2018, 319, 1901. [Google Scholar] [CrossRef]
- Fenton, J.J.; Weyrich, M.S.; Durbin, S.; Liu, Y.; Bang, H.; Melnikow, J. Prostate-Specific Antigen–Based Screening for Prostate Cancer. JAMA 2018, 319, 1914. [Google Scholar] [CrossRef]
- Etzioni, R.; Gulati, R.; Cooperberg, M.R.; Penson, D.M.; Weiss, N.S.; Thompson, I.M. Limitations of Basing Screening Policies on Screening Trials. Med. Care 2013, 51, 295–300. [Google Scholar] [CrossRef]
- Hayes, J.H.; Barry, M.J. Screening for Prostate Cancer With the Prostate-Specific Antigen Test. JAMA 2014, 311, 1143. [Google Scholar] [CrossRef]
- Schröder, F.H.; Hugosson, J.; Roobol, M.J.; Tammela, T.L.J.; Ciatto, S.; Nelen, V.; Kwiatkowski, M.; Lujan, M.; Lilja, H.; Zappa, M.; et al. Screening and Prostate-Cancer Mortality in a Randomized European Study. N. Engl. J. Med. 2009, 360, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- Andriole, G.L.; Crawford, E.D.; Grubb, R.L.; Buys, S.S.; Chia, D.; Church, T.R.; Fouad, M.N.; Gelmann, E.P.; Kvale, P.A.; Reding, D.J.; et al. Mortality Results from a Randomized Prostate-Cancer Screening Trial. N. Engl. J. Med. 2009, 360, 1310–1319. [Google Scholar] [CrossRef]
- Sandblom, G.; Varenhorst, E.; Rosell, J.; Lofman, O.; Carlsson, P. Randomised prostate cancer screening trial: 20 year follow-up. BMJ 2011, 342, d1539. [Google Scholar] [CrossRef]
- Den, R.B.; Yousefi, K.; Trabulsi, E.J.; Abdollah, F.; Choeurng, V.; Feng, F.Y.; Dicker, A.P.; Lallas, C.D.; Gomella, L.G.; Davicioni, E.; et al. Genomic classifier identifies men with adverse pathology after radical prostatectomy who benefit from adjuvant radiation therapy. J. Clin. Oncol. 2015, 33, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Spratt, D.E.; Yousefi, K.; Deheshi, S.; Ross, A.E.; Den, R.B.; Schaeffer, E.M.; Trock, B.J.; Zhang, J.; Glass, A.G.; Dicker, A.P.; et al. Individual patient-level meta-Analysis of the performance of the decipher genomic classifier in high-risk men after prostatectomy to predict development of metastatic disease. J. Clin. Oncol. 2017, 35, 1991–1998. [Google Scholar] [CrossRef] [PubMed]
- Cullen, J.; Rosner, I.L.; Brand, T.C.; Zhang, N.; Tsiatis, A.C.; Moncur, J.; Ali, A.; Chen, Y.; Knezevic, D.; Maddala, T.; et al. A biopsy-based 17-gene genomic prostate score predicts recurrence after radical prostatectomy and adverse surgical pathology in a racially diverse population of men with clinically low- and intermediate-risk prostate cancer. Eur. Urol. 2015, 68, 123–131. [Google Scholar] [CrossRef]
- Badani, K.K.; Kemeter, M.J.; Febbo, P.G.; Lawrence, H.J.; Denes, B.S.; Rothney, M.P.; Rothberg, M.B.; Brown, G.A. The Impact of a Biopsy Based 17-Gene Genomic Prostate Score on Treatment Recommendations in Men with Newly Diagnosed Clinically Prostate Cancer Who are Candidates for Active Surveillance. Urol. Pract. 2015, 2, 181–189. [Google Scholar] [CrossRef]
- Spohn, S.K.B.; Draulans, C.; Kishan, A.U.; Spratt, D.; Ross, A.; Maurer, T.; Tilki, D.; Berlin, A.; Blanchard, P.; Collins, S.; et al. Genomic Classifiers in Personalized Prostate Cancer Radiation Therapy Approaches: A Systematic Review and Future Perspectives Based on International Consensus. Int. J. Radiat. Oncol. 2023, 116, 503–520. [Google Scholar] [CrossRef]
- Shore, N.D.; Kella, N.; Moran, B.; Boczko, J.; Bianco, F.J.; Crawford, E.D.; Davis, T.; Roundy, K.M.; Rushton, K.; Grier, C.; et al. Impact of the Cell Cycle Progression Test on Physician and Patient Treatment Selection for Localized Prostate Cancer. J. Urol. 2016, 195, 612–618. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer. Version 4.2024. 2024. Available online: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (accessed on 22 June 2025).
- Turkbey, B.; Pinto, P.A.; Choyke, P.L. Imaging techniques for prostate cancer: Implications for focal therapy. Nat. Rev. Urol. 2009, 6, 191–203. [Google Scholar] [CrossRef]
- Ahmed, H.U.; El-Shater Bosaily, A.; Brown, L.C.; Gabe, R.; Kaplan, R.; Parmar, M.K.; Collaco-Moraes, Y.; Ward, K.; Hindley, R.G.; Freeman, A.; et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): A paired validating confirmatory study. Lancet 2017, 389, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Turkbey, B.; Albert, P.S.; Kurdziel, K.; Choyke, P.L. Imaging Localized Prostate Cancer: Current Approaches and New Developments. Am. J. Roentgenol. 2009, 192, 1471–1480. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, L.; Chen, Y.; Cai, Y.; Jiang, H.; Ding, Q. The predictive efficacy of hypoechoic lesion in ultrasound for prostate cancer in Chinese people: Five-year experience in a moderated 10-core transperineal prostate biopsy procedure. Oncotarget 2017, 8, 79433–79440. [Google Scholar] [CrossRef]
- Uno, H.; Taniguchi, T.; Seike, K.; Kato, D.; Takai, M.; Iinuma, K.; Horie, K.; Nakane, K.; Koie, T. The accuracy of prostate cancer diagnosis in biopsy-naive patients using combined magnetic resonance imaging and transrectal ultrasound fusion-targeted prostate biopsy. Transl. Androl. Urol. 2021, 10, 2982–2989. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.K.; Song, W.H.; Lee, S.S.; Lee, H.J.; Kim, T.U.; Park, S.-W. Impact of Ultrasonographic Findings on Cancer Detection Rate during Magnetic Resonance Image/Ultrasonography Fusion-Targeted Prostate Biopsy. World J. Mens. Health 2023, 41, 743. [Google Scholar] [CrossRef]
- Jahanandish, H.; Sang, S.; Li, C.X.; Vesal, S.; Bhattacharya, I.; Lee, J.H.; Fan, R.; Sonna, G.A.; Rusu, M. Multimodal MRI-Ultrasound AI for Prostate Cancer Detection Outperforms Radiologist MRI Interpretation: A Multi-Center Study. arXiv 2025. [Google Scholar] [CrossRef]
- Chen, F.K.; de Castro Abreu, A.L.; Palmer, S.L. Utility of Ultrasound in the Diagnosis, Treatment, and Follow-up of Prostate Cancer: State of the Art. J. Nucl. Med. 2016, 57, 13S–18S. [Google Scholar] [CrossRef]
- Noh, T.I.L.; Shin, Y.S.; Shim, J.S.; Yoon, J.H.; Kim, J.H.; Bae, J.H.; Moon, D.G.; Park, J.Y. Are Hypoechoic Lesions on Transrectal Ultrasonography a Marker for Clinically Significant Prostate Cancer? Korean J. Urol. 2013, 54, 666. [Google Scholar] [CrossRef] [PubMed]
- Gomella, L.G.; El-Gabry, E.A.; Strup, S.E.; Halpern, E. Ultrasound contrast agents for prostate imaging and biopsy. Urol. Oncol. Semin. Orig. Investig. 2001, 6, 189–192. [Google Scholar] [CrossRef]
- Harvey, C.J.; Pilcher, J.; Richenberg, J.; Patel, U.; Frauscher, F. Applications of transrectal ultrasound in prostate cancer. Br. J. Radiol. 2012, 85, S3–S17. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, P.; Geng, H.; Liu, Y.; Wang, E. Ultrasound and advanced imaging techniques in prostate cancer diagnosis: A comparative study of mpMRI, TRUS, and PET/CT. J. X-Ray Sci. Technol. Clin. Appl. Diagn. Ther. 2025, 33, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Hendrikx, A.; Safarik, L.; Hammerer, P. TRUS and Biopsy: Practical Aspects. Eur. Urol. 2002, 41, I–X. [Google Scholar] [CrossRef]
- Chavoshi, M.; Mirshahvalad, S.A.; Zamani, S.; Radmard, A.R.; Fallahi, B.; Mousavi, S.A. Whole-body low-dose CT can be of value in prostate cancer decision-making: A retrospective study on 601 patients. Insights Imaging 2023, 14, 124. [Google Scholar] [CrossRef]
- Schlemmer, H.-P.; Krause, B.J.; Schütz, V.; Bonekamp, D.; Schwarzenböck, S.M.; Hohenfellner, M. Imaging of prostate cancer. Dtsch. Arztebl. Int. 2021, 118, 713–719. [Google Scholar] [CrossRef]
- Turpin, A.; Girard, E.; Baillet, C.; Pasquier, D.; Olivier, J.; Villers, A.; Puech, P.; Penel, N. Imaging for Metastasis in Prostate Cancer: A Review of the Literature. Front. Oncol. 2020, 10, 55. [Google Scholar] [CrossRef]
- Manfredi, M.; Mele, F.; Garrou, D.; Walz, J.; Fütterer, J.J.; Russo, F.; Vassallo, L.; Villers, A.; Emberton, M.; Valerio, M. Multiparametric prostate MRI: Technical conduct, standardized report and clinical use. Minerva Urol. Nephrol. 2018, 70, 9–21. [Google Scholar] [CrossRef]
- Gold, S.A.; Shih, J.H.; Rais-Bahrami, S.; Bloom, J.B.; Vourganti, S.; Singla, N.; Baroni, R.H.; Coker, M.A.; Fialkoff, J.; Noschang, J.; et al. When to Biopsy the Seminal Vesicles: A Validated Multiparametric Magnetic Resonance Imaging and Target Driven Model to Detect Seminal Vesicle Invasion of Prostate Cancer. J. Urol. 2019, 201, 943–949. [Google Scholar] [CrossRef] [PubMed]
- de Rooij, M.; Hamoen, E.H.J.; Witjes, J.A.; Barentsz, J.O.; Rovers, M.M. Accuracy of Magnetic Resonance Imaging for Local Staging of Prostate Cancer: A Diagnostic Meta-analysis. Eur. Urol. 2016, 70, 233–245. [Google Scholar] [CrossRef] [PubMed]
- American College of Radiology®; Committee on PI-RADS®. PI-RADS 2019 v2.1. Available online: https://www.acr.org/-/media/ACR/Files/RADS/PI-RADS/PIRADS-V2-1.pdf (accessed on 1 January 2025).
- Perera, M.; Papa, N.; Roberts, M.; Williams, M.; Udovicich, C.; Vela, I.; Christidis, D.; Bolton, D.; Hofman, M.S.; Lawrentschuk, N.; et al. Gallium-68 Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer—Updated Diagnostic Utility, Sensitivity, Specificity, and Distribution of Prostate-specific Membrane Antigen-avid Lesions: A Systematic Review and Meta-. Eur. Urol. 2020, 77, 403–417. [Google Scholar] [CrossRef]
- Turkbey, B.; Mani, H.; Shah, V.; Rastinehad, A.R.; Bernardo, M.; Pohida, T.; Pang, Y.; Daar, D.; Benjamin, C.; McKinney, Y.L.; et al. Multiparametric 3T Prostate Magnetic Resonance Imaging to Detect Cancer: Histopathological Correlation Using Prostatectomy Specimens Processed in Customized Magnetic Resonance Imaging Based Molds. J. Urol. 2011, 186, 1818–1824. [Google Scholar] [CrossRef]
- Oerther, B.; Engel, H.; Bamberg, F.; Sigle, A.; Gratzke, C.; Benndorf, M. Cancer detection rates of the PI-RADSv2.1 assessment categories: Systematic review and meta-analysis on lesion level and patient level. Prostate Cancer Prostatic Dis. 2022, 25, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Sklinda, K.; Dąbrowska, A.; Olejnik, P.; Walecki, J. Komentarz dotyczący oceny i opisu badania proponowanego w PI-RADS v2: Komentarz dotyczący sposobu przeprowadzania badania multiparametrycznego MR w PI-RADS v2 Część 4. Przegląd Urol. 2017, 103, 8–11. [Google Scholar]
- Muller, B.G.; van den Bos, W.; Brausi, M.; Fütterer, J.J.; Ghai, S.; Pinto, P.A.; Popeneciu, I.V.; de Reijke, T.M.; Robertson, C.; de la Rosette, J.J.M.C.H.; et al. Follow-up modalities in focal therapy for prostate cancer: Results from a Delphi consensus project. World J. Urol. 2015, 33, 1503–1509. [Google Scholar] [CrossRef]
- Turkbey, B.; Rosenkrantz, A.B.; Haider, M.A.; Padhani, A.R.; Villeirs, G.; Macura, K.J.; Tempany, C.M.; Choyke, P.L.; Cornud, F.; Margolis, D.J.; et al. Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur. Urol. 2019, 76, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Zabihollahy, F.; Raman, S.; Wibulpolprasert, P.; Reiter, R.; Wu, H.; Sung, K. Localization of Prostate Cancer at 3-T Multiparametric Magnetic Resonance Imaging using Prostate Sector Map. In Proceedings of the Joint Annual Meeting ISMRM-ESMRMB ISMRT 31st Annual Meeting, London, UK, 7–12 May 2022. [Google Scholar]
- Hegde, J.V.; Mulkern, R.V.; Panych, L.P.; Fennessy, F.M.; Fedorov, A.; Maier, S.E.; Tempany, C.M.C. Multiparametric MRI of prostate cancer: An update on state-of-the-art techniques and their performance in detecting and localizing prostate cancer. J. Magn. Reson. Imaging 2013, 37, 1035–1054. [Google Scholar] [CrossRef]
- Saba, P.; Melnyk, R.; Holler, T.; Oppenheimer, D.; Schuler, N.; Tabayoyong, W.; Bloom, J.; Bandari, J.; Frye, T.; Joseph, J.; et al. Comparison of Multi-Parametric MRI of the Prostate to 3D Prostate Computer Aided Designs and 3D-Printed Prostate Models for Pre-Operative Planning of Radical Prostatectomies: A Pilot Study. Urology 2021, 158, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Valerio, M.; Donaldson, I.; Emberton, M.; Ehdaie, B.; Hadaschik, B.A.; Marks, L.S.; Mozer, P.; Rastinehad, A.R.; Ahmed, H.U. Detection of Clinically Significant Prostate Cancer Using Magnetic Resonance Imaging–Ultrasound Fusion Targeted Biopsy: A Systematic Review. Eur. Urol. 2015, 68, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Haack, M.; Reisen, K.; Ghazy, A.; Stroh, K.; Frey, L.; Sparwasser, P.; Duwe, G.; Mager, R.; Haferkamp, A.; Borgmann, H. Understanding tumor localization in multiparametric MRI of the prostate—Effectiveness of 3D printed models. Front. Surg. 2023, 10, 1264164. [Google Scholar] [CrossRef]
- Johnson, D.C.; Raman, S.S.; Mirak, S.A.; Kwan, L.; Bajgiran, A.M.; Hsu, W.; Maehara, C.K.; Ahuja, P.; Faiena, I.; Pooli, A.; et al. Detection of Individual Prostate Cancer Foci via Multiparametric Magnetic Resonance Imaging. Eur. Urol. 2019, 75, 712–720. [Google Scholar] [CrossRef]
- Bratan, F.; Niaf, E.; Melodelima, C.; Chesnais, A.L.; Souchon, R.; Mège-Lechevallier, F.; Colombel, M.; Rouvière, O. Influence of imaging and histological factors on prostate cancer detection and localisation on multiparametric MRI: A prospective study. Eur. Radiol. 2013, 23, 2019–2029. [Google Scholar] [CrossRef]
- Stabile, A.; Giganti, F.; Kasivisvanathan, V.; Giannarini, G.; Moore, C.M.; Padhani, A.R.; Panebianco, V.; Rosenkrantz, A.B.; Salomon, G.; Turkbey, B.; et al. Factors Influencing Variability in the Performance of Multiparametric Magnetic Resonance Imaging in Detecting Clinically Significant Prostate Cancer: A Systematic Literature Review. Eur. Urol. Oncol. 2020, 3, 145–167. [Google Scholar] [CrossRef] [PubMed]
- Kohestani, K.; Wallström, J.; Dehlfors, N.; Sponga, O.M.; Månsson, M.; Josefsson, A.; Carlsson, S.; Hellström, M.; Hugosson, J. Performance and inter-observer variability of prostate MRI (PI-RADS version 2) outside high-volume centres. Scand. J. Urol. 2019, 53, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Padhani, A.R.; Barentsz, J.; Villeirs, G.; Rosenkrantz, A.B.; Margolis, D.J.; Turkbey, B.; Thoeny, H.C.; Cornud, F.; Haider, M.A.; Macura, K.J.; et al. PI-RADS Steering Committee: The PI-RADS Multiparametric MRI and MRI-directed Biopsy Pathway. Radiology 2019, 292, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Schoots, I.G.; Padhani, A.R.; Rouvière, O.; Barentsz, J.O.; Richenberg, J. Analysis of Magnetic Resonance Imaging–directed Biopsy Strategies for Changing the Paradigm of Prostate Cancer Diagnosis. Eur. Urol. Oncol. 2020, 3, 32–41. [Google Scholar] [CrossRef]
- Waibel, P.M.A.; Glavynskyi, I.; Fechter, T.; Mix, M.; Kind, F.; Sigle, A.; Jilg, C.A.; Gratzke, C.; Werner, M.; Schilling, O.; et al. Can PSMA PET detect intratumour heterogeneity in histological PSMA expression of primary prostate cancer? Analysis of [68Ga]Ga-PSMA-11 and [18F]PSMA-1007. Eur. J. Nucl. Med. Mol. Imaging 2025, 52, 2023–2033. [Google Scholar] [CrossRef]
- Rosales, J.J.; Betech-Antar, V.; Mínguez, F.; Guillén, E.F.; Prieto, E.; Quincoces, G.; Beorlegui, C.; Fenor de la Maza, M.D.; Díez-Caballero, F.; Miñana, B.; et al. Association of [68Ga]Ga-PSMA-11 PET/CT Metrics with PSA Persistence Following Radical Prostatectomy in Patients with Intermediate- and High-Risk Prostate Cancer. Diagnostics 2025, 15, 301. [Google Scholar] [CrossRef]
- dos Santos Loureiro, G.G.; Duarte Couto, P.; Gambini Gonzalez, J.P.; Alonso Nuñez, O. Comparative Evaluation of (18F)AlF-PSMA-HBED-CC and 68Ga-PSMA-HBED-CC in Staging Intermediate-/High-Risk Prostate Cancer: A Prospective Study. World J. Nucl. Med. 2025, 24, 118–127. [Google Scholar] [CrossRef]
- Kesch, C.; Franiel, T.; Berliner, C.; Fendler, W.P.; Herrmann, K.; Hadaschik, B. Stellenwert der PSMA-PET/CT („prostate-specific membrane antigen positron emission tomography/computed tomography“) im Rahmen des Stagings. Die Urol. 2025, 64, 220–228. [Google Scholar] [CrossRef]
- Islam, M.Z.; Spiro, E.; Yap, P.-T.; Gorin, M.A.; Rowe, S.P. The potential of generative AI with prostate-specific membrane antigen (PSMA) PET/CT: Challenges and future directions. Med. Rev. 2025. [Google Scholar] [CrossRef]
- Eldred-Evans, D.; Burak, P.; Connor, M.J.; Day, E.; Evans, M.; Fiorentino, F.; Gammon, M.; Hosking-Jervis, F.; Klimowska-Nassar, N.; McGuire, W.; et al. Population-Based Prostate Cancer Screening With Magnetic Resonance Imaging or Ultrasonography. JAMA Oncol. 2021, 7, 395. [Google Scholar] [CrossRef]
- Marenco Jimenez, J.L.; Claps, F.; Ramón-Borja, J.C.; Mascarós Martinez, J.M.; Gutierrez, A.W.; Lozano, Á.G.F.; Ramírez-Backhaus, M.; Domìnguez Escrig, J.L.; Serra, A.C.; Rubio-Briones, J. Rebiopsy rate after transperineal or transrectal prostate biopsy. Prostate Int. 2021, 9, 78–81. [Google Scholar] [CrossRef]
- Guo, L.-H.; Wu, R.; Xu, H.-X.; Xu, J.-M.; Wu, J.; Wang, S.; Bo, X.-W.; Liu, B.-J. Comparison between Ultrasound Guided Transperineal and Transrectal Prostate Biopsy: A Prospective, Randomized and Controlled Trial. Sci. Rep. 2015, 5, 16089. [Google Scholar] [CrossRef]
- Ward, A.D.; Crukley, C.; McKenzie, C.A.; Montreuil, J.; Gibson, E.; Romagnoli, C.; Gomez, J.A.; Moussa, M.; Chin, J.; Bauman, G.; et al. Prostate: Registration of Digital Histopathologic Images to in Vivo MR Images Acquired by Using Endorectal Receive Coil. Radiology 2012, 263, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.T.S.; Challacombe, B.; Lawrentschuk, N. Transperineal biopsy of the prostate—Is this the future? Nat. Rev. Urol. 2013, 10, 690–702. [Google Scholar] [CrossRef]
- Schmeusser, B.; Levin, B.; Lama, D.; Sidana, A. Hundred years of transperineal prostate biopsy. Ther. Adv. Urol. 2022, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hübner, N.; Shariat, S.; Remzi, M. Prostate biopsy. Curr. Opin. Urol. 2018, 28, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Peltier, A.; Aoun, F.; Lemort, M.; Kwizera, F.; Paesmans, M.; Van Velthoven, R. MRI-Targeted Biopsies versus Systematic Transrectal Ultrasound Guided Biopsies for the Diagnosis of Localized Prostate Cancer in Biopsy Naïve Men. Biomed. Res. Int. 2015, 2015, 571708. [Google Scholar] [CrossRef]
- Yamada, Y.; Ukimura, O.; Kaneko, M.; Matsugasumi, T.; Fujihara, A.; Vourganti, S.; Marks, L.; Sidana, A.; Klotz, L.; Salomon, G.; et al. Moving away from systematic biopsies: Image-guided prostate biopsy (in-bore biopsy, cognitive fusion biopsy, MRUS fusion biopsy) —Literature review. World J. Urol. 2021, 39, 677–686. [Google Scholar] [CrossRef]
- Osses, D.F.; van Asten, J.J.; Tijsterman, J.D. Cognitive-Targeted versus Magnetic Resonance Imaging-Guided Prostate Biopsy in Prostate Cancer Detection. Curr. Urol. 2018, 11, 182–188. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Z.; Liu, M.; Zhu, G.; Roobol, M.J. Comparison of clinically significant prostate cancer detection by MRI cognitive biopsy and in-bore MRI-targeted biopsy for naïve biopsy patients. Transl. Androl. Urol. 2020, 9, 243–249. [Google Scholar] [CrossRef]
- Wysock, J.S.; Rosenkrantz, A.B.; Huang, W.C.; Stifelman, M.D.; Lepor, H.; Deng, F.-M.; Melamed, J.; Taneja, S.S. A Prospective, Blinded Comparison of Magnetic Resonance (MR) Imaging–Ultrasound Fusion and Visual Estimation in the Performance of MR-targeted Prostate Biopsy: The PROFUS Trial. Eur. Urol. 2014, 66, 343–351. [Google Scholar] [CrossRef]
- Bando, Y.; Teishima, J.; Ueno, Y.; Chiba, K.; Miyake, H. Predictive ability of lesion localization using real-time three-dimensional magnetic resonance imaging/ultrasound fusion prostate biopsy in robot-assisted laparoscopic prostatectomy. Int. J. Urol. 2025, 32, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Bignante, G.; Katz, D.O.; Langbo, W.A.; Orsini, A.; Lasorsa, F.; Cherullo, E.E.; Autorino, R.; Vourganti, S. Robot-assisted MRI/US fusion transperineal prostate biopsy using the Biobot system: A single-centre experience. BJU Int. 2025, 136, 165–168. [Google Scholar] [CrossRef]
- Brodsky, C.N.; Daignault-Newton, S.; Davenport, M.S.; Marchetti, K.A.; Goh, M.; Wei, J.T. How Many Cores Should Be Collected per Region of Interest in Fusion Targeted Prostate Biopsy? A Retrospective Single Institution Statistical Simulation. Urology 2025, 197, 133–140. [Google Scholar] [CrossRef]
- Borde, T.; Varble, N.A.; Hazen, L.A.; Saccenti, L.; Garcia, C.; Digennaro, M.; Gurram, S.; Pinto, P.A.; Turkbey, B.; Wood, B.J. Impact of Discordance Between Magnetic Resonance Imaging and Ultrasound Volume Measurements on Prostate Fusion Biopsy Outcomes. J. Urol. 2025, 213, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Oderda, M.; Dematteis, A.; Calleris, G.; Diamand, R.; Gatti, M.; Marra, G.; Adans-Dester, G.; Al Salhi, Y.; Pastore, A.; Faletti, R.; et al. MRI-Targeted Prostate Fusion Biopsy: What Are We Missing outside the Target? Implications for Treatment Planning. Curr. Oncol. 2024, 31, 4133–4140. [Google Scholar] [CrossRef] [PubMed]
- Falagario, U.G.; Pellegrino, F.; Fanelli, A.; Guzzi, F.; Bartoletti, R.; Cash, H.; Pavlovich, C.; Emberton, M.; Carrieri, G.; Giannarini, G. Prostate cancer detection and complications of MRI-targeted prostate biopsy using cognitive registration, software-assisted image fusion or in-bore guidance: A systematic review and meta-analysis of comparative studies. Prostate Cancer Prostatic Dis. 2024, 28, 270–279. [Google Scholar] [CrossRef]
- Izadpanahi, M.-H.; Elahian, A.; Gholipour, F.; Khorrami, M.-H.; Zargham, M.; Mohammadi Sichani, M.; Alizadeh, F.; Khorrami, F. Diagnostic yield of fusion magnetic resonance-guided prostate biopsy versus cognitive-guided biopsy in biopsy-naive patients: A head-to-head randomized controlled trial. Prostate Cancer Prostatic Dis. 2021, 24, 1103–1109. [Google Scholar] [CrossRef]
- Simmons, L.A.M.; Kanthabalan, A.; Arya, M.; Briggs, T.; Barratt, D.; Charman, S.C.; Freeman, A.; Hawkes, D.; Hu, Y.; Jameson, C.; et al. Accuracy of Transperineal Targeted Prostate Biopsies, Visual Estimation and Image Fusion in Men Needing Repeat Biopsy in the PICTURE Trial. J. Urol. 2018, 200, 1227–1234. [Google Scholar] [CrossRef]
- Bjurlin, M.A.; Meng, X.; Le Nobin, J.; Wysock, J.S.; Lepor, H.; Rosenkrantz, A.B.; Taneja, S.S. Optimization of Prostate Biopsy: The Role of Magnetic Resonance Imaging Targeted Biopsy in Detection, Localization and Risk Assessment. J. Urol. 2014, 192, 648–658. [Google Scholar] [CrossRef]
- Kilic, M.; Vural, M.; Coskun, B.; Acar, Ö.; Saglican, Y.; Akpek, S.; Esen, T. Accuracy of Sampling PI-RADS 4–5 Index Lesions Alone by MRI-guided In-bore Biopsy in Biopsy–naive Patients Undergoing Radical Prostatectomy. Eur. Urol. Focus 2020, 6, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.M.; Rais-Bahrami, S.; Turkbey, B.; George, A.K.; Rothwax, J.; Shakir, N.; Okoro, C.; Raskolnikov, D.; Parnes, H.L.; Linehan, W.M.; et al. Comparison of MR/Ultrasound Fusion–Guided Biopsy With Ultrasound-Guided Biopsy for the Diagnosis of Prostate Cancer. JAMA 2015, 313, 390. [Google Scholar] [CrossRef]
- Serefoglu, E.C.; Altinova, S.; Ugras, N.S.; Akincioglu, E.; Asil, E.; Balbay, D. How reliable is 12-core prostate biopsy procedure in the detection of prostate cancer? Can. Urol. Assoc. J. 2012, 6, E293–E298. [Google Scholar] [CrossRef]
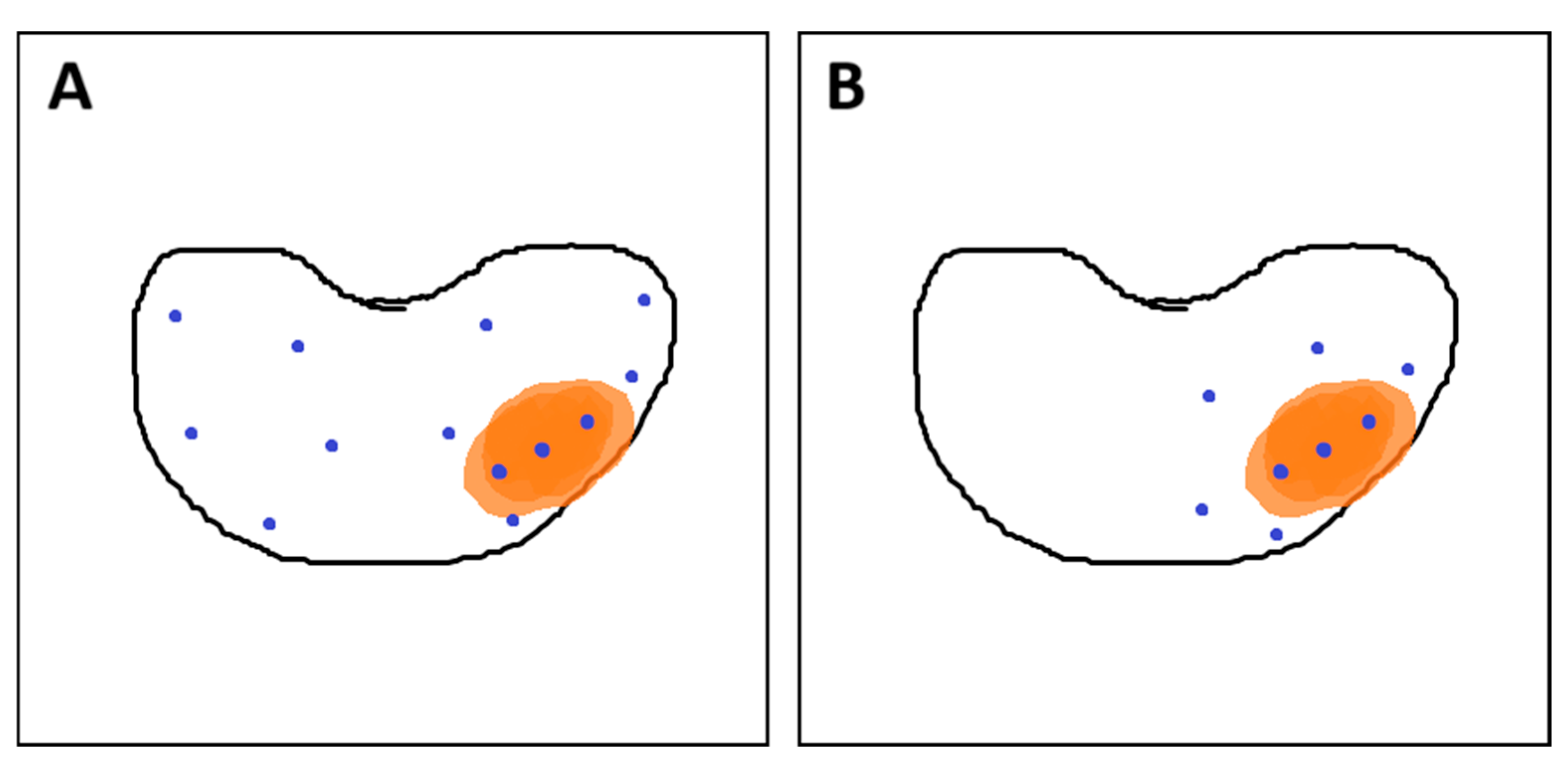
- Hansen, N.L.; Barrett, T.; Lloyd, T.; Warren, A.; Samel, C.; Bratt, O.; Kastner, C. Optimising the number of cores for magnetic resonance imaging-guided targeted and systematic transperineal prostate biopsy. BJU Int. 2020, 125, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Calio, B.P.; Sidana, A.; Sugano, D.; Gaur, S.; Maruf, M.; Jain, A.L.; Merino, M.J.; Choyke, P.L.; Wood, B.J.; Pinto, P.A.; et al. Risk of Upgrading from Prostate Biopsy to Radical Prostatectomy Pathology—Does Saturation Biopsy of Index Lesion during Multiparametric Magnetic Resonance Imaging-Transrectal Ultrasound Fusion Biopsy Help? J. Urol. 2018, 199, 976–982. [Google Scholar] [CrossRef] [PubMed]
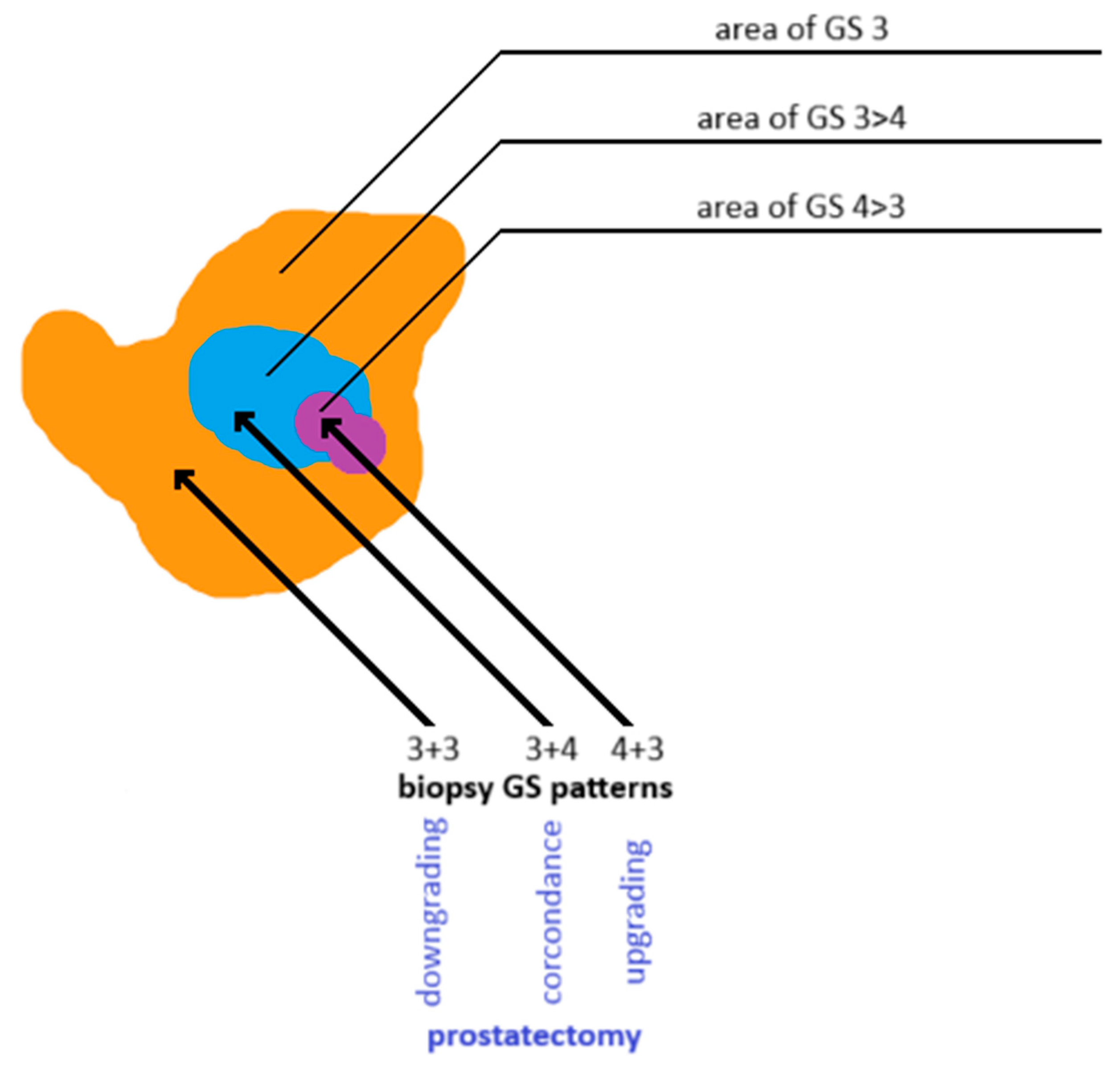
- Goel, S.; Shoag, J.E.; Gross, M.D.; Al Hussein Al Awamlh, B.; Robinson, B.; Khani, F.; Baltich Nelson, B.; Margolis, D.J.; Hu, J.C. Concordance Between Biopsy and Radical Prostatectomy Pathology in the Era of Targeted Biopsy: A Systematic Review and Meta-analysis. Eur. Urol. Oncol. 2020, 3, 10–20. [Google Scholar] [CrossRef]
- Suer, E.; Gokce, M.I.; Gulpinar, O.; Gucal Guclu, A.; Haciyev, P.; Gogus, C.; Turkolmez, K.; Baltaci, S. How significant is upgrade in Gleason score between prostate biopsy and radical prostatectomy pathology while discussing less invasive treatment options? Scand. J. Urol. 2014, 48, 177–182. [Google Scholar] [CrossRef]
- Lardas, M.; Liew, M.; van den Bergh, R.C.; De Santis, M.; Bellmunt, J.; Van den Broeck, T.; Cornford, P.; Cumberbatch, M.G.; Fossati, N.; Gross, T.; et al. Quality of Life Outcomes after Primary Treatment for Clinically Localised Prostate Cancer: A Systematic Review. Eur. Urol. 2017, 72, 869–885. [Google Scholar] [CrossRef]
- Klotz, L.; Vesprini, D.; Sethukavalan, P.; Jethava, V.; Zhang, L.; Jain, S.; Yamamoto, T.; Mamedov, A.; Loblaw, A. Long-Term Follow-Up of a Large Active Surveillance Cohort of Patients With Prostate Cancer. J. Clin. Oncol. 2015, 33, 272–277. [Google Scholar] [CrossRef]
- Diamand, R.; Hollans, M.; Lefebvre, Y.; Sirtaine, N.; Limani, K.; Hawaux, E.; Abou Zahr, R.; Mattlet, A.; Albisinni, S.; Roumeguère, T.; et al. The role of perilesional and multiparametric resonance imaging-targeted biopsies to reduce the risk of upgrading at radical prostatectomy pathology: A retrospective monocentric study. Urol. Oncol. Semin. Orig. Investig. 2022, 40, 192.e11–192.e17. [Google Scholar] [CrossRef]
- Brisbane, W.G.; Priester, A.M.; Ballon, J.; Kwan, L.; Delfin, M.K.; Felker, E.R.; Sisk, A.E.; Hu, J.C.; Marks, L.S. Targeted Prostate Biopsy: Umbra, Penumbra, and Value of Perilesional Sampling. Eur. Urol. 2022, 82, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Romero-Otero, J.; García-Gómez, B.; Duarte-Ojeda, J.M.; Rodríguez-Antolín, A.; Vilaseca, A.; Carlsson, S.V.; Touijer, K.A. Active surveillance for prostate cancer. Int. J. Urol. 2016, 23, 211–218. [Google Scholar] [CrossRef]
- Brawley, S.; Mohan, R.; Nein, C.D. Localized Prostate Cancer: Treatment Options. Am. Fam. Physician 2018, 97, 798–805. [Google Scholar] [PubMed]
- Monni, F.; Fontanella, P.; Grasso, A.; Wiklund, P.; Ou, Y.-C.; Randazzo, M.; Rocco, B.; Montanari, E.; Bianchi, G. Magnetic resonance imaging in prostate cancer detection and management: A systematic review. Minerva Urol. Nephrol. 2017, 69, 567–578. [Google Scholar] [CrossRef]
- Sanda, M.G.; Cadeddu, J.A.; Kirkby, E.; Chen, R.C.; Crispino, T.; Fontanarosa, J.; Freedland, S.J.; Greene, K.; Klotz, L.H.; Makarov, D.V.; et al. Clinically Localized Prostate Cancer: AUA/ASTRO/SUO Guideline. Part I: Risk Stratification, Shared Decision Making, and Care Options. J. Urol. 2018, 199, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Keyes, M.; Crook, J.; Morton, G.; Vigneault, E.; Usmani, N.; Morris, W.J. Treatment options for localized prostate cancer. Can. Fam. Physician 2013, 59, 1269–1274. [Google Scholar] [PubMed]
- Liu, D.; Lehmann, H.P.; Frick, K.D.; Carter, H.B. Active Surveillance Versus Surgery for Low Risk Prostate Cancer: A Clinical Decision Analysis. J. Urol. 2012, 187, 1241–1246. [Google Scholar] [CrossRef]
- Holmberg, L.; Bill-Axelson, A.; Steineck, G.; Garmo, H.; Palmgren, J.; Johansson, E.; Adami, H.-O.; Johansson, J.-E. Results From the Scandinavian Prostate Cancer Group Trial Number 4: A Randomized Controlled Trial of Radical Prostatectomy Versus Watchful Waiting. JNCI Monogr. 2012, 2012, 230–233. [Google Scholar] [CrossRef]
- Jereczek-Fossa, B.A.; Curigliano, G.; Orecchia, R. Systemic Therapies for Non-Metastatic Prostate Cancer: Review of the Literature. Onkologie 2009, 32, 359–363. [Google Scholar] [CrossRef]

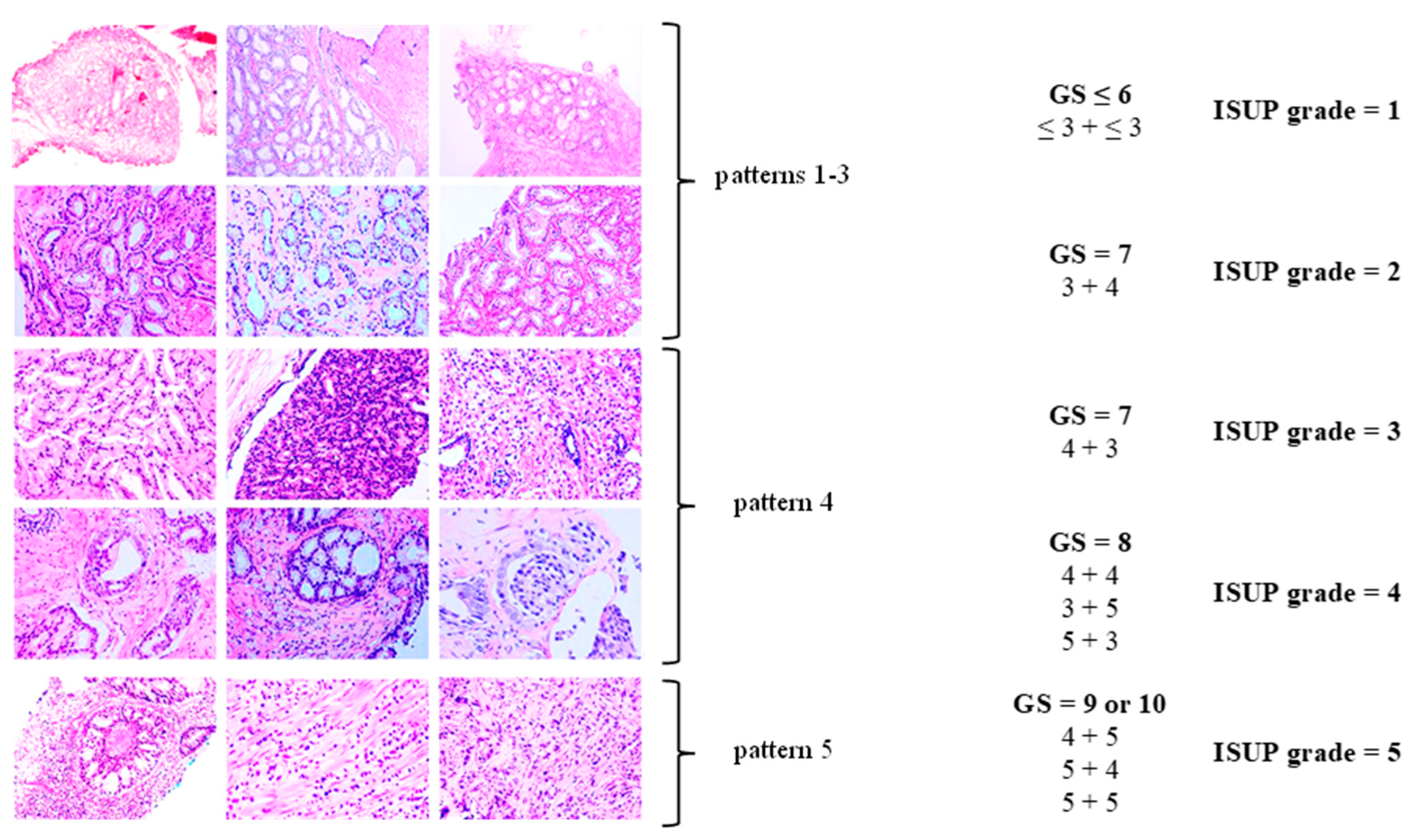

| International Society of Urological Pathology (ISUP) Grade Group | Gleason Score (GS; the Sum of the Scores of the Most Dominant Pattern, as Well as the Second Most Common Pattern) |
|---|---|
| 1 | GS ≤ 6 (≤ 3 + ≤ 3) |
| 2 | GS = 7 (3 + 4) |
| 3 | GS = 7 (4 + 3) |
| 4 | GS = 8 (4 + 4, 3 + 5, or 5 + 3) |
| 5 | GS = 9 or 10 (4 + 5, 5 + 4, or 5 + 5) |
| Low-Risk Group | Intermediate-Risk Group | High-Risk Group | |
|---|---|---|---|
| PSA < 10 ng/mL and GS < 7 (ISUP grade = 1), and T1–T2a | PSA = 10–20 ng/mL or GS = 7 (ISUP grade = 2 or 3), or T2b | PSA > 20 ng/mL or GS > 7 (ISUP grade = 4 or 5), or T2c | T3–T4 or N1 |
| localized disease | locally advanced disease | ||
| PI-RADS Grade | Risk of Malignant Neoplasm |
|---|---|
| 1 | very low (clinically significant cancer is highly unlikely to be present) |
| 2 | low (clinically significant cancer is unlikely to be present) |
| 3 | intermediate (the presence of clinically significant cancer is equivocal) |
| 4 | high (clinically significant cancer is likely to be present) |
| 5 | very high (clinically significant cancer is highly likely to be present) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kania, E.; Janica, M.; Nesterowicz, M.; Modzelewski, W.; Cybulski, M.; Janica, J. Advances and Challenges in Prostate Cancer Diagnosis: A Comprehensive Review. Cancers 2025, 17, 2137. https://doi.org/10.3390/cancers17132137
Kania E, Janica M, Nesterowicz M, Modzelewski W, Cybulski M, Janica J. Advances and Challenges in Prostate Cancer Diagnosis: A Comprehensive Review. Cancers. 2025; 17(13):2137. https://doi.org/10.3390/cancers17132137
Chicago/Turabian StyleKania, Emil, Maciej Janica, Miłosz Nesterowicz, Wojciech Modzelewski, Mateusz Cybulski, and Jacek Janica. 2025. "Advances and Challenges in Prostate Cancer Diagnosis: A Comprehensive Review" Cancers 17, no. 13: 2137. https://doi.org/10.3390/cancers17132137
APA StyleKania, E., Janica, M., Nesterowicz, M., Modzelewski, W., Cybulski, M., & Janica, J. (2025). Advances and Challenges in Prostate Cancer Diagnosis: A Comprehensive Review. Cancers, 17(13), 2137. https://doi.org/10.3390/cancers17132137







