Penile Cancer Profile in a Central European Context: Clinical Characteristics, Prognosis, and Outcomes—Insights from a Polish Tertiary Medical Center
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Statistical Methods
4. Results
4.1. Patient Characteristics
4.2. Surgical Treatments
4.3. Radiotherapy and Chemotherapy
4.4. Histopathological Findings
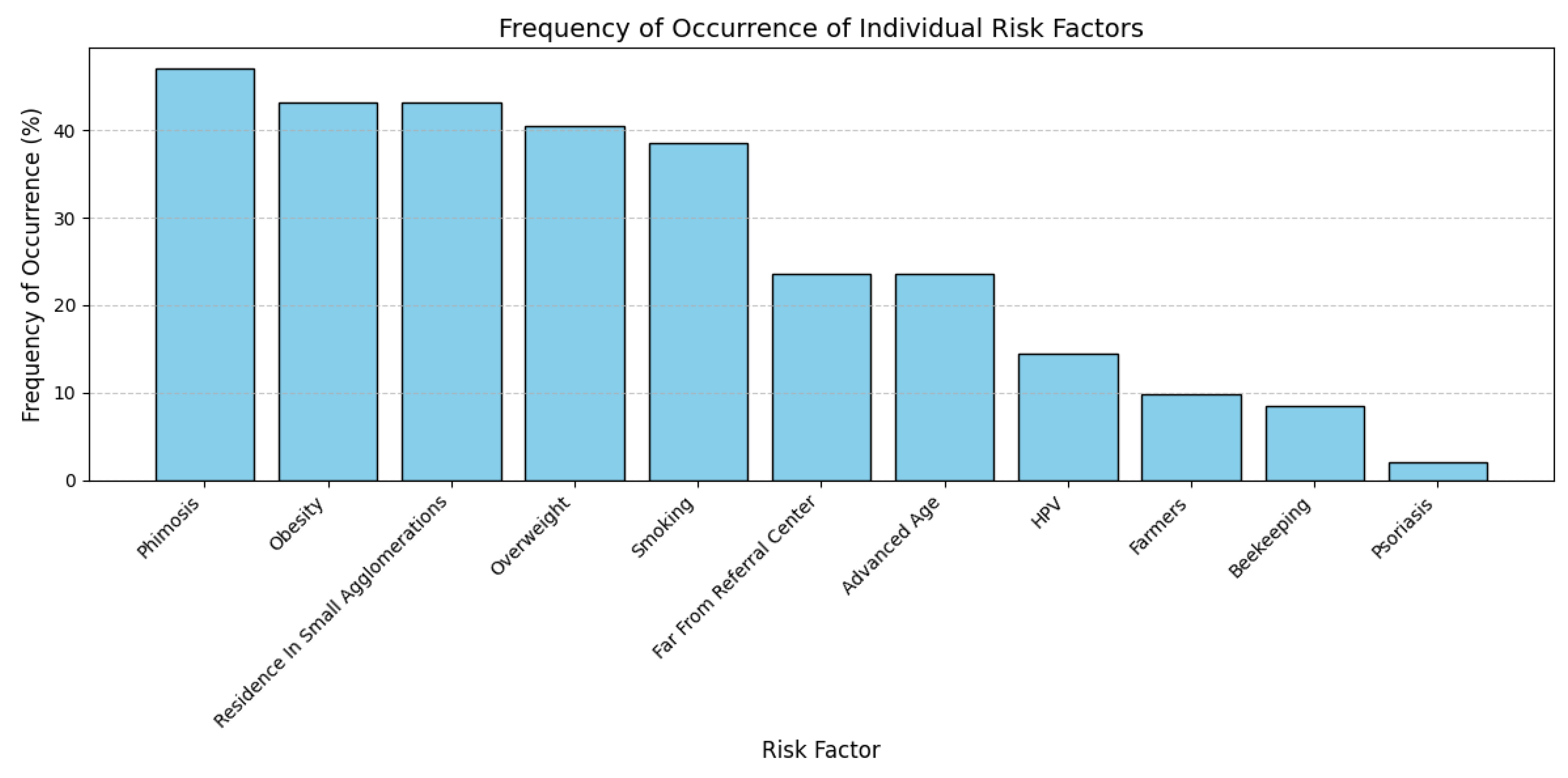
4.5. Clinical and Behavioral Characteristics
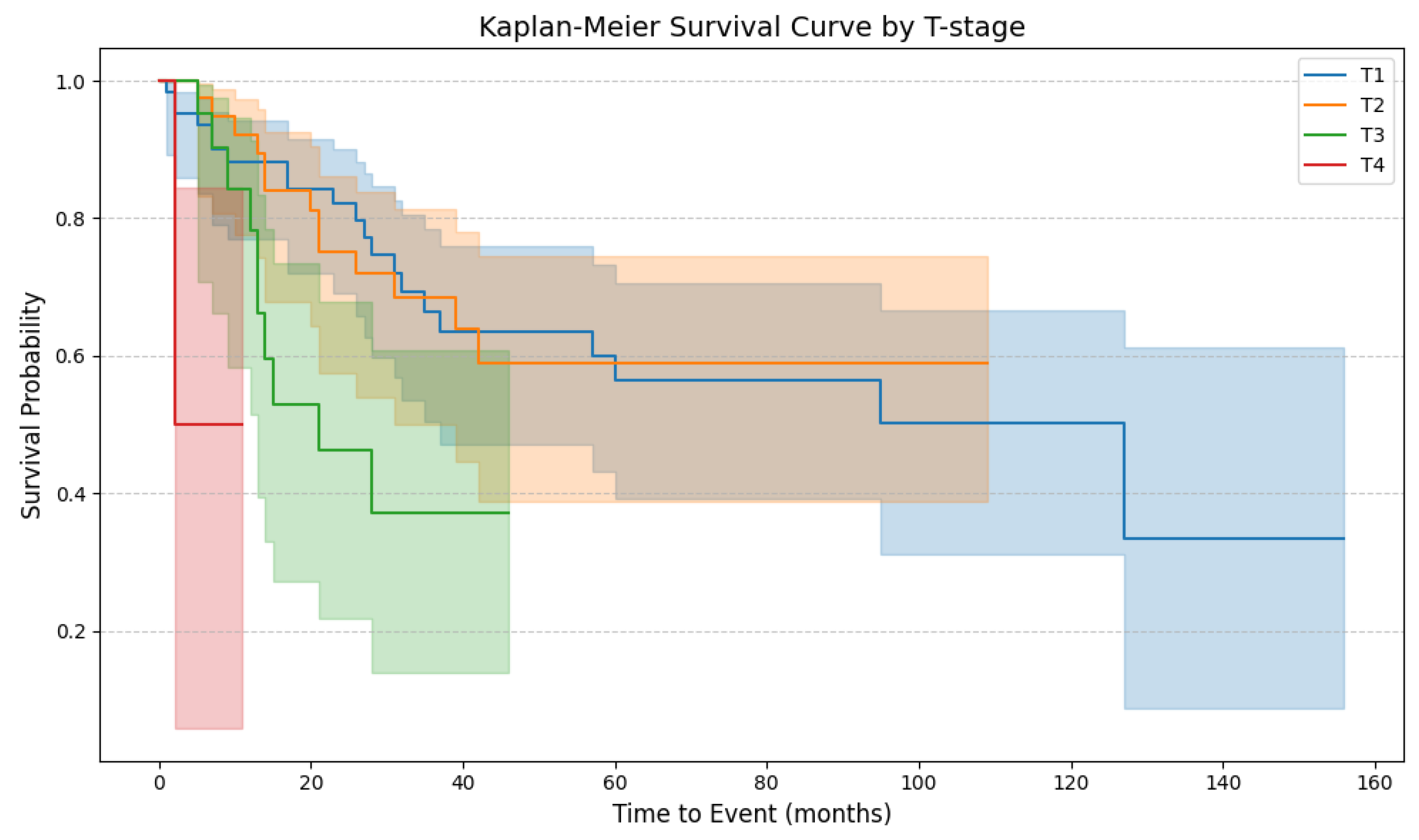
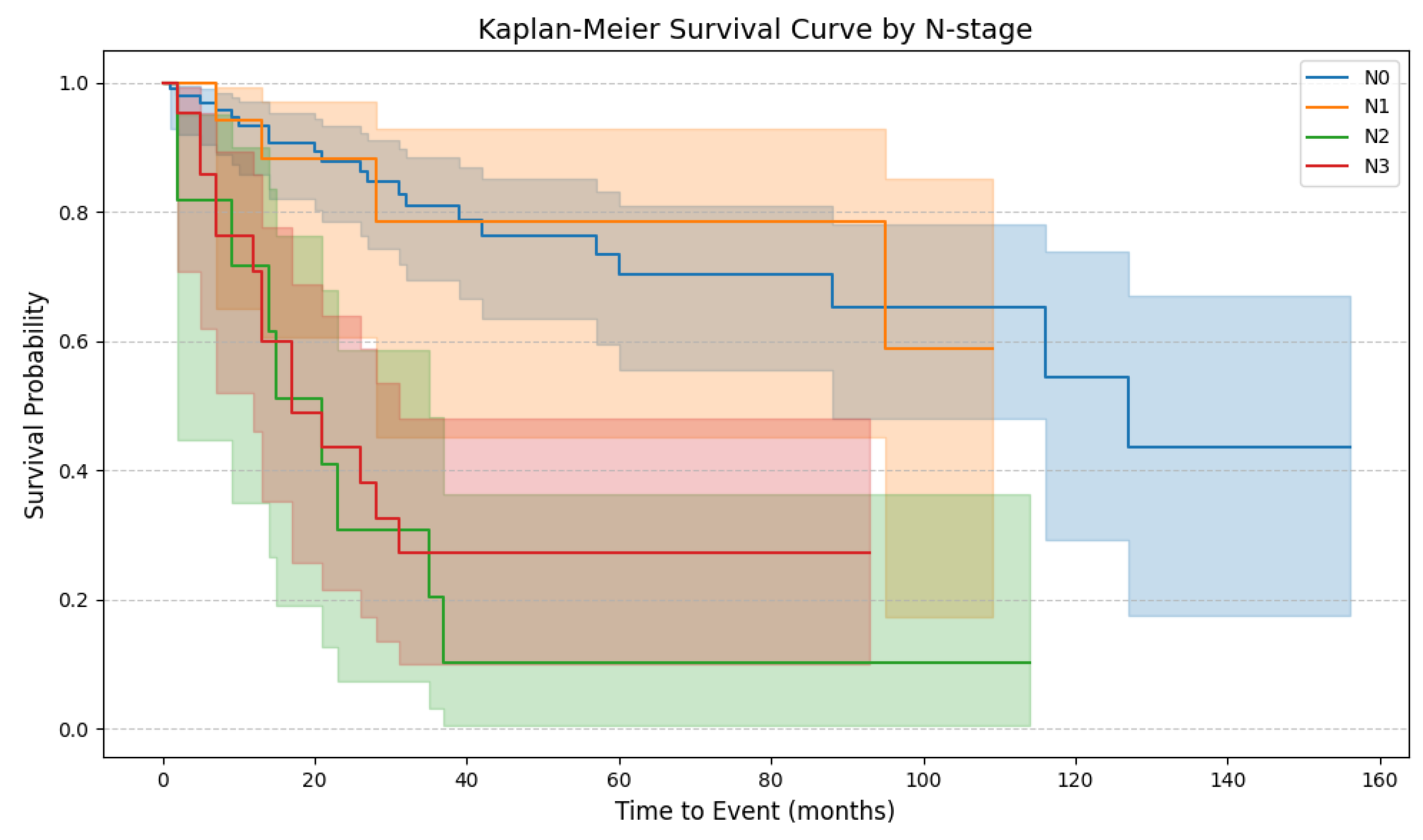
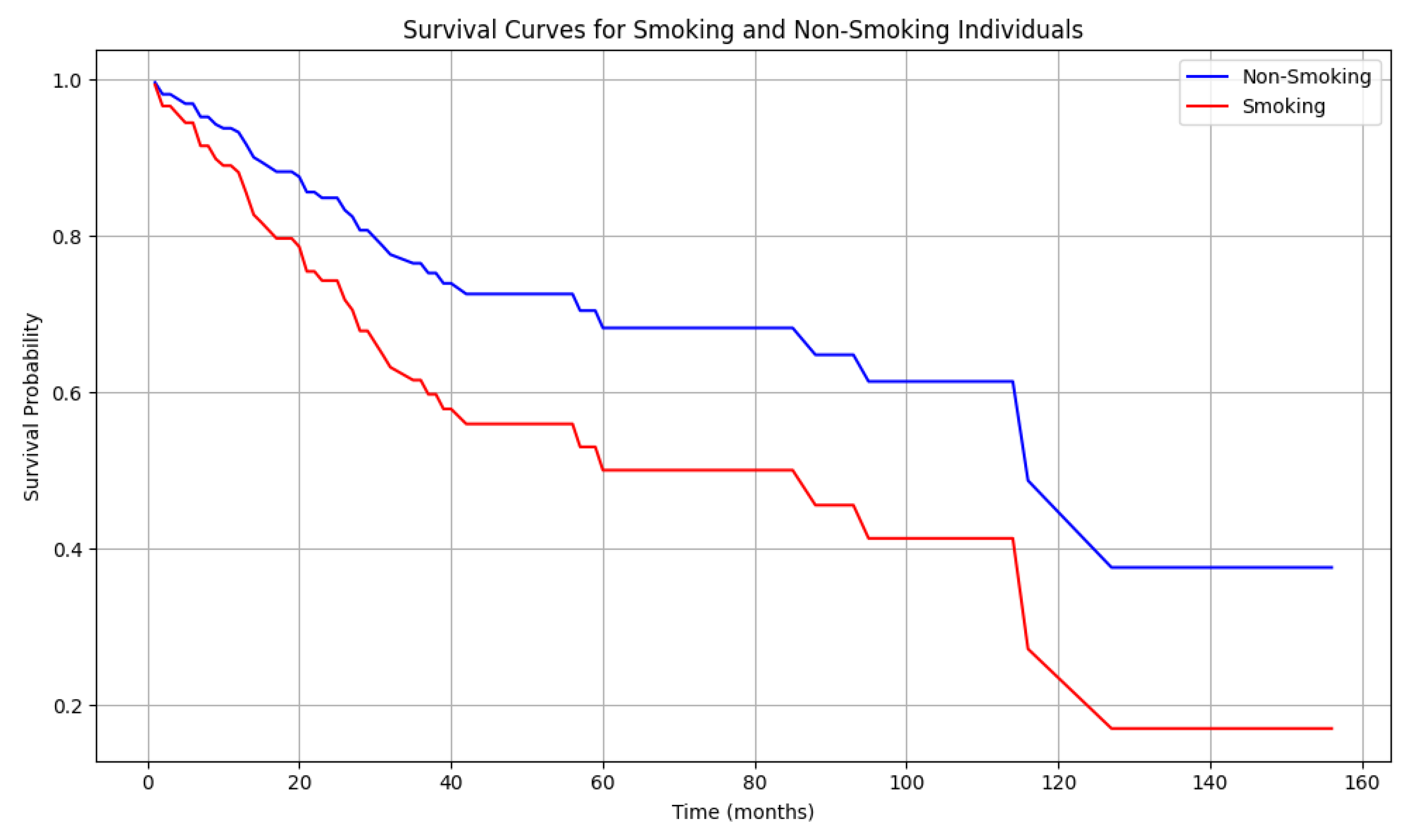
4.6. Patient Survival
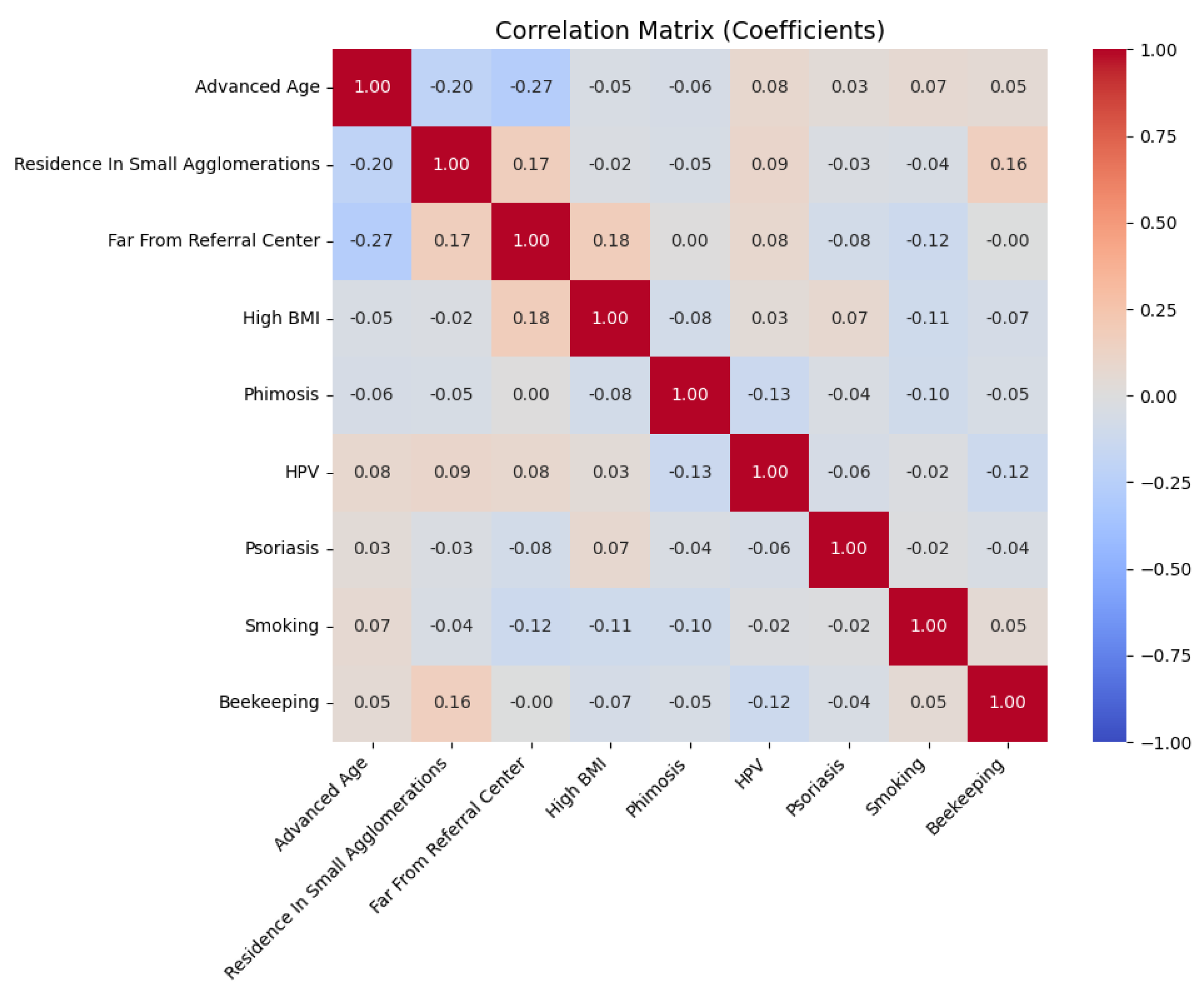
4.7. Risk of Death
5. Discussion
5.1. Uncircumcised Males and Penile Cancer Risk: The Influence of Phimosis
5.2. Elevated BMI and Penile Cancer Risk
5.3. Rural Living and Penile Cancer Incidence
5.4. The Role of Tobacco in Penile Cancer
5.5. The Role of HPV in Penile Carcinogenesis: From Infection to Prognosis
5.6. Centralization of Penile Cancer Care
5.7. Limitations and Future Research Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Douglawi, A.; Masterson, T.A. Updates on the Epidemiology and Risk Factors for Penile Cancer. Transl. Androl. Urol. 2017, 6, 785–790. [Google Scholar] [CrossRef]
- Thomas, A.; Necchi, A.; Muneer, A.; Tobias-Machado, M.; Tran, A.T.H.; Van Rompuy, A.-S.; Spiess, P.E.; Albersen, M. Penile Cancer. Nat. Rev. Dis. Primers 2021, 7, 1–24. [Google Scholar] [CrossRef]
- Coelho, R.W.P.; Pinho, J.D.; Moreno, J.S.; Garbis, D.V.e.O.; do Nascimento, A.M.T.; Larges, J.S.; Calixto, J.R.R.; Ramalho, L.N.Z.; da Silva, A.A.M.; Nogueira, L.R.; et al. Penile Cancer in Maranhão, Northeast Brazil: The Highest Incidence Globally? BMC Urol. 2018, 18, 50. [Google Scholar] [CrossRef] [PubMed]
- Strona Główna|Krajowy Rejestr Nowotworów. Available online: http://onkologia.org.pl/pl (accessed on 15 May 2025).
- Wnętrzak, I.; Czajkowski, M.; Barańska, K.; Miklewska, M.; Wojciechowska, U.; Sosnowski, R.; Didkowska, J.A. Epidemiology of Penile Cancer in Poland Compared to Other European Countries. Cancer Med. 2024, 13, e70092. [Google Scholar] [CrossRef]
- Morris, B.J.; Wamai, R.G.; Henebeng, E.B.; Tobian, A.A.; Klausner, J.D.; Banerjee, J.; Hankins, C.A. Estimation of Country-Specific and Global Prevalence of Male Circumcision. Popul. Health Metr. 2016, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Engelsgjerd, J.S.; Leslie, S.W.; LaGrange, C.A. Penile Cancer and Penile Intraepithelial Neoplasia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Mosquera Angulo, H.; Nieva-Posso, D.A.; García-Perdomo, H.A. Sexuality in Penile Cancer Survivors: A Rarely Discussed Problem in Uro-Oncology. Int. J. Urol. Nurs. 2024, 18, e12390. [Google Scholar] [CrossRef]
- Huang, J.; Chan, S.C.; Pang, W.S.; Liu, X.; Zhang, L.; Lucero-Prisno, D.E., III; Xu, W.; Zheng, Z.-J.; Ng, A.C.-F.; Necchi, A.; et al. Incidence, Risk Factors, and Temporal Trends of Penile Cancer: A Global Population-Based Study. BJU Int. 2024, 133, 314–323. [Google Scholar] [CrossRef]
- Rosato, E.; Miano, R.; Germani, S.; Asimakopoulos, A.D. Phimosis in Adults: Narrative Review of the New Available Devices and the Standard Treatments. Clin. Pract. 2024, 14, 361–376. [Google Scholar] [CrossRef]
- Czajkowski, M.; Wierzbicki, P.M.; Dolny, M.; Matuszewski, M.; Hakenberg, O.W. Inflammation in Penile Squamous Cell Carcinoma: A Comprehensive Review. Int. J. Mol. Sci. 2025, 26, 2785. [Google Scholar] [CrossRef]
- Hellberg, D.; Valentin, J.; Eklund, T.; Nilsson, S. Penile Cancer: Is There an Epidemiological Role for Smoking and Sexual Behaviour? Br. Med. J. (Clin. Res. Ed.) 1987, 295, 1306–1308. [Google Scholar] [CrossRef]
- Hernandez, M.; Gibb, J.K. Culture, Behavior and Health. Evol. Med. Public Health 2020, 2020, 12–13. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours Editorial Board. Urinary and Male Genital Tumours: WHO Classification of Tumours, 5th ed.; Moch, H., Ed.; IARC Publications: Lyon, France, 2022; Volume 8, ISBN 978-92-832-4512-4. [Google Scholar]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours; John Wiley & Sons: Hoboken, NJ, USA, 2017; ISBN 978-1-119-26357-9. [Google Scholar]
- Olesen, T.B.; Sand, F.L.; Rasmussen, C.L.; Albieri, V.; Toft, B.G.; Norrild, B.; Munk, C.; Kjær, S.K. Prevalence of Human Papillomavirus DNA and p16INK4a in Penile Cancer and Penile Intraepithelial Neoplasia: A Systematic Review and Meta-Analysis. Lancet Oncol. 2019, 20, 145–158. [Google Scholar] [CrossRef]
- Kikiros, C.S.; Beasley, S.W.; Woodward, A.A. The Response of Phimosis to Local Steroid Application. Pediatr. Surg. Int. 1993, 8, 329–332. [Google Scholar] [CrossRef]
- Morris, B.J.; Matthews, J.G.; Krieger, J.N. Prevalence of Phimosis in Males of All Ages: Systematic Review. Urology 2020, 135, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.E.; Khalil, M.I.; Kamel, M.H.; Karnes, R.J.; Spiess, P.E. Progress on Management of Penile Cancer in 2020. Curr. Treat. Options Oncol. 2021, 22, 4. [Google Scholar] [CrossRef]
- Dillner, J.; von Krogh, G.; Horenblas, S.; Meijer, C.J. Etiology of Squamous Cell Carcinoma of the Penis. Scand. J. Urol. Nephrol. Suppl. 2000, 34, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Chaturvedi, A.; Misra, N.C. Penile Carcinoma: A Challenge for the Developing World. Lancet Oncol. 2004, 5, 240–247. [Google Scholar] [CrossRef]
- Daling, J.R.; Madeleine, M.M.; Johnson, L.G.; Schwartz, S.M.; Shera, K.A.; Wurscher, M.A.; Carter, J.J.; Porter, P.L.; Galloway, D.A.; McDougall, J.K.; et al. Penile Cancer: Importance of Circumcision, Human Papillomavirus and Smoking in in Situ and Invasive Disease. Int. J. Cancer 2005, 116, 606–616. [Google Scholar] [CrossRef]
- Tseng, H.-F.; Morgenstern, H.; Mack, T.; Peters, R.K. Risk Factors for Penile Cancer: Results of a Population-Based Case–Control Study in Los Angeles County (United States). Cancer Causes Control. 2001, 12, 267–277. [Google Scholar] [CrossRef]
- Brinton, L.A.; Li, J.-Y.; Rong, S.D.; Huang, S.; Sheng, X.B.; Shi, B.G.; Zhu, Z.J.; Schiffman, M.H.; Dawsey, S. Risk Factors for Penile Cancer: Results from a Case-Control Study in China. Int. J. Cancer 1991, 47, 504–509. [Google Scholar] [CrossRef]
- Madsen, B.S.; van den Brule, A.J.C.; Jensen, H.L.; Wohlfahrt, J.; Frisch, M. Risk Factors for Squamous Cell Carcinoma of the Penis—Population-Based Case-Control Study in Denmark. Cancer Epidemiol. Biomark. Prev. 2008, 17, 2683–2691. [Google Scholar] [CrossRef] [PubMed]
- Borque-Fernando, Á.; Gaya, J.M.; Esteban-Escaño, L.M.; Gómez-Rivas, J.; García-Baquero, R.; Agreda-Castañeda, F.; Gallioli, A.; Verri, P.; Ortiz-Vico, F.J.; Amir-Nicolau, B.F.; et al. Epidemiology, Diagnosis and Management of Penile Cancer: Results from the Spanish National Registry of Penile Cancer. Cancers 2023, 15, 616. [Google Scholar] [CrossRef] [PubMed]
- Daryanto, B.; Firdaus, M.M.; Nurhadi, P. The Relationship between Phimosis, Smegma, and Preputial Bacteria with Inflammatory Status of Circumcised Patient. J. Pediatr. Urol. 2020, 16, S12. [Google Scholar] [CrossRef]
- Falcão, B.P.; Stegani, M.; Matias, J.E.F. Phimosis and Circumcision: Concepts, History, and Evolution. Int. J. Med. Rev. 2018, 5, 6–18. [Google Scholar] [CrossRef]
- Murata, M. Inflammation and Cancer. Environ. Health Prev. Med. 2018, 23, 50. [Google Scholar] [CrossRef]
- Korniluk, A.; Koper, O.; Kemona, H.; Dymicka-Piekarska, V. From Inflammation to Cancer. Ir. J. Med. Sci. 2017, 186, 57–62. [Google Scholar] [CrossRef]
- Czajkowski, M.; Wierzbicki, P.M.; Kotulak-Chrząszcz, A.; Małkiewicz, B.; Sosnowski, R.; Kmieć, Z.; Matuszewski, M. Pro-Inflammatory Cytokine Gene Expression in Penile Cancer: Preliminary Studies. Medicina 2023, 59, 1623. [Google Scholar] [CrossRef]
- Van Howe, R.; Hodges, F. The Carcinogenicity of Smegma: Debunking a Myth. Acad. Dermatol. Venereol. 2006, 20, 1046–1054. [Google Scholar] [CrossRef]
- Pawlikowska-Gorzelańczyk, A.; Szuster, E.; Kostrzewska, P.; Mandera-Grygierzec, A. Obesity—Lifestyle Choice or a Disease? Changes in Perception of Obesity. J. Educ. Health Sport 2022, 12, 660–666. [Google Scholar] [CrossRef]
- Pati, S.; Irfan, W.; Jameel, A.; Ahmed, S.; Shahid, R.K. Obesity and Cancer: A Current Overview of Epidemiology, Pathogenesis, Outcomes, and Management. Cancers 2023, 15, 485. [Google Scholar] [CrossRef]
- Aune, D.; Nordsletten, M.; Myklebust, T.Å.; Robsahm, T.E.; Skålhegg, B.S.; Mala, T.; Yaqub, S.; Saeed, U. Body Mass Index and Penile Cancer Incidence: Results from a Norwegian Cohort Study of 829,081 Men. BMC Urol. 2024, 24, 260. [Google Scholar] [CrossRef] [PubMed]
- Barnes, K.T.; Smith, B.J.; Lynch, C.F.; Gupta, A. Obesity and Invasive Penile Cancer. Eur. Urol. 2013, 63, 588–589. [Google Scholar] [CrossRef] [PubMed]
- Barnes, K.T.; McDowell, B.D.; Button, A.; Smith, B.J.; Lynch, C.F.; Gupta, A. Obesity Is Associated with Increased Risk of Invasive Penile Cancer. BMC Urol. 2016, 16, 42. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.R. Adult Acquired Buried Penis: A Hidden Problem in Obese Men. Cureus 2021, 13, e13067. [Google Scholar] [CrossRef]
- Higuchi, T.T.; Yamaguchi, Y.; Wood, H.M.; Angermeier, K.W. Evaluation and Treatment of Adult Concealed Penis. Curr. Urol. Rep. 2012, 13, 277–284. [Google Scholar] [CrossRef]
- Pekala, K.R.; Pelzman, D.; Theisen, K.M.; Rogers, D.; Maganty, A.; Fuller, T.W.; Rusilko, P.J. The Prevalence of Penile Cancer in Patients With Adult Acquired Buried Penis. Urology 2019, 133, 229–233. [Google Scholar] [CrossRef]
- Czajkowski, M.; Wierzbicki, P.; Kotulak-Chrząszcz, A.; Czajkowska, K.; Bolcewicz, M.; Kłącz, J.; Kreft, K.; Lewandowska, A.; Nedoszytko, B.; Sokołowska-Wojdyło, M.; et al. The Role of Occlusion and Micro-Incontinence in the Pathogenesis of Penile Lichen Sclerosus: An Observational Study of pro-Inflammatory Cytokines’ Gene Expression. Int. Urol. Nephrol. 2022, 54, 763–772. [Google Scholar] [CrossRef]
- Ho, T.S.; Gelman, J. Evaluation and Management of Adult Acquired Buried Penis. Transl. Androl. Urol. 2018, 7, 618–627. [Google Scholar] [CrossRef]
- Kershaw, E.E.; Flier, J.S. Adipose Tissue as an Endocrine Organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef]
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and Cancer Risk: Emerging Biological Mechanisms and Perspectives. Metabolism 2019, 92, 121–135. [Google Scholar] [CrossRef]
- Wang, T.; He, C. Pro-Inflammatory Cytokines: The Link between Obesity and Osteoarthritis. Cytokine Growth Factor Rev. 2018, 44, 38–50. [Google Scholar] [CrossRef]
- Khanna, D.; Peltzer, C.; Kahar, P.; Parmar, M.S.; Khanna, D.; Peltzer, C.; Kahar, P.; Parmar, M.S. Body Mass Index (BMI): A Screening Tool Analysis. Cureus 2022, 14, e22119. [Google Scholar] [CrossRef]
- Mantovani, A. Inflammation by Remote Control. Nature 2005, 435, 752–753. [Google Scholar] [CrossRef]
- Wesseling, C.; Antich, D.; Hogstedt, C.; Rodriguez, A.C.; Ahlbom, A. Geographical Differences of Cancer Incidence in Costa Rica in Relation to Environmental and Occupational Pesticide Exposure. Int. J. Epidemiol. 1999, 28, 365–374. [Google Scholar] [CrossRef]
- Pinho-França, J.D.R.; Chein, M.B.D.C.; Thuler, L.C.S. Patterns of Cervical Cytological Abnormalities According to the Human Development Index in the Northeast Region of Brazil. BMC Women’s Health 2016, 16, 54. [Google Scholar] [CrossRef]
- Vieira, C.B.; Feitoza, L.; Pinho, J.; Teixeira-Júnior, A.; Lages, J.; Calixto, J.; Coelho, R.; Nogueira, L.; Cunha, I.; Soares, F.; et al. Profile of Patients with Penile Cancer in the Region with the Highest Worldwide Incidence. Sci. Rep. 2020, 10, 2965. [Google Scholar] [CrossRef]
- Nepal, U.; Jalan, A.; Gharti, B.B.; Pandey, G.; Lamichhane, N. A Retrospective Analysis of Carcinoma Penis Patients Treated at a Cancer Center in Nepal in a Period of Five Years. Nep. J. Cancer 2019, 3, 19–23. [Google Scholar] [CrossRef]
- GUS Rural Areas in Poland 2020. Available online: https://stat.gov.pl/en/topics/agriculture-forestry/agriculture/rural-areas-in-poland-2020,3,5.html (accessed on 18 January 2025).
- Noonan, D.; Frisbee, S.; Bittencourt, L.; Rubenstein, D.; McClernon, F.J.; Carroll, D.M. Rural-Urban Differences in Smoking Quit Ratios and Cessation-Related Factors: Results from a Nationally Representative Sample. J. Rural Health 2024, 41, e12870. [Google Scholar] [CrossRef]
- Brandt, H.M.; Vanderpool, R.C.; Pilar, M.; Zubizarreta, M.; Stradtman, L.R. A Narrative Review of HPV Vaccination Interventions in Rural U.S. Communities. Prev. Med. 2021, 145, 106407. [Google Scholar] [CrossRef]
- Júnior, P.F.d.M.; Silva, E.H.V.; Moura, K.L.; de Aquino, Y.F.; Weller, M. Increased Risk of Penile Cancer among Men Working in Agriculture. Asian Pac. J. Cancer Prev. 2018, 19, 237–241. [Google Scholar] [CrossRef]
- Harish, K.; Ravi, R. The Role of Tobacco in Penile Carcinoma. Br. J. Urol. 1995, 75, 375–377. [Google Scholar] [CrossRef]
- Chalya, P.L.; Rambau, P.F.; Masalu, N.; Simbila, S. Ten-Year Surgical Experiences with Penile Cancer at a Tertiary Care Hospital in Northwestern Tanzania: A Retrospective Study of 236 Patients. World J. Surg. Onc 2015, 13, 71. [Google Scholar] [CrossRef]
- Barton, S.E.; Jenkins, D.; Cuzick, J.; Maddox, P.H.; Edwards, R.; Singer, A. . Effect of cigarette smoking on cervical epithelial immunity: A mechanism for neoplastic change? Lancet 1988, 332, 652–654. [Google Scholar] [CrossRef]
- Winkelstein, W. Smoking and Cancer of the Uterine Cervix: Hypothesis. Am. J. Epidemiol. 1977, 106, 257–259. [Google Scholar] [CrossRef]
- Aden, D.; Zaheer, S.; Khan, S.; Jairajpuri, Z.S.; Jetley, S. Navigating the Landscape of HPV-Associated Cancers: From Epidemiology to Prevention. Pathol.—Res. Pract. 2024, 263, 155574. [Google Scholar] [CrossRef]
- Onon, T.S. History of Human Papillomavirus, Warts and Cancer: What Do We Know Today? Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 565–574. [Google Scholar] [CrossRef]
- Bruni, L.; Albero, G.; Rowley, J.; Alemany, L.; Arbyn, M.; Giuliano, A.R.; Markowitz, L.E.; Broutet, N.; Taylor, M. Global and Regional Estimates of Genital Human Papillomavirus Prevalence among Men: A Systematic Review and Meta-Analysis. Lancet Glob. Health 2023, 11, e1345–e1362. [Google Scholar] [CrossRef]
- Kravvas, G.; Ge, L.; Ng, J.; Shim, T.N.; Doiron, P.R.; Watchorn, R.; Kentley, J.; Panou, E.; Dinneen, M.; Freeman, A.; et al. The Management of Penile Intraepithelial Neoplasia (PeIN): Clinical and Histological Features and Treatment of 345 Patients and a Review of the Literature. J. Dermatolog. Treat. 2022, 33, 1047–1062. [Google Scholar] [CrossRef]
- Gu, W.; Zhang, P.; Zhang, G.; Zhou, J.; Ding, X.; Wang, Q.; Wang, B.; Wei, Y.; Jin, S.; Ye, D.; et al. Importance of HPV in Chinese Penile Cancer: A Contemporary Multicenter Study. Front. Oncol. 2020, 10, 1521. [Google Scholar] [CrossRef]
- Mustasam, A.; Parza, K.; Ionescu, F.; Gullapalli, K.; Paravathaneni, M.; Kim, Y.; Sandstrom, R.E.; Assaad, M.A.; Grass, G.D.; Johnstone, P.; et al. The Prognostic Role of HPV or p16INK4a Status in Penile Squamous Cell Carcinoma: A Meta-Analysis. J. Natl. Compr. Cancer Netw. 2025, 23, e247078. [Google Scholar] [CrossRef]
- Lont, A.P.; Kroon, B.K.; Horenblas, S.; Gallee, M.P.W.; Berkhof, J.; Meijer, C.J.L.M.; Snijders, P.J.F. Presence of High-Risk Human Papillomavirus DNA in Penile Carcinoma Predicts Favorable Outcome in Survival. Int. J. Cancer 2006, 119, 1078–1081. [Google Scholar] [CrossRef]
- Bezerra, A.L.R.; Lopes, A.; Landman, G.; Alencar, G.N.; Torloni, H.; Villa, L.L. Clinicopathologic Features and Human Papillomavirus DNA Prevalence of Warty and Squamous Cell Carcinoma of the Penis. Am. J. Surg. Pathol. 2001, 25, 673. [Google Scholar] [CrossRef]
- Vreeburg, M.T.A.; de Vries, H.-M.; van der Noort, V.; Horenblas, S.; van Rhijn, B.W.G.; Hendricksen, K.; Graafland, N.; van der Poel, H.G.; Brouwer, O.R. Penile Cancer Care in the Netherlands: Increased Incidence, Centralisation, and Improved Survival. BJU Int. 2024, 133, 596–603. [Google Scholar] [CrossRef]
- Pecoraro, A.; Elst, L.; Roussel, E.; Miletić, M.; Vanthoor, J.; De Ridder, D.; Van Rompuy, A.-S.; De Cuyper, E.; Dumez, H.; De Meerleer, G.; et al. Impact of the Standardization of Penile Cancer Care on the Quality of Care, Outcomes, and Academic-Driven Centralization in a Single eUROGEN Referral Center. Eur. Urol. Focus. 2024, 10, 57–65. [Google Scholar] [CrossRef]
- Brouwer, O.R.; Albersen, M.; Parnham, A.; Protzel, C.; Pettaway, C.A.; Ayres, B.; Antunes-Lopes, T.; Barreto, L.; Campi, R.; Crook, J.; et al. European Association of Urology-American Society of Clinical Oncology Collaborative Guideline on Penile Cancer: 2023 Update. Eur. Urol. 2023, 83, 548–560. [Google Scholar] [CrossRef]

| Patients | Criteria | Count | Percent [%] | |
|---|---|---|---|---|
| Age (Years) | Minimum | 30 | - | |
| Maximum | 87 | |||
| Median | 64 | |||
| BMI [kg/m2] | Minimum | 16 | - | |
| Maximum | 38 | |||
| Median | 29 | |||
| Religious faith | Jehovah’s Witnesses | 3 | 1.96 | |
| Catholicism | 150 | 98.04 | ||
| Circumcised (before diagnosis) | Yes | 0 | 0 | |
| No | 153 | 100 | ||
| Residential distribution | Rural areas | 42 | 27.45 | |
| Small urban centers | 24 | 15.95 | ||
| Medium-sized cities | 41 | 26.80 | ||
| Large metropolitan areas | 46 | 30.07 | ||
| TNM—local stage | Tis | 22 | 14.38 | |
| pT1a | 45 | 29.41 | ||
| pT1b | 20 | 13.07 | ||
| pT2 | 38 | 24.84 | ||
| pT3 | 23 | 15.03 | ||
| pT4 | 5 | 3.27 | ||
| TNM—lymph nodes | pN0 | 99 | 64.71 | |
| pN1 | 20 | 13.07 | ||
| pN2 | 11 | 7.19 | ||
| pN3 | 23 | 15.03 | ||
| TNM—distant metastases | M0 | 150 | 98.04 | |
| M1 | 3 | 1.96 | ||
| Grading | PeIN | 22 | 14.38 | |
| G1 | 35 | 22.88 | ||
| G2 | 68 | 44.44 | ||
| G3 | 28 | 18.30 | ||
| Histopathological type | Squamous cell carcinoma | 147 | 96.08 | |
| Verrucous squamous cell carcinoma | 6 | 3.92 | ||
| Surgical treatment | Circumcision | 20 | 13.07 | |
| Wide local excision | 12 | 7.84 | ||
| Glansectomy with reconstruction using a split-thickness skin graft | 55 | 35.95 | ||
| Partial penectomy with reconstruction using a split-thickness skin graft | 49 | 32.03 | ||
| Partial penectomy without reconstruction | 10 | 6.54 | ||
| Total penectomy | 7 | 4.57 | ||
| Radiotherapy | Primary tumor radiotherapy | 2 | 1.3 | |
| Adjuvant radiotherapy | 6 | 3.91 | ||
| Chemotherapy | Neoadjuvant chemotherapy (TIP *) | 7 | 4.6 | |
| Adjuvant chemotherapy | TIP * | 32 | 20.92 | |
| PF ** | 10 | 6.54 | ||
| Immunotherapy | 8 | 5.23 | ||
| Parameter | Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | Statistical Power | |
| Age (centered) | 1.02 | 1.02–1.05 | 0.08787 | 1.02 | 1.00–1.05 | 0.098 | 0.38 |
| Smoking | 1.76 | 1.00–3.11 | 0.052 | 1.81 | 1.01–3.25 | 0.047 * | 0.51 |
| HPV | 0.15 | 0.02–1.11 | 0.063 | 0.15 | 0.02–1.11 | 0.063 | 0.46 |
| BMI | 0.19 | 0.03–1.08 | 0.060 | - | - | - | - |
| Phimosis | 0.79 | 0.44–1.42 | 0.428 | - | - | - | - |
| Beekeeping | 0.39 | 0.09–1.62 | 0.195 | - | - | - | - |
| Residence in small agglomerations | 0.97 | 0.77–1.23 | 0.808 | - | - | - | - |
| Psoriasis | 2.40 | 0.74–7.77 | 0.143 | - | - | - | - |
| Lymph node stages | 3.36 | 1.86–6.06 | <0.001 | 2.10 | 1.09–4.06 | 0.027 * | 0.60 |
| Tumor grade | 2.10 | 1.46–3.03 | <0.001 | 1.85 | 1.23–2.78 | 0.003 * | 0.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czajkowski, M.; Falis, M.; Błaczkowska, A.; Rybarczyk, A.; Wierzbicki, P.M.; Gondek, J.; Matuszewski, M.; Hakenberg, O.W. Penile Cancer Profile in a Central European Context: Clinical Characteristics, Prognosis, and Outcomes—Insights from a Polish Tertiary Medical Center. Cancers 2025, 17, 2140. https://doi.org/10.3390/cancers17132140
Czajkowski M, Falis M, Błaczkowska A, Rybarczyk A, Wierzbicki PM, Gondek J, Matuszewski M, Hakenberg OW. Penile Cancer Profile in a Central European Context: Clinical Characteristics, Prognosis, and Outcomes—Insights from a Polish Tertiary Medical Center. Cancers. 2025; 17(13):2140. https://doi.org/10.3390/cancers17132140
Chicago/Turabian StyleCzajkowski, Mateusz, Michał Falis, Agata Błaczkowska, Agnieszka Rybarczyk, Piotr M. Wierzbicki, Jakub Gondek, Marcin Matuszewski, and Oliver W. Hakenberg. 2025. "Penile Cancer Profile in a Central European Context: Clinical Characteristics, Prognosis, and Outcomes—Insights from a Polish Tertiary Medical Center" Cancers 17, no. 13: 2140. https://doi.org/10.3390/cancers17132140
APA StyleCzajkowski, M., Falis, M., Błaczkowska, A., Rybarczyk, A., Wierzbicki, P. M., Gondek, J., Matuszewski, M., & Hakenberg, O. W. (2025). Penile Cancer Profile in a Central European Context: Clinical Characteristics, Prognosis, and Outcomes—Insights from a Polish Tertiary Medical Center. Cancers, 17(13), 2140. https://doi.org/10.3390/cancers17132140








