Systematic Review of Intraoperative Radiotherapy (IORT) in Head and Neck Oncology: Past, Present, and Future Perspectives
Simple Summary
Abstract
1. Introduction
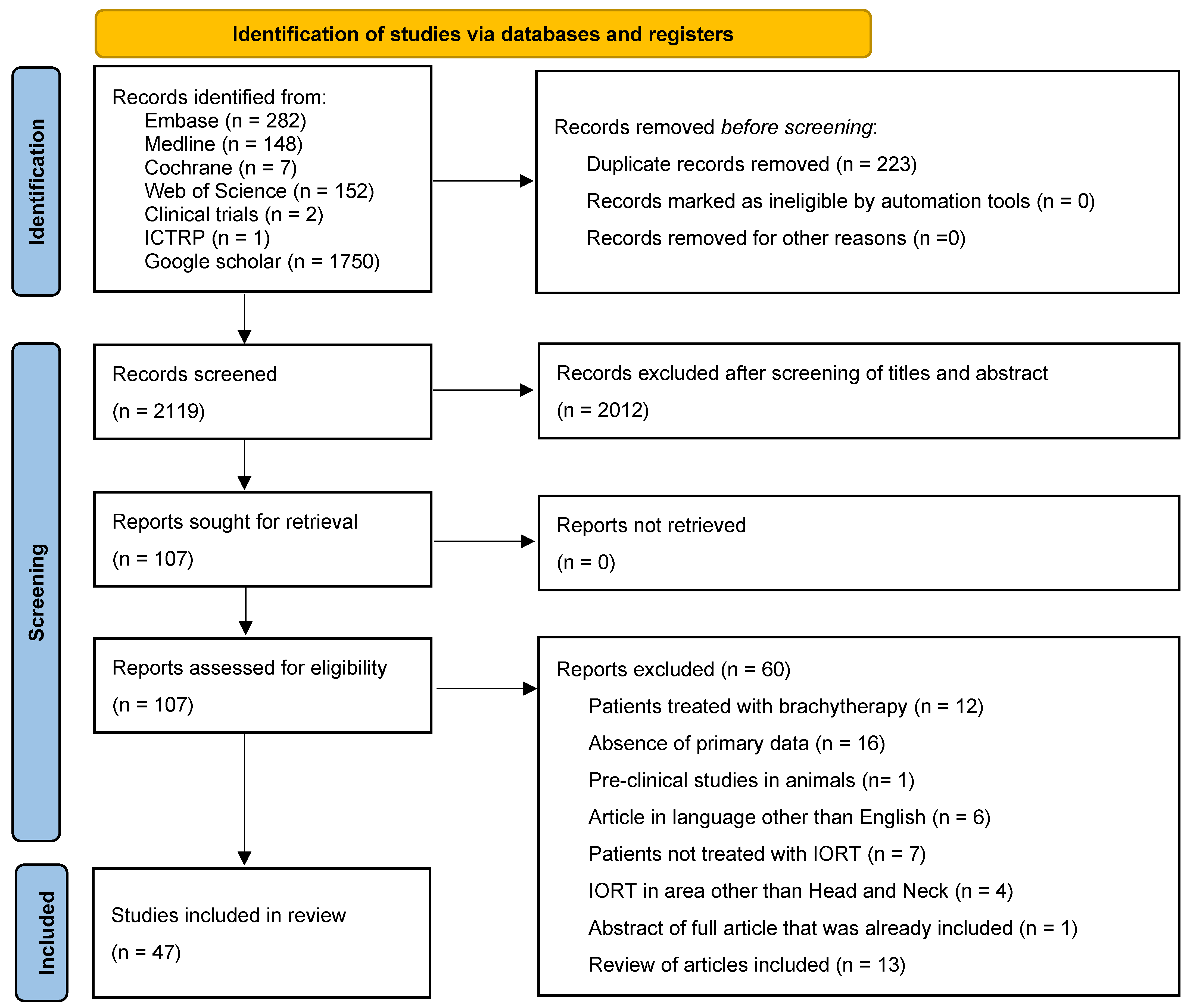
2. Methods
3. Results
3.1. Patients’ Characteristics (Table 2, Figure 3)
| Patients’ Characteristics: | ||
| Location (Data from 1640 irradiated fields, representing 68% of the total) | ||
| 466 279 263 192 144 126 73 66 43 23 16 11 10 8 5 3 2 | 45% 27% 25% 18% 14% 12% 7% 6% 4% 2% 2% 1% 1% 1% 0.5% 0.3% 0.2% |
| Primary vs. Recurrence (Data from 804 patients, representing 35% of the total) | ||
| 132 675 | 16% 84% |
| Margins (Data from 398 patients, representing 17% of the total) | ||
| 113 165 120 | 28% 41% 30% |
| Stage (Data from 731 patients, representing 31% of the total) | ||
| 186 545 | 25% 75% |
| Histology (Data from 1397 patients, representing 60% of the total) | ||
| 1197 144 22 14 7 4 2 2 1 1 1 1 1 | 85.7% 10.3% 1.6% 1.0% 0.5% 0.3% 0.1% 0.1% 0.1% 0.1% 0.1% 0.1% 0.1% |

3.2. Radiation Characteristics
3.3. Outcomes
3.4. Complications (Table 3)
| Complications, p Value in Comparison to the Literature Without IORT | ||
|---|---|---|
| - Wound infection/soft tissue necrosis | 130 | 22.6% |
| - Oro, tracheo or pharyngo-cutaneous fistula | 102 | 17.8% |
| - Carotid or innominate artery blow-out;
In comparison with 4.5% in the literature: p = 0.0006 | 83 | 14.5% |
| - Facial osteoradionecrosis (mandible, hard palate) | 58 | 10.1% |
| - Haematological | 57 | 9.9% |
| - Xerostomia/mucitis | 48 | 8.4% |
| - Radiation-induced neuropathy (vagus nerve, facial and hypogloss) | 32 | 5.6% |
| - Trismus/loss of oral competence | 15 | 2.6% |
| - Flap necrosis | 14 | 2.4% |
| - Carotid occlusion with or without stroke | 8 | 1.4% |
| - Hematoma | 5 | 0.9% |
| - Respiratory failure | 3 | 0.5% |
| - Vertebral osteoradionecrosis (one case with quadriplegia) | 2 | 0.3% |
| - Meningeal fistula with or without CSF leak | 2 | 0.3% |
| - Epistaxis | 2 | 0.3% |
| - Brain necrosis | 2 | 0.3% |
| - Hypopituitarism | 2 | 0.3% |
| - Otitis/hearing loss | 2 | 0.3% |
| - Late laryngeal necrosis | 2 | 0.3% |
| - Skull base necrosis | 1 | 0.2% |
| - Supraglottic edema | 1 | 0.2% |
| - Seroma | 1 | 0.2% |
| - Pleural effusion | 1 | 0.2% |
| - Esophageal stenosis | 1 | 0.2% |
4. Discussion
4.1. Indications
Advanced
4.2. Recurrence/Prior Irradiation
4.3. Radiation Dose
4.4. Outcomes and Complications
4.5. Limitations
4.6. Risk of Bias Assessment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wenz, F.; Blank, E.; Welzel, G.; Hofmann, F.; Astor, D.; Neumaier, C.; Herskind, C.; Gerhardt, A.; Suetterlin, M.; Kraus-Tiefenbacher, U. Intraoperative radiotherapy during breast-conserving surgery using a miniature x-ray generator (Intrabeam®): Theoretical and experimental background and clinical experience. Women’s Health 2012, 8, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; De Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Fastner, G.; Gaisberger, C.; Kaiser, J.; Scherer, P.; Ciabattoni, A.; Petoukhova, A.; Sperk, E.; Poortmans, P.; Calvo, F.A.; Sedlmayer, F.; et al. ESTRO IORT Task Force/ACROP recommendations for intraoperative radiation therapy with electrons (IOERT) in breast cancer. Radiother. Oncol. 2020, 149, 150–157. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). 2021. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (accessed on 1 August 2024).
- Emami, B.; Borrowdale, R.W.; Sethi, A.; Thorpe, E.; Chinsky, B.; Small, W. Intraoperative radiation therapy in head and neck cancers. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, E335–E336. [Google Scholar] [CrossRef][Green Version]
- Villafuerte, C.V.L., III; Ylananb, A.M.D.; Wong, H.V.T.; Cañal, J.P.A.; Fragante, E.J.V., Jr. Systematic review of intraoperative radiation therapy for head and neck cancer. Ecancermedicalscience 2022, 16, 1488. [Google Scholar] [CrossRef]
- Freeman, S.B.; Hamaker, R.C.; Singer, M.I.; Pugh, N.; Garrett, P.; Ross, D. Intraoperative Radiotherapy of Head and Neck Cancer. Arch. Otolaryngol. Head Neck Surg. 1990, 116, 165–168. [Google Scholar] [CrossRef]
- Garrett, P.; Pugh, N.; Ross, D.; Hamaker, R.; Singer, M. Intraoperative radiation therapy for advanced or recurrent head and neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 1987, 13, 785–788. [Google Scholar] [CrossRef]
- Garrett, P.; Hamaker, R.; Pugh, N.O., Jr.; Ross, D.; Singer, M. Intraoperative radiation therapy in head and neck cancer. Indiana Med. 1988, 81, 780–782. [Google Scholar]
- Schmitt, T.; Puel, G.; Prades, J.M.; Barbet, N.; Perron, X.; Mere, P. Intraoperative radiotherapy (IORT) for locally advanced carcinoma of the base of tongue. Technique—Preliminary results. Ann. Radiol. 1989, 32, 505–508. [Google Scholar]
- Rate, W.R.; Garrett, P.; Hamaker, R.; Singer, M.; Pugh, N.; Ross, D.; Charles, G. Intraoperative radiation therapy for recurrent head and neck cancer. Cancer 1991, 67, 2738–2740. [Google Scholar] [CrossRef]
- Braun, S.D.; Gemer, L.S.; McIntee, R.A.; Baugh, R.; Thomas, J.H.; Evans, R.G. Intraoperative radiation therapy in the treatment of recurrent carcinoma of the head and neck. Kansas Med. 1991, 92, 47–49. [Google Scholar]
- Toita, T.; Nakano, M.; Takizawa, Y.; Sueyama, H.; Kakihana, Y.; Kushi, A.; Ogawa, K.; Hara, R.; Sunakawa, H.; Arasaki, A.; et al. Intraoperative radiation therapy (IORT) for head and neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 1994, 30, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G.; Geyer, E.; Langsteger, W.; Ramschak, S.; Rosanelli, G. Intraoperative radiation therapy in advanced thyroid cancer. Eur. J. Surg. Oncol. 1995, 21, 357–359. [Google Scholar] [CrossRef]
- Freeman, S.B.; Hamaker, R.C.; Rate, W.R.; Garrett, P.G.; Pugh, N.; Huntley, T.C.; Borrowdale, R. Management of advanced cervical metastasis using intraoperative radiotherapy. Laryngoscope 1995, 105, 575–578. [Google Scholar] [CrossRef]
- Nag, S.; Schuller, D.E.; Martinez-Monge, R.; Rodriguez-Villalba, S.; Grecula, J.; Bauer, C. Intraoperative electron beam radio-therapy for previously irradiated advanced head and neck malignancies. Int. J. Radiat. Oncol. Biol. Phys. 1998, 42, 1085–1089. [Google Scholar] [CrossRef]
- Ling, S.M.; Roach, M., 3rd; Fu, K.K.; Coleman, C.; Chan, A.; Singer, M. Local control after the use of adjuvant electron beam intraoperative radiotherapy in patients with high-risk head and neck cancer: The UCSF experience. Cancer J. Sci. Am. 1996, 2, 321–329. [Google Scholar]
- Coleman, C.W.; Roach, M., 3rd; Ling, S.M.; Kroll, S.M.; Kaplan, M.J.; Chan, A.S.; Fu, K.K.; Singer, M.I. Adjuvant electron-beam IORT in high-risk head and neck cancer patients. Front. Radiat. Ther. Oncol. 1997, 31, 105–111. [Google Scholar]
- Spaeth, J.; Andreopoulos, D.; Unger, T.; Beckman, J.; Ammon, J.; Schlöndorff, G. Intra-operative radiotherapy—5 years of experience in the palliative treatment of recurrent and advanced head and neck cancers. Oncology 1997, 54, 208–213. [Google Scholar] [CrossRef]
- Schmitt, T.; Prades, J.M.; Favrel, V.; Mayaud, R.; Puel, G.; Barbet, N.; Pinto, N.; Calloc’h, F.; Trombert-Paviot, B.; Martin, C. IORT for locally advanced oropharyngeal carcinomas with major extension to the base of the tongue: 5-year results of a prospective study. Front. Radiat. Ther. Oncol. 1997, 31, 117–121. [Google Scholar]
- Nilles-Schendera, A.; Bruggmoser, G.; Stoll, P.; Frommhold, H.; Schilli, W. IORT in floor of the mouth cancer. Front. Radiat. Ther. Oncol. 1997, 31, 102–104. [Google Scholar]
- Martínez-Monge, R.; Azinovic, I.; Alcalde, J.; Aristu, J.; Paloma, V.; García-Tapia, R.; Calvo, F.A. IORT in the management of locally advanced or recurrent head and neck cancer. Front. Radiat. Ther. Oncol. 1997, 31, 122–125. [Google Scholar]
- Schleicher, U.M.; Phonias, C.; Spaeth, J.; Schlöndorff, G.; Ammon, J.; Andreopoulos, D. Intraoperative radiotherapy for pre-irradiated head and neck cancer. Radiother. Oncol. 2001, 58, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Schuller, D.E.; Grecula, J.C.; Agrawal, A.; Rhoades, C.A.; Orr, D.A.; Young, D.C.; Merz, M. Multimodal intensification therapy for previously untreated advanced resectable squamous cell carcinoma of the oral cavity, oropharynx, or hypopharynx. Cancer 2002, 94, 3169–3178. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, A.D.; Foote, R.L.; McCaffrey, T.V.; Kasperbauer, J.L.; Bonner, J.A.; Olsen, K.D.; Cha, S.S.; Sargent, D.J. Intraoperative radiotherapy for head and neck and skull base cancer. Head Neck 2003, 25, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Ozer, E.; Grecula, J.C.; Agrawal, A.; Rhoades, C.A.; Young, D.C.; Schuller, D.E. Long-term results of a multimodal intensification regimen for previously untreated advanced resectable squamous cell cancer of the oral cavity, oropharynx, or hypopharynx. Laryngoscope 2006, 116, 607–612. [Google Scholar] [CrossRef]
- Chen, A.M.; Bucci, M.K.; Singer, M.I.; Garcia, J.; Kaplan, M.J.; Chan, A.S.; Phillips, T.L. Intraoperative radiation therapy for recurrent head-and-neck cancer: The UCSF experience. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 122–129. [Google Scholar] [CrossRef]
- Schuller, D.E.; Ozer, E.; Agrawal, A.; Grecula, J.C.; Rhoades, C.A.; Young, D.C. Multimodal intensification regimens for advanced, resectable, previously untreated squamous cell cancer of the oral cavity, oropharynx, or hypopharynx: A 12-year experience. Arch. Otolaryngol. Head Neck Surg. 2007, 133, 320–326. [Google Scholar] [CrossRef]
- Mendenhall, W.M.; Mendenhall, C.M.; Malyapa, R.S.; Palta, J.R.; Mendenhall, N.P. Re-irradiation of head and neck carcinoma. Am. J. Clin. Oncol. 2008, 31, 393–398. [Google Scholar] [CrossRef]
- Marucci, L.; Pichi, B.; Iaccarino, G.; Ruscito, P.; Spriano, G.; Arcangeli, G. Intraoperative radiation therapy as an “early boost” in locally advanced head and neck cancer: Preliminary results of a feasibility study. Head Neck 2008, 30, 701–708. [Google Scholar] [CrossRef]
- Chen, A.M.; Garcia, J.; Bucci, M.K.; Chan, A.S.; Kaplan, M.J.; Singer, M.I.; Phillips, T.L. Recurrent salivary gland carcinomas treated by surgery with or without intraoperative radiation therapy. Head Neck 2008, 30, 2–9. [Google Scholar] [CrossRef]
- Most, M.D.; Allori, A.C.; Hu, K.; Urken, M.L.; Persky, M.S.; Sessions, R.B.; Nussbaum, M.; Harrison, L.B.; Frank, D.K.; Smith, M.L. Feasibility of flap reconstruction in conjunction with intraoperative radiation therapy for advanced and recurrent head and neck cancer. Laryngoscope 2008, 118, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Kopp, M.; Meco, S.; Oberascher, G. Intraoperative radiotherapy in advanced tumours of the anterior skull base. The results of 30 patients over an eight-year period. Radiother. Oncol. 2009, 91, S7–S8. [Google Scholar] [CrossRef]
- Perry, D.J.; Chan, K.; Wolden, S.; Zelefsky, M.J.; Chiu, J.; Cohen, G.; Zaider, M.; Kraus, D.; Shah, J.; Lee, N. High-dose-rate intraoperative radiation therapy for recurrent head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Joos, N.; Coleman, B.; McNulty, B.; Bumpous, J. Outcomes of intraoperative radiation therapy in the treatment of advanced head and neck cancer. Laryngoscope 2010, 120 (Suppl. S3), S37. [Google Scholar] [CrossRef]
- Rutkowski, T.; Wygoda, A.; Hutnik, M.; Skladowski, K.; Wydmanski, J.; Maciejewski, A.; Szymczyk, C.; Wierzgoń, J.; Orlef, A.; Maciejewski, B. Intraoperative radiotherapy (IORT) with low-energy photons as a boost in patients with early-stage oral cancer with the indications for postoperative radiotherapy: Treatment feasibility and preliminary results. Strahlenther. Onkol. 2010, 186, 496–501. [Google Scholar] [CrossRef]
- Harrison, L.B.; Mourad, W.F.; Perksy, M.; Urken, M.; Jacobson, A.; Culliney, B.; Tran, T.; Schantz, S.P.; Costantino, P.; Hu, K. High dose rate intraoperative radiation therapy for recurrent head and neck cancer: The importance of in-field control on survival. J. Clin. Oncol. 2011, 29, 5593. [Google Scholar] [CrossRef]
- Zeidan, Y.H.; Yeh, A.; Weed, D.; Terry, C.; Freeman, S.; Krowiak, E.; Borrowdale, R.; Huntley, T. Intraoperative radiation therapy for advanced cervical metastasis: A single institution experience. Radiat. Oncol. 2011, 6, 72. [Google Scholar] [CrossRef]
- Zeidan, Y.H.; Shiue, K.; Weed, D.; Johnstone, P.A.; Terry, C.; Freeman, S.; Krowiak, E.; Borrowdale, R.; Huntley, T.; Yeh, A. Intraoperative radiotherapy for parotid cancer: A single-institution experience. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 1831–1836. [Google Scholar] [CrossRef]
- Scala, L.M.; Hu, K.; Urken, M.L.; Jacobson, A.S.; Persky, M.S.; Tran, T.N.; Smith, M.L.; Schantz, S.; Harrison, L.B. Intraoperative high-dose-rate radiotherapy in the management of locoregionally recurrent head and neck cancer†. Head Neck 2013, 35, 485–492. [Google Scholar] [CrossRef]
- Majercakova, K.; Isern Verdum, J.; Alegre, M.; Garcés, J.R.; Craven-Bartle, J. “Intraoperative-like” single dose X-ray radiotherapy in squamous cell skin carcinoma in elderly patients. Radiother. Oncol. 2014, 111, S110. [Google Scholar] [CrossRef]
- Cristalli, G.; Mercante, G.; Marucci, L.; Soriani, A.; Telera, S.; Spriano, G. Intraoperative radiation therapy as adjuvant treatment in locally advanced stage tumours involving the middle ear: A hypothesis-generating retrospective study. Acta Otorhinolaryngol. Ital. 2016, 36, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Yi, P.Q.; Nie, F.F.; Fan, Y.B.; Yu, W.W.; Hu, C.S.; Guo, X.M.; Fu, J. Intraoperative radiotherapy for the treatment of thyroid cancer: A pilot study. Oncotarget 2017, 8, 29355–29360. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moubayed, S.P.; Machado, R.; Osorio, M.; Khorsandi, A.; Hernandez-Prera, J.; Urken, M.L. Metastatic squamous cell carcinoma to the superior cervical ganglion mimicking a retropharyngeal lymph node. Am. J. Otolaryngol. 2017, 38, 720–723. [Google Scholar] [CrossRef]
- Wald, P.; Grecula, J.; Bhatt, A.; Diavolitsis, V.; Teknos, T.; Old, M.; Rocco, J.; Agarwal, A.; Ozer, E.; Carrau, R.; et al. Intraoperative electron beam radiotherapy for locoregionally recurrent head and neck cancer. Radiother. Oncol. 2017, 123, S573. [Google Scholar] [CrossRef]
- Pierik, A.S.; Leemans, C.R.; Brakenhoff, R.H. Resection Margins in Head and Neck Cancer Surgery: An Update of Residual Disease and Field Cancerization. Cancers 2021, 13, 2635. [Google Scholar] [CrossRef]
- Zhang, S.; Zeng, N.; Yang, J.; He, J.; Zhu, F.; Liao, W.; Xiong, M.; Li, Y. Advancements of radiotherapy for recurrent head and neck cancer in modern era. Radiat. Oncol. 2023, 18, 166. [Google Scholar] [CrossRef]
- Hilal, L.; Al Feghali, K.A.; Ramia, P.; Abu Gheida, I.; Obeid, J.P.; Jalbout, W.; Youssef, B.; Geara, F.; Zeidan, Y.H. Intraoperative Radiation Therapy: A Promising Treatment Modality in Head and Neck Cancer. Front. Oncol. 2017, 7, 148. [Google Scholar] [CrossRef]
- Vaidya, J.S.; Vaidya, U.J.; Baum, M.; Bulsara, M.K.; Joseph, D.; Tobias, J.S. Global adoption of single-shot targeted intraoperative radiotherapy (TARGIT-IORT) for breast cancer—Better for patients, better for healthcare systems. Front. Oncol. 2022, 12, 786515. [Google Scholar] [CrossRef]
- Hensley, F.W. Present state and issues in IORT Physics. Radiat. Oncol. 2017, 12, 37. [Google Scholar] [CrossRef]
- Chang, F.C.; Lirng, J.F.; Luo, C.B.; Guo, W.Y.; Teng, M.M.; Tai, S.K.; Chang, C.-Y. Carotid blowout syndrome in patients with head-and-neck cancers: Reconstructive management by self-expandable stent-grafts. AJNR Am. J. Neuroradiol. 2007, 28, 181–188. [Google Scholar]
- Chan, R.J.; Webster, J.; Chung, B.; Marquart, L.; Ahmed, M.; Garantziotis, S. Prevention and treatment of acute radiation-induced skin reactions: A systematic review and meta-analysis of randomized controlled trials. BMC Cancer 2014, 14, 53. [Google Scholar] [CrossRef] [PubMed]
- Grobmyer, S.R.; Lightsey, J.L.; Bryant, C.M.; Shaw, C.; Yeung, A.; Bhandare, N.; Hitchingham, B.; Copeland, E.M. Low-kilovoltage, single-dose intraoperative radiation therapy for breast cancer: Results and impact on a multidisciplinary breast cancer program. J. Am. Coll. Surg. 2013, 216, 617–623; discussion 23–24. [Google Scholar] [CrossRef]

| Reference | Year Publication | Number of Patients (Sites) | Median Follow-Up (mo) | Histology 1 | IORT Median Dose (Range), Gy | Depth; Energy; Photon/Electron; | Surgical Margins | Previous RT, % | Loco-Regional Failure, % | DFS, % | OS, % | Rate and Major Complications | Additionnal EBRT, % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Garett et al. [8] | 1987 | 28 (30) | 14 | SCC | 20 (10–25) | P | R2: 23%; R1: 27%; CM: 50% | 61% | R2: 100%; R1: 275; CM: 50% | - | - | 3/28: 2 V; 1 ON | - |
| Garett et al. [9] | 1988 | 67 | N/A | SCC, AD. ME, Other | 1–10 | P | - | - | R2: 83; R1: 16; CM: 26; R0: 0 | - | - | 8/67: 4 CB; 4 ON | 40 |
| Schmitt et al. [10] | 1989 | 15 | 6–12 | SCC | 17.5–20 | E; 6–9 MeV | - | - | R2: 100; R1: 25 | - | - | CB | 100 |
| Rate et al. [11] | 1989 | 47 | 24 | 20 (15–25) | P | - | 100 | 38 (14mo) | - | 55 (2y) | 8/47: 1 V; 2 ON, 2 F | - | |
| Freeman et al. [7] | 1990 | 104 (109) | 24 | SCC, SG, S, M | 15–20 | E; 4 MeV | R2: 7/35; R1: 28/35 | 62% of SCC | R2: 43; R1: 44; CM: 30 | - | - | 21/104: 6% ON; 6% F; 3% CB | 42 |
| Rate et al. [11] | 1991 | 47 | 14 | SCC, AC | 20 (15–25) | P | R2: 4/47; R1: 41/47 | 100 | 38.5 (2 years) | 55 (29 months) | - | 1 CB; 2 ON; 3 F | - |
| Braun et al. [12] | 1991 | 1 | 20 | P | - | 100 | - | - | - | ||||
| Freeman et al. [12] | 1991 | 25 | 12 | 15–20 | E; 4 MeV | - | yes without precision | 33% | - | - | 2 ON | - | |
| Toita et al. [13] | 1994 | 25 (30) | 19 (3–59) | SCC |
| E; 6–18 MeV (median 9 MeV) | R2: 9.3%; R1: 33.3% | - | - |
|
-R2: 0;
-R1: 54.5; -CM: 81.8. -Overall: 54.1 (2y) | 5/23: 3 CB; 6 ON | 66 |
| Wolf et al. [14] | 1995 | 5 | 20–48 | T | 4–6 | P | 1 R2; 2 R1; 2 CM | - | R2: 50; R1: 0 | - | 1 F | 100 | |
| Freeman et al. [15] | 1995 | 75 | 24 | SCC; M; ME; AC | 17.5 (10–25) | E: 5–11 MeV | - | 61 | overall: 32 (2y); R2 75; R1: 27; CM: 24 | - | 45 (2y) | 19/75: 1 CB; 4 V; 4 F; 2 N; x ON | 33 |
| Nag et al., [16] | 1995 | 29 | 21 | 7.5–15 | P | - | - | overall 11; 67 if only IORT, (21mo) | - | overall 72, 100 if IORT + EBRT, 17 if only IORT, | CSF leak | 58 | |
| Ling et al. [17] | 1996 | 30 | 30 | SCC, ME, AC, AD, A | 15 | E: 6–9 MeV | - | overall: 40 (3y) | - | 70 (3y) | - | ||
| Coleman et al. [18] | 1997 | 44 (46) | 24 | SCC, ME, AD, AC, PD; A, chrondrom | 14–18 | E: 2.5–9.5 MeV; 2.5–9.5 mm | - | 39 | 39 (20mo) | 61.7 (2y) | 66 (2y) | 8/44: 3W; 1CB; 1 ON, 3N | 37 |
| Spaeth et al. [19] | 1997 | 95 (120) | 11 | SCC, AD, M, S, ME, A, LE, H, N-HL | 20 (10–40) | P | - | 95 | 89 (11mo) | - | 20 (2y) | 11/120: 8W; 3F | |
| Schmitt et al. [20] | 1997 | 43 | 24 | SCC | 20–25 | E: 6 MeV; 1–2.7 cm | _ | _ | overall 66 (3y); 51 (5y) | - | 60 (5y) | 3FN; 1LN; 1LS | 100 |
| Nilles-Schendera et al. [21] | 1997 | 42 | _ | SCC | 12–15 | P | _ | _ | 8/42 (6y) | - | - | 0 | 66 |
| Martinez-Monge et al. [22] | 1997 | 31 | 24 | SCC mostly | 10–15 | P | - | 47 | 67 (2y) | - | 20 (2y) | 7/31: 5 F; 1 FN | - |
| Nag et al. [16] | 1998 | 38 (40) | 30 | SCC, ADK, LC | 15 (15–20) | P | - | 100 | 79 (30mo) | - | 21 (2y) | 6/38: 1W; 2 V; 2 F; 1 CO | - |
| Schendera et al. [21] | 1998 | 58 | 60 | 12–25 | P | - | overall: 19; Gp III: 100 | - | - | Gp I: 32 (5y); Gp II: 90 (5y); Gp III: 14 (3mo) | - | - | |
| Schleicher et al. [23] | 2001 | 84 (113) | 6.8 | SCC, T, SG | 20 (10–20) | P | - | 100 | R2: 76; R1: 58; R0: 50 | - | 37 (1y) | 21/84: 3.5%F, 2%N; 9%W | - |
| Schuller et al. [24] | 2002 | 43 | 14.6 | SCC | 7.5–10 | 6 MeV, P and E, 5 mm | - | - | overall 7 | - | 6 F, 1FN, 1 N | 100 | |
| Pinheiro et al. [25] | 2003 | 44 (50) | 75.6 for survivors | SCC, non SCC | 12.5–22.5 | P | - | - | 54 SCC, 48 non-SCC (2y) | - | 32 SCC; 50 non-SCC (2y) | 23/44: 8 W; 2 CB; 3 F; 5 N | - |
| Ozer et al. [26] | 2006 | 32 | 89 (3.4–140) | SCC | 7.5–10 | 6 MeV, P and E, 5 mm | - | - | 9 (5y) | - | Overall 56 (5y) | - | yes, not specified |
| Chen et al. [27] | 2007 | 137 (191) | 41 (3–122) | SCC, AC, ME, AD, S, M; AcC | 15 (10–18) | E | - | - | in field: 38; locoregional: 49 (3y) | - | overall 36 (44 if primary recurrence, 19 if neck recurrence) | 4/137: 2 F, 1 Ne | 26 |
| Schuller et al. [28] | 2007 | 123 | 60 | SCC | 7.5–10 | E | - | - | 73 (5y) | 57 (5y) | 29/123: 4W; 19F; 1 FN; 2ON | yes, not specified | |
| Mendenhall et al. [29] | 2008 | 86 | Nasopharynx 60; H&N: 8-39 | NP, SCC, AD, LC | 4–12.5 | P | - | - | Nasopharynx 53 (5y) H&N: 87 in field; 96 locoregional (2y) | - | Nasopharynx: 47 (5y); H&N: 8 (2y) | 2 F; 1 CB; 7 BN; 2 Hy; 6 N; 2ON | |
| Marucci et al. [30] | 2008 | 25 | 10 | SCC, DC | 12 | 7–9 MeV; P; 4–8 cm | - | - | 41.5 (2y) | 50.6 (2y) | 64.5 (2y) | 6/25: 1W; 1 ON; 3 F; 1 FN | |
| Chen et al. [31] | 2008 | 37 | 44.4 | SG | 15 (15–18) | P | - | - | 18 (w) vs. 40 (w/o IORT), p = 0.001 | - | 54 (3y); 34 (5y) | 4/37: 2W, 1N | 15 |
| Most et al. [32] | 2008 | 21 | SCC, AD, ME, S | 10–15 | P | - | - | - | 1 FN; 1 CB; 1F | 28 | |||
| Kopp et al. [33] | 2009 | 47 | SCC, AD, undifferentiated, AC, EB, NC, ME, other | 10 | P | - | - | - | - | 41 if primary, 33 if recurrence (5.7y) | - | 70 | |
| Perry et al. [34] | 2010 | 34 | 15 | P | - | - | 34 (1y); 44 (2y) | - | 27 (1y); 45 (2y) | - | |||
| Joos et al. [35] | 2010 | 60 (30 w IORT, 30 w/o IORT) | 2y | SCC mostly | 15 (10–18) | - | - | - | - | - | IORT group: 23.3 if R0, 50 if R1, 26.6 if R2 (2y). No difference w or w/o IORT | - | - |
| Rutkowski et al. [36] | 2010 | 16 | 30 (0–66) | SCC | 5–7.5Gy | P; 20 KeV | - | 0 | Local: 0; Locoregional: 18 | - | 81 | 3/16: Other | 100 |
| Harrison et al. [37] | 2011 | 90 | 11 | SCC, SG, S, Skin, EB | 12 (10–17.5) | P; 1 cm | 51% positive margins | - | in field: 35; out of field: 22 (1y) | 51 (1y) | 66 (1y) | 1 ON, 1 CB | 100 |
| Zeidan et al. [38] | 2011 | 46 | 67.2 | SG | 15–20 | P | - | - | - | 51.9 (5y) | 59 (3y); 48 (5y) | 4% ON, 4% FN, 1% N | 57 |
| Zieden et al. [39] | 2012 | 96 | 67.2 | ME, SCC, AC, AD | 15–20 | P | - | 35.5 | - | 82 (1y); 68.5 (3y); 65.2 (5y) | 88.4 (1y), 66.1 (3y); 56.2 (5y) | 26/96: 2 W; 7V; 4 F; 4 ON; 1 N | - |
| Zieden et al. [38] | 2012 | 231 | 15–20 | P | - | - | - | 66 (1y); 55 (3y), 49 (5y) | 58 (1y);34 (3y); 26 (5y) | 20/231: 23V; 8ON; 20 F; 2FN | - | ||
| Scala et al. [40] | 2013 | 76 | 12–60 | SCC, AD, other | 10–17.5 | P | - | - | 44 (1y); 48 (2y) | - | 64 (1y); 42 (2y) | 4% CB, 1% N | N/A |
| Majercakova et al. [41] | 2014 | 9 | SCC | 8 (8–12) | P; 5 mm | - | - | - | 85% (w) vs. 66% (w/o). | none | - | ||
| Enami et al. [5] | 2016 | 12 | 5–18 | parotid and other | 6 (5–14) | P; 5 mm | CM or clinical impression of CM | 42 | 5 mm; P; 50 keV | - | - | none | 50 |
| Cristalli et al. [42] | 2016 | 13 | 33 | 12 | P; 12–17 mm | No R2 | - | - | 68 (5y) | - | 1 FN, 1 F | 100 | |
| Emami et al. [5] | 2017 | 22 | 16 (3–33) | SCC, SG | 12–14 | P; 4–6 cm | - | - | 13 (1.5) | - | 2 N | 45 | |
| Yi et al. [43] | 2017 | 9 | 14–22 (2 lost) | T | 3–8 | P; 50 KeV | - | - | - | 89 (5mo) | - | 1 F, 1 W | 11 |
| Moubayed et al. [44] | 2017 | 1 | - | SCC | 6 | P | - | - | - | - | - | 1 F | - |
| Wald et al. [45] | 2019 | 61 | 15.9 (4.9–74.4) | SCC | 12.5 (10–17.5) | P | 46% R3, 44% R-, 10% unkmown | 95 (1–277 mo) | 41 (1y) | 39 (1y) | 62 (1y); 42 (2y) | 1 CB | 38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pincet, L.; Fanchette, A.; Elmers, J.; Bourhis, J.; Lambercy, K.; Romano, E. Systematic Review of Intraoperative Radiotherapy (IORT) in Head and Neck Oncology: Past, Present, and Future Perspectives. Cancers 2025, 17, 2124. https://doi.org/10.3390/cancers17132124
Pincet L, Fanchette A, Elmers J, Bourhis J, Lambercy K, Romano E. Systematic Review of Intraoperative Radiotherapy (IORT) in Head and Neck Oncology: Past, Present, and Future Perspectives. Cancers. 2025; 17(13):2124. https://doi.org/10.3390/cancers17132124
Chicago/Turabian StylePincet, Laurence, Aurelie Fanchette, Jolanda Elmers, Jean Bourhis, Karma Lambercy, and Edouard Romano. 2025. "Systematic Review of Intraoperative Radiotherapy (IORT) in Head and Neck Oncology: Past, Present, and Future Perspectives" Cancers 17, no. 13: 2124. https://doi.org/10.3390/cancers17132124
APA StylePincet, L., Fanchette, A., Elmers, J., Bourhis, J., Lambercy, K., & Romano, E. (2025). Systematic Review of Intraoperative Radiotherapy (IORT) in Head and Neck Oncology: Past, Present, and Future Perspectives. Cancers, 17(13), 2124. https://doi.org/10.3390/cancers17132124







