The Impact of Treatment Delay on Endometrial and Ovarian Cancer Patients: A Systematic Review
Simple Summary
Abstract
1. Introduction
1.1. Rationale
1.2. Objectives
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources
2.3. Search Strategy
- PubMed (999 studies):
- Cochrane (386 studies):
- Scopus (1767 studies):
- ClinicalTrials.org (134 studies):
2.4. Selection Process
2.5. Data Collection Process
2.6. Data Items
2.7. Study Risk of Bias Assessment
2.8. Effect Measures
2.9. Synthesis Methods
2.10. Reporting Bias Assessment
2.11. Certainty Assessment
3. Results
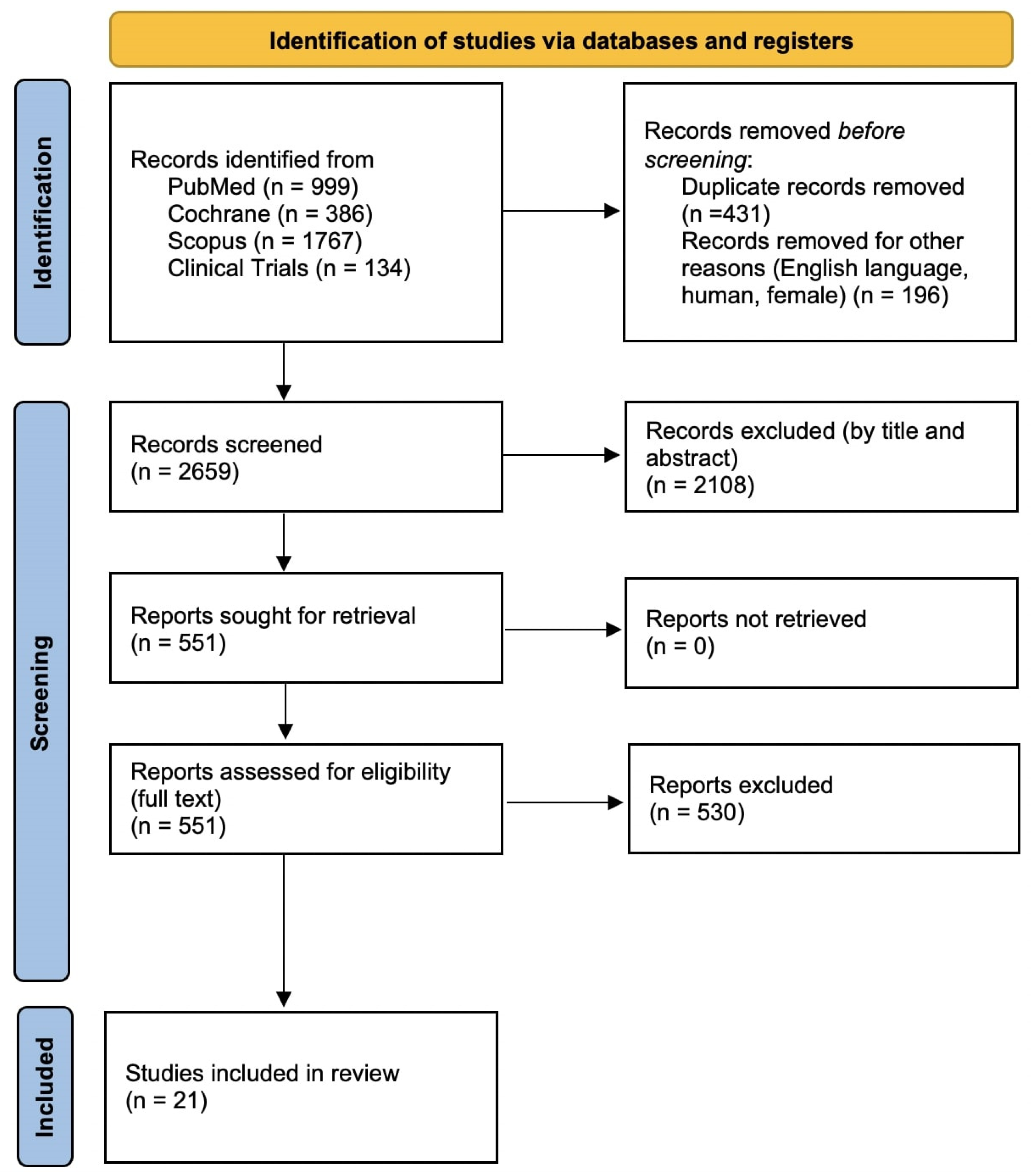
3.1. Study Selection
3.2. Study Characteristics
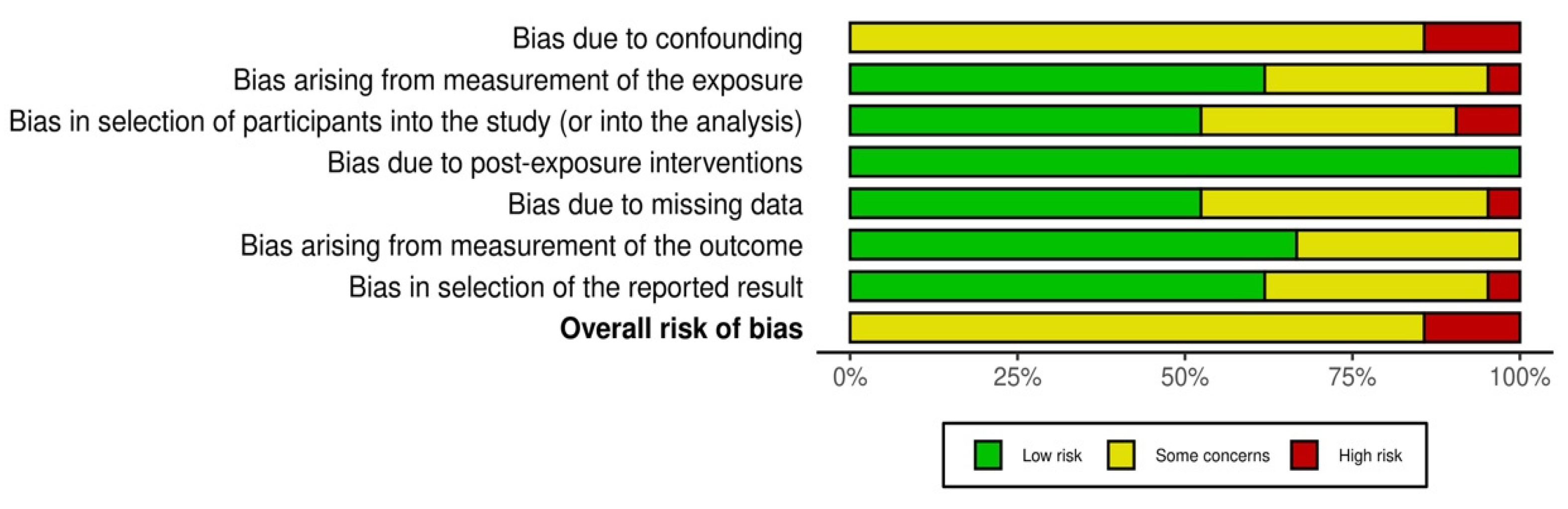
3.3. Risk of Bias in Studies
3.4. Results of Individual Studies
3.4.1. Quantitative Data
3.4.2. Delay Intervals and Measurement Methods
3.4.3. Impact of Delay in Survival Outcomes
3.4.4. Impact of Delay on Disease Progression and Cancer Stage
3.4.5. Delay and Patient-Reported Outcomes
3.4.6. Healthcare System Factors and Treatment Delays
3.4.7. COVID-19 Pandemic and Treatment Delay
3.4.8. Comparisons Between Ovarian and Endometrial Cancer
3.4.9. Discrepancies and Conflicting Findings
3.4.10. Reporting Biases
3.4.11. Certainty of Evidence
4. Discussion
4.1. Summary of Main Findings
4.2. Clinical Implications of Treatment Delays
4.3. Patient-Reported Outcomes and Psychological Impacts
4.4. Influence of Healthcare System Factors on Treatment Timeliness
4.5. Impact of COVID-19 Pandemic on Cancer Care Delivery
4.6. Methodological Variability and Study Heterogeneity
4.7. Risk of Bias and Strength of Evidence
4.8. Strengths and Limitations of the Review
4.9. Recommendations for Clinical Practice and Policy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| QoL | Quality of Life |
| COVID-19 | Coronavirus Disease 2019 |
| ROBINS-E | Risk Of Bias In Non-randomized Studies of Exposures |
| HR | Hazard Ratio |
| OR | Odds Ratio |
| CIs | Confidence Intervals |
| GRADE | Grading of Recommendations Assessment, Development, and Evaluation |
| Dx | Diagnosis |
| Tx | Treatment |
| Sx | Symptoms |
| OS | Overall Survival |
| DFS | Disease-Free Survival |
References
- Hummeida, M.E.; Hamad, K.; Abdel Gadir, A.F.; Ali, A.A. Ovarian cancer during pregnancy: A case report and literature review. Clin. Pract. 2015, 5, 1–4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Olson, S.H.; Mignone, L.; Nakraseive, C.; Caputo, T.A.; Barakat, R.R.; Harlap, S. Symptoms of ovarian cancer. Obstet. Gynecol. 2001, 98, 212–217. [Google Scholar] [CrossRef]
- Felix, A.S.; Weissfeld, J.L.; Stone, R.A.; Bowser, R.; Chivukula, M.; Edwards, R.P.; Linkov, F. Factors associated with Type I and Type II endometrial cancer. Cancer Causes Control 2010, 21, 1851–1856. [Google Scholar] [CrossRef] [PubMed]
- Neal, R.D.; Tharmanathan, P.; France, B.; Din, N.U.; Cotton, S.; Fallon-Ferguson, J.; Hamilton, W.; Hendry, A.; Hendry, M.; Lewis, R.; et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br. J. Cancer 2015, 112 (Suppl. 1), S92–S107. [Google Scholar] [CrossRef]
- Pergialiotis, V.; Haidopoulos, D.; Tzortzis, A.S.; Antonopoulos, I.; Thomakos, N.; Rodolakis, A. The impact of waiting intervals on survival outcomes of patients with endometrial cancer: A systematic review of the literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 246, 1–6. [Google Scholar] [CrossRef]
- Prendergast, E.N.; Elvin, J.A. Genomic profiling of gynecologic cancers and implications for clinical practice. Curr. Opin. Obstet. Gynecol. 2017, 29, 18–25. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Morgan, R.L.; Rooney, A.A.; Taylor, K.W.; Thayer, K.A.; Silva, R.A.; Lemeris, C.; Akl, E.A.; Bateson, T.F.; Berkman, N.D.; et al. A tool to assess risk of bias in non-randomized follow-up studies of exposure effects (ROBINS-E). Environ. Int. 2024, 186, 108602. [Google Scholar] [CrossRef]
- GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University and Evidence Prime. Available online: https://www.gradepro.org/ (accessed on 26 May 2025).
- AlHilli, M.M.; Elson, P.; Rybicki, L.; Khorana, A.A.; Rose, P.G. Time to surgery and its impact on survival in patients with endometrial cancer: A National cancer database study. Gynecol. Oncol. 2019, 153, 511–516. [Google Scholar] [CrossRef]
- Crawford, S.C.; Davis, J.A.; Siddiqui, N.A.; de Caestecker, L.; Gillis, C.R.; Hole, D.; Penney, G. The waiting time paradox: Population based retrospective study of treatment delay and survival of women with endometrial cancer in Scotland. BMJ 2002, 325, 196. [Google Scholar] [CrossRef]
- Elit, L.M.; O’Leary, E.M.; Pond, G.R.; Seow, H.-Y. Impact of wait times on survival for women with uterine cancer. J. Clin. Oncol. 2014, 32, 27–33. [Google Scholar] [CrossRef]
- Frey, M.K.; Moss, H.A.; Musa, F.; Rolnitzky, L.; David-West, G.; Chern, J.-Y.; Boyd, L.R.; Curtin, J.P. Preoperative experience for public hospital patients with gynecologic cancer: Do structural barriers widen the gap? Cancer 2016, 122, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Frey, M.K.; Ellis, A.E.; Zeligs, K.; Chapman-Davis, E.; Thomas, C.; Christos, P.J.; Kolev, V.; Prasad-Hayes, M.; Cohen, S.; Holcomb, K.; et al. Impact of the coronavirus disease 2019 pandemic on the quality of life for women with ovarian cancer. Am. J. Obstet. Gynecol. 2020, 223, 725.e1–725.e9. [Google Scholar] [CrossRef]
- Huepenbecker, S.P.; Sun, C.C.; Fu, S.; Zhao, H.; Primm, K.; Rauh-Hain, J.A.; Fleming, N.D.; Giordano, S.H.; Meyer, L.A. Association between time to diagnosis, time to treatment, and ovarian cancer survival in the United States. Int. J. Gynecol. Cancer 2022, 32, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Kadan, Y.; Asali, A.; Fishman, A.; Helpman, L.; Perri, T.; Korach, J.; Beiner, M. Time interval from biopsy to surgery and risk for adjuvant therapy in patients with low-risk endometrial cancer. Surg. Oncol. 2020, 35, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Levin, G.; Matanes, E.; Salvador, S.; Lau, S.; Gotlieb, W. Time interval from biopsy of endometrial atypical hyperplasia to surgery and risk for concurrent endometrial carcinoma—A retrospective study. BJOG 2024, 131, 1320–1321. [Google Scholar] [CrossRef]
- Marcickiewicz, J.; Åvall-Lundqvist, E.; Holmberg, E.C.V.; Borgfeldt, C.; Bjurberg, M.; Dahm-Kähler, P.; Flöter-Rådestad, A.; Hellman, K.; Högberg, T.; Rosenberg, P.; et al. The wait time to primary surgery in endometrial cancer - impact on survival and predictive factors: A population-based SweGCG study. Acta Oncol. 2022, 61, 30–37. [Google Scholar] [CrossRef]
- Matsuo, K.; Opper, N.R.; Ciccone, M.A.; Garcia, J.; Tierney, K.E.; Baba, T.; Muderspach, L.I.; Roman, L.D. Time interval between endometrial biopsy and surgical staging for type I endometrial cancer: Association between tumor characteristics and survival outcome. Obstet. Gynecol. 2015, 125, 424–433. [Google Scholar] [CrossRef]
- Mitric, C.; Matanes, E.; Wissing, M.; Amajoud, Z.; Abitbol, J.; Yasmeen, A.; López-Ozuna, V.; Eisenberg, N.; Laskov, I.; Lau, S.; et al. The impact of wait times on oncological outcome in high-risk patients with endometrial cancer. J. Surg. Oncol. 2020, 122, 306–314. [Google Scholar] [CrossRef]
- Nica, A.; Sutradhar, R.; Kupets, R.; Covens, A.; Vicus, D.; Li, Q.; Ferguson, S.E.; Gien, L.T. Pre-operative wait times in high-grade non-endometrioid endometrial cancer: Do surgical delays impact patient survival? Gynecol. Oncol. 2022, 164, 333–340. [Google Scholar] [CrossRef]
- O’Leary, E.; Elit, L.; Pond, G.; Seow, H. The wait time creep: Changes in the surgical wait time for women with uterine cancer in Ontario, Canada, during 2000–2009. Gynecol. Oncol. 2013, 131, 151–157. [Google Scholar] [CrossRef]
- Rabiu, K.A.; Akinola, O.I.; Adewunmi, A.A.; Fabamwo, A.O.; Adedeji, M.O.; Popoola, A.O. Delays in presentation and management of ovarian cancer in Lagos, Nigeria. J. Obstet. Gynaecol. 2013, 33, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.M.; Christensen, K.B.; Ottesen, B.; Krasnik, A. Diagnostic delay, quality of life and patient satisfaction among women diagnosed with endometrial or ovarian cancer: A nationwide Danish study. Qual. Life Res. 2012, 21, 1519–1525. [Google Scholar] [CrossRef]
- Sabourin, J.N.; Glaze, S. The impact of time between histologic diagnosis of endometrial cancer and surgical treatment on stage and survival. Gynecol. Oncol. 2015, 137, 88. [Google Scholar] [CrossRef]
- Shalowitz, D.I.; Epstein, A.J.; Buckingham, L.; Ko, E.M.; Giuntoli, R.L. Survival implications of time to surgical treatment of endometrial cancers. Am. J. Obstet. Gynecol. 2017, 216, 268.e1–268.e18. [Google Scholar] [CrossRef] [PubMed]
- Strohl, A.E.; Feinglass, J.M.; Shahabi, S.; Simon, M.A. Surgical wait time: A new health indicator in women with endometrial cancer. Gynecol. Oncol. 2016, 141, 511–515. [Google Scholar] [CrossRef]
- Hansen, R.P.; Vedsted, P.; Sokolowski, I.; Søndergaard, J.; Olesen, F. Time intervals from first symptom to treatment of cancer: A cohort study of 2212 newly diagnosed cancer patients. BMC Health Serv. Res. 2011, 11, 284. [Google Scholar] [CrossRef]
- Zouzoulas, D.; Tsolakidis, D.; Karalis, T.; Aristotelidis, M.; Topalidou, M.; Grimbizis, G. The impact of delay from diagnosis to surgery in endometrial cancer. Arch. Gynecol. Obstet. 2025, 311, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Zouzoulas, D.; Tsolakidis, D.; Sofianou, I.; Karalis, T.; Aristotelidis, M.; Tzitzis, P.; Deligeoroglou, E.; Topalidou, M.; Timotheadou, E.; Grimbizis, G. The Impact of Surgery Delay on Early-Stage Ovarian Cancer. Life 2025, 15, 122. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]

| Study Author | Year | Country | Study Design | Sample Size | Study Setting | Delay Measurement | Outcomes Evaluated |
|---|---|---|---|---|---|---|---|
| AlHilli et al. [9] | 2019 | USA | Retrospective cohort | 284,499 | Population-based | Diagnosis to surgery | Survival outcomes, stage progression |
| Crawford et al. [10] | 2002 | UK | Retrospective cohort | 703 | Hospital-based | Referral/diagnosis to treatment | Survival outcomes |
| Elit et al. [11] | 2014 | Canada | Population-based cohort | 9417 | Population-based | Histologic diagnosis to hysterectomy | Survival outcomes, stage progression |
| Frey et al. [12] | 2016 | Australia | Retrospective cohort | 143 (Endometrial subgroup) | Hospital-based | Diagnosis to surgery | Delay intervals (public vs. private hospitals) |
| Kadan et al. [15] | 2020 | Israel | Retrospective cohort | 468 | Hospital-based | Biopsy diagnosis to surgery | Adjuvant therapy needs, survival outcomes |
| Levin et al. [16] | 2024 | Canada | Retrospective cohort | 160 | Hospital-based | Biopsy diagnosis (atypical hyperplasia) to surgery | Presence of concurrent carcinoma |
| Marcickiewicz et al. [17] | 2022 | Sweden | Retrospective cohort | 7366 | Population-based | Diagnosis to primary surgery | Survival outcomes, sociodemographic predictors of delay |
| Matsuo et al. [18] | 2015 | USA | Retrospective cohort | 435 | Hospital-based | Biopsy diagnosis to surgical staging | Survival outcomes, tumor grade upgrading |
| Mitric et al. [19] | 2020 | Canada | Retrospective cohort | 136 | Hospital-based | Biopsy diagnosis to surgery | Survival outcomes, tumor aggressiveness |
| Nica et al. [20] | 2022 | Canada | Retrospective cohort | 3518 | Population-based | Diagnosis and oncology consultation to surgery | Survival outcomes |
| O’Leary et al. [21] | 2013 | Canada | Population-based cohort | 9330 | Population-based | Diagnosis to surgery | Delay intervals trends, predictors of delay |
| Robinson et al. [23] | 2012 | Denmark | Nationwide cohort | 165 (Endometrial subgroup) | Population-based | Symptoms to diagnosis/treatment | Quality of life, patient satisfaction, survival |
| Sabourin et al. [24] | 2015 | Canada | Retrospective cohort | 2809 | Population-based | Histologic diagnosis to surgery | Survival outcomes, stage progression |
| Shalowitz et al. [25] | 2017 | USA | Retrospective cohort | 208,438 | Population-based | Diagnosis to surgery | Survival outcomes, optimal surgical timing |
| Strohl et al. [26] | 2016 | USA | Retrospective cohort | 112,041 | Population-based | Diagnosis to definitive surgery | Survival outcomes, demographic disparities |
| Zouzoulas et al. [28] | 2024 | Greece | Retrospective cohort | 259 | Hospital-based | Biopsy diagnosis to surgery | Survival outcomes |
| Study Author | Year | Country | Study Design | Sample Size | Study Setting | Delay Measurement | Outcomes Evaluated |
|---|---|---|---|---|---|---|---|
| Frey et al. [12] | 2016 | Australia | Retrospective cohort | 114 (Ovarian subgroup) | Hospital-based | Diagnosis to surgery | Delay intervals (public vs. private hospitals) |
| Frey et al. [13] | 2020 | Australia | Retrospective cohort | 555 | Hospital-based | COVID-19 related delay in planned treatment | Patient anxiety, QoL impacts |
| Hansen et al. [27] | 2011 | Denmark | Population-based cohort | 59 | Population-based | Symptoms to definitive treatment | Delay intervals, survival outcomes |
| Huepenbecker et al. [14] | 2022 | Germany | Retrospective cohort | 13,872 | Population-based | Symptoms to diagnosis and diagnosis to treatment | Survival outcomes |
| Rabiu et al. [22] | 2013 | Nigeria | Retrospective cohort | 37 | Hospital-based | Symptoms to definitive treatment | Stage at diagnosis, treatment compliance |
| Robinson et al. [23] | 2012 | Denmark | Nationwide cohort | 188 (Ovarian subgroup) | Population-based | Symptoms to diagnosis/treatment | Quality of life, patient satisfaction, survival |
| Zouzoulas et al. [29] | 2025 | Greece | Retrospective cohort | 72 | Hospital-based | Symptoms to definitive treatment | Survival outcomes |
| Study Author | Year | Cancer Type | Sample Size | Delay Interval | Delay Measurement | Survival Outcomes | Disease Progression/Stage Outcomes | Patient-Reported Outcomes | Healthcare System Factors |
|---|---|---|---|---|---|---|---|---|---|
| AlHilli et al. [9] | 2019 | Endometrial | 284,499 | Dx–Tx | >6 weeks associated with poorer survival | ↓ survival | Stage progression linked to delays | - | - |
| Crawford et al. [10] | 2002 | Endometrial | 703 | Referral–Tx | Median delay: 43 days | ↓ survival with longer delays | Advanced stage at longer delays | - | Potential systemic delays |
| Elit et al. [11] | 2014 | Endometrial | 9417 | Dx–Tx | Median delay: 36 days | ↓ survival with increased delay | Advanced stage correlated with increased delay | - | Population-based delays |
| Frey et al. [12] | 2016 | Endometrial, ovarian | 257 | Dx–Tx | Median delay (endo): 34 days; (ov): 28 days | - | - | Reduced satisfaction in public hospitals | Longer delays in public hospitals |
| Frey et al. [13] | 2020 | Ovarian | 555 | COVID-related | Significant delays due to COVID-19 | - | - | ↑ anxiety, ↓ QoL | Healthcare disruptions due to pandemic |
| Hansen et al. [27] | 2011 | Ovarian | 59 | Sx–Tx | Median delay: 100 days | ↓ survival, delays detrimental | Advanced stage at diagnosis due to delays | - | Systemic and patient delays |
| Huepenbecker et al. [14] | 2022 | Ovarian | 13,872 | Sx–Dx–Tx | Sx–Dx (median 66 days) Dx–Tx (median 29 days) | ↓ survival linked to symptom delays | Advanced disease linked to symptom delays | - | German healthcare registry data |
| Kadan et al. [15] | 2020 | Endometrial | 468 | Dx–Tx | Median delay: 30 days | Minimal survival impact | Minimal impact on stage progression | - | - |
| Levin et al. [16] | 2024 | Endometrial | 160 | Dx–Tx | Median delay: 42 days | - | Minimal stage progression | - | - |
| Marcickiewicz et al. [17] | 2022 | Endometrial | 7366 | Dx–Tx | Optimal interval: 2–6 weeks | ↓ survival at extremes | - | - | Socioeconomic disparities affecting delays |
| Matsuo et al. [18] | 2015 | Endometrial | 435 | Dx–Tx | Median delay: 34 days | No clear survival disadvantage | Minimal grade/stage progression | - | - |
| Mitric et al. [19] | 2020 | Endometrial | 136 | Dx–Tx | Median delay: 42 days | No significant survival disadvantage | Minimal disease aggressiveness impact | - | - |
| Nica et al. [20] | 2022 | Endometrial | 3518 | Dx–Tx | Median delay: 45 days | ↓ survival with increased delay | - | - | Regional disparities, healthcare system impacts |
| O’Leary et al. [21] | 2013 | Endometrial | 9,33 | Dx–Tx | Increased delay trends | ↓ survival with longer delays | Increased advanced-stage cases with delays | - | Regional variations, resource availability |
| Rabiu et al. [22] | 2013 | Ovarian | 37 | Sx–Tx | Severe delays (>3 months) | - | Advanced stage at presentation | - | Socioeconomic factors affecting delay |
| Robinson et al. [23] | 2012 | Endometrial, ovarian | 453 | Sx–Tx | Median delay (endo): 77 days; (ov): 92 days | Poorer survival in ovarian cancer | Advanced stage due to delays | ↓ QoL, patient dissatisfaction | System delays, socioeconomic factors |
| Sabourin et al. [24] | 2015 | Endometrial | 2809 | Dx–Tx | Median delay: 43 days | ↓ survival with delays | Increased stage progression | - | Canadian provincial healthcare |
| Shalowitz et al. [25] | 2017 | Endometrial | 208,438 | Dx–Tx | Optimal interval: 3–8 weeks | ↓ survival outside optimal interval | - | - | Socioeconomic disparities, demographic impacts |
| Strohl et al. [26] | 2016 | Endometrial | 112,041 | Dx–Tx | Delays associated with demographic disparities | ↓ survival with longer delays | - | - | Disparities based on socioeconomic/racial factors |
| Zouzoulas et al. [28] | 2024 | Endometrial | 259 | Dx–Surgery | >8 weeks | Worse DFS, no difference in OS | Increased need for adjuvant pelvic radiation | - | Older age, higher BMI, and more comorbidities in delayed group |
| Zouzoulas et al. [29] | 2025 | Ovarian (early stage) | 72 | Dx–Surgery | >5 weeks | No significant differences in DFS or OS | No significant differences in stage progression | - | No significant differences in postoperative complications |
| Study Author | Year | Cancer Type | Delay Interval | Survival Outcomes/Quantitative Data |
|---|---|---|---|---|
| AlHilli et al. [9] | 2019 | Endometrial | Dx–Tx (>6 weeks) | Stage I-II: HR 1.22 (95% CI 1.16–1.29) Stage III: HR 0.99 (95% CI 0.91–1.08, non-significant) Stage IV: improved survival (HR 0.89; 95% CI 0.80–0.99) |
| Shalowitz et al. [25] | 2017 | Endometrial | Dx–Tx (>8 weeks or <2 weeks) | Surgery within 1 week HR 1.4 (95% CI 1.3–1.5); within 2 weeks HR 1.1 (95% CI 1.0–1.2). Increased mortality risk. |
| Strohl et al. [26] | 2016 | Endometrial | Dx–Tx (>6 weeks) | Overall survival decrease: HR 1.14 (95% CI 1.09–1.20) |
| Crawford et al. [10] | 2002 | Endometrial | Referral–Tx (>40 days) | 62–91 days HR 0.47 (95% CI 0.27–0.83); >92 days HR 0.53 (95% CI 0.30–0.93) |
| Elit et al. [11] | 2014 | Endometrial | Dx–Surgery (>12 weeks or ≤2 weeks) | Significantly worse 5-year OS for delays >12 weeks; for delays ≤2 weeks, 5-year OS 71.1% (also poorer outcome) |
| Matsuo et al. [18] | 2015 | Endometrial | Dx–Tx intervals (1–14, 15–42, 43–84, and ≥85 days) | No significant differences in survival (5-year OS rates 62.5%, 93.6%, 95.2%, and 100%, respectively) |
| Mitric et al. [19] | 2020 | Endometrial | Dx–Tx (>12 weeks) | No significant impact on DFS (HR 1.2; 95% CI 0.6–2.5), OS (HR 1.1; 95% CI 0.6–2.1), or PFS (HR 0.9; 95% CI 0.5–1.7) |
| Nica et al. [20] | 2022 | Endometrial | First oncology appointment to Surgery (>45 days) | 46–60 days HR 1.19 (95% CI 1.04–1.36); 61–75 days HR 1.42 (95% CI 1.11–1.83) |
| Sabourin et al. [24] | 2015 | Endometrial | Dx–Surgery (>12 weeks) | 5-year OS: ≤6 weeks 87.1%, 6–12 weeks 84.1%, >12 weeks 79.8%; delay >12 weeks HR 1.41 (95% CI 1.03–1.93) |
| Zouzoulas et al. [28] | 2024 | Endometrial | Dx–Surgery (>8 weeks) | DFS significantly worse with delays >8 weeks (p = 0.0312); OS not significantly impacted (p = 0.146) |
| Zouzoulas et al. [29] | 2025 | Ovarian (early stage) | Dx–Surgery (>5 weeks) | No significant impact on DFS (p = 0.48) or OS (p = 0.703) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zouzoulas, D.; Karalis, T.; Sofianou, I.; Anthoulakis, C.; Tzika, K.; Zafrakas, M.; Timotheadou, E.; Grimbizis, G.; Tsolakidis, D. The Impact of Treatment Delay on Endometrial and Ovarian Cancer Patients: A Systematic Review. Cancers 2025, 17, 2076. https://doi.org/10.3390/cancers17132076
Zouzoulas D, Karalis T, Sofianou I, Anthoulakis C, Tzika K, Zafrakas M, Timotheadou E, Grimbizis G, Tsolakidis D. The Impact of Treatment Delay on Endometrial and Ovarian Cancer Patients: A Systematic Review. Cancers. 2025; 17(13):2076. https://doi.org/10.3390/cancers17132076
Chicago/Turabian StyleZouzoulas, Dimitrios, Tilemachos Karalis, Iliana Sofianou, Christos Anthoulakis, Katerina Tzika, Menelaos Zafrakas, Eleni Timotheadou, Grigoris Grimbizis, and Dimitrios Tsolakidis. 2025. "The Impact of Treatment Delay on Endometrial and Ovarian Cancer Patients: A Systematic Review" Cancers 17, no. 13: 2076. https://doi.org/10.3390/cancers17132076
APA StyleZouzoulas, D., Karalis, T., Sofianou, I., Anthoulakis, C., Tzika, K., Zafrakas, M., Timotheadou, E., Grimbizis, G., & Tsolakidis, D. (2025). The Impact of Treatment Delay on Endometrial and Ovarian Cancer Patients: A Systematic Review. Cancers, 17(13), 2076. https://doi.org/10.3390/cancers17132076







