Subclassification-Specific Tumor Immune Microenvironment in Intrahepatic Cholangiocarcinoma: Implications for Appropriate Pharmacotherapy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
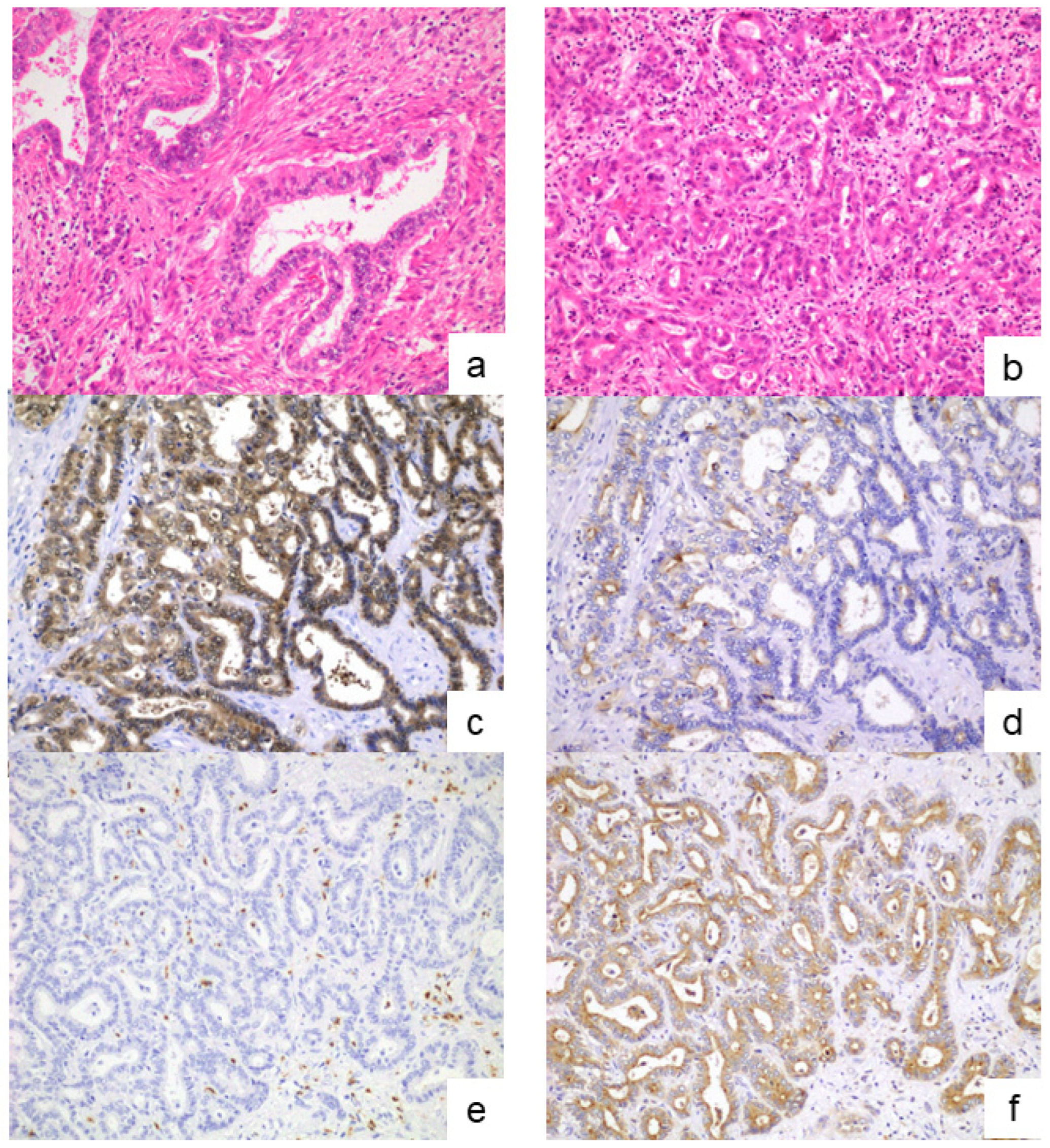
2.2. Classification of Large- and Small-Duct-Type iCCAs
2.3. Analyses of the TIME
2.4. Statistical Analysis
3. Results
3.1. Diagnosis of the iCCA Subclassifications
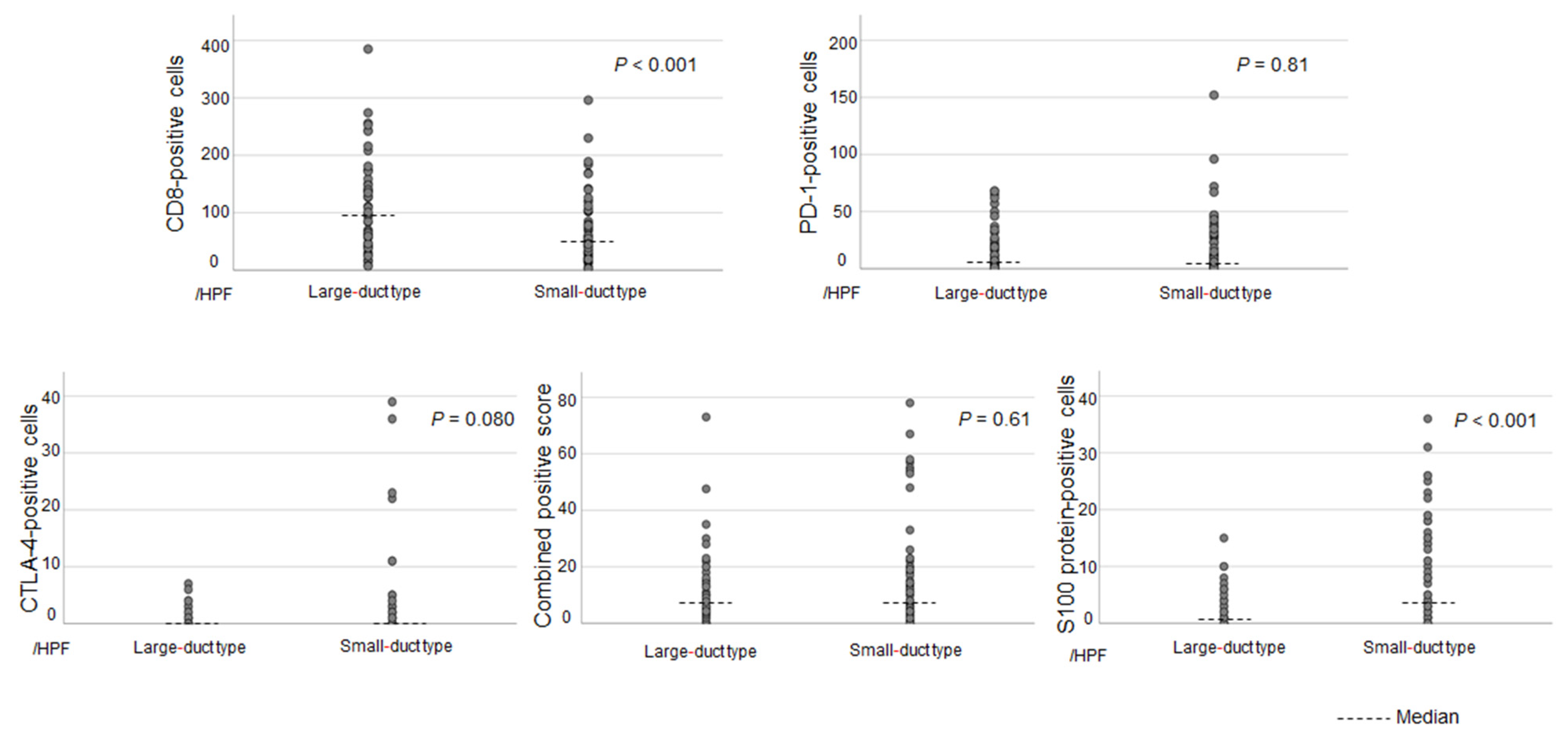
3.2. Differences in the TIME Between Each Subclassification
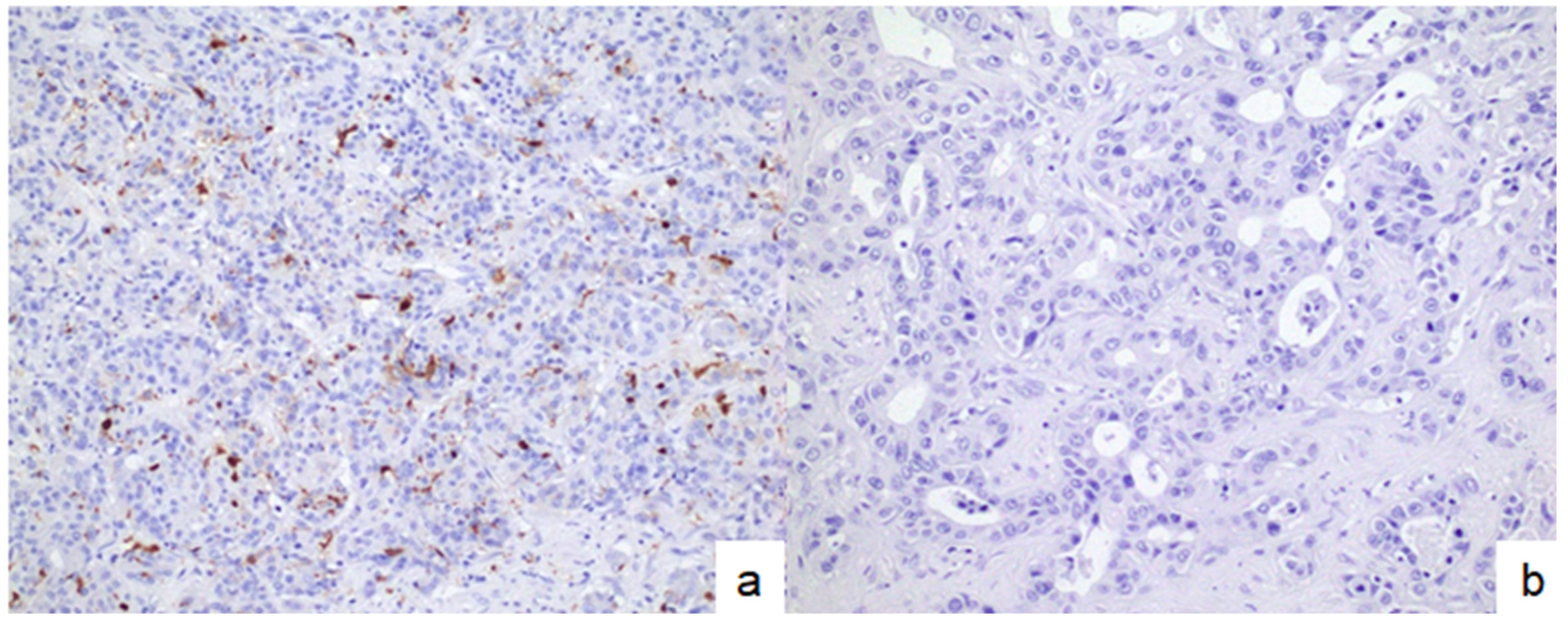
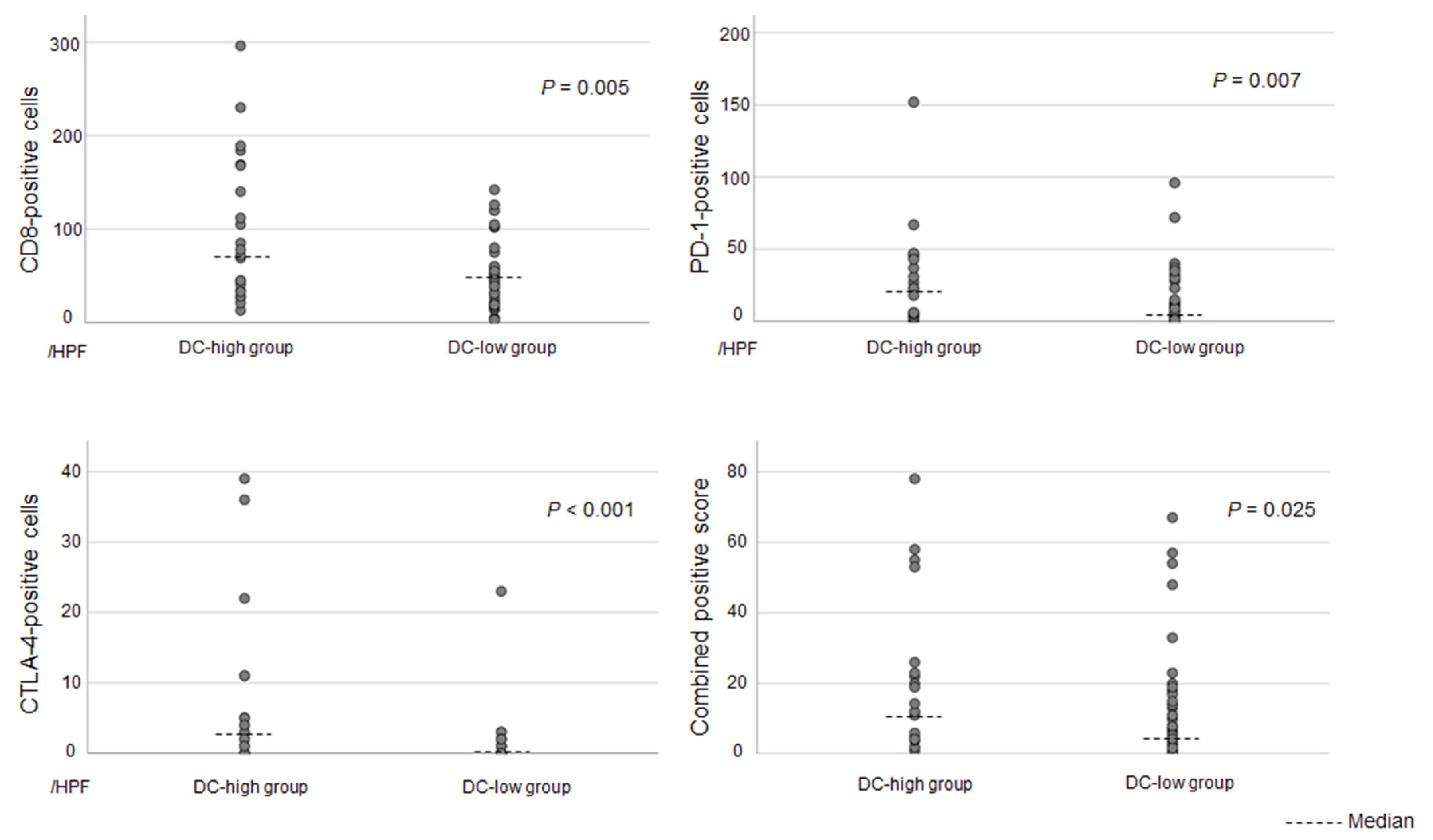
3.3. Associations Between DC Infiltration and Other Immune-Related Molecules in Patients with Small-Duct-Type iCCAs
3.4. Clinicopathological Differences Between DC-High and DC-Low Tumors in Patients with Small-Duct-Type iCCAs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DC | Dendritic cell |
| H&E | Hematoxylin and eosin |
| iCCA | Intrahepatic cholangiocarcinoma |
| ICI | Immune checkpoint inhibitor |
| TIME | Tumor immune microenvironment |
| TMB | Tumor mutation burden |
References
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A.; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Liau, J.Y.; Tsai, J.H.; Yuan, R.H.; Chang, C.N.; Lee, H.J.; Jeng, Y.M. Morphological subclassification of intrahepatic cholangiocarcinoma: Etiological, clinicopathological, and molecular features. Mod. Pathol. 2014, 27, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Aishima, S.; Oda, Y. Pathogenesis and classification of intrahepatic cholangiocarcinoma: Different characters of perihilar large duct type versus peripheral small duct type. J. Hepato-Biliary-Pancreat. Sci. 2015, 22, 94–100. [Google Scholar] [CrossRef]
- Hayashi, A.; Misumi, K.; Shibahara, J.; Arita, J.; Sakamoto, Y.; Hasegawa, K.; Kokudo, N.; Fukayama, M. Distinct clinicopathologic and genetic features of 2 histologic subtypes of intrahepatic cholangiocarcinoma. Am. J. Surg. Pathol. 2016, 40, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Kubo, S.; Nakanuma, Y.; Takemura, S.; Sakata, C.; Urata, Y.; Nozawa, A.; Nishioka, T.; Kinoshita, M.; Hamano, G.; Terajima, H.; et al. Case series of 17 patients with cholangiocarcinoma among young adult workers of a printing company in Japan. J. Hepatobiliary Pancreat. Sci. 2014, 21, 479–488. [Google Scholar] [CrossRef]
- Akita, M.; Fujikura, K.; Ajiki, T.; Fukumoto, T.; Otani, K.; Azuma, T.; Itoh, T.; Ku, Y.; Zen, Y. Dichotomy in intrahepatic cholangiocarcinomas based on histologic similarities to hilar cholangiocarcinomas. Mod. Pathol. 2017, 30, 986–997. [Google Scholar] [CrossRef]
- Akita, M.; Sofue, K.; Fujikura, K.; Otani, K.; Itoh, T.; Ajiki, T.; Fukumoto, T.; Zen, Y. Histological and molecular characterization of intrahepatic bile duct cancers suggests an expanded definition of perihilar cholangiocarcinoma. HPB 2019, 21, 226–234. [Google Scholar] [CrossRef]
- Kinoshita, M.; Sato, Y.; Shinkawa, H.; Kimura, K.; Ohira, G.; Nishio, K.; Tanaka, R.; Kurihara, S.; Kushiyama, S.; Tani, N.; et al. Impact of tumor subclassifications for identifying an appropriate surgical strategy in patients with intrahepatic cholangiocarcinoma. Ann. Surg. Oncol. 2024, 31, 2579–2590. [Google Scholar] [CrossRef]
- Shah, N.J.; Della Pia, A.; Wu, T.; Williams, A.; Weber, M.; Sinclaire, B.; Gourna Paleoudis, E.; Alaoui, A.; Lev-Ari, S.; Adams, S.; et al. Clinical outcomes of immune checkpoint inhibitors in unique cohorts underrepresented in clinical trials. Cancers 2024, 16, 2223. [Google Scholar] [CrossRef]
- Fitzsimmons, T.S.; Singh, N.; Walker, T.D.J.; Newton, C.; Evans, D.G.R.; Crosbie, E.J.; Ryan, N.A.J. Immune checkpoint inhibitors efficacy across solid cancers and the utility of PD-L1 as a biomarker of response: A systematic review and meta-analysis. Front. Med. 2023, 10, 1192762. [Google Scholar] [CrossRef]
- Wang, S.L.; Chan, T.A. Navigating established and emerging biomarkers for immune checkpoint inhibitor therapy. Cancer Cell 2025, 43, 641–664. [Google Scholar] [CrossRef]
- Oh, D.Y.; Ruth He, A.; Qin, S.; Chen, L.T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Ah Lee, M.; Kitano, M.; et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid. 2022, 1, EVIDoa2200015. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.K.; Ueno, M.; Yoo, C.; Finn, R.S.; Furuse, J.; Ren, Z.; Yau, T.; Klümpen, H.J.; Chan, S.L.; Ozaka, M.; et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023, 401, 1853–1865. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.G.; Kim, M.H.; Jang, M.; Kim, H.; Hwang, H.K.; Kang, C.M.; Lee, W.J.; Kang, B.; Lee, C.K.; Lee, M.G.; et al. Molecular characterization of biliary tract cancer predicts chemotherapy and programmed death 1/programmed death-ligand 1 blockade responses. Hepatology 2021, 74, 1914–1931. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Amoozgar, Z.; Liu, X.; Aoki, S.; Liu, Z.; Shin, S.M.; Matsui, A.; Hernandez, A.; Pu, Z.; Halvorsen, S.; et al. Reprogramming the intrahepatic cholangiocarcinoma immune microenvironment by chemotherapy and CTLA-4 blockade enhances anti-PD-1 therapy. Cancer Immunol. Res. 2024, 12, 400–412. [Google Scholar] [CrossRef]
- Ricci, A.D.; Rizzo, A.; Schirizzi, A.; D’Alessandro, R.; Frega, G.; Brandi, G.; Shahini, E.; Cozzolongo, R.; Lotesoriere, C.; Giannelli, G. Tumor immune microenvironment in intrahepatic cholangiocarcinoma: Regulatory mechanisms, functions, and therapeutic implications. Cancers 2024, 16, 3542. [Google Scholar] [CrossRef]
- Yu, X.; Zhu, L.; Wang, T.; Chen, J. Immune microenvironment of cholangiocarcinoma: Biological concepts and treatment strategies. Front. Immunol. 2023, 14, 1037945. [Google Scholar] [CrossRef]
- Kinoshita, M.; Kubo, S.; Nakanuma, Y.; Sato, Y.; Takemura, S.; Tanaka, S.; Hamano, G.; Ito, T.; Terajima, H.; Yamada, T.; et al. Pathological spectrum of bile duct lesions from chronic bile duct injury to invasive cholangiocarcinoma corresponding to bile duct imaging findings of occupational cholangiocarcinoma. J. Hepatobiliary Pancreat. Sci. 2016, 23, 92–101. [Google Scholar] [CrossRef]
- Kubo, S.; Tanaka, S.; Kinoshita, M.; Shinkawa, H.; Ishizawa, T.; Sato, Y. Development of intraductal papillary neoplasm of the bile duct in patients with occupational cholangiocarcinoma. Virchows Arch. 2023, 482, 745–753. [Google Scholar] [CrossRef]
- Aishima, S.; Fujita, N.; Mano, Y.; Kubo, Y.; Tanaka, Y.; Taketomi, A.; Shirabe, K.; Maehara, Y.; Oda, Y. Different roles of S100P overexpression in intrahepatic cholangiocarcinoma: Carcinogenesis of perihilar type and aggressive behavior of peripheral type. Am. J. Surg. Pathol. 2011, 35, 590–598. [Google Scholar] [CrossRef]
- Song, G.; Shi, Y.; Meng, L.; Ma, J.; Huang, S.; Zhang, J.; Wu, Y.; Li, J.; Lin, Y.; Yang, S.; et al. Single-cell transcriptomic analysis suggests two molecularly subtypes of intrahepatic cholangiocarcinoma. Nat. Commun. 2022, 13, 1642. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, T.; Uehara, T.; Iwaya, M.; Nakajima, T.; Shimizu, A.; Kubota, K.; Notake, T.; Kitagawa, N.; Masuo, H.; Sakai, H.; et al. An immunohistochemical analysis of osteopontin and S100 calcium-binding protein P is useful for subclassifying large- and small-duct type intrahepatic cholangiocarcinomas. Am. J. Surg. Pathol. 2024, 48, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Kulangara, K.; Zhang, N.; Corigliano, E.; Guerrero, L.; Waldroup, S.; Jaiswal, D.; Ms, M.J.; Shah, S.; Hanks, D.; Wang, J.; et al. Clinical utility of the combined positive score for programmed death Ligand-1 expression and the approval of Pembrolizumab for treatment of gastric cancer. Arch. Pathol. Lab. Med. 2019, 143, 330–337. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef]
- Ferguson, T.A.; Choi, J.; Green, D.R. Armed response: How dying cells influence T-cell functions. Immunol. Rev. 2011, 241, 77–88. [Google Scholar] [CrossRef]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef]
- Franciszkiewicz, K.; Boissonnas, A.; Boutet, M.; Combadière, C.; Mami-Chouaib, F. Role of chemokines and chemokine receptors in shaping the effector phase of the antitumor immune response. Cancer Res. 2012, 72, 6325–6332. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Irving, B.A.; Hodi, F.S. Molecular pathways: Next-generation immunotherapy—Inhibiting programmed death-ligand 1 and programmed death-1. Clin. Cancer Res. 2012, 18, 6580–6587. [Google Scholar] [CrossRef]
- Jardim, D.L.; Goodman, A.; de Melo Gagliato, D.; Kurzrock, R. The challenges of tumor mutational burden as an immunotherapy biomarker. Cancer Cell 2021, 39, 154–173. [Google Scholar] [CrossRef]
- Xie, N.; Shen, G.; Gao, W.; Huang, Z.; Huang, C.; Fu, L. Neoantigens: Promising targets for cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 9. [Google Scholar] [CrossRef]
- Job, S.; Rapoud, D.; Dos Santos, A.; Gonzalez, P.; Desterke, C.; Pascal, G.; Elarouci, N.; Ayadi, M.; Adam, R.; Azoulay, D.; et al. Identification of four immune subtypes characterized by distinct composition and functions of tumor microenvironment in intrahepatic cholangiocarcinoma. Hepatology 2020, 72, 965–981. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Kunita, A.; Yamagishi, M.; Katoh, H.; Ishikawa, S.; Yamamoto, H.; Abe, J.; Arita, J.; Hasegawa, K.; Shibata, T.; et al. KRAS mutation in intrahepatic cholangiocarcinoma: Linkage with metastasis-free survival and reduced E-cadherin expression. Liver Int. 2022, 42, 2329–2340. [Google Scholar] [CrossRef]
- Chung, T.; Park, Y.N. Up-to-date pathologic classification and molecular characteristics of intrahepatic cholangiocarcinoma. Front. Med. 2022, 9, 857140. [Google Scholar] [CrossRef]
- Zen, Y. Intrahepatic cholangiocarcinoma: Typical features, uncommon variants, and controversial related entities. Hum. Pathol. 2023, 132, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Testa, U.; Pelosi, E.; Castelli, G. Cholangiocarcinoma: Molecular abnormalities and cells of origin. Technol. Cancer Res. Treat. 2023, 22, 15330338221128689. [Google Scholar] [CrossRef]
- Lu, J.C.; Zeng, H.Y.; Sun, Q.M.; Meng, Q.N.; Huang, X.Y.; Zhang, P.F.; Yang, X.; Peng, R.; Gao, C.; Wei, C.Y.; et al. Distinct PD-L1/PD1 profiles and clinical implications in intrahepatic cholangiocarcinoma patients with different risk factors. Theranostics 2019, 9, 4678–4687. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, H.S.; Lee, S.H.; Woo, S.M.; Kim, D.U.; Bang, S. Efficacy and safety of Pembrolizumab for gemcitabine/cisplatin-refractory biliary tract cancer: A multicenter retrospective study. J. Clin. Med. 2020, 9, 1769. [Google Scholar] [CrossRef]
- Cheng, Z.; Zeng, T.; Yang, G.; Liu, D.; Zheng, Z.; Yuan, Z. Genomic and immune features in an intrahepatic cholangiocarcinoma patient with microsatellite instability-high suffered rapid acquired resistance to PD-1 inhibitor. Liver Cancer 2023, 12, 281–288. [Google Scholar] [CrossRef]
- Sato, Y.; Tanaka, S.; Kinoshita, M.; Takemura, S.; Shinkawa, H.; Kokudo, T.; Hasegawa, K.; Tanaka, H.; Yoshimoto, H.; Mori, A.; et al. Immunosuppressive tumor microenvironment in occupational cholangiocarcinoma: Supportive evidence for the efficacy of immune checkpoint inhibitor therapy. J. Hepatobiliary Pancreat. Sci. 2020, 27, 860–869. [Google Scholar] [CrossRef]
- Choi, S.B.; Kim, K.S.; Choi, J.Y.; Park, S.W.; Choi, J.S.; Lee, W.J.; Chung, J.B. The prognosis and survival outcome of intrahepatic cholangiocarcinoma following surgical resection: Association of lymph node metastasis and lymph node dissection with survival. Ann. Surg. Oncol. 2009, 16, 3048–3056. [Google Scholar] [CrossRef]
- Shimada, M.; Yamashita, Y.; Aishima, S.; Shirabe, K.; Takenaka, K.; Sugimachi, K. Value of lymph node dissection during resection of intrahepatic cholangiocarcinoma. Br. J. Surg. 2001, 88, 1463–1466. [Google Scholar] [CrossRef] [PubMed]


| Variables | DC-High Group (n = 22) | DC-Low Group (n = 51) | p-Value |
|---|---|---|---|
| Age, years | 70 (55–89) | 68 (32–82) | 0.38 |
| Sex, male/female | 18/4 | 37/14 | 0.56 |
| Laboratory data results | |||
| T-Bil level, mg/dL | 0.6 (0.3–1.6) | 0.6 (0.1–22.7) | 0.43 |
| Albumin level, g/dL | 4.0 (3.3–4.5) | 4.2 (2.7–4.8) | 0.35 |
| AST level, U/L | 33 (17–164) | 29 (11–164) | 0.60 |
| ALT level, U/L | 24 (9–208) | 27 (7–208) | 0.76 |
| CEA level, ng/mL | 3.4 (1.1–9.2) | 3.4 (0.7–56.9) | 0.88 |
| CA19-9 level, U/mL | 16 (5–1554) | 28 (2–1392) | 0.82 |
| Chronic liver disease | 13 | 27 | 0.75 |
| Viral hepatitis | 5 | 15 | 0.58 |
| Positive for HBsAg | 1 | 4 | 0.99 |
| Positive for HCVAb | 4 | 11 | 0.99 |
| Metabolic-associated steatohepatitis | 5 | 6 | 0.23 |
| Alcoholic hepatitis | 3 | 8 | 0.99 |
| Development of synchronous or metachronous HCC | 1 | 9 | 0.26 |
| Preoperative chemotherapy | 2 | 3 | 0.63 |
| Tumor diameter, cm | 3.4 (1.2–7.3) | 3.8 (0.4–12.5) | 0.50 |
| Macroscopic classification, MF/PI | 21/1 | 51/0 | 0.30 |
| Multiple lesions, n | 3 | 14 | 0.15 |
| Perihilar invasion | 10 | 12 | 0.17 |
| Adjuvant chemotherapy | 9 | 19 | 0.77 |
| Major hepatectomy | 15 | 12 | 0.043 |
| Pathological findings | |||
| Liver cirrhosis | 1 | 6 | 0.67 |
| Perineural invasion | 3 | 5 | 0.69 |
| Microvascular invasion | 10 | 18 | 0.41 |
| Lymph node metastasis | 7 | 6 | 0.040 |
| Tumor invasion at the surgical margin | 2 | 5 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kinoshita, M.; Sato, Y.; Kubo, S.; Shinkawa, H.; Kimura, K.; Nishio, K.; Tanaka, R.; Kurihara, S.; Ishizawa, T. Subclassification-Specific Tumor Immune Microenvironment in Intrahepatic Cholangiocarcinoma: Implications for Appropriate Pharmacotherapy. Cancers 2025, 17, 2082. https://doi.org/10.3390/cancers17132082
Kinoshita M, Sato Y, Kubo S, Shinkawa H, Kimura K, Nishio K, Tanaka R, Kurihara S, Ishizawa T. Subclassification-Specific Tumor Immune Microenvironment in Intrahepatic Cholangiocarcinoma: Implications for Appropriate Pharmacotherapy. Cancers. 2025; 17(13):2082. https://doi.org/10.3390/cancers17132082
Chicago/Turabian StyleKinoshita, Masahiko, Yasunori Sato, Shoji Kubo, Hiroji Shinkawa, Kenjiro Kimura, Kohei Nishio, Ryota Tanaka, Shigeaki Kurihara, and Takeaki Ishizawa. 2025. "Subclassification-Specific Tumor Immune Microenvironment in Intrahepatic Cholangiocarcinoma: Implications for Appropriate Pharmacotherapy" Cancers 17, no. 13: 2082. https://doi.org/10.3390/cancers17132082
APA StyleKinoshita, M., Sato, Y., Kubo, S., Shinkawa, H., Kimura, K., Nishio, K., Tanaka, R., Kurihara, S., & Ishizawa, T. (2025). Subclassification-Specific Tumor Immune Microenvironment in Intrahepatic Cholangiocarcinoma: Implications for Appropriate Pharmacotherapy. Cancers, 17(13), 2082. https://doi.org/10.3390/cancers17132082






