Unveiling the Antioxidant Role of Hemp Oils in Cancer Prevention and Treatment
Simple Summary
Abstract
1. Introduction
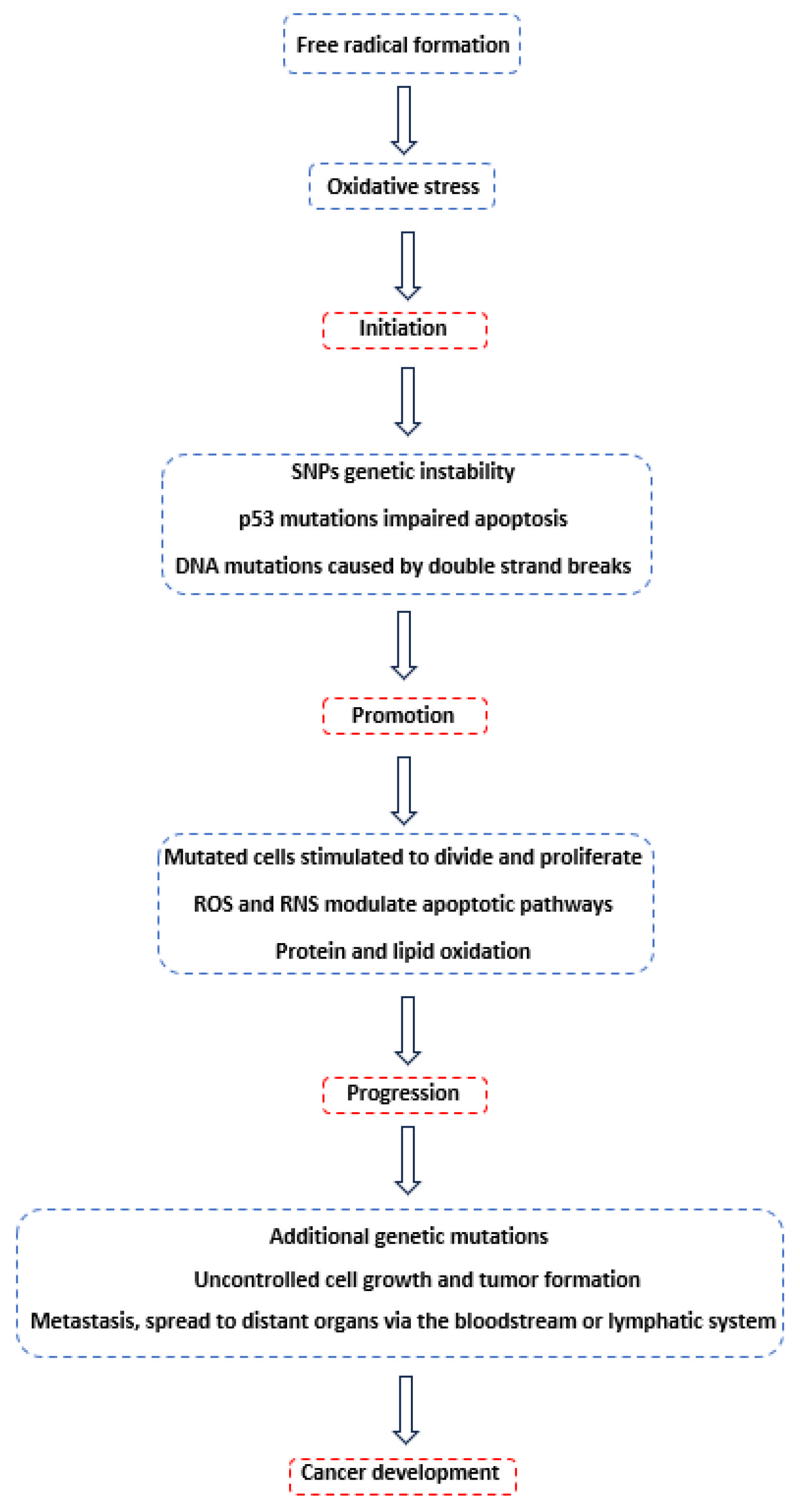
2. Carcinogenesis
3. Legislation for Cannabis-Based Edibles
3.1. Hemp Oils
3.2. Hashish Oil
3.3. Hemp Seed Oil
3.4. Hemp Essential Oil
3.5. CBD Oil
4. Medicinal Cannabis
4.1. The Endocannabinoid System
4.2. Antioxidant and Anticancer Mechanisms
4.2.1. The Role of THC and CBD
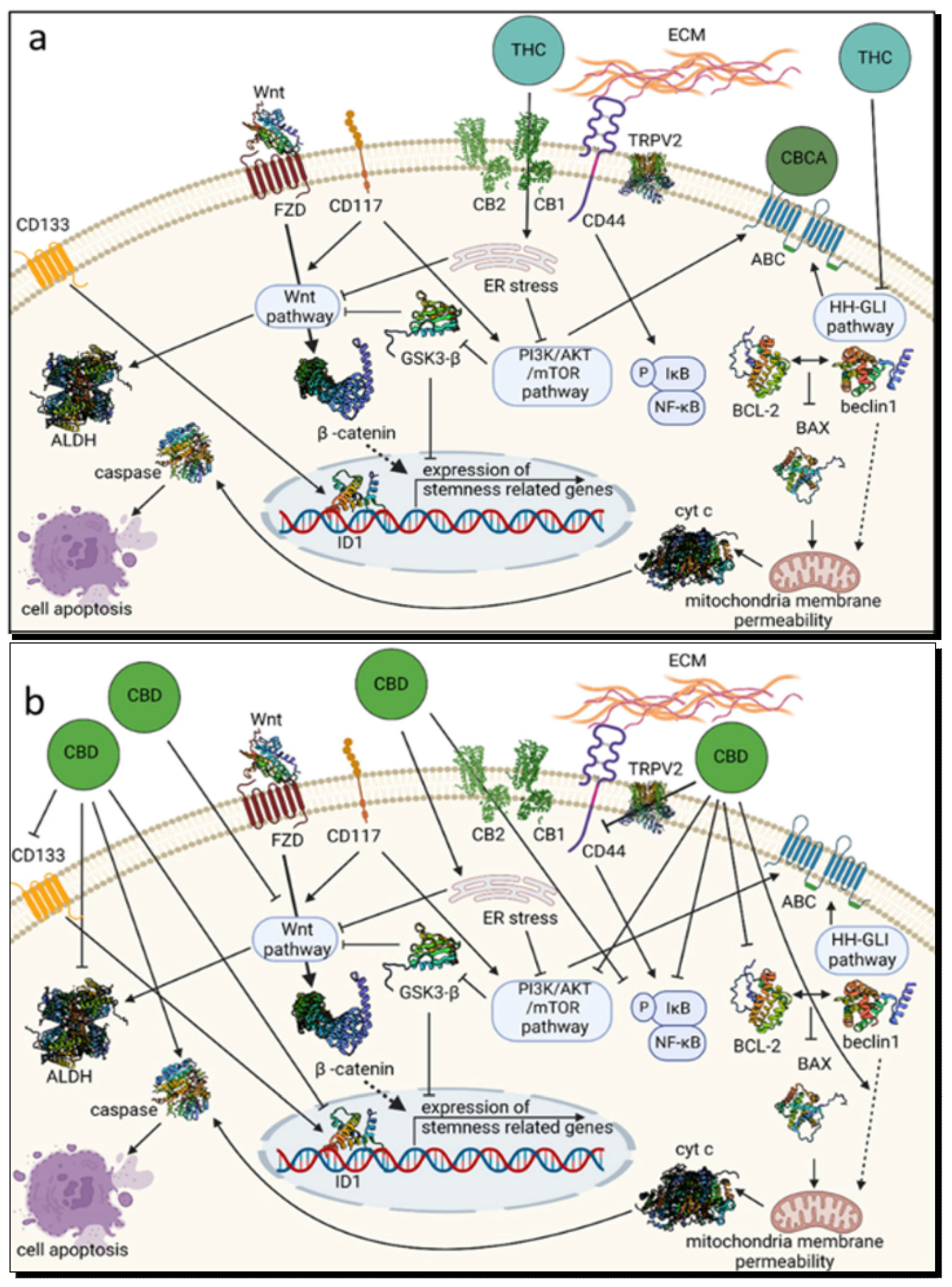
4.2.2. Synergetic Effects
5. Hemp Oil Treatments
6. Discussion and Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ROS | Reactive Oxygen Species |
| RNS | Reactive Nitrogen Species |
| UV | Ultraviolet |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate (Reduced) |
| LOOH | Lipid Hydroperoxide |
| SOD | Superoxide Dismutase |
| CAT | Catalase |
| GPx | Glutathione Peroxidase |
| L-Arginine | L-Arginine |
| L-Citrulline | L-Citrulline |
| CBD | Cannabidiol |
| CBDA | Cannabidiolic Acid |
| CBDV | Cannabidivarin |
| Δ9-THC | Δ9-Tetrahydrocannabinol |
| THCA-A | Tetrahydrocannabinolic Acid A |
| THCA-B | Tetrahydrocannabinolic Acid B |
| CBN | Cannabinol |
| THCV | Tetrahydrocannabivarin |
| CBG | Cannabigerol |
| FDA | U.S. Food and Drug Administration |
| EFSA | European Food Safety Authority |
| BfR | Federal Institute for Risk Assessment |
| DNFI | Danish National Food Institute |
| WHO | World Health Organization |
| ARfD | Acute Reference Dose |
| bw | Body Weight |
| UB | Upper Bound |
| EU | European Union |
| SNP | Single Nucleotide Polymorphism |
| CYP | Cytochrome P450 |
| 8-OHdG | 8-Hydroxy-2′-deoxyguanosine |
| BER | Base Excision Repair |
| NER | Nucleotide Excision Repair |
| OGG1 | 8-oxo-Guanine DNA Glycosylase |
| ASK1 | Apoptosis Signal-Regulating Kinase 1 |
| JNK | c-Jun N-terminal Kinase |
| p38 | p38 Mitogen-Activated Protein Kinase |
| MAPK | Mitogen-Activated Protein Kinase |
| AC | Adenylate Cyclase |
| cAMP | Cyclic Adenosine Monophosphate |
| PDE | Phosphodiesterase |
| PDE4 | Phosphodiesterase Type 4 |
| PKA | Protein Kinase A |
| CREB | cAMP Response Element-Binding Protein |
| Bim | Bcl-2-interacting mediator of cell death |
| BAD | Bcl-2-associated Agonist of Cell Death |
| IAP-2 | Inhibitor of Apoptosis Protein 2 |
| p21(Cip1) | Cyclin-Dependent Kinase Inhibitor 1A |
| p27(Kip1) | Cyclin-Dependent Kinase Inhibitor 1B |
| ATP | Adenosine Triphosphate |
| 5-AMP | 5-Adenosine Monophosphate |
| TRPV1 | Transient Receptor Potential Vanilloid 1 |
| 5-HT1A | 5-Hydroxytryptamine Receptor 1A |
| GPR55 | G-Protein Coupled Receptor 55 |
| A2A | Adenosine A2A Receptor |
| CANNUSE | Cannabis Use Database |
| ECS | Endocannabinoid System |
| GPCR | G-Protein Coupled Receptor |
| CRC | Colorectal Cancer |
| CDK | Cyclin-Dependent Kinase |
| G1 Phase | Gap 1 Phase |
| Neutraceutical | Nutritional + Pharmaceutical |
| pERK | Phosphorylated Extracellular Signal-Regulated Kinase |
| p53 | Tumor Protein p53 |
| GBM | Glioblastoma Multiforme |
| SPP1 | Secreted Phosphoprotein 1 (Osteopontin) |
| CD44 | Cluster of Differentiation 44 |
| ER | Endoplasmic Reticulum |
| PI3K | Phosphoinositide 3-Kinase |
| AKT | Protein Kinase B |
| mTOR | Mechanistic Target of Rapamycin |
| Wnt | Wingless/Integrated |
| Β-catenin | Beta-catenin |
| GSK3-Β | Glycogen Synthase Kinase 3 Beta |
| ID1 | Inhibitor of DNA Binding 1 |
| Bax | Bcl-2-associated X Protein |
| Bcl-2 | B-cell Lymphoma 2 |
| Caspase-9 | Cysteine-aspartic Protease 9 |
| CD44-TRPV2 | CD44 and Transient Receptor Potential Vanilloid 2 Complex |
| CD133 | Prominin-1 |
| CD117 | c-Kit |
| ALDH | Aldehyde Dehydrogenase |
| TRPV1 | Transient Receptor Potential Vanilloid 1 |
| PPARÎγ | Peroxisome Proliferator-Activated Receptor Gamma |
| 5-HT1A | 5-Hydroxytryptamine Receptor 1A |
| COX-2 | Cyclooxygenase-2 |
| TRAIL | TNF-related Apoptosis-Inducing Ligand |
| TRAIL-R2 | TRAIL Receptor 2 |
| DUSP1 | Dual Specificity Phosphatase 1 |
| ATM | Ataxia Telangiectasia Mutated |
| MMP2 | Matrix Metalloproteinase 2 |
| MMP9 | Matrix Metalloproteinase 9 |
| ET-1 | Endothelin-1 |
| PDGF-AA | Platelet-Derived Growth Factor AA |
| VEGF | Vascular Endothelial Growth Factor |
| HIF-1α | Hypoxia-Inducible Factor 1-alpha |
| ICAM-1 | Intercellular Adhesion Molecule 1 |
| Id-1 | Inhibitor of DNA Binding 1 |
| RXR | Retinoid X Receptor |
| PPREs | PPAR Response Elements |
| Bcl-xL | B-cell Lymphoma-extra Large |
| Bak | Bcl-2 homologous antagonist/killer |
| Caspases | Cysteine-aspartic Proteases |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B-cells |
| Hedgehog | Hedgehog Signaling Pathway |
| A549 | A549 Lung Carcinoma Cell Line |
| H538 | H538 Lung Cancer Cell Line |
| H460 | NCI-H460 Lung Cancer Cell Line |
| MDA-MB-231 | MDA-MB-231 Breast Cancer Cell Line |
| MDA-MB-436 | MDA-MB-436 Breast Cancer Cell Line |
| CD133 | Cluster of Differentiation 133 |
| FZD | Frizzled Receptor |
| ABC | ATP-Binding Cassette Transporters |
| IκB | Inhibitor of kappa B |
| TME | Tumor Microenvironment |
| CAFs | Cancer-Associated Fibroblasts |
| ECM | Extracellular Matrix |
| TGF-beta | Transforming Growth Factor Beta |
| EMT | Epithelial to Mesenchymal Transition |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor Necrosis Factor Alpha |
| M1/M2 | Macrophage Polarization States |
| THCA | Tetrahydrocannabinolic Acid |
| CBCA | Cannabichromenic Acid |
| CBC | Cannabichromene |
| CBDV | Cannabidivarin |
| CBGA | Cannabigerolic Acid |
| CGRP | Calcitonin Gene-Related Peptide |
| SNP | Sodium Nitroprusside |
| TMZ | Temozolomide |
| ERAD | Endoplasmic Reticulum-Associated Degradation |
| UPR | Unfolded Protein Response |
| PARP-1 | Poly (ADP-ribose) Polymerase 1 |
| Canniprene | Canniprene |
| Cannastilbenes | Cannastilbenes I, IIa, IIb |
| β-Caryophyllene | Beta-Caryophyllene |
References
- Weinberg, R.A.; Weinberg, R.A. The Biology of Cancer; WW Norton & Company: New York, NY, USA, 2006; p. 864, eBook; ISBN 9780203852569. [Google Scholar] [CrossRef]
- de Morais, E.F.; de Oliveira, L.Q.R.; de Farias Morais, H.G.; de Souto Medeiros, M.R.; Freitas, R.; de Almeida Freitas, R.; Rodini, C.O.; Coletta, R.D. The Anticancer Potential of Kaempferol: A Systematic Review Based on In Vitro Studies. Cancers 2024, 16, 585. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, M.C.; Orellana Palacios, J.C.; Hesami, G.; Jafarzadeh, S.; Lorenzo, J.M.; Domínguez, R.; Moreno, A.; Hadidi, M. Spectrophotometric Methods for Measurement of Antioxidant Activity in Food and Pharmaceuticals. Antioxidants 2022, 11, 2213. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free Radicals, Antioxidants and Functional Foods: Impact on Human Health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Willcox, J.K.; Ash, S.L.; Catignani, G.L. Antioxidants and Prevention of Chronic Disease. Crit. Rev. Food Sci. Nutr. 2004, 44, 275–295. [Google Scholar] [CrossRef]
- Wiseman, A. Dietary Alkyl Thiol Free Radicals (RSS) Can Be as Toxic as Reactive Oxygen Species (ROS). Med. Hypotheses 2004, 63, 667–670. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Azad, N.; Rojanasakul, Y.; Vallyathan, V. Inflammation and Lung Cancer: Roles of Reactive Oxygen/Nitrogen Species. J. Toxicol. Environ. Health—Part B Crit. Rev. 2008, 11, 1–15. [Google Scholar] [CrossRef]
- Sainz, R.M.; Lombo, F.; Mayo, J.C. Radical Decisions in Cancer: Redox Control of Cell Growth and Death. Cancers 2012, 4, 442–474. [Google Scholar] [CrossRef]
- Ahmed, O.M.; Mohammed, M.T. Oxidative Stress: The Role of Reactive Oxygen Species (Ros) and Antioxidants in Human Diseases. Plant Arch. 2020, 20, 4089–4095. [Google Scholar]
- Haklar, G.; Sayin-Özveri, E.; Yüksel, M.; Aktan, A.Ö.; Yalçin, A.S. Different Kinds of Reactive Oxygen and Nitrogen Species Were Detected in Colon and Breast Tumors. Cancer Lett. 2001, 165, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Koul, S.; Khandrika, L.; Meacham, R.B.; Koul, H.K. Oxidative Stress Is Inherent in Prostate Cancer Cells and Is Required for Aggressive Phenotype. Cancer Res. 2008, 68, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.D.; Sun, C.; Lambeth, J.D.; Marshall, F.; Amin, M.; Chung, L.; Petros, J.A.; Arnold, R.S. Increased Nox1 and Hydrogen Peroxide in Prostate Cancer. Prostate 2005, 62, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Betteridge, D.J. What Is Oxidative Stress? Metabolism 2000, 49, 3–8. [Google Scholar] [CrossRef]
- Droge, W. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Gregoriou, G.; Neophytou, C.M.; Vasincu, A.; Gregoriou, Y.; Hadjipakkou, H.; Pinakoulaki, E.; Christodoulou, M.C.; Ioannou, G.D.; Stavrou, I.J.; Christou, A.; et al. Anti-Cancer Activity and Phenolic Content of Extracts Derived from Cypriot Carob (Ceratonia siliqua L.) Pods Using Different Solvents. Molecules 2021, 26, 5017. [Google Scholar] [CrossRef]
- Gueffai, A.; Gonzalez-serrano, D.J.; Christodoulou, M.C.; Orellana-palacios, J.C.; Ortega, M.L.S.; Ouldmoumna, A.; Kiari, F.Z.; Ioannou, G.D.; Kapnissi-christodoulou, C.P.; Moreno, A.; et al. Phenolics from Defatted Black Cumin Seeds (Nigella sativa L.): Ultrasound-Assisted Extraction Optimization, Comparison, and Antioxidant Activity. Biomolecules 2022, 12, 1311. [Google Scholar] [CrossRef]
- Arias, A.; Feijoo, G.; Moreira, M.T. Exploring the Potential of Antioxidants from Fruits and Vegetables and Strategies for Their Recovery. Innov. Food Sci. Emerg. Technol. 2022, 77, 102974. [Google Scholar] [CrossRef]
- Amarowicz, R.; Pegg, R.B. Natural Antioxidants of Plant Origin, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Volume 90, ISBN 9780128165676. [Google Scholar]
- King, J.W. The Relationship between Cannabis/Hemp Use in Foods and Processing Methodology. Curr. Opin. Food Sci. 2019, 28, 32–40. [Google Scholar] [CrossRef]
- Chandra, S.; Lata, H.; ElSohly, M.A. Cannabis sativa L.—Botany and Biotechnology; Springer: Cham, Switzerland, 2017; pp. 1–474. [Google Scholar] [CrossRef]
- Salami, S.A.; Martinelli, F.; Giovino, A.; Bachari, A.; Arad, N.; Mantri, N. It Is Our Turn to Get Cannabis High: Put Cannabinoids in Food and Health Baskets. Molecules 2020, 25, 4036. [Google Scholar] [CrossRef]
- Afrin, F.; Chi, M.; Eamens, A.L.; Duchatel, R.J.; Douglas, A.M.; Schneider, J.; Gedye, C.; Woldu, A.S.; Dun, M.D. Can Hemp Help? Low-THC Cannabis and Non-THC Cannabinoids for the Treatment of Cancer. Cancers 2020, 12, 1033. [Google Scholar] [CrossRef] [PubMed]
- Grotenhermen, F.; Müller-Vahl, K. Das Therapeutische Potenzial von Cannabis Und Cannabinoiden. Dtsch. Arztebl. Int. 2012, 109, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Brien, K.O. Cannabidiol (CBD) in Cancer Management. Cancers 2022, 14, 885. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B. Taming THC: Potential Cannabis Synergy and Phytocannabinoid-Terpenoid Entourage Effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef]
- Christodoulou, M.C.; Christou, A.; Stavrou, I.J.; Kapnissi-Christodoulou, C.P. Evaluation of Different Extraction Procedures for the Quantification of Seven Cannabinoids in Cannabis-Based Edibles by the Use of LC-MS. J. Food Compos. Anal. 2023, 115, 104915. [Google Scholar] [CrossRef]
- Food and Drug Administration. What You Need to Know (And What We’re Working to Find Out) About Products Containing Cannabis or Cannabis-Derived Compounds, Including CBD. Available online: https://www.fda.gov/consumers/consumer-updates/what-you-need-know-and-what-were-working-find-out-about-products-containing-cannabis-or-cannabis (accessed on 3 May 2020).
- Stewart, Z.A.; Pietenpol, J.A. P53 Signaling and Cell Cycle Checkpoints. Chem. Res. Toxicol. 2001, 14, 243–263. [Google Scholar] [CrossRef]
- Franklin, W.A.; Gazdar, A.F.; Haney, J.; Wistuba, I.I.; La Rosa, F.G.; Kennedy, T.; Ritchey, D.M.; Miller, Y.E. Widely Dispersed P53 Mutation in Respiratory Epithelium. A Novel Mechanism for Field Carcinogenesis. J. Clin. Investig. 1997, 100, 2133. [Google Scholar] [CrossRef]
- Sergentanis, T.N.; Economopoulos, K.P.; Choussein, S.; Vlahos, N.F. Cytochrome P450 1A1 Gene Polymorphisms and Endometrial Cancer Risk: A Meta-Analysis. Int. J. Gynecol. Cancer 2011, 21, 323–331. [Google Scholar] [CrossRef]
- Nock, N.L.; Cicek, M.S.; Li, L.; Liu, X.; Rybicki, B.A.; Moreira, A.; Plummer, S.J.; Casey, G.; Witte, J.S. Polymorphisms in Estrogen Bioactivation, Detoxification and Oxidative DNA Base Excision Repair Genes and Prostate Cancer Risk. Carcinogenesis 2006, 27, 1842–1848. [Google Scholar] [CrossRef]
- Compton, C. Cancer Initiation, Promotion, and Progression and the Acquisition of Key Behavioral Traits. In Cancer Enemy from Within; Springer: Cham, Switzerland, 2020; pp. 25–48. [Google Scholar] [CrossRef]
- Karin, M. Tracking the Road from Inflammation to Cancer: The Critical Role of IκB Kinase (IKK). In The Harvey Lectues: Delivered Under the Auspices of The Harvey Society of New York, 2006–2007; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 133–151. [Google Scholar] [CrossRef]
- Reed, J.C. Cytochrome c: Can’t Live with It—Can’t Live without It. Cell 1997, 91, 559–562. Available online: https://www.cell.com/cell/pdf/S0092-8674(00)80442-0.pdf (accessed on 23 April 2025). [CrossRef]
- Benhar, M.; Dalyot, I.; Engelberg, D.; Levitzki, A. Enhanced ROS Production in Oncogenically Transformed Cells Potentiates C-Jun N-Terminal Kinase and P38 Mitogen-Activated Protein Kinase Activation and Sensitization to Genotoxic Stress. Mol. Cell. Biol. 2001, 21, 6913–6926. [Google Scholar] [CrossRef] [PubMed]
- Heigold, S.; Sers, C.; Bechtel, W.; Ivanovas, B.; Schäfer, R.; Bauer, G. Nitric Oxide Mediates Apoptosis Induction Selectively in Transformed Fibroblasts Compared to Nontransformed Fibroblasts. Carcinogenesis 2002, 23, 929–941. [Google Scholar] [CrossRef] [PubMed]
- Borutaite, V.; Brown, G.C. Nitric Oxide Induces Apoptosis via Hydrogen Peroxide, but Necrosis via Energy and Thiol Depletion. Free Radic. Biol. Med. 2003, 35, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Nicoll, M.; Ingham, R.J. AP-1 Family Transcription Factors: A Diverse Family of Proteins That Regulate Varied Cellular Activities in Classical Hodgkin Lymphoma and ALK+ ALCL. Exp. Hematol. Oncol. 2021, 10, 4. [Google Scholar] [CrossRef]
- Tanaka, M.; Kishimoto, Y.; Sasaki, M.; Sato, A.; Kamiya, T.; Kondo, K.; Iida, K. Terminalia bellirica (Gaertn.) Roxb. Extract and Gallic Acid Attenuate LPS-Induced Inflammation and Oxidative Stress via MAPK/NF- κ B and Akt/AMPK/Nrf2 Pathways. Oxid. Med. Cell. Longev. 2018, 2018, 9364364. [Google Scholar] [CrossRef]
- Liebert, M.A.; Fessel, J.P.; Ii, L.J.R. Isofurans: Novel Products of Lipid Peroxidation That Define the Occurrence of Oxidant Injury in Settings of Elevated Oxygen Tension. Antioxid. Redox Signal. 2005, 7, 202–209. [Google Scholar]
- Yokota, J. Tumor Progression and Metastasis. Carcinogenesis 2000, 21, 497–503. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Huysentruyt, L.C. On the Origin of Cancer Metastasis. Crit. Rev. Oncog. 2013, 18, 43–73. [Google Scholar] [CrossRef]
- European Monitoring Centre for Drugs and Drug Addiction. European Drug Report 2022: Trends and Developments; Publications Office of the European Union: Lisbon, Portugal, 2022. Available online: https://www.euda.europa.eu/publications/edr/trends-developments/2022_en (accessed on 23 April 2025)ISBN 9789294977694.
- Di Marco Pisciottano, I.; Guadagnuolo, G.; Soprano, V.; Esposito, M.; Gallo, P. A Survey of Δ9-THC and Relevant Cannabinoids in Products from the Italian Market: A Study by LC–MS/MS of Food, Beverages and Feed. Food Chem. 2021, 346, 128898. [Google Scholar] [CrossRef]
- Panel, E.; Chain, F. Scientific Opinion on the Risks for Human Health Related to the Presence of Tetrahydrocannabinol (THC) in Milk and Other Food of Animal Origin. EFSA J. 2015, 13, 4141. [Google Scholar] [CrossRef]
- Beitzke, B.; Pate, D.W. A Broader View on Deriving a Reference Dose for THC Traces in Foods. Crit. Rev. Toxicol. 2021, 51, 695–722. [Google Scholar] [CrossRef] [PubMed]
- Arcella, D.; Cascio, C.; Mackay, K. Acute Human Exposure Assessment to Tetrahydrocannabinol (Δ9-THC). EFSA J. 2020, 18, 5953. [Google Scholar] [CrossRef]
- Kladar, N.; Čonić, B.S.; Božin, B.; Torović, L. European Hemp-Based Food Products—Health Concerning Cannabinoids Exposure Assessment. Food Control 2021, 129, 108233. [Google Scholar] [CrossRef]
- Borodovsky, J.T.; Lee, D.C.; Crosier, B.S.; Gabrielli, J.L.; Sargent, J.D.; Budney, A.J. U.S. Cannabis Legalization and Use of Vaping and Edible Products among Youth. Drug Alcohol Depend. 2017, 177, 299–306. [Google Scholar] [CrossRef]
- Goundar, P.; Macaulay, T.; Szafron, M. A Comparative Analysis of Laws on Recreational Cannabis Edibles between Canada and the United States of America. Int. J. Drug Policy 2021, 94, 103191. [Google Scholar] [CrossRef]
- Tallon, M.J. Cannabis sativa L. and Its Extracts: Regulation of Cannabidiol in the European Union and United Kingdom. J. Diet. Suppl. 2020, 17, 503–516. [Google Scholar] [CrossRef]
- Fordjour, E.; Armah, A.; Thomas, R. Cannabis-Infused Foods: Phytonutrients, Health, and Safe Product Innovations. Compr. Rev. Food Sci. Food Saf. 2024, 23, e70021. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Kroener, L.; Musshoff, F.; Madea, B. Determination of Cannabinoids in Hemp Food Products by Use of Headspace Solid-Phase Microextraction and Gas Chromatography-Mass Spectrometry. Anal. Bioanal. Chem. 2004, 378, 183–189. [Google Scholar] [CrossRef]
- Peng, H.; Shahidi, F. Cannabis and Cannabis Edibles: A Review. J. Agric. Food Chem. 2021, 69, 1751–1774. [Google Scholar] [CrossRef]
- Orcutt, M.; Verrecchia, R.; Abubakar, I. The UK National Health Service Regulations for Overseas Visitors. Lancet 2019, 394, 734–735. [Google Scholar] [CrossRef]
- Coombes, R. Cannabis Regulation: High Time for Change? BMJ 2014, 348, g3382. [Google Scholar] [CrossRef] [PubMed]
- Caulkins, J.P.; Kilborn, M.L. Cannabis Legalization, Regulation, & Control: A Review of Key Challenges for Local, State, and Provincial Officials. Am. J. Drug Alcohol Abuse 2019, 45, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Flengmark, P.; Deleuran, L. Yield Potential of Hemp (Cannabis sativa L.) Cultivars in Denmark. J. Ind. Hemp 2005, 10, 5–17. [Google Scholar] [CrossRef]
- Hammond, D. Communicating THC Levels and ‘Dose’ to Consumers: Implications for Product Labelling and Packaging of Cannabis Products in Regulated Markets. Int. J. Drug Policy 2021, 91, 102509. [Google Scholar] [CrossRef]
- Lachenmeier, D.; Walch, S. Analysis and Toxicological Evaluation of Cannabinoids in Hemp Food Products—A Review. Electron. J. Environ. Agric. Food Chem. 2005, 4, 812–826. [Google Scholar] [CrossRef]
- Deville, M.; Dubois, N.; Denooz, R.; Charlier, C. Validation of an UHPLC/DAD Method for the Determination of Cannabinoids in Seized Materials: Analysis of 213 Samples Sold in Belgian CBD Shops. Forensic Sci. Int. 2020, 310, 110234. [Google Scholar] [CrossRef]
- Christinat, N.; Savoy, M.C.; Mottier, P. Development, Validation and Application of a LC-MS/MS Method for Quantification of 15 Cannabinoids in Food. Food Chem. 2020, 318, 126469. [Google Scholar] [CrossRef]
- Christodoulou, M.C.; Ioannou, G.D.; Ioannou, K.A.; Stavrou, I.J.; Kapnissi-christodoulou, C.P. Optimization of Ionic Liquid-Based Ultrasound- Assisted Extraction to Enhance Cannabinoid Recovery from Hemp Teas. Sep. Sci. Technol. 2024, 59, 1583–1600. [Google Scholar] [CrossRef]
- Hazekamp, A. The Trouble with CBD Oil. Med. Cannabis Cannabinoids 2018, 1, 65–72. [Google Scholar] [CrossRef]
- Steigerwald, S.; Wong, P.O.; Khorasani, A.; Keyhani, S. The Form and Content of Cannabis Products in the United States. J. Gen. Intern. Med. 2018, 33, 1426–1428. [Google Scholar] [CrossRef]
- Piccolella, S.; Crescente, G.; Formato, M.; Pacifico, S. A Cup of Hemp Coffee by Moka Pot from Southern Italy: An Uhplc-Hrms Investigation. Foods 2020, 9, 1123. [Google Scholar] [CrossRef] [PubMed]
- McGregor, I.S.; Cairns, E.A.; Abelev, S.; Cohen, R.; Henderson, M.; Couch, D.; Arnold, J.C.; Gauld, N. Access to Cannabidiol without a Prescription: A Cross-Country Comparison and Analysis. Int. J. Drug Policy 2020, 85, 102935. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, C.M.; Abel, W.D.; Jones-Edwards, E.E.; Brown, P.D.; Bernard, K.K.; Taylor, T.T. Form and Content of Jamaican Cannabis Edibles. J. Cannabis Res. 2021, 3, 29. [Google Scholar] [CrossRef] [PubMed]
- Nyland, C.R.; Moyer, D.C. Regulating for Safety: Cannabidiol Dose in Food: A Review. J. Food Prot. 2022, 85, 1355–1369. [Google Scholar] [CrossRef]
- Knopf, A. From the FDA: Cannabis Edibles Copycatting Cereals, Candy. Brown Univ. Child Adolesc. Behav. Lett. 2022, 38, 9–10. [Google Scholar] [CrossRef]
- Mead, A. The Legal Status of Cannabis (Marijuana) and Cannabidiol (CBD) under U.S. Law. Epilepsy Behav. 2017, 70, 288–291. [Google Scholar] [CrossRef]
- Birenboim, M.; Chalupowicz, D.; Maurer, D.; Barel, S.; Chen, Y.; Fallik, E.; Paz-Kagan, T.; Rapaport, T.; Sadeh, A.; Kengisbuch, D.; et al. Multivariate classification of cannabis chemovars based on their terpene and cannabinoid profiles. Phytochemistry 2022, 200, 113215. [Google Scholar] [CrossRef]
- Farag, S. Chapter 1—The Cannabis Plant: Botanical Aspects. In Handbook of Cannabis and Related Pathologies; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780128007563. [Google Scholar]
- Escrivá, Ú.; Andrés-Costa, M.J.; Andreu, V.; Picó, Y. Analysis of Cannabinoids by Liquid Chromatography–Mass Spectrometry in Milk, Liver and Hemp Seed to Ensure Food Safety. Food Chem. 2017, 228, 177–185. [Google Scholar] [CrossRef]
- United Nations Office on Drugs and Crime. Recommended Methods for the Identification and Analysis of Cannabis and Cannabis Products: Manual for Use by National Drug Analysis Laboratories, Revised and Updated; United Nations: Vienna, Austria, March 2022; Available online: https://www.unodc.org/documents/scientific/Recommended_methods_for_the_Identification_and_Analysis_of_Cannabis_and_Cannabis_products.pdf (accessed on 23 April 2025).
- Komarnytsky, S.; Rathinasabapathy, T.; Wagner, C.; Metzger, B.; Carlisle, C.; Panda, C.; Le Brun-blashka, S.; Troup, J.P.; Varadharaj, S. Endocannabinoid System and Its Regulation by Polyunsaturated Fatty Acids and Full Spectrum Hemp Oils. Int. J. Mol. Sci. 2021, 22, 5479. [Google Scholar] [CrossRef]
- Vigil, J.M.; Montera, M.A.; Pentkowski, N.S.; Diviant, J.P.; Orozco, J.; Ortiz, A.L.; Rael, L.J.; Westlund, K.N. The Therapeutic E Ff Ectiveness of Full Spectrum Hemp Oil Using a Chronic Neuropathic Pain Model. Life 2020, 10, 69. [Google Scholar] [CrossRef]
- Souza, T.E.A.; Bertol, G.; Santos, P.M. New Analytical Screening Method for Fast Classification of Hemp Oil Based on THC Content. ACS Omega 2025, 10, 15143–15147. [Google Scholar] [CrossRef] [PubMed]
- Starks, M.; Starks, M. Marijuana Chemistry: Genetics, Processing & Potency; Ronin Publishing: Berkeley, CA, USA, 1990. [Google Scholar]
- Stogner, J.M.; Miller, B.L. Assessing the Dangers of ‘Dabbing’: Mere Marijuana or Harmful New Trend? Pediatrics 2015, 136, 1–3. [Google Scholar] [CrossRef] [PubMed]
- MacCoun, R.J.; Mello, M.M. Half-Baked—The Retail Promotion of Marijuana Edibles. N. Engl. J. Med. 2015, 372, 989–991. [Google Scholar] [CrossRef] [PubMed]
- Hazekamp, A.; Romano, L.L. Cannabis Oil: Chemical Evaluation of an Upcoming Cannabis-Based Medicine. Cannabinoids 2013, 1, 1–11. [Google Scholar]
- Brenneisen, R. Chemistry and Analysis of Phytocannabinoids and Other Cannabis Constituents. In Marijuana and the Cannabinoids; Humana Press: Totowa, NJ, USA, 2007; pp. 17–49. [Google Scholar] [CrossRef]
- Casiraghi, A.; Roda, G.; Casagni, E.; Cristina, C.; Musazzi, U.M.; Franzè, S.; Rocco, P.; Giuliani, C.; Fico, G.; Minghetti, P.; et al. Extraction Method and Analysis of Cannabinoids in Cannabis Olive Oil Preparations. Planta Med. 2018, 84, 242–249. [Google Scholar] [CrossRef]
- Callaway, J.C. Hempseed as a Nutritional Resource: An Overview. Euphytica 2004, 140, 65–72. [Google Scholar] [CrossRef]
- Xia, T.; Shi, S.; Wan, X. Impact of Ultrasonic-Assisted Extraction on the Chemical and Sensory Quality of Tea Infusion. J. Food Eng. 2006, 74, 557–560. [Google Scholar] [CrossRef]
- VanDolah, H.J.; Bauer, B.A.; Mauck, K.F. Clinicians’ Guide to Cannabidiol and Hemp Oils. Mayo Clin. Proc. 2019, 94, 1840–1851. [Google Scholar] [CrossRef]
- Citti, C.; Pacchetti, B.; Vandelli, M.A.; Forni, F.; Cannazza, G. Analysis of Cannabinoids in Commercial Hemp Seed Oil and Decarboxylation Kinetics Studies of Cannabidiolic Acid (CBDA). J. Pharm. Biomed. Anal. 2018, 149, 532–540. [Google Scholar] [CrossRef]
- Dimić, E.; Romanić, R.; Vujasinović, V. Essential Fatty Acids, Nutritive Value and Oxidative Stability of Cold Pressed Hempseed (Cannabis sativa L.) Oil from Different Varieties. Acta Aliment. 2009, 38, 229–236. [Google Scholar] [CrossRef]
- Rezvankhah, A.; Emam-Djomeh, Z.; Safari, M.; Askari, G.; Salami, M. Microwave-Assisted Extraction of Hempseed Oil: Studying and Comparing of Fatty Acid Composition, Antioxidant Activity, Physiochemical and Thermal Properties with Soxhlet Extraction. J. Food Sci. Technol. 2019, 56, 4198–4210. [Google Scholar] [CrossRef] [PubMed]
- De Vita, D.; Madia, V.N.; Tudino, V.; Saccoliti, F.; De Leo, A.; Messore, A.; Roscilli, P.; Botto, A.; Pindinello, I.; Santilli, G.; et al. Comparison of Different Methods for the Extraction of Cannabinoids from Cannabis. Nat. Prod. Res. 2020, 34, 2952–2958. [Google Scholar] [CrossRef] [PubMed]
- Kostić, M.D.; Joković, N.M.; Stamenković, O.S.; Rajković, K.M.; Milić, P.S.; Veljković, V.B. Optimization of Hempseed Oil Extraction by N-Hexane. Ind. Crops Prod. 2013, 48, 133–143. [Google Scholar] [CrossRef]
- Aladić, K.; Jarni, K.; Barbir, T.; Vidović, S.; Vladić, J.; Bilić, M.; Jokić, S. Supercritical CO2 Extraction of Hemp (Cannabis sativa L.) Seed Oil. Ind. Crops Prod. 2015, 76, 472–478. [Google Scholar] [CrossRef]
- Joki, S.; Pavi, V.; Jakovljevi, M. Terpenes and Cannabinoids in Supercritical CO2 Extracts of Industrial Hemp Inflorescences: Optimization of Extraction, Antiradical and Antibacterial Activity. Pharmaceuticals 2022, 15, 1117. [Google Scholar] [CrossRef]
- Leizer, C.; Ribnicky, D.M.; Poulev, A.; Dushenkov, D.; Raskin, I. The Composition of Hemp Seed Oil and Its Potential as an Important Source of Nutrition. J. Nutraceuticals Funct. Med. Foods 2000, 2, 35–53. [Google Scholar] [CrossRef]
- Cerino, P.; Buonerba, C.; Cannazza, G.; D’Auria, J.; Ottoni, E.; Fulgione, A.; Di Stasio, A.; Pierri, B.; Gallo, A. A Review of Hemp as Food and Nutritional Supplement. Cannabis Cannabinoid Res. 2021, 6, 19–27. [Google Scholar] [CrossRef]
- Grigoriev, O.V. Application of Hempseed (Cannabis sativa L.) Oil in the Treatment of Ear, Nose and Throat (Ent) Disorders. J. Ind. Hemp 2002, 7, 5–15. [Google Scholar] [CrossRef]
- Baral, P.; Bagul, V.; Gajbhiye, S. Hemp Seed Oil for Skin Care (Non-Drug Cannabis sativa. World J. Pharm. Res. 2020, 9, 2534–2556. [Google Scholar] [CrossRef]
- Farinon, B.; Molinari, R.; Costantini, L.; Merendino, N. The Seed of Industrial Hemp (Cannabis sativa L.): Nutritional Quality and Potential Functionality for Human Health and Nutrition. Nutrients 2020, 12, 1935. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Walch, S.G. Current Status of THC in German Hemp Food Products PEER-REVIEWED PAPERS Current Status of THC in German Hemp Food Products. J. Ind. Hemp 2005, 10, 5–17. [Google Scholar] [CrossRef]
- Jang, E.; Kim, H.; Jang, S.; Lee, J.; Baeck, S.; In, S.; Kim, E.; Kim, Y.-U.; Han, E. Concentrations of THC, CBD, and CBN in Commercial Hemp Seeds and Hempseed Oil Sold in Korea. Forensic Sci. Int. 2020, 306, 110064. [Google Scholar] [CrossRef] [PubMed]
- Fiorini, D.; Molle, A.; Nabissi, M.; Santini, G.; Benelli, G.; Maggi, F. Valorizing Industrial Hemp (Cannabis sativa L.) by-Products: Cannabidiol Enrichment in the Inflorescence Essential Oil Optimizing Sample Pre-Treatment Prior to Distillation. Ind. Crops Prod. 2019, 128, 581–589. [Google Scholar] [CrossRef]
- Palmieri, S.; Maggio, F.; Pellegrini, M.; Ricci, A.; Serio, A.; Paparella, A.; Lo Sterzo, C. Effect of the Distillation Time on the Chemical Composition, Antioxidant Potential and Antimicrobial Activity of Essential Oils from Different Cannabis sativa L. Cultivars. Molecules 2021, 26, 4770. [Google Scholar] [CrossRef]
- Hughston, L.; Conarton, M. Terpenes and Flavonoids: Cannabis Essential Oil. In Cannabis Therapy in Veterinary Medicine; Springer: Cham, Switzerland, 2021; pp. 85–115. [Google Scholar] [CrossRef]
- Leas, E.C.; Moy, N.; McMenamin, S.B.; Shi, Y.; Benmarhnia, T.; Stone, M.D.; Trinidad, D.R.; White, M. Availability and Promotion of Cannabidiol (Cbd) Products in Online Vape Shops. Int. J. Environ. Res. Public Health 2021, 18, 6719. [Google Scholar] [CrossRef]
- Pezantes-Orellana, C.; German Bermúdez, F.; Matías De la Cruz, C.; Montalvo, J.L.; Orellana-Manzano, A. Essential Oils: A Systematic Review on Revolutionizing Health, Nutrition, and Omics for Optimal Well-Being. Front. Med. 2024, 11, 1337785. [Google Scholar] [CrossRef]
- Rapin, L.; Gamaoun, R.; El Hage, C.; Arboleda, M.F.; Prosk, E. Cannabidiol Use and Effectiveness: Real-World Evidence from a Canadian Medical Cannabis Clinic. J. Cannabis Res. 2021, 3, 19. [Google Scholar] [CrossRef]
- Bhaskar, A.; Bell, A.; Boivin, M.; Briques, W.; Brown, M.; Clarke, H.; Cyr, C.; Eisenberg, E.; de Oliveira Silva, R.F.; Frohlich, E.; et al. Consensus Recommendations on Dosing and Administration of Medical Cannabis to Treat Chronic Pain: Results of a Modified Delphi Process. J. Cannabis Res. 2021, 3, 22. [Google Scholar] [CrossRef]
- Newton, M.; Newton, D.W. Cannabidiol or CBD Oil: Help, Hope, and Hype for Psychiatric and Neurologic Conditions. J. Am. Psychiatr. Nurses Assoc. 2020, 26, 447–457. [Google Scholar] [CrossRef]
- Ross, S.A.; Elsohly, M.A. The Volatile Oil Composition of Fresh and Air-Dried Buds of Cannabis sativa. J. Nat. Prod. 1996, 59, 49–51. [Google Scholar] [CrossRef]
- Sexton, M.; Shelton, K.; Haley, P.; West, M. Evaluation of Cannabinoid and Terpenoid Content: Cannabis Flower Compared to Supercritical CO2 Concentrate. Planta Med. 2017, 84, 234–241, Erratum in Planta Med. 2018, 84, E3. https://doi.org/10.1055/s-0043-122272. [Google Scholar] [CrossRef] [PubMed]
- Hazekamp, A.; Ware, M.A.; Muller-Vahl, K.R.; Abrams, D.; Grotenhermen, F. The Medicinal Use of Cannabis and Cannabinoids-An International Cross-Sectional Survey on Administration Forms. J. Psychoactive Drugs 2013, 45, 199–210. [Google Scholar] [CrossRef] [PubMed]
- European Monitoring Centre for Drugs and Drug Addiction; Europol. EU Drug Markets Analysis: Key Insights for Policy and Practice; Publications Office of the European Union: Luxembourg, 2024. Available online: https://www.europol.europa.eu/cms/sites/default/files/documents/EU%20Drug%20Markets%20Analysis%202024.pdf (accessed on 23 April 2025).
- EMCDDA. Cannabis Laws in Europe; European Monitoring Centre for Drugs and Drug Addiction: Cais do Sodré, Lisbon, 2023; ISBN 9789294978578.
- Gulluni, N.; Re, T.; Loiacono, I.; Lanzo, G.; Gori, L.; MacChi, C.; Epifani, F.; Bragazzi, N.; Firenzuoli, F. Cannabis Essential Oil: A Preliminary Study for the Evaluation of the Brain Effects. Evid.-Based Complement. Altern. Med. 2018, 2018, 1709182. [Google Scholar] [CrossRef] [PubMed]
- Gicewicz, E.; Gatewood, S.S.; Kaefer, T.N.; Nadpara, P.; Goode, J.V.R. Assessment of Hemp Oil-Based Cannabidiol Use in a Community-Based Pharmacy Setting. J. Am. Pharm. Assoc. 2021, 61, S49–S56. [Google Scholar] [CrossRef]
- Morales, P.; Hurst, D.P.; Reggio, P.H. Molecular Targets of the Phytocannabinoids: A Complex Picture. Prog. Chem. Org. Nat. Prod. 2017, 103, 103–131. [Google Scholar] [CrossRef]
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A Comprehensive Ethnopharmacological Review of a Medicinal Plant with a Long History. J. Ethnopharmacol. 2018, 227, 300–315. [Google Scholar] [CrossRef]
- Favell, J.W.; Hayward, R.; O’Brien, E.; Riordan-Short, S.; Sagar, N.; O’Brien, R.; Noestheden, M. Quantitating Cannabinoids in Edible Chocolates Using Heated Ultrasonic-Assisted Extraction, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2020; Volume 90, ISBN 9780444643414. [Google Scholar]
- Lazarjani, M.P.; Young, O.; Kebede, L.; Seyfoddin, A. Processing and Extraction Methods of Medicinal Cannabis: A Narrative Review. J. Cannabis Res. 2021, 3, 32. [Google Scholar] [CrossRef]
- Ražić, S.; Bakić, T.; Topić, A.; Lukić, J.; Onjia, A. Deep Eutectic Solvent Based Reversed-Phase Dispersive Liquid–Liquid Microextraction and High-Performance Liquid Chromatography for the Determination of Free Tryptophan in Cold-Pressed Oils. Molecules 2023, 28, 2395. [Google Scholar] [CrossRef]
- Pieracci, Y.; Ascrizzi, R.; Terreni, V.; Pistelli, L.; Flamini, G.; Bassolino, L.; Fulvio, F.; Montanari, M.; Paris, R. Essential Oil of Cannabis sativa l: Comparison of Yield and Chemical Composition of 11 Hemp Genotypes. Molecules 2021, 26, 4080. [Google Scholar] [CrossRef]
- Christodoulou, M.C.; Gonzalez-Serrano, D.J.; Christou, A.; Stavrou, I.J.; Hadidi, M.; Moreno, A.; Kapnissi-Christodoulou, C.P. Optimization of Microwave-Assisted Extraction for Quantification of Cannabinoids in Hemp Tea by Liquid Chromatography-Mass Spectrometry. Sep. Sci. Plus 2024, 7, e202300220. [Google Scholar] [CrossRef]
- Toloza, H.; Buitrago, O.Y.; Orjuela, A.; Santaella, M.A.; Hurtado, A.M.; Arturo, D.E. Solvent Extraction of Cannabis sativa under Cryogenic Conditions. Sep. Purif. Technol. 2024, 329, 124906. [Google Scholar] [CrossRef]
- Fairbairn, J.W.; Liebmann, J.A.; Rowan, M.G. The Stability of Cannabis and Its Preparations on Storage. J. Pharm. Pharmacol. 1976, 28, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, R.; Nenna, G.; Calvi, L.; Panseri, S.; Borgonovo, G.; Giupponi, L.; Cannazza, G.; Giorgi, A. Quality Traits of “Cannabidiol Oils”: Cannabinoids Content, Terpene Fingerprint and Oxidation Stability of European Commercially Available Preparations. Molecules 2018, 23, 1230. [Google Scholar] [CrossRef]
- Siol, M.; Chołuj, N.; Mańko-Jurkowska, D.; Bryś, J. Assessment of the Stability and Nutritional Quality of Hemp Oil and Pumpkin Seed Oil Blends. Foods 2024, 13, 3813. [Google Scholar] [CrossRef] [PubMed]
- Bonazza, F.; Monti, L.; Povolo, M.; Gasparini, A.; Pelizzola, V.; Cabassi, G. Monitoring the Shelf Life of Hemp Seed Oil Stored at Two Temperatures in Different Materials via Near-Infrared (NIR) Spectroscopy. Molecules 2024, 29, 5577. [Google Scholar] [CrossRef]
- Da Porto, C.; Natolino, A.; Decorti, D. Effect of Ultrasound Pre-Treatment of Hemp (Cannabis sativa L.) Seed on Supercritical CO2 Extraction of Oil. J. Food Sci. Technol. 2015, 52, 1748–1753. [Google Scholar] [CrossRef]
- Morano, C.; Dei Cas, M.; Roda, G.; Fabbriconi, A.; Casagni, E.; Pallavicini, M.; Bolchi, C.; Pallotti, G.; Romaniello, F.; Rovellini, P. The Antioxidant Role of Hemp Phytocomplex in Cannabis Oil-Based Extracts. Pharmaceuticals 2022, 15, 1102. [Google Scholar] [CrossRef]
- Degrave, V. Effects of Five Cannabis Oils with Different CBD: THC Ratios and Terpenes on Hypertension, Dyslipidemia, Hepatic Steatosis, Oxidative Stress, and CB1 Receptor in an Experimental Model. J. Cannabis Res. 2025. [Google Scholar] [CrossRef]
- Gebregzi, H.H.; Zeiger, J.S.; Smith, J.P.; Stuyt, L.; Cullen, L.; Carsella, J.; Rogers, D.C.; Lafebre, J.; Knalfec, J.; Vargas, A.; et al. Oral Cannabidiol Did Not Impair Learning and Memory in Healthy Adults. J. Cannabis Res. 2025, 7, 5. [Google Scholar] [CrossRef]
- Wiraswati, H.L.; Calina, D. Piperine: An Emerging Biofactor with Anticancer Efficacy and Therapeutic Potential. BioFactors 2025, 51, e2134. [Google Scholar] [CrossRef]
- Cital, S.; Kramer, K.; Hughston, L.; Gaynor, J.S. Cannabis Therapy in Veterinary Medicine; Springer: Cham, Switzerland, 2021; ISBN 9783030683160. [Google Scholar]
- Sławińska, N.; Olas, B. Selected Seeds as Sources of Bioactive Compounds with Diverse Biological Activities. Nutrients 2023, 15, 187. [Google Scholar] [CrossRef] [PubMed]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Gammazza, A.M.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential Health Benefits of Olive Oil and Plant Polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef] [PubMed]
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). Cannabis Legislation in Europe; EMCDDA: Lisbon, Portugal, 2018; ISBN 978-92-9497-328-3.
- Huestis, M.A.; Solimini, R.; Pichini, S.; Pacifici, R.; Carlier, J.; Busardò, F.P. Cannabidiol Adverse Effects and Toxicity. Curr. Neuropharmacol. 2019, 17, 974–989. [Google Scholar] [CrossRef] [PubMed]
- Balant, M.; Gras, A.; Ruz, M.; Vallès, J.; Vitales, D.; Garnatje, T. Traditional Uses of Cannabis: An Analysis of the CANNUSE Database. J. Ethnopharmacol. 2021, 279, 114362. [Google Scholar] [CrossRef]
- Josefina, I.; Verpoorte, F.Æ.R. Secondary Metabolism in Cannabis. Phytochem. Rev. 2008, 7, 615–639. [Google Scholar] [CrossRef]
- Bijelić, K.; Srdjenović Čonić, B.; Prpa, B.; Pilija, V.; Vukmirović, S.; Kladar, N. The Potential of Hemp Extracts to Modify the Course of Oxidative-Stress Related Conditions. The Potential of Hemp Extracts to Modify the Course of Oxidative-Stress Related Conditions. Plants 2024, 13, 1630. [Google Scholar] [CrossRef]
- Koltai, H.; Shalev, N. Anti-Cancer Activity of Cannabis sativa Phytocannabinoids: Molecular Mechanisms and Potential in the Fight against Ovarian Cancer and Stem Cells. Cancers 2022, 14, 4299. [Google Scholar] [CrossRef]
- Alves, V.L.; Gonçalves, J.L.; Aguiar, J.; Teixeira, H.M.; Câmara, J.S. The Synthetic Cannabinoids Phenomenon: From Structure to Toxicological Properties. A Review. Crit. Rev. Toxicol. 2020, 50, 359–382. [Google Scholar] [CrossRef]
- Piomelli, D.; Russo, E.B. The Cannabis sativa Versus Cannabis indica Debate: An Interview with Ethan Russo, MD. Cannabis Cannabinoid Res. 2016, 1, 44–46. [Google Scholar] [CrossRef]
- Lorimier, L.-P.; Hazzah, T.; Almeida, E.; Cital, S. Cannabinoids in Oncology and Immune Response. In Cannabis Therapy in Veterinary Medicine: A Complete Guide; Springer International Publishing: Cham, Switzerland, 2021; pp. 231–269. ISBN 978-3-030-68316-0. [Google Scholar]
- Pertwee, R.G. Emerging Strategies for Exploiting Cannabinoid Receptor Agonists as Medicines. Br. J. Pharmacol. 2009, 156, 397–411. [Google Scholar] [CrossRef]
- Pertwee, R.G. Pharmacology of Cannabinoid CB1 and CB2 Receptors. Pharmacol. Ther. 1997, 74, 129–180. [Google Scholar] [CrossRef] [PubMed]
- White, C.M. A Review of Human Studies Assessing Cannabidiol’s (CBD) Therapeutic Actions and Potential. J. Clin. Pharmacol. 2019, 59, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Guy, G.W. A Tale of Two Cannabinoids: The Therapeutic Rationale for Combining Tetrahydrocannabinol and Cannabidiol. Med. Hypotheses 2006, 66, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Atakan, Z. Cannabis, a Complex Plant: Different Compounds and Different Effects on Individuals. Ther. Adv. Psychopharmacol. 2012, 2, 241–254. [Google Scholar] [CrossRef]
- Brown, J.D.; Rivera Rivera, K.J.; Hernandez, L.Y.C.; Doenges, M.R.; Auchey, I.; Pham, T.; Goodin, A.J. Natural and Synthetic Cannabinoids: Pharmacology, Uses, Adverse Drug Events, and Drug Interactions. J. Clin. Pharmacol. 2021, 61, S37–S52. [Google Scholar] [CrossRef]
- Englund, A.; Atakan, Z.; Kralj, A.; Tunstall, N.; Murray, R.; Morrison, P. The Effect of Five Day Dosing with THCV on THC-Induced Cognitive, Psychological and Physiological Effects in Healthy Male Human Volunteers: A Placebo-Controlled, Double-Blind, Crossover Pilot Trial. J. Psychopharmacol. 2016, 30, 140–151. [Google Scholar] [CrossRef]
- Bie, B.; Wu, J.; Foss, J.F.; Naguib, M. An Overview of the Cannabinoid Type 2 Receptor System and Its Therapeutic Potential. Curr. Opin. Anesthesiol. 2018, 31, 407–414. [Google Scholar] [CrossRef]
- Santarpia, L.; Lippman, S.M.; El-Naggar, A.K. Targeting the MAPK-RAS-RAF Signaling Pathway in Cancer Therapy. Expert Opin. Ther. Targets 2012, 16, 103–119. [Google Scholar] [CrossRef]
- Tutino, V.; Caruso, M.G.; De Nunzio, V.; Lorusso, D.; Veronese, N.; Gigante, I.; Notarnicola, M.; Giannelli, G. Down-Regulation of Cannabinoid Type 1 (CB1) Receptor and its Downstream Signaling Pathways in Metastatic Colorectal Cancer. Cancers 2019, 11, 708. [Google Scholar] [CrossRef]
- Donders, Z.; Skorupska, I.J.; Willems, E.; Mussen, F.; Van Broeckhoven, J.; Carlier, A.; Schepers, M.; Vanmierlo, T. Beyond PDE4 Inhibition: A Comprehensive Review on Downstream CAMP Signaling in the Central Nervous System. Biomed. Pharmacother. 2024, 177, 117009. [Google Scholar] [CrossRef]
- Zhang, H.; Kong, Q.; Wang, J.; Jiang, Y.; Hua, H. Complex Roles of CAMP–PKA–CREB Signaling in Cancer. Exp. Hematol. Oncol. 2020, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Kilanowska, A.; Ziółkowska, A.; Stasiak, P.; Gibas-Dorna, M. CAMP-Dependent Signaling and Ovarian Cancer. Cells 2022, 11, 3835. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.C.; Wadsten, P.; Su, S.; Rawlinson, N.; Hofman, F.M.; Hill, C.K.; Schönthal, A.H. The Type IV Phosphodiesterase Inhibitor Rolipram Induces Expression of the Cell Cycle Inhibitors P21(Cip1) and P27(Kip1), Resulting in Growth Inhibition, Increased Differentiation, and Subsequent Apoptosis of Malignant A-172 Glioma Cells. Cancer Biol. Ther. 2002, 1, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Y.; Liu, J.; Chen, J.; Wang, J.; Hua, H.; Jiang, Y. CAMP-PKA/EPAC Signaling and Cancer: The Interplay in Tumor Microenvironment. J. Hematol. Oncol. 2024, 17, 5. [Google Scholar] [CrossRef]
- Oz, M.; Yang, K.H.S.; Mahgoub, M.O. Effects of Cannabinoids on Ligand-Gated Ion Channels. Front. Physiol. 2022, 13, 1041833. [Google Scholar] [CrossRef]
- Petrovici, A.R.; Simionescu, N.; Sandu, A.I.; Paraschiv, V.; Silion, M.; Pinteala, M. New Insights on Hemp Oil Enriched in Cannabidiol: Decarboxylation, Antioxidant Properties and In Vitro Anticancer Effect. Antioxidants 2021, 10, 738. [Google Scholar] [CrossRef]
- Łyczko, J.; Pluta, M.; Wietrzyk, J.; Haczkiewicz, M.; Switalska, M. Extraction of Cannabinoids and Terpenes from Hemp Flowers and Leaves (Cannabis sativa L., Futura 75): Chemical Profiling and Evaluation of Anticancer Properties. Molecules 2025, 30, 1325. [Google Scholar] [CrossRef]
- Yu, H.; Chen, Y.; Deng, J.; Cai, G.; Fu, W.; Shentu, C.; Xu, Y.; Liu, J.; Zhou, Y.; Luo, Y.; et al. Integrated Metabolomics and Proteomics Analyses to Reveal Anticancer Mechanism of Hemp Oil Extract in Colorectal Cancer. J. Pharm. Biomed. Anal. 2024, 249, 116379. [Google Scholar] [CrossRef]
- Herrera, B.; Carracedo, A.; Diez-Zaera, M.; Guzmán, M.; Velasco, G. P38 MAPK Is Involved in CB2 Receptor-Induced Apoptosis of Human Leukaemia Cells. FEBS Lett. 2005, 579, 5084–5088. [Google Scholar] [CrossRef]
- Powles, T.; Te Poele, R.; Shamash, J.; Chaplin, T.; Propper, D.; Joel, S.; Oliver, T.; Wai, M.L. Cannabis-Induced Cytotoxicity in Leukemic Cell Lines: The Role of the Cannabinoid Receptors and the MAPK Pathway. Blood 2005, 105, 1214–1221. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, T.; Su, W.; Dou, Z.; Zhao, D.; Jin, X.; Lei, H.; Wang, J.; Xie, X.; Cheng, B.; et al. Mutant P53 in Cancer: From Molecular Mechanism to Therapeutic Modulation. Cell Death Dis. 2022, 13, 974. [Google Scholar] [CrossRef] [PubMed]
- Peeri, H.; Shalev, N.; Vinayaka, A.C.; Nizar, R.; Kazimirsky, G.; Namdar, D.; Anil, S.M.; Belausov, E.; Brodie, C.; Koltai, H. Specific Compositions of Cannabis sativa Compounds Have Cytotoxic Activity and Inhibit Motility and Colony Formation of Human Glioblastoma Cells In Vitro. Cancers 2021, 13, 1720. [Google Scholar] [CrossRef] [PubMed]
- Peeri, H.; Koltai, H. Cannabis Biomolecule Effects on Cancer Cells and Cancer Stem Cells: Cytotoxic, Anti-Proliferative, and Anti-Migratory Activities. Biomolecules 2022, 12, 491. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, D.; Fiselier, A.; Kovalchuk, I.; Kovalchuk, O. High-CBD Cannabis Extracts Inhibit the Expression of Proinflammatory Factors via MiRNA-Mediated Silencing in Human Small Intestinal Epithelial Cells. Heliyon 2023, 9, e18817. [Google Scholar] [CrossRef]
- Zhou, M.; Li, R.; Lian, G.; Yang, M.; Li, L.; Yin, Z.; Li, G.; Zhao, J.; Tan, R. Tetrahydrocurcumin Alleviates Colorectal Tumorigenesis by Modulating the SPP1/CD44 Axis and Preventing M2 Tumor-Associated Macrophage Polarization. Phytomedicine 2025, 141, 156674. [Google Scholar] [CrossRef]
- Mashabela, M.D.; Kappo, A.P. Anti-Cancer and Anti-Proliferative Potential of Cannabidiol: A Cellular and Molecular Perspective. Int. J. Mol. Sci. 2024, 25, 5659. [Google Scholar] [CrossRef]
- Wang, F.; Multhoff, G. Repurposing Cannabidiol as a Potential Drug Candidate for Anti-Tumor Therapies. Biomolecules 2021, 11, 582. [Google Scholar] [CrossRef]
- Shrivastava, A.; Kuzontkoski, P.M.; Groopman, J.E.; Prasad, A. Cannabidiol Induces Programmed Cell Death in Breast Cancer Cells by Coordinating the Cross-Talk between Apoptosis and Autophagy. Mol. Cancer Ther. 2011, 10, 1161–1172. [Google Scholar] [CrossRef]
- Ramer, R.; Heinemann, K.; Merkord, J.; Rohde, H.; Salamon, A.; Linnebacher, M.; Hinz, B. COX-2 and PPAR-γ Confer Cannabidiol-Induced Apoptosis of Human Lung Cancer Cells. Mol. Cancer Ther. 2013, 12, 69–82. [Google Scholar] [CrossRef]
- Ivanov, V.N.; Wu, J.; Hei, T.K. Regulation of Human Glioblastoma Cell Death by Combined Treatment of Cannabidiol, γ-Radiation and Small Molecule Inhibitors of Cell Signaling Pathways. Oncotarget 2017, 8, 74068–74095. [Google Scholar] [CrossRef]
- Motadi, L.R.; Moleya, N.B. Abstract 3714: The Antitumor Activity of Cannabis sativa and CBD in Prostate Cancer PC3 Cells. Cancer Res. 2022, 82, 3714. [Google Scholar] [CrossRef]
- Go, Y.Y.; Kim, S.R.; Kim, D.Y.; Chae, S.-W.; Song, J.-J. Cannabidiol Enhances Cytotoxicity of Anti-Cancer Drugs in Human Head and Neck Squamous Cell Carcinoma. Sci. Rep. 2020, 10, 20622. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qin, Y.; Pan, Z.; Li, M.; Liu, X.; Chen, X.; Qu, G.; Zhou, L.; Xu, M.; Zheng, Q.; et al. Cannabidiol Induces Cell Cycle Arrest and Cell Apoptosis in Human Gastric Cancer SGC-7901 Cells. Biomolecules 2019, 9, 302. [Google Scholar] [CrossRef] [PubMed]
- Massi, P.; Solinas, M.; Cinquina, V.; Parolaro, D. Cannabidiol as Potential Anticancer Drug. Br. J. Clin. Pharmacol. 2013, 75, 303–312. [Google Scholar] [CrossRef]
- Maia, J.; Fonseca, B.M.; Teixeira, N.; Correia-da-Silva, G. Unveiling the Angiogenic Effects of Cannabinoids: Enhancers or Inhibitors? Biochem. Pharmacol. 2023, 215, 115686. [Google Scholar] [CrossRef]
- Ramer, R.; Bublitz, K.; Freimuth, N.; Merkord, J.; Rohde, H.; Haustein, M.; Borchert, P.; Schmuhl, E.; Linnebacher, M.; Hinz, B. Cannabidiol Inhibits Lung Cancer Cell Invasion and Metastasis via Intercellular Adhesion Molecule-1. FASEB J. 2012, 26, 1535–1548. [Google Scholar] [CrossRef]
- Mcallister, S.D.; Murase, R.; Christian, R.T.; Lau, D.; Desprez, P.; Erk, C.Á.; Lung, Á.R.O.S.Á. Pathways Mediating the Effects of Cannabidiol on the Reduction of Breast Cancer Cell Proliferation, Invasion, and Metastasis. Breast Cancer Res. Treat. 2011, 129, 37–47. [Google Scholar] [CrossRef]
- Shalata, W.; Abu Saleh, O.; Tourkey, L.L.; Shalata, S.; Neime, A.E.; Abu Juma’a, A.; Soklakova, A.; Tourkey, L.L.; Jama, A.A.; Yakobson, A. The Efficacy of Cannabis in Oncology Patient Care and Its Anti-Tumor Effects. Cancers 2024, 16, 2909. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, B.; Liu, Y.; Wu, S.; Xiang, Q.; Xiao, Y.; Zhao, J.; Yuan, R.; Xie, K.; Li, L. Roles of PPAR Activation in Cancer Therapeutic Resistance: Implications for Combination Therapy and Drug Development. Eur. J. Pharmacol. 2024, 964, 176304. [Google Scholar] [CrossRef]
- Pellati, F.; Borgonetti, V.; Brighenti, V.; Biagi, M.; Benvenuti, S.; Corsi, L. Cannabis sativa L. and Nonpsychoactive Cannabinoids: Their Chemistry and Role against Oxidative Stress, Inflammation, and Cancer. Biomed. Res. Int. 2018, 2018, 1691428. [Google Scholar] [CrossRef]
- O’Sullivan, S.E. An Update on PPAR Activation by Cannabinoids. Br. J. Pharmacol. 2016, 173, 1899–1910. [Google Scholar] [CrossRef] [PubMed]
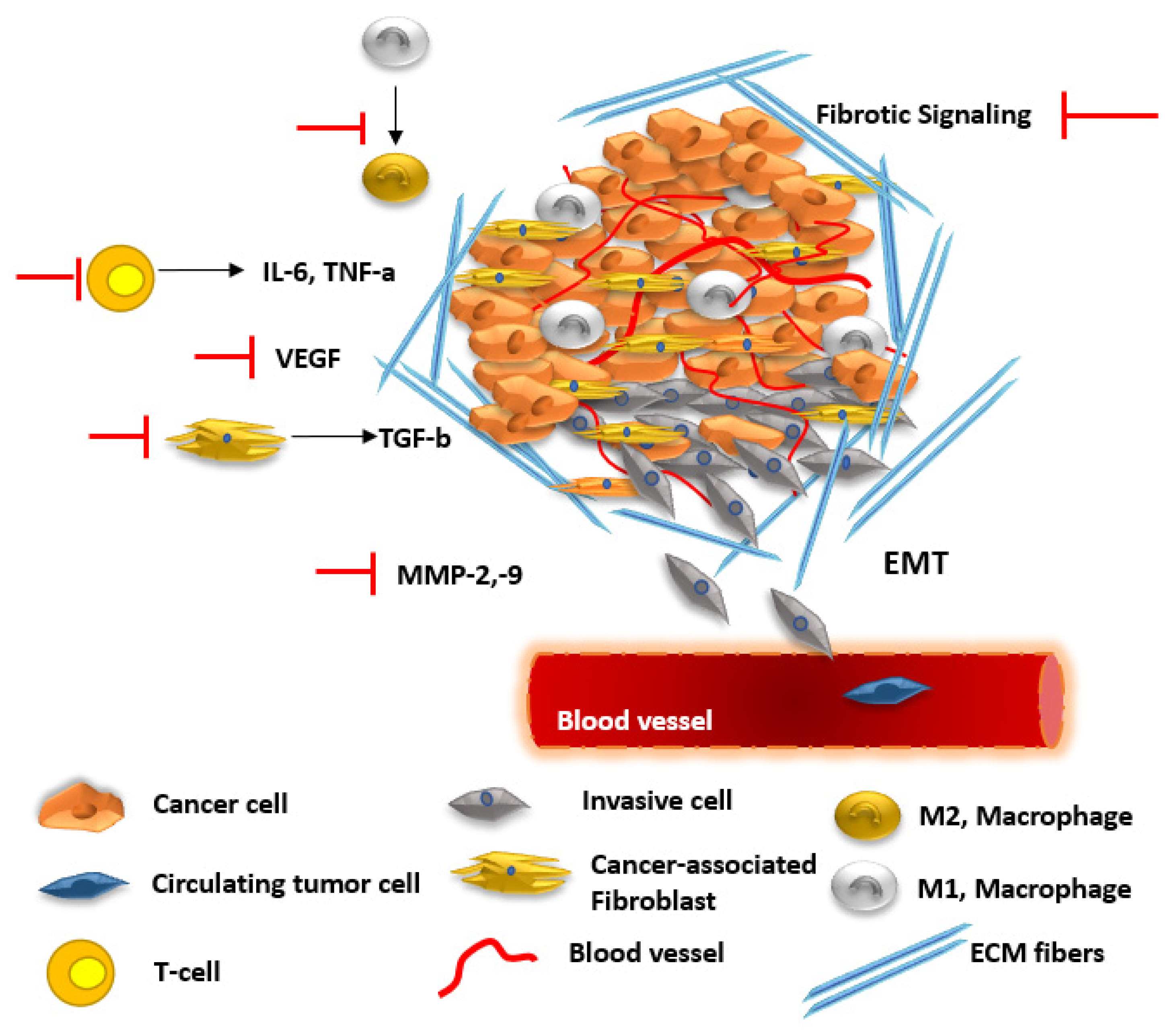
- Neophytou, C.M.; Panagi, M.; Stylianopoulos, T.; Papageorgis, P. The Role of Tumor Microenvironment in Cancer Metastasis: Molecular Mechanisms and Therapeutic Opportunities. Cancers 2021, 13, 2053. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hassan, W.; Jabeen, Q.; Khan, G.J.; Iqbal, F. Interdependent and Independent Multidimensional Role of Tumor Microenvironment on Hepatocellular Carcinoma. Cytokine 2018, 103, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Khan, G.J.; Sun, L.; Khan, S.; Yuan, S.; Nongyue, H. Versatility of Cancer Associated Fibroblasts: Commendable Targets for Anti-Tumor Therapy. Curr. Drug Targets 2018, 19, 1573–1588. [Google Scholar] [CrossRef]
- Poyia, F.; Neophytou, C.M.; Christodoulou, M. The Role of Tumor Microenvironment in Pancreatic Cancer Immunotherapy: Current Status and Future Perspectives. Int. J. Mol. Sci. 2024, 25, 9555. [Google Scholar] [CrossRef]
- Proto, M.C.; Fiore, D.; Bifulco, M.; Gazzerro, P. Rimonabant and Cannabidiol Rewrite the Interactions between Breast Cancer Cells and Tumor Microenvironment. Int. J. Mol. Sci. 2023, 24, 13427. [Google Scholar] [CrossRef]
- Gao, Y.; Khan, G.J.; Wei, X.; Zhai, K.-F.; Sun, L.; Yuan, S. DT-13 Inhibits Breast Cancer Cell Migration via Non-Muscle Myosin II-A Regulation in Tumor Microenvironment Synchronized Adaptations. Clin. Transl. Oncol. 2020, 22, 1591–1602. [Google Scholar] [CrossRef]
- Sheik, A.; Farani, M.R.; Kim, E.; Kim, S.; Gupta, V.K.; Kumar, K.; Huh, Y.S. Therapeutic Targeting of the Tumor Microenvironments with Cannabinoids and Their Analogs: Update on Clinical Trials. Environ. Res. 2023, 231, 115862. [Google Scholar] [CrossRef]
- Khan, G.J.; Gao, Y.; Gu, M.; Wang, L.; Khan, S.; Naeem, F.; Semukunzi, H.; Roy, D.; Yuan, S.; Sun, L. TGF-Β1 Causes EMT by Regulating N-Acetyl Glucosaminyl Transferases via Downregulation of Non Muscle Myosin II-A through JNK/P38/PI3K Pathway in Lung Cancer. Curr. Cancer Drug Targets 2018, 18, 209–219. [Google Scholar] [CrossRef]
- Milián, L.; Monleón-Guinot, I.; Sancho-Tello, M.; Galbis, J.M.; Cremades, A.; Almenar-Ordaz, M.; Peñaroja-Martinez, J.; Farras, R.; Martín de Llano, J.J.; Carda, C.; et al. In Vitro Effect of Δ9-Tetrahydrocannabinol and Cannabidiol on Cancer-Associated Fibroblasts Isolated from Lung Cancer. Int. J. Mol. Sci. 2022, 23, 6766. [Google Scholar] [CrossRef]
- Do, E.P.B. Medicinal Properties of Cannabinoids, Terpenes, and Flavonoids in Cannabis, and Benefits in Migraine, Headache, and Pain: An Update on Current Evidence and Cannabis Science. Headache J. Head Face Pain 2018, 58, 1139–1186. [Google Scholar]
- Dawidowicz, A.L.; Olszowy-tomczyk, M.; Typek, R. Fitoterapia CBG, CBD, Δ9-THC, CBN, CBGA, CBDA and Δ9-THCA as Antioxidant Agents and Their Intervention Abilities in Antioxidant Action. Fitoterapia 2021, 152, 104915. [Google Scholar] [CrossRef] [PubMed]
- Marcu, J.P.; Christian, R.T.; Lau, D.; Zielinski, A.J.; Horowitz, M.P.; Lee, J.; Pakdel, A.; Allison, J.; Limbad, C.; Moore, D.H.; et al. Cannabidiol Enhances the Inhibitory Effects of Δ9-Tetrahydrocannabinol on Human Glioblastoma Cell Proliferation and Survival. Mol. Cancer Ther. 2010, 9, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Lah, T.T.; Novak, M.; Pena Almidon, M.A.; Marinelli, O.; Baškovič, B.Ž.; Majc, B.; Mlinar, M.; Bošnjak, R.; Breznik, B.; Zomer, R.; et al. Cannabigerol Is a Potential Therapeutic Agent in a Novel Combined Therapy for Glioblastoma. Cells 2021, 10, 340. [Google Scholar] [CrossRef]
- Sarfaraz, S.; Afaq, F.; Adhami, V.M.; Malik, A.; Mukhtar, H. Cannabinoid Receptor Agonist-Induced Apoptosis of Human Prostate Cancer Cells LNCaP Proceeds through Sustained Activation of ERK1/2 Leading to G1 Cell Cycle Arrest. J. Biol. Chem. 2006, 281, 39480–39491. [Google Scholar] [CrossRef]
- Ligresti, A.; Moriello, A.S.; Starowicz, K.; Matias, I.; Pisanti, S.; De Petrocellis, L.; Laezza, C.; Portella, G.; Bifulco, M.; Di Marzo, V. Antitumor Activity of Plant Cannabinoids with Emphasis on the Effect of Cannabidiol on Human Breast Carcinoma. J. Pharmacol. Exp. Ther. 2006, 318, 1375–1387. [Google Scholar] [CrossRef]
- Anis, O.; Vinayaka, A.C.; Shalev, N.; Namdar, D.; Nadarajan, S.; Anil, S.M.; Cohen, O.; Belausov, E.; Ramon, J.; Gati, E.M.; et al. Cannabis-Derived Compounds Cannabichromene and ∆9-Tetrahydrocannabinol Interact and Exhibit Cytotoxic Activity against Urothelial Cell Carcinoma Correlated with Inhibition of Cell Migration and Cytoskeleton Organization. Molecules 2021, 26, 465. [Google Scholar] [CrossRef]
- Zhou, H.-M.; Zhang, J.-G.; Zhang, X.; Li, Q. Targeting Cancer Stem Cells for Reversing Therapy Resistance: Mechanism, Signaling, and Prospective Agents. Signal Transduct. Target. Ther. 2021, 6, 62. [Google Scholar] [CrossRef]
- Mesas, C.; Moreno, J.; Doello, K.; Peña, M.; López-Romero, J.M.; Prados, J.; Melguizo, C. Cannabidiol Effects in Stem Cells: A Systematic Review. Biofactors 2025, 51, e2148. [Google Scholar] [CrossRef]
- Zhong, T.; Zhang, W.; Guo, H.; Pan, X.; Chen, X.; He, Q.; Yang, B.; Ding, L. The Regulatory and Modulatory Roles of TRP Family Channels in Malignant Tumors and Relevant Therapeutic Strategies. Acta Pharm. Sin. B 2022, 12, 1761–1780. [Google Scholar] [CrossRef]
- Zhao, Z.; Bo, Z.; Gong, W.; Guo, Y. Inhibitor of Differentiation 1 (Id1) in Cancer and Cancer Therapy. Int. J. Med. Sci. 2020, 17, 995–1005. [Google Scholar] [CrossRef]
- McAllister, S.D.; Soroceanu, L.; Desprez, P.-Y. The Antitumor Activity of Plant-Derived Non-Psychoactive Cannabinoids. J. Neuroimmune Pharmacol. 2015, 10, 255–267. [Google Scholar] [CrossRef]
- Zorrilla, E.; Krivoshein, G.; Kuburas, A.; Schenke, M.; Piña, C.L.; van Heiningen, S.H.; Waite, J.S.; Dehghani, A.; Castonguay, W.C.; Flinn, H.C.; et al. Combined Effects of Cannabidiol and Δ9-Tetrahydrocannabinol Alleviate Migraine-like Symptoms in Mice. Cephalalgia 2025, 45, 03331024251314487. [Google Scholar] [CrossRef]
- Tomagra, G.; Gandlevskiy, N.; Rosso, E.; Bonardi, M.; Binello, A.; Carabelli, V.; Barge, A. THC, CBD and Minor Cannabinoid CBDV Differently Modulate Hippocampal Neurons Firing. Neurotoxicology 2025, 108, 180–190. [Google Scholar] [CrossRef]
- Mosley, C.; Gaynor, J.; Cital, S.; Brassard, J. Cannabinoids for Pain Management BT. In Cannabis Therapy in Veterinary Medicine: A Complete Guide; Cital, S., Kramer, K., Hughston, L., Gaynor, J.S., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 117–141. ISBN 978-3-030-68317-7. [Google Scholar]
- U.S. Food and Drug Administration (FDA). Safety of CBD in Humans: A Literature Review; FDA: Silver Spring, MD, USA, 12 December 2019. Available online: https://www.fda.gov/media/152317/download (accessed on 23 April 2025).
- Erzurumlu, Y.; Catakli, D. Cannabidiol Enhances the Anticancer Activity of Etoposide on Prostate Cancer Cells. Cannabis Cannabinoid Res. 2024, 10, 258–276. [Google Scholar] [CrossRef]
- Guo, T.; Liu, Q.; Hou, P.; Li, F.; Guo, S.; Song, W.; Zhang, H.; Liu, X.; Zhang, S.; Zhang, J.; et al. Stilbenoids and Cannabinoids from the Leaves of Cannabis sativa f. sativa with Potential Reverse Cholesterol Transport Activity. Food Funct. 2018, 9, 6608–6617. [Google Scholar] [CrossRef]
- Favero, G.R.; de Melo Pereira, G.V.; de Carvalho, J.C.; de Carvalho Neto, D.P.; Soccol, C.R. Converting Sugars into Cannabinoids—The State-of-the-Art of Heterologous Production in Microorganisms. Fermentation 2022, 8, 84. [Google Scholar] [CrossRef]
- Robak, J.; Gryglewski, R.J. Flavonoids Are Scavengers of Superoxide Anions. Biochem. Pharmacol. 1988, 37, 837–841. [Google Scholar] [CrossRef]
- Ofir, R. Cannabis-Derived Terpenes and Flavonoids as Potential Pharmaceuticals. Isr. J. Plant Sci. 2021, 68, 29–37. [Google Scholar] [CrossRef]
- Ani, V.; Varadaraj, M.C.; Naidu, K.A. Antioxidant and Antibacterial Activities of Polyphenolic Compounds from Bitter Cumin (Cuminum nigrum L.). Eur. Food Res. Technol. 2006, 224, 109–115. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic Content, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef]
- Hazra, B.; Biswas, S.; Mandal, N. Antioxidant and Free Radical Scavenging Activity of Spondias Pinnata. BMC Complement. Altern. Med. 2008, 8, 63. [Google Scholar] [CrossRef]
- Khan, B.A.; Warner, P.; Wang, H. Antibacterial Properties of Hemp and Other Natural Fibre Plants: A Review. BioResources 2014, 9, 3642–3659. [Google Scholar] [CrossRef]
- Marciniuk, J.; Sadowska, B.; Wi, M. Macro- and Microelement Composition, Antioxidant Activity, and Biological Effect of Cold-Pressed Edible Oils from Commercial and Amateur Companies. Molecules 2025, 30, 1425. [Google Scholar] [CrossRef]
- Raucci, U.; Pietrafusa, N.; Paolino, M.C.; Di Nardo, G.; Villa, M.P.; Pavone, P.; Terrin, G.; Specchio, N.; Striano, P.; Parisi, P. Cannabidiol Treatment for Refractory Epilepsies in Pediatrics. Front. Pharmacol. 2020, 11, 586110. [Google Scholar] [CrossRef]
- Devinsky, O.; Nabbout, R.; Miller, I.; Laux, L.; Zolnowska, M.; Wright, S.; Roberts, C. Long-Term Cannabidiol Treatment in Patients with Dravet Syndrome: An Open-Label Extension Trial. Epilepsia 2019, 60, 294–302. [Google Scholar] [CrossRef]
- Pino, S.; Espinoza, L.; Jara-Gutiérrez, C.; Villena, J.; Olea, A.F.; Díaz, K. Study of Cannabis Oils Obtained from Three Varieties of C. sativa and by Two Different Extraction Methods: Phytochemical Characterization and Biological Activities. Plants 2023, 12, 1772. [Google Scholar] [CrossRef]
- Han, J.-Y.; Lee, Y.J.; Lim, D.-W.; Jung, H.-J.; Kwon, E.; Hong, J.; Lee, Y.-M. Cheungsam Seed Husk Extract Reduces Skin Inflammation through Regulation of Inflammatory Mediator in TNF-α/IFN-γ-Induced HaCaT Cells. Plants 2024, 13, 1704. [Google Scholar] [CrossRef]
- Khosravi, M.; Asadikaram, G.; Musavi Mehdiabadi, F.; Nikvarz, N. The Effect of Hempseed Oil on Markers of Systemic Inflammation in Hemodialysis Patients: A Single-Blind Randomized Trial. J. Kerman Univ. Med. Sci. 2021, 28, 236–242. [Google Scholar] [CrossRef]
- Chinello, M.; Scommegna, S.; Shardlow, A.; Mazzoli, F.; De Giovanni, N.; Fucci, N.; Borgiani, P.; Ciccacci, C.; Locasciulli, A.; Calvani, M. Cannabinoid Poisoning by Hemp Seed Oil in a Child. Pediatr. Emerg. Care 2017, 33, 344–345. [Google Scholar] [CrossRef]
- Iffland, K.; Grotenhermen, F. An Update on Safety and Side Effects of Cannabidiol: A Review of Clinical Data and Relevant Animal Studies. Cannabis Cannabinoid Res. 2017, 2, 139–154. [Google Scholar] [CrossRef]
- Souza, D.R.; Guimar, F.S.; Campos, A.C.; Hallak, J.E.C.; Crippa, A.S. Research and Clinical Practice Involving the Use of Cannabis Products, with Emphasis on Cannabidiol: A Narrative Review. Pharmaceuticals 2024, 17, 1644. [Google Scholar] [CrossRef]
- Ferreira, L.L.; Gomes, F.S.; Nascimento, B.G.; Corsini, W.; dos Reis, L.F.C.; Oliveira-Silva, J.M.; da Silva, J.R.T.; da Silva, M.L.; Gamero, A.M.C.; de Almeida Hermes, T. Treatment With Full-Spectrum Cannabidiol Oil Improved the Pathological Findings of Dystrophic Mutant Mice. Muscle Nerve 2025, 71, 651–661. [Google Scholar] [CrossRef]
- Metouekel, A.; Zejli, H.; Chebaibi, M.; Lefrioui, Y.; Bousta, D.; El Amri, H.; El Fahime, E.; El Kazzouli, S.; El Brahmi, N. Formulation and Physicochemical Characterization of Terpenocannabinoid-Functionalized Hemp Oil Emulsifier: Assessment of Topical Anti-Inflammatory, Antinociceptive, Wound Healing Activity and Cutaneous Toxicity Effects. Sci. Pharm. 2024, 92, 36. [Google Scholar] [CrossRef]
- Rodriguez-Almaraz, J.E.; Butowski, N. Therapeutic and Supportive Effects of Cannabinoids in Patients with Brain Tumors (CBD Oil and Cannabis). Curr. Treat. Options Oncol. 2023, 24, 30–44. [Google Scholar] [CrossRef]
- Ismail, J.; Shebaby, W.; Atallah, S.A.; Taleb, R.I.; Kawrani, S.; Faour, W.; Mroueh, M. Combination of Cannabidiol with Cisplatin or Paclitaxel Analysis Using the Chou—Talalay Method and Chemo-Sensitization Evaluation in Platinum-Resistant Ovarian Cancer Cells. Biomedicines 2025, 13, 520. [Google Scholar] [CrossRef]
- Hazekamp, A.; Epifanova, S. Grote Variatie in Samenstelling Cannabisolie Noopt Tot Regels. Pharm. Weekbl. 2017, 152, 16–18. [Google Scholar]
| Country | Allowable Limit for THC | Reference |
|---|---|---|
| Germany | 5 mg/kg | [47,62] |
| Canada | 10 mg per package | [61] |
| Switzerland | 20 mg/kg | [49] |
| United Kingdom | 1 mg per product | [60,61] |
| Australia | 2 mg per serving | [63] |
| Belgium | 10 mg/kg | [64] |
| Cyprus | Zero tolerance | [65] |
| Netherlands | 5 mg per product | [66] |
| United States | 5–10 mg per serving | [58,67] |
| Italy | 5 mg/kg | [68] |
| New Zealand | 2 mg per serving | [69] |
| Jamaica | 0.01–100 mg/100 g | [70] |
| Hemp Seed Oil | CBD Oil | Hashish Oil | Essential Oil | References | |
|---|---|---|---|---|---|
| Plant parts extracted | Seeds (achene) | Aerial parts (flowers, leaves, sometimes stems) | Resinous parts of hemp (trichome-rich flowers/leaves), often processed into “hash” prior to oil extraction | Primarily flowers and leaves (terpene-rich plant material) | [77,98] |
| THC levels CBD levels |
Negligible (<0.03%) * It can reach up to 20 w/w% |
Up to legal limit (e.g., <0.3% w/w% in many jurisdictions) Usually 10–20 w/w% and sometimes even higher due to post-extraction enrichment |
Variable; can be near legal limit (<0.3% for hemp-based) or higher if from non-hemp Cannabis ** 10–15 w/w%, but it can vary significantly |
Trace quantities. Trace amounts (focus is on volatile terpenes rather than cannabinoids) | [59,89,98,115,116] |
| Applications | Edible food supplement and cosmetics |
Medicinal uses and especially muscle relaxation, anti-inflammatory and antioxidant properties, cosmetics | Primarily for recreational use, some medicinal applications | Aromatherapy, perfumery, cosmetics | [23,28,56,89,117,118,119,120,121] |
| Extraction approaches |
Cold-pressed or CO2 extraction, UAE, MAE and Soxhlet can also be used |
CO2 extraction or SLE (organic solvent extraction utilizing petroleum ether, ethanol, methanol, acetone) | SLE (organic solvent extraction utilizing petroleum ether, ethanol, methanol, acetone) | Hydrodistillation and steam distillation | [91,93,94,104,122,123,124,125,126] |
| Shelf life | 6–12 months | 1–2 years, store in a cool place | 1–2 years under airtight conditions | 6–12 months | [127,128,129,130] |
| Category | Representative Compounds | Typical Content | Biological Activity | References |
|---|---|---|---|---|
| Fatty acids | Linoleic Acid (Omega-6) | 50–60% | Skin barrier, anti-inflammatory | [87,89,97] |
| α-Linolenic Acid (Omega-3) | 15–25% | Cardiovascular, cognitive health | ||
| γ-Linolenic Acid (GLA) | ~1–6% | Hormonal balance, eczema relief | ||
| Vitamins and antioxidants | γ-Tocopherol (Vitamin E) | 80–100 mg/100 g | Antioxidant, skin repair | [6,131] |
| Phytosterols (β-sitosterol, campesterol) | ~0.5–1% | Cholesterol-lowering, anti-inflammatory | ||
| Terpenes | β-Caryophyllene, Myrcene, Limonene, Pinene, Linalool, Humulene | Trace < 0.5% (varies) | Entourage effect, anti-inflammatory, sedative, anxiolytic | [85,132,133] |
| Aromatic compounds and additives | Piperine, Curcumin, Cumarin, Coffee, other furans and pyrazines | Trace < 0.5% (added or infused) | Bioavailability enhancer, antioxidant, stimulant | [134,135] |
| Stilbenes (e.g., canniprene, cannastilbenes) | Trace <0.5% | Antioxidant, anti-inflammatory, ECS synergy | ||
| Flavonoids and lignanamides | Apigenin, Quercetin, Kaempferol, Cannflavins A/B N-caffeoyltyramine, grossamide, cannabisin B, cannabisin F | ~0.01–3% | Anti-inflammatory, antioxidant, neuroprotective | [106,136,137] |
| Carrier oil | Sunflower Oil (Helianthus Annuus), Hemp Seed Oil (Cannabis Sativa L.), Olive Oil (Olea Europaea) | Usually is up to 90–99% of the oil product (9–9.9 mL of a 10 mL cbd oil bottle) | Skin hydration and barrier repair, contains antioxidant ingridients, improves cannabinoid solubility and stability, supports anti-inflammatory and cardiovascular health, enhances the synergistic effect when used with CBD | [47,66,87,138] |
| CB1 Receptor | CB2 Receptor | Cannabinoid and Binding Activity to CB Receptors | |
|---|---|---|---|
| Primarily concentrated in the central nerve system and brain | Located primarily in immune cells and the peripheral nerve system | Δ9-THC | Partial agonist of CB1 and CB2 receptors |
| Responsible for psychoactive effects of cannabinoids | Not in charge of the psychoactive effects of cannabinoids | CBD | Low affinity for CB receptors and inverse agonist of CB2 receptor |
| Activation results in inhibition of neurotransmitter release | Activation results in inhibition of immune cell function and inflammatory response | CBN | Weak agonist for CB1 and higher affinity towards CB2 receptor |
| Plays a part in controlling hunger, mood, pain, and memory | Plays a part in controlling inflammation and immunological response | CBG | Low affinity for CB1 and CB2 receptors, but it affects the endocannabinoid system because of its ability to inhibit anandamide (AEA) uptake. |
| Associated with addiction and dependence on Cannabis | Has potential as a treatment for autoimmune disorders like multiple sclerosis | THCV | Partial agonist and in high doses, antagonist for CB1 and CB2 receptors |
| Highly expressed in regions including the cerebellum, basal ganglia, and hippocampus | Expressed at high levels in immune cells, such as B-cells and T cells | CBC | Low affinity for the cannabinoid receptors, but it affects the endocannabinoid system because of its ability to inhibit anandamide (AEA) uptake. It is also the most potent agonist of the transient receptor potential ankyrin subtype 1 protein (TRPA1) |
| Can be activated by endogenous cannabinoids, such as anandamide and 2-arachidonoylglycerol | Activated by endogenous cannabinoids, such as 2-arachidonoylglycerol, but less responsive to anandamide | Δ8-THC | Moderate partial agonistic effects on CB1 and CB2 receptors |
| Can be targeted by drugs that mimic or block cannabinoid activity | Can be targeted by drugs that modulate immune function and inflammation | CBDV | Very weak affinity for CB1 and CB2 receptors |
| Benefit/Drawback | Dosage | Frequency | Type of Hemp Oil | Reference |
|---|---|---|---|---|
| Mild cannabinoid poisoning, including neurological symptoms (stupor, poor reactivity) | 1 teaspoon (~5 mL) | Twice daily | CBD isolate | [230] |
| Reduce oxidative damage and cellular stress | 10–40 mg | Daily | Full-spectrum | [164] |
| Reduce blood pressure and inflammation | 600 mg (acute dose) | Single use or low daily dose | CBD isolate and full-spectrum | [232] |
| Anticancer effects via G1 cell cycle arrest and metabolic disruption in CRC | 3–6 µg/mL (in vitro) 30 mg/kg (in vivo) | In vitro: 24–72 h In vivo: every 3 days | Full-spectrum | [166] |
| Antioxidant, antifungal, and anticancer activity | 15–300 µg/mL (in vitro) | Single dose (72 h in vitro) | Full-spectrum | [227] |
| Anti-inflammatory effect in keratinocytes (reduce cytokines and improved skin barrier) | 25–200 ng/mL (in vitro) | Single dose (24 h) | Hemp seed oil | [228] |
| Reduce inflammation, fibrosis, and necrosis and improve muscle function | 10 mg/kg (in vivo) 18.75–300 µM (in vitro) | Daily for 14 days (in vivo) | Full-spectrum | [233] |
| Promising topical anti-inflammatory, analgesic, wound healing, and antioxidant effects | 10–20% TC in hemp oil emulsifier | Daily for 14–28 days | Full-spectrum | [234] |
| No reduction in IL-6 or TNF-α in hemodialysis patients (does not decrease inflammation) | 20 mL/day (3.68 g ALA) | Daily for 8 weeks | Hemp seed oil | [229] |
| Cause muscle spasms and hallucinations | Not specified | Single dose per day | Oil- extracts of Cannabis based on a 1:1 and 4:1 ratio of THC:CBD | [235] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christodoulou, M.C.; Rodosthenous, P.; Neophytou, C.M. Unveiling the Antioxidant Role of Hemp Oils in Cancer Prevention and Treatment. Cancers 2025, 17, 2128. https://doi.org/10.3390/cancers17132128
Christodoulou MC, Rodosthenous P, Neophytou CM. Unveiling the Antioxidant Role of Hemp Oils in Cancer Prevention and Treatment. Cancers. 2025; 17(13):2128. https://doi.org/10.3390/cancers17132128
Chicago/Turabian StyleChristodoulou, Marios C., Panagiotis Rodosthenous, and Christiana M. Neophytou. 2025. "Unveiling the Antioxidant Role of Hemp Oils in Cancer Prevention and Treatment" Cancers 17, no. 13: 2128. https://doi.org/10.3390/cancers17132128
APA StyleChristodoulou, M. C., Rodosthenous, P., & Neophytou, C. M. (2025). Unveiling the Antioxidant Role of Hemp Oils in Cancer Prevention and Treatment. Cancers, 17(13), 2128. https://doi.org/10.3390/cancers17132128










