High HER2 Intratumoral Heterogeneity Is Resistant to Anti-HER2 Neoadjuvant Chemotherapy in Early Stage and Locally Advanced HER2-Positive Breast Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. HER2 FISH Assay
2.2. HER2 ITH Assessment
2.3. Statistical Analyses
2.4. Data Availability
3. Results
3.1. Association Between HER2 ITH and Patient Characteristics
3.2. HER2 ITH and Patient Prognosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DFS | disease-free survival |
| ER | estrogen receptor |
| FISH | fluorescence in situ hybridization |
| GMM | Gaussian mixture model |
| GPA | gene–protein assay |
| HER2 | human epidermal growth factor receptor 2 |
| HH | high heterogeneity |
| HR | hormone receptor |
| IHC | immunohistochemistry |
| ITH | intratumoral heterogeneity |
| LH | low heterogeneity |
| NAC | neoadjuvant chemotherapy |
| OCR | optical character recognition |
| OS | overall survival |
| pCR | pathological complete response |
| PFS | progression-free survival |
| PgR | progesterone receptor |
References
- Turashvili, G.; Brogi, E. Tumor Heterogeneity in Breast Cancer. Front. Med. 2017, 4, 227. [Google Scholar] [CrossRef] [PubMed]
- Aleskandarany, M.A.; Vandenberghe, M.E.; Marchio, C.; Ellis, I.O.; Sapino, A.; Rakha, E.A. Tumour Heterogeneity of Breast Cancer: From Morphology to Personalised Medicine. Pathobiology 2018, 85, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Beca, F.; Polyak, K. Intratumor Heterogeneity in Breast Cancer. Adv. Exp. Med. Biol. 2016, 882, 169–189. [Google Scholar] [PubMed]
- Nitta, H.; Li, Z. Breast HER2 Intratumoral Heterogeneity as a Biomarker for Improving HER2-Targeted Therapy. Crit. Rev. Oncog. 2020, 25, 233–240. [Google Scholar] [CrossRef]
- Lu, W.; Lashen, A.G.; Wahab, N.; Miligy, I.M.; Jahanifar, M.; Toss, M.; Graham, S.; Bilal, M.; Bhalerao, A.; Atallah, N.M.; et al. AI-based intra-tumor heterogeneity score of Ki67 expression as a prognostic marker for early-stage ER+/HER2- breast cancer. J. Pathol. Clin. Res. 2024, 10, e346. [Google Scholar] [CrossRef]
- Wang, Y.; Ali, M.A.; Vallon-Christersson, J.; Humphreys, K.; Hartman, J.; Rantalainen, M. Transcriptional intra-tumour heterogeneity predicted by deep learning in routine breast histopathology slides provides independent prognostic information. Eur. J. Cancer 2023, 191, 112953. [Google Scholar] [CrossRef]
- Li, Y.; Wang, M.; Yang, S.; Kuang, L.; Tao, X.; Yang, J.; Zhao, W.; Zhang, J. Intratumoral heterogeneity contributes to the chemotherapy prognosis of breast cancer. J. Cancer Res. Ther. 2022, 18, 1268–1275. [Google Scholar] [CrossRef]
- Martelotto, L.G.; Ng, C.K.Y.; Piscuoglio, S.; Weigelt, B.; Reis, J.S. Breast cancer intra-tumor heterogeneity. Breast Cancer Res. 2014, 16, 210. [Google Scholar] [CrossRef]
- Muller, K.E.; Marotti, J.D.; Tafe, L.J. Pathologic Features and Clinical Implications of Breast Cancer with HER2 Intratumoral Genetic Heterogeneity. Am. J. Clin. Pathol. 2019, 152, 7–16. [Google Scholar] [CrossRef]
- Miglietta, F.; Dieci, M.V.; Griguolo, G.; Guarneri, V.; Conte, P.F. Chemotherapy for advanced HER2-negative breast cancer: Can one algorithm fit all? Cancer Treat. Rev. 2017, 60, 100–108. [Google Scholar] [CrossRef]
- Martinez-Saez, O.; Waks, A.G. Individualizing Curative-Intent Therapy in HER2-Positive Early-Stage Breast Cancer. Curr. Treat. Options Oncol. 2023, 24, 479–495. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.C.; Qian, X.K.; Guo, Z.B.; Ren, C.Y.; Yao, M.; Li, X.R.; Wang, K.; Zu, J.; Liao, N. Pre-treatment hormonal receptor status and Ki67 index predict pathologic complete response to neoadjuvant trastuzumab/taxanes but not disease-free survival in HER2-positive breast cancer patients. Med. Oncol. 2012, 29, 3222–3231. [Google Scholar] [CrossRef] [PubMed]
- Teruya, N.; Inoue, H.; Horii, R.; Akiyama, F.; Ueno, T.; Ohno, S.; Takahashi, S. Intratumoral heterogeneity, treatment response, and survival outcome of ER-positive HER2-positive breast cancer. Cancer Med. 2023, 12, 10526–10535. [Google Scholar] [CrossRef] [PubMed]
- Tanei, T.; Seno, S.; Sota, Y.; Hatano, T.; Kitahara, Y.; Abe, K.; Masunaga, N.; Tsukabe, M.; Yoshinami, T.; Miyake, T.; et al. High HER2 Intratumoral Heterogeneity Is a Predictive Factor for Poor Prognosis in Early-Stage and Locally Advanced HER2-Positive Breast Cancer. Cancers 2024, 16, 1062. [Google Scholar] [CrossRef]
- Hanna, W.M.; Ruschoff, J.; Bilous, M.; Coudry, R.A.; Dowsett, M.; Osamura, R.Y.; Penault-Llorca, F.; van de Vijver, M.; Viale, G. HER2 in situ hybridization in breast cancer: Clinical implications of polysomy 17 and genetic heterogeneity. Mod. Pathol. 2014, 27, 4–18. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.; Schwartz, J.N.; Hagerty, K.L.; Allred, D.C.; Cote, R.J.; Dowsett, M.; Fitzgibbons, P.L.; Hanna, W.M.; Langer, A.; et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J. Clin. Oncol. 2007, 25, 118–145. [Google Scholar] [CrossRef]
- Allison, K.H.; Dintzis, S.M.; Schmidt, R.A. Frequency of HER2 heterogeneity by fluorescence in situ hybridization according to CAP expert panel recommendations: Time for a new look at how to report heterogeneity. Am. J. Clin. Pathol. 2011, 136, 864–871. [Google Scholar] [CrossRef]
- Chang, M.C.; Malowany, J.I.; Mazurkiewicz, J.; Wood, M. ‘Genetic heterogeneity’ in HER2/neu testing by fluorescence in situ hybridization: A study of 2,522 cases. Mod. Pathol. 2012, 25, 683–688. [Google Scholar] [CrossRef]
- Shen, T.; Nitta, H.; Wei, L.; Parwani, A.V.; Li, Z. HER2 intratumoral heterogeneity is independently associated with distal metastasis and overall survival in HER2-positive breast carcinomas. Breast Cancer Res. Treat. 2020, 181, 519–527. [Google Scholar] [CrossRef]
- Van Bockstal, M.R.; Agahozo, M.C.; van Marion, R.; Atmodimedjo, P.N.; Sleddens, H.; Dinjens, W.N.M.; Visser, L.L.; Lips, E.H.; Wesseling, J.; van Deurzen, C.H.M. Somatic mutations and copy number variations in breast cancers with heterogeneous HER2 amplification. Mol. Oncol. 2020, 14, 671–685. [Google Scholar] [CrossRef]
- Gianni, L.; Pienkowski, T.; Im, Y.H.; Roman, L.; Tseng, L.M.; Liu, M.C.; Lluch, A.; Staroslawska, E.; de la Haba-Rodriguez, J.; Im, S.A.; et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012, 13, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Gianni, L.; Pienkowski, T.; Im, Y.H.; Tseng, L.M.; Liu, M.C.; Lluch, A.; Staroslawska, E.; de la Haba-Rodriguez, J.; Im, S.A.; Pedrini, J.L.; et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): A multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016, 17, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Pang, D.; Yang, H.; Li, W.; Wang, S.; Cui, S.; Liao, N.; Wang, Y.; Wang, C.; Chang, Y.C.; et al. Efficacy, Safety, and Tolerability of Pertuzumab, Trastuzumab, and Docetaxel for Patients with Early or Locally Advanced ERBB2-Positive Breast Cancer in Asia: The PEONY Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, e193692. [Google Scholar] [CrossRef]
- Swain, S.M.; Baselga, J.; Kim, S.B.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.M.; Schneeweiss, A.; Heeson, S.; et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N. Engl. J. Med. 2015, 372, 724–734. [Google Scholar] [CrossRef]
- Swain, S.M.; Miles, D.; Kim, S.B.; Im, Y.H.; Im, S.A.; Semiglazov, V.; Ciruelos, E.; Schneeweiss, A.; Loi, S.; Monturus, E.; et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): End-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 519–530. [Google Scholar] [CrossRef]
- Okines, A.F.C.; Turner, N.C. Heterogeneous HER2 Amplification-a New Clinical Category of HER2-Positive Breast Cancer? Cancer Discov. 2021, 11, 2369–2371. [Google Scholar] [CrossRef]
- Pavlenko, I.A.; Zavalishina, L.E.; Povilaitite, P.E. HER2/neu gene amplification as a mechanism of clonal heterogeneity in breast cancer. Arkh Patol. 2019, 81, 49–55. [Google Scholar] [CrossRef]
- Jensen, S.G.; Thomas, P.E.; Christensen, I.J.; Balslev, E.; Hansen, A.; Hogdall, E. Evaluation of analytical accuracy of HER2 status in patients with breast cancer: Comparison of HER2 GPAwith HER2, I.H.C.; HER2, F.I.S.H. APMIS 2020, 128, 573–582. [Google Scholar] [CrossRef]
- Horii, R.; Nitta, H.; Nojima, M.; Maruyama, R.; Ueno, T.; Ito, Y.; Ohno, S.; Banks, P.; Kanda, H.; Akiyama, F. Predictive significance of HER2 intratumoral heterogeneity, determined by simultaneous gene and protein analysis, for resistance to trastuzumab-based treatments for HER2-positive breast cancer. Virchows Arch. 2021, 479, 13–21. [Google Scholar] [CrossRef]
- Hou, Y.; Nitta, H.; Wei, L.; Banks, P.M.; Portier, B.; Parwani, A.V.; Li, Z. HER2 intratumoral heterogeneity is independently associated with incomplete response to anti-HER2 neoadjuvant chemotherapy in HER2-positive breast carcinoma. Breast Cancer Res. Treat. 2017, 166, 447–457. [Google Scholar] [CrossRef]
- Gu, L.; Lau, S.K.; Loera, S.; Somlo, G.; Kane, S.E. Protein kinase A activation confers resistance to trastuzumab in human breast cancer cell lines. Clin. Cancer Res. 2009, 15, 7196–7206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tai, L.K.; Wong, L.L.; Chiu, L.L.; Sethi, S.K.; Koay, E.S. Proteomic study reveals that proteins involved in metabolic and detoxification pathways are highly expressed in HER-2/neu-positive breast cancer. Mol. Cell. Proteom. 2005, 4, 1686–1696. [Google Scholar] [CrossRef] [PubMed]
- Steiner, C.; Tille, J.C.; Lamerz, J.; Kux van Geijtenbeek, S.; McKee, T.A.; Venturi, M.; Rubbia-Brandt, L.; Hochstrasser, D.; Cutler, P.; Lescuyer, P.; et al. Quantification of HER2 by Targeted Mass Spectrometry in Formalin-Fixed Paraffin-Embedded (FFPE) Breast Cancer Tissues. Mol. Cell. Proteom. 2015, 14, 2786–2799. [Google Scholar] [CrossRef] [PubMed]
- Debets, D.O.; Stecker, K.E.; Piskopou, A.; Liefaard, M.C.; Wesseling, J.; Sonke, G.S.; Lips, E.H.; Altelaar, M. Deep (phospho)proteomics profiling of pre- treatment needle biopsies identifies signatures of treatment resistance in HER2+ breast cancer. Cell Rep. Med. 2023, 4, 101203. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef]
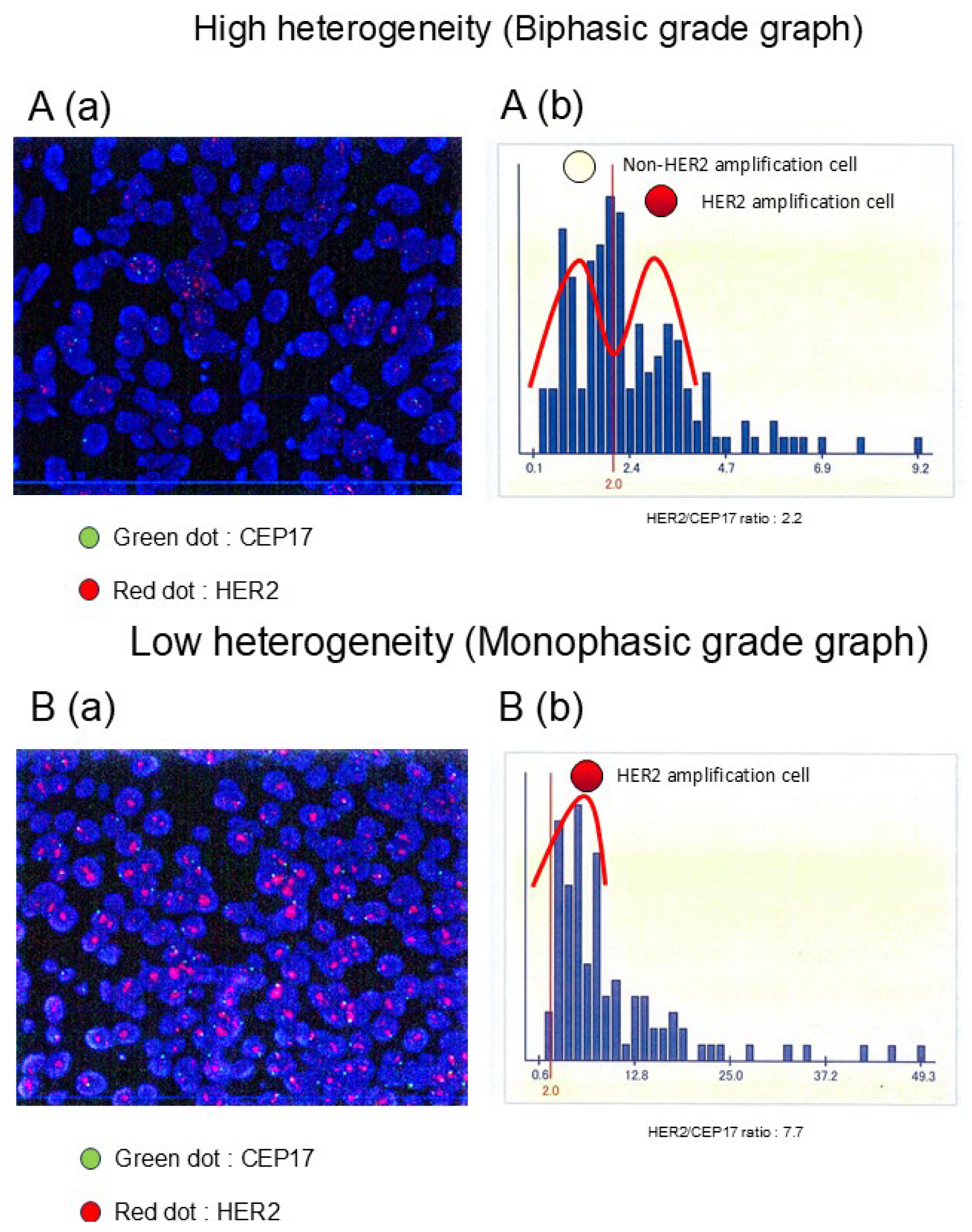
| HER2 Heterogeneity | ||||
|---|---|---|---|---|
| Total | High | Low | p Value | |
| n = 97% | n = 18% | n = 79% | ||
| Menopause | ||||
| pre | 47 (48.5%) | 12 (66.7%) | 35 (44.3%) | 0.12 |
| post | 50 (51.5%) | 6 (33.3%) | 44 (55.7%) | |
| Histological type | ||||
| IDC | 95 (97.9%) | 17 (94.4%) | 78 (98.8%) | 0.34 |
| ILC | 2 (2.1%) | 1 (5.6%) | 1 (1.3%) | |
| Histological grade | ||||
| 1 | 5 (5.2%) | 0 (0.0%) | 5 (6.3%) | 0.25 |
| 2 | 33 (34.0%) | 9 (50.0%) | 24 (30.4%) | |
| 3 | 59 (60.8%) | 9 (50.0%) | 50 (63.3%) | |
| Tumor size | ||||
| T1 | 7 (7.2%) | 1 (5.6%) | 6 (7.6%) | 1.00 |
| T2–T4 | 90 (92.8%) | 17 (94.4%) | 73 (92.4%) | |
| Pretreatment LN metastasis | ||||
| Negative | 34 (35.1%) | 3 (16.7%) | 31 (39.2%) | 0.10 |
| Positive | 63 (64.9%) | 15 (83.3%) | 48 (60.8%) | |
| ER | ||||
| Positive | 52 (53.6%) | 13 (72.2%) | 40 (50.6%) | 0.12 |
| Negative | 45 (46.4%) | 5 (27.8%) | 39 (49.4%) | |
| PgR | ||||
| Positive | 28 (28.9%) | 7 (38.9%) | 21 (26.6%) | 0.39 |
| Negative | 69 (71.1%) | 11 (61.1%) | 58 (73.4%) | |
| HER2 IHC | ||||
| 3+ | 90 (92.8%) | 16 (88.9%) | 74 (93.7%) | 0.61 |
| 2+ | 7 (7.2%) | 2 (11.1%) | 5 (6.3%) | |
| HER2 ratio | ||||
| Mean | 4.8 ± 2.2 | 2.5 ± 0.5 | 5.3 ± 2.2 | 1.23 × 10−17 |
| Stage | ||||
| I | 4 (5.1%) | 0 (0.0%) | 4 (5.1%) | 1.00 |
| II | 74 (76.3%) | 14 (77.8%) | 60 (75.9%) | |
| III | 19 (19.6%) | 4 (22.2%) | 15 (19.0%) | |
| Univariate Analysis | Multivariate Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | pCR Rate(%) | N | pCR(N) | OR | 95% CI | p Value | OR | 95% CI | p Value |
| Total | 58 | 97 | 56 | ||||||
| ER | |||||||||
| Positive | 40 | 52 | 21 | ||||||
| Negative | 78 | 45 | 35 | 0.194 | 0.0791–0.474 | 0.0003 | 0.321 | 0.1130–0.912 | 0.03 |
| PgR | |||||||||
| Positive | 32 | 28 | 9 | ||||||
| Negative | 68 | 69 | 47 | 0.222 | 0.0865–0.568 | 0.001 | 0.38 | 0.1230–1.170 | 0.09 |
| Histological grade | |||||||||
| 1 or 2 | 45 | 38 | 17 | ||||||
| 3 | 66 | 59 | 39 | 1.79 | 0.8970–3.59 | 0.09 | 1.84 | 0.8560–3.970 | 0.11 |
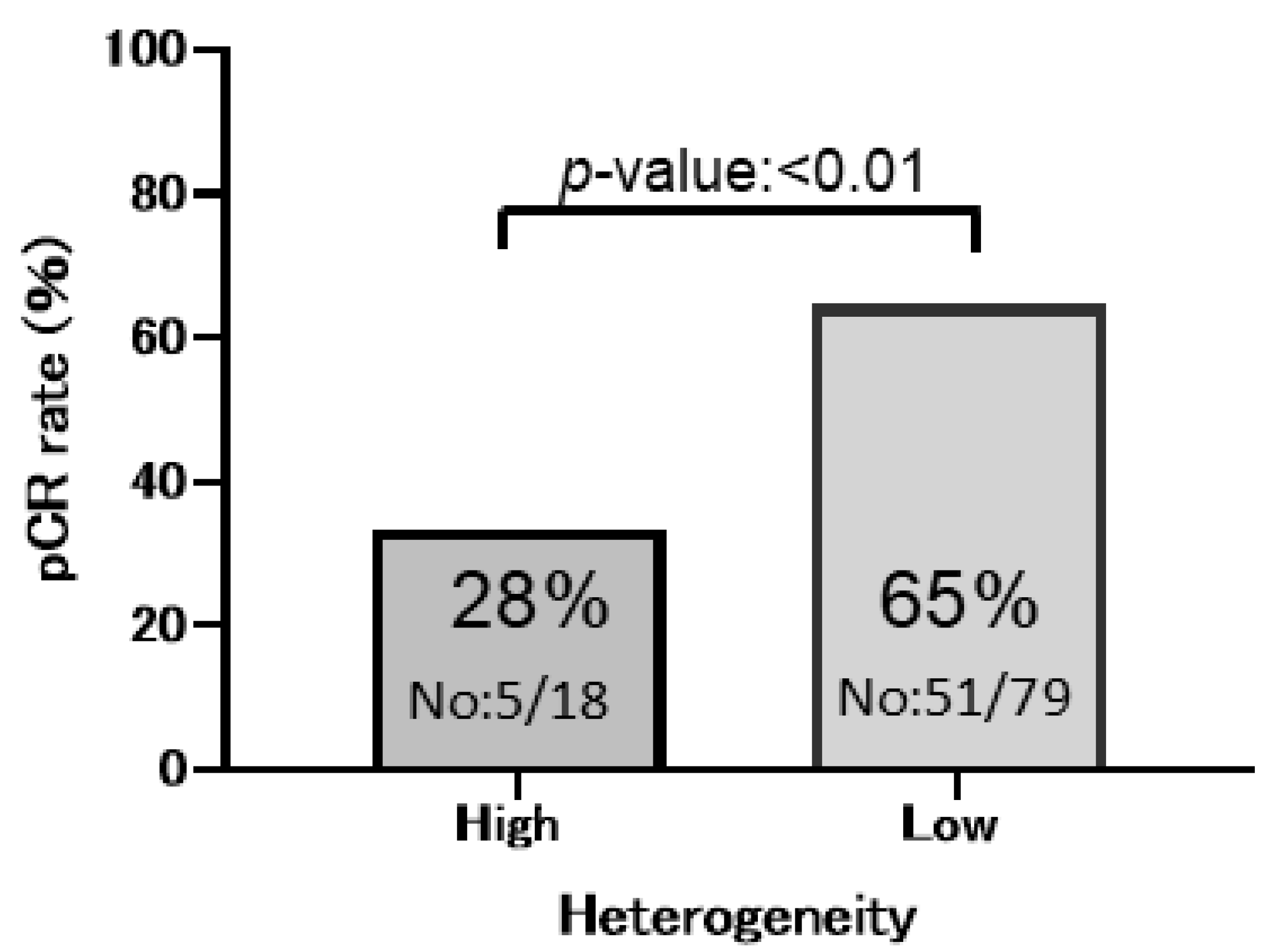
| HER2 heterogeneity | |||||||||
| Low | 65 | 79 | 51 | ||||||
| High | 28 | 18 | 5 | 0.211 | 0.0682–0.654 | 0.006 | 0.241 | 0.0699–0.828 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hatano, T.; Tanei, T.; Seno, S.; Sota, Y.; Masunaga, N.; Mishima, C.; Tsukabe, M.; Yoshinami, T.; Miyake, T.; Shimoda, M.; et al. High HER2 Intratumoral Heterogeneity Is Resistant to Anti-HER2 Neoadjuvant Chemotherapy in Early Stage and Locally Advanced HER2-Positive Breast Cancer. Cancers 2025, 17, 2126. https://doi.org/10.3390/cancers17132126
Hatano T, Tanei T, Seno S, Sota Y, Masunaga N, Mishima C, Tsukabe M, Yoshinami T, Miyake T, Shimoda M, et al. High HER2 Intratumoral Heterogeneity Is Resistant to Anti-HER2 Neoadjuvant Chemotherapy in Early Stage and Locally Advanced HER2-Positive Breast Cancer. Cancers. 2025; 17(13):2126. https://doi.org/10.3390/cancers17132126
Chicago/Turabian StyleHatano, Takaaki, Tomonori Tanei, Shigeto Seno, Yoshiaki Sota, Nanae Masunaga, Chieko Mishima, Masami Tsukabe, Tetsuhiro Yoshinami, Tomohiro Miyake, Masafumi Shimoda, and et al. 2025. "High HER2 Intratumoral Heterogeneity Is Resistant to Anti-HER2 Neoadjuvant Chemotherapy in Early Stage and Locally Advanced HER2-Positive Breast Cancer" Cancers 17, no. 13: 2126. https://doi.org/10.3390/cancers17132126
APA StyleHatano, T., Tanei, T., Seno, S., Sota, Y., Masunaga, N., Mishima, C., Tsukabe, M., Yoshinami, T., Miyake, T., Shimoda, M., & Shimazu, K. (2025). High HER2 Intratumoral Heterogeneity Is Resistant to Anti-HER2 Neoadjuvant Chemotherapy in Early Stage and Locally Advanced HER2-Positive Breast Cancer. Cancers, 17(13), 2126. https://doi.org/10.3390/cancers17132126






