Socioeconomic and Healthcare Indicators and Colorectal Cancer Burden: Analysis of Eurostat and Global Burden of Disease Study 2021 Data
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Sources
2.2. Statistical Analysis
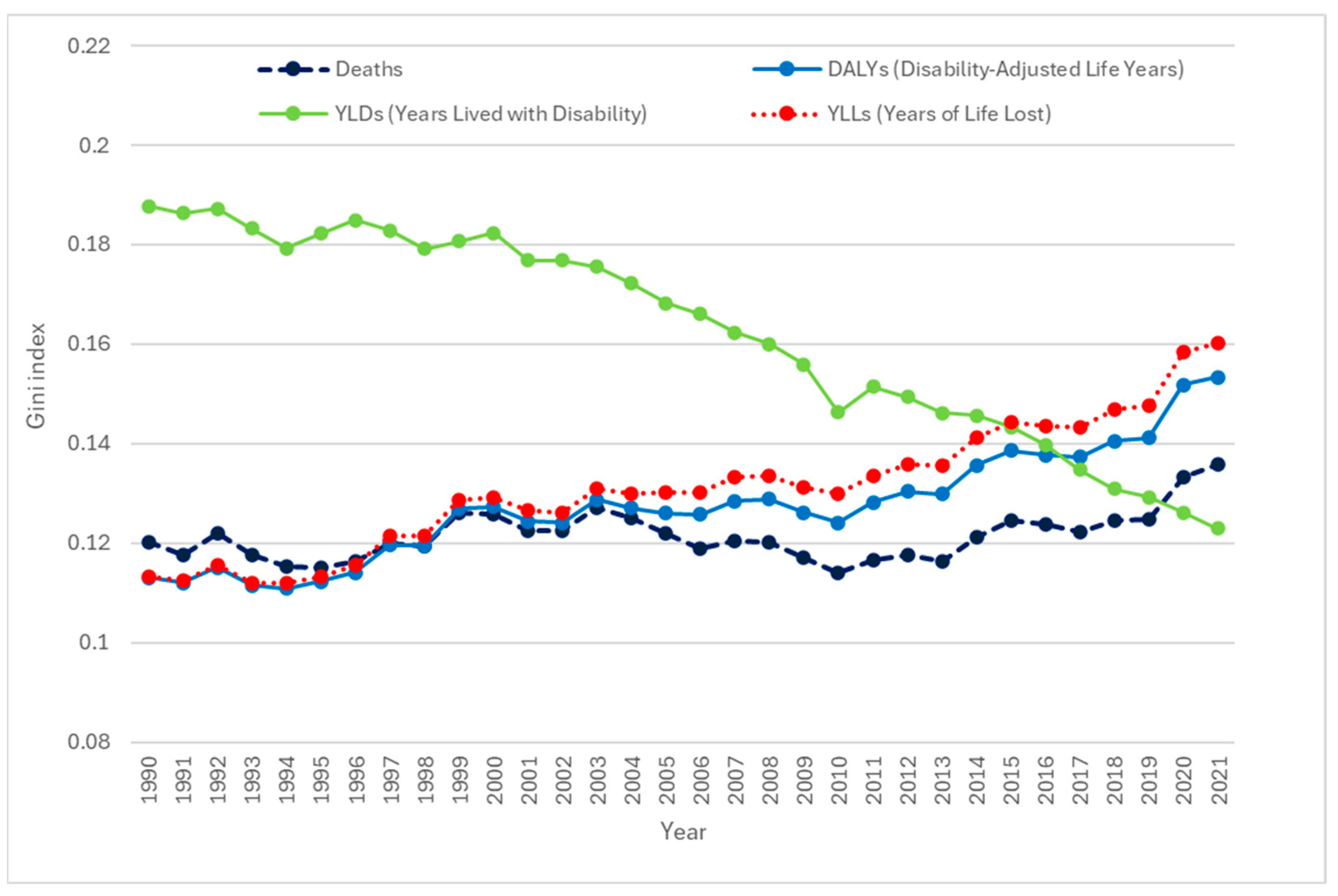
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAPC | average annual percent change |
| CRC | Colorectal cancer |
| DALY | Disability-adjusted life years |
| EU | European Union |
| GBD | Global Burden of Disease |
| DALY | Disability-adjusted life years |
| YLD | Years lived with disability |
| YLL | Years of life lost |
References
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global Burden of Colorectal Cancer in 2020 and 2040: Incidence and Mortality Estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Cancer Statistics—Specific Cancers. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Cancer_statistics_-_specific_cancers (accessed on 21 May 2025).
- Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2021 (GBD 2021) Results; Institute for Health Metrics and Evaluation (IHME): Seattle, WA, USA, 2021. [Google Scholar]
- Hofmarcher, T.; Lindgren, P. The Cost of Cancers of the Digestive System in Europe; IHE Report: Lund, Sweden, 2020. [Google Scholar]
- Carethers, J.M.; Doubeni, C.A. Causes of Socioeconomic Disparities in Colorectal Cancer and Intervention Framework and Strategies. Gastroenterology 2020, 158, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.; Guo, F.; Heisser, T.; Hackl, M.; Ihle, P.; Schutter, H.D.; Damme, N.V.; Valerianova, Z.; Atanasov, T.; Májek, O.; et al. Colorectal Cancer Incidence, Mortality, and Stage Distribution in European Countries in the Colorectal Cancer Screening Era: An International Population-Based Study. Lancet Oncol. 2021, 22, 1002–1013. [Google Scholar] [CrossRef]
- Ola, I.; Cardoso, R.; Hoffmeister, M.; Brenner, H. Utilization of Colorectal Cancer Screening Tests across European Countries: A Cross-Sectional Analysis of the European Health Interview Survey 2018-2020. Lancet Reg. Health Eur. 2024, 41, 100920. [Google Scholar] [CrossRef] [PubMed]
- Bozhar, H.; McKee, M.; Spadea, T.; Veerus, P.; Heinävaara, S.; Anttila, A.; Senore, C.; Zielonke, N.; de Kok, I.M.C.M.; van Ravesteyn, N.T.; et al. Socio-Economic Inequality of Utilization of Cancer Testing in Europe: A Cross-Sectional Study. Prev. Med. Rep. 2022, 26, 101733. [Google Scholar] [CrossRef]
- Hayes, L.; Adams, J.; McCallum, I.; Forrest, L.; Hidajat, M.; White, M.; Sharp, L. Age-Related and Socioeconomic Inequalities in Timeliness of Referral and Start of Treatment in Colorectal Cancer: A Population-Based Analysis. J. Epidemiol. Community Health 2021, 75, 1–9. [Google Scholar] [CrossRef]
- Aarts, M.J.; Lemmens, V.E.P.P.; Louwman, M.W.J.; Kunst, A.E.; Coebergh, J.W.W. Socioeconomic Status and Changing Inequalities in Colorectal Cancer? A Review of the Associations with Risk, Treatment and Outcome. Eur. J. Cancer 2010, 46, 2681–2695. [Google Scholar] [CrossRef]
- Feller, A.; Schmidlin, K.; Bordoni, A.; Bouchardy, C.; Bulliard, J.; Camey, B.; Konzelmann, I.; Maspoli, M.; Wanner, M.; Zwahlen, M.; et al. Socioeconomic and Demographic Inequalities in Stage at Diagnosis and Survival among Colorectal Cancer Patients: Evidence from a Swiss Population-based Study. Cancer Med. 2018, 7, 1498–1510. [Google Scholar] [CrossRef]
- Jansen, L.; Behrens, G.; Finke, I.; Maier, W.; Gerken, M.; Pritzkuleit, R.; Holleczek, B.; Brenner, H. Area-Based Socioeconomic Inequalities in Colorectal Cancer Survival in Germany: Investigation Based on Population-Based Clinical Cancer Registration. Front. Oncol. 2020, 10, 857. [Google Scholar] [CrossRef]
- Ungvari, Z.; Fekete, M.; Fekete, J.T.; Lehoczki, A.; Buda, A.; Munkácsy, G.; Varga, P.; Ungvari, A.; Győrffy, B. Treatment Delay Significantly Increases Mortality in Colorectal Cancer: A Meta-Analysis. GeroScience 2025. [Google Scholar] [CrossRef] [PubMed]
- Hollis, R.H.; Chu, D.I. Healthcare Disparities and Colorectal Cancer. Surg. Oncol. Clin. N. Am. 2022, 31, 157–169. [Google Scholar] [CrossRef]
- Lejeune, C.; Sassi, F.; Ellis, L.; Godward, S.; Mak, V.; Day, M.; Rachet, B. Socio-Economic Disparities in Access to Treatment and Their Impact on Colorectal Cancer Survival. Int. J. Epidemiol. 2010, 39, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Campbell, N.C.; Elliott, A.M.; Sharp, L.; Ritchie, L.D.; Cassidy, J.; Little, J. Rural and Urban Differences in Stage at Diagnosis of Colorectal and Lung Cancers. Br. J. Cancer 2001, 84, 910–914. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, M.; Ong, K.L.; Aali, A.; Ababneh, H.S.; Abate, Y.H.; Abbafati, C.; Abbasgholizadeh, R.; Abbasian, M.; Abbasi-Kangevari, M.; Abbastabar, H.; et al. Global Burden of 288 Causes of Death and Life Expectancy Decomposition in 204 Countries and Territories and 811 Subnational Locations, 1990–2021: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2100–2132. [Google Scholar] [CrossRef]
- Kim, Y.-E.; Jung, Y.-S.; Ock, M.; Yoon, S.-J. DALY Estimation Approaches: Understanding and Using the Incidence-Based Approach and the Prevalence-Based Approach. J. Prev. Med. Public Health 2022, 55, 10–18. [Google Scholar] [CrossRef]
- Ferrari, A.J.; Santomauro, D.F.; Aali, A.; Abate, Y.H.; Abbafati, C.; Abbastabar, H.; ElHafeez, S.A.; Abdelmasseh, M.; Abd-Elsalam, S.; Abdollahi, A.; et al. Global Incidence, Prevalence, Years Lived with Disability (YLDs), Disability-Adjusted Life-Years (DALYs), and Healthy Life Expectancy (HALE) for 371 Diseases and Injuries in 204 Countries and Territories and 811 Subnational Locations, 1990–2021: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2133–2161. [Google Scholar] [CrossRef]
- World Health Organization. Department of Data and Analytics Division of Data, Analytics and Delivery for Impact WHO Methods and Data Sources for Global Burden of Disease Estimates 2000–2019; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- National Cancer Institute. Surveillance Research Program, Statistical Methodology and Applications Branch. In Joinpoint Regression Program, Version 5.4.0.0—April 2025; National Cancer Institute: Bethesda, MD, USA, 2025. [Google Scholar]
- Bleichrodt, H.; van Doorslaer, E. A Welfare Economics Foundation for Health Inequality Measurement. J. Health Econ. 2006, 25, 945–957. [Google Scholar] [CrossRef]
- Pan American Health Organization. Measuring Health Inequalities: Gini Coefficient and Concentration Index. Epidemiol. Bull. 2001, 22, 3–4. [Google Scholar]
- Cardoso, R.; Guo, F.; Heisser, T.; De Schutter, H.; Van Damme, N.; Nilbert, M.C.; Tybjerg, A.J.; Bouvier, A.-M.; Bouvier, V.; Launoy, G.; et al. Proportion and Stage Distribution of Screen-Detected and Non-Screen-Detected Colorectal Cancer in Nine European Countries: An International, Population-Based Study. Lancet Gastroenterol. Hepatol. 2022, 7, 711–723. [Google Scholar] [CrossRef]
- Draganic, D.; Wangen, K.R. Spatial Analysis of Colorectal Cancer Outcomes: Investigating the Impact of Place-Specific Factors Using Causal Inference Methods for Spatial Data. J. Public Health 2025, fdaf044. [Google Scholar] [CrossRef]
- Sharma, R.; Abbasi-Kangevari, M.; Abd-Rabu, R.; Abidi, H.; Abu-Gharbieh, E.; Acuna, J.M.; Adhikari, S.; Advani, S.M.; Afzal, M.S.; Meybodi, M.A.; et al. Global, Regional, and National Burden of Colorectal Cancer and Its Risk Factors, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol. Hepatol. 2022, 7, 627–647. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO Consensus Guidelines for the Management of Patients with Metastatic Colorectal Cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal Cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- OECD. EU Country Cancer Profile: Bulgaria 2023, EU Country Cancer Profiles; OECD Publishing: Paris, France, 2023. [Google Scholar]
- OECD. EU Country Cancer Profile: Romania 2023, EU Country Cancer Profiles; OECD Publishing: Paris, France, 2023. [Google Scholar]
- O’Gorman, C.; Stack, J.; O’Ceilleachair, A.; Denieffe, S.; Gooney, M.; McKnight, M.; Sharp, L. Colorectal Cancer Survivors: An Investigation of Symptom Burden and Influencing Factors. BMC Cancer 2018, 18, 1022. [Google Scholar] [CrossRef]
- Nikoletti, S.; Young, J.; Levitt, M.; King, M.; Chidlow, C.; Hollingsworth, S. Bowel Problems, Self-Care Practices, and Information Needs of Colorectal Cancer Survivors at 6 to 24 Months after Sphincter-Saving Surgery. Cancer Nurs. 2008, 31, 389–398. [Google Scholar] [CrossRef]
- Yabroff, K.R.; Lawrence, W.F.; Clauser, S.; Davis, W.W.; Brown, M.L. Burden of Illness in Cancer Survivors: Findings From a Population-Based National Sample. JNCI J. Natl. Cancer Inst. 2004, 96, 1322–1330. [Google Scholar] [CrossRef]
- Santin, O.; Murray, L.; Prue, G.; Gavin, A.; Gormley, G.; Donnelly, M. Self-Reported Psychosocial Needs and Health-Related Quality of Life of Colorectal Cancer Survivors. Eur. J. Oncol. Nurs. 2015, 19, 336–342. [Google Scholar] [CrossRef]
- Walling, A.M.; Weeks, J.C.; Kahn, K.L.; Tisnado, D.; Keating, N.L.; Dy, S.M.; Arora, N.K.; Mack, J.W.; Pantoja, P.M.; Malin, J.L. Symptom Prevalence in Lung and Colorectal Cancer Patients. J Pain Symptom Manag. 2015, 49, 192–202. [Google Scholar] [CrossRef]
- Wu, H.-S.; Harden, J.K. Symptom Burden and Quality of Life in Survivorship: A Review of the Literature. Cancer Nurs. 2015, 38, E29. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Hoffmann, R.G.; Saeian, K. Higher Physician Density Is Associated with Lower Incidence of Late-Stage Colorectal Cancer. J. Gen. Intern. Med. 2010, 25, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- van der Heide, I.; Uiters, E.; Jantine Schuit, A.; Rademakers, J.; Fransen, M. Health Literacy and Informed Decision Making Regarding Colorectal Cancer Screening: A Systematic Review. Eur. J. Public Health 2015, 25, 575–582. [Google Scholar] [CrossRef]
- Qin, P.-P.; Jin, J.-Y.; Min, S.; Wang, W.-J.; Shen, Y.-W. Association Between Health Literacy and Enhanced Recovery After Surgery Protocol Adherence and Postoperative Outcomes Among Patients Undergoing Colorectal Cancer Surgery: A Prospective Cohort Study. Anesth. Analg. 2022, 134, 330. [Google Scholar] [CrossRef]
- Husson, O.; Mols, F.; Fransen, M.P.; van de Poll-Franse, L.V.; Ezendam, N.P.M. Low Subjective Health Literacy Is Associated with Adverse Health Behaviors and Worse Health-Related Quality of Life among Colorectal Cancer Survivors: Results from the Profiles Registry. Psycho-Oncol. 2015, 24, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.; Sembajwe, G.; Kawachi, I.; Van Dam, R.M.; Subramanian, S.V.; Yamagata, Z. Income Inequality, Mortality, and Self Rated Health: Meta-Analysis of Multilevel Studies. BMJ 2009, 339, b4471. [Google Scholar] [CrossRef] [PubMed]
- Syriopoulou, E.; Osterman, E.; Miething, A.; Nordenvall, C.; Andersson, T.M.-L. Income Disparities in Loss in Life Expectancy after Colon and Rectal Cancers: A Swedish Register-Based Study. J. Epidemiol. Community Health 2024, 78, 402–408. [Google Scholar] [CrossRef]
- Shibata, T.; Shinjo, D.; Fushimi, K. The Impact of Deprivation on Colorectal Cancer-stage Distribution in a Setting with High Hospital Bed Density: A Japanese Multilevel Study. Cancer Med. 2024, 13, e70042. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, K.; Kim, B.C.; Jun, J.K.; Choi, K.S.; Suh, M. Effectiveness of the Korean National Cancer Screening Program in Reducing Colorectal Cancer Mortality. Cancers 2024, 16, 4278. [Google Scholar] [CrossRef]
- Zorzi, M.; Hassan, C.; Selby, K.; Rugge, M. Do Not Leave FIT Positives Alone! Off. J. Am. Coll. Gastroenterol. ACG 2018, 113, 913. [Google Scholar] [CrossRef]
- Tinmouth, J.; Lansdorp-Vogelaar, I.; Allison, J.E. Faecal Immunochemical Tests versus Guaiac Faecal Occult Blood Tests: What Clinicians and Colorectal Cancer Screening Programme Organisers Need to Know. Gut 2015, 64, 1327–1337. [Google Scholar] [CrossRef]

| Death | DALY | YLD | YLL | |||||
|---|---|---|---|---|---|---|---|---|
| β (95% CI) | p-Value | β (95% CI) | p-Value | β (95% CI) | p-Value | β (95% CI) | p-Value | |
| Healthcare indicators | ||||||||
| Current healthcare expenditure in share of GDP (%) | –0.004 (–0.012, 0.004) | 0.283 | –0.005 (–0.013, 0.003) | 0.195 | –0.007 (–0.016, 0.002) | 0.132 | –0.005 (–0.012, 0.003) | 0.224 |
| Practicing physicians (per 1000) | –0.036 (–0.066, –0.005) | 0.022 | –0.035 (–0.064, –0.006) | 0.019 | –0.060 (–0.094, –0.026) | <0.001 | –0.034 (–0.063, –0.005) | 0.022 |
| Hospital bed (per 1000) | –0.009 (–0.024, 0.005) | 0.193 | –0.002 (–0.016, 0.011) | 0.762 | –0.011 (–0.027, 0.005) | 0.186 | –0.001 (–0.015, 0.012) | 0.847 |
| Socioeconomic indicators | ||||||||
| Proportion of population with tertiary education (%) | –0.009 (–0.012, –0.006) | <0.001 | –0.009 (–0.012, –0.006) | <0.001 | –0.008 (–0.012, –0.005) | <0.001 | –0.009 (–0.012, –0.006) | <0.001 |
| Income inequality | 0.015 (0.001, 0.029) | 0.035 | 0.019 (0.006, 0.032) | 0.005 | 0.014 (–0.001, 0.030) | 0.068 | 0.019 (0.006, 0.033) | 0.004 |
| Unemployment rate (%) | –0.000 (–0.002, 0.002) | 0.920 | –0.000 (–0.002, 0.002) | 0.723 | 0.000 (–0.002, 0.003) | 0.775 | –0.000 (–0.002, 0.002) | 0.638 |
| Intercept | 12.430 (5.785, 19.076) | <0.001 | 17.175 (10.840, 23.509) | <0.001 | –10.866 (–18.317, –3.415) | 0.004 | 18.550 (12.190, 24.911) | <0.001 |
| Country intercept | 0.038 (0.022, 0.068) | <0.001 | 0.043 (0.024, 0.077) | <0.001 | 0.072 (0.040, 0.129) | <0.001 | 0.042 (0.023, 0.074) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovács, N.; Varga, O. Socioeconomic and Healthcare Indicators and Colorectal Cancer Burden: Analysis of Eurostat and Global Burden of Disease Study 2021 Data. Cancers 2025, 17, 2075. https://doi.org/10.3390/cancers17132075
Kovács N, Varga O. Socioeconomic and Healthcare Indicators and Colorectal Cancer Burden: Analysis of Eurostat and Global Burden of Disease Study 2021 Data. Cancers. 2025; 17(13):2075. https://doi.org/10.3390/cancers17132075
Chicago/Turabian StyleKovács, Nóra, and Orsolya Varga. 2025. "Socioeconomic and Healthcare Indicators and Colorectal Cancer Burden: Analysis of Eurostat and Global Burden of Disease Study 2021 Data" Cancers 17, no. 13: 2075. https://doi.org/10.3390/cancers17132075
APA StyleKovács, N., & Varga, O. (2025). Socioeconomic and Healthcare Indicators and Colorectal Cancer Burden: Analysis of Eurostat and Global Burden of Disease Study 2021 Data. Cancers, 17(13), 2075. https://doi.org/10.3390/cancers17132075





