Impact of Chemotherapy on Implant-Based Breast Reconstruction in Breast Cancer Patients: A Nationwide, Retrospective, Cohort Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Enrollment Criteria
2.2. Study Outcomes
2.3. Statistical Analysis
3. Results
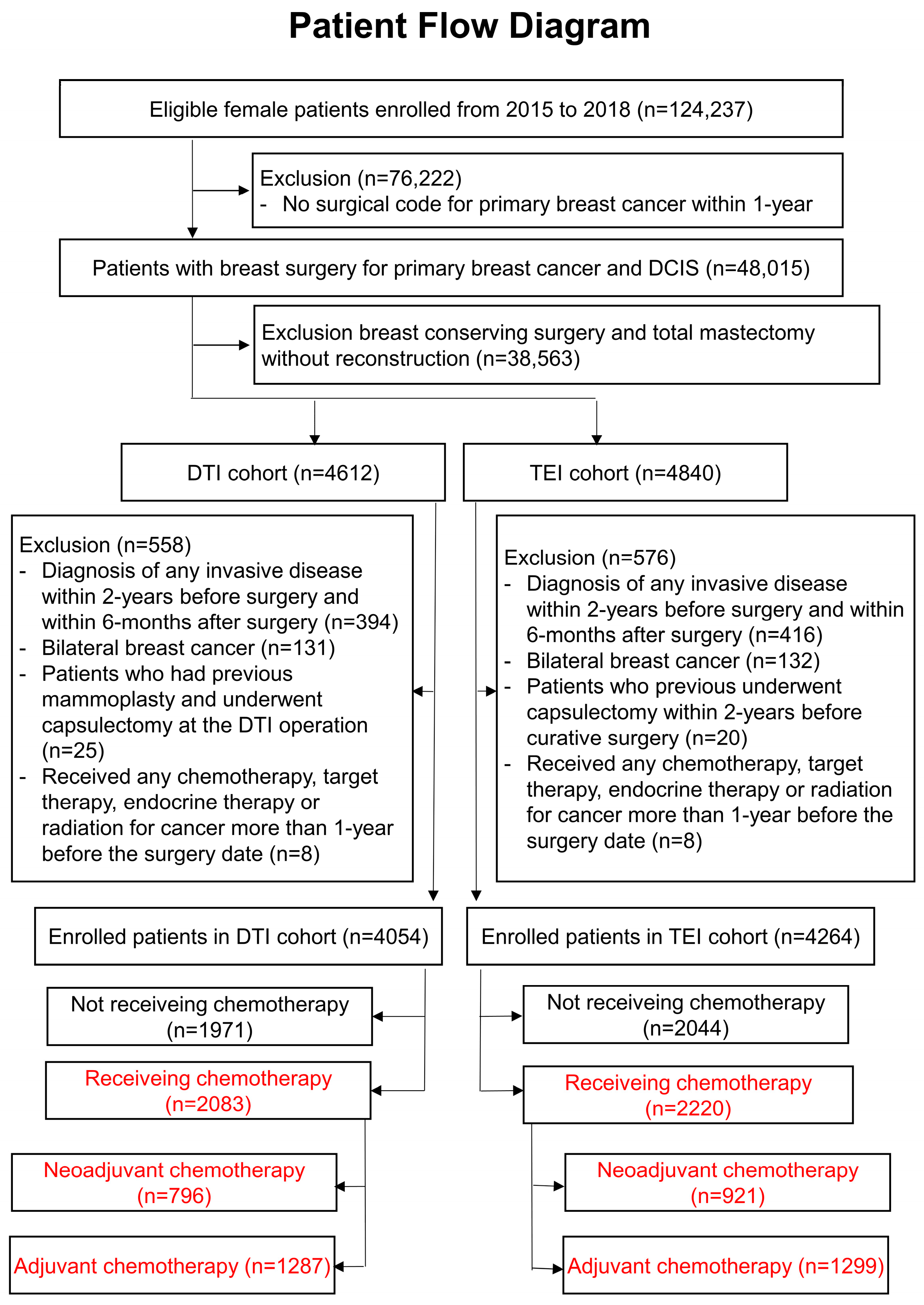
3.1. Patient Flow Diagram of the Cohort
3.2. Demographics and Incidence by Chemotherapy Type
3.2.1. DTI Cohort
3.2.2. TEI Cohort
3.3. Risk Factors for Capsular Contracture by Chemotherapy Type
3.3.1. DTI Cohort
3.3.2. TEI Cohort
3.4. Demographics and Incidence of Contracture Based on Chemotherapy Duration
3.5. Risk Factors for Capsular Contracture Based on Chemotherapy Duration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IBBR | Implant-based breast reconstruction |
| HIRA | Health Insurance Review and Assessment Service |
| IRB | Institutional Review Board |
| DCIS | Ductal carcinoma in situ |
| ICD-10 | International Classification of Disease, 10th revision |
| DTI | Direct-to-implant |
| TEI | Tissue-expander insertion |
| HER2 | Human epidermal growth factor receptor 2 |
| CCI | Charlson Comorbidity index |
| DM | Diabetes mellitus |
| ALND | Axillary lymph node dissection |
References
- Roy, N.; Downes, M.H.; Ibelli, T.; Amakiri, U.O.; Li, T.; Tebha, S.S.; Balija, T.M.; Schnur, J.B.; Montgomery, G.H.; Henderson, P.W. The psychological impacts of post-mastectomy breast reconstruction: A systematic review. Ann. Breast Surg. 2024, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Albornoz, C.R.; Bach, P.B.; Mehrara, B.J.; Disa, J.J.; Pusic, A.L.; McCarthy, C.M.; Cordeiro, P.G.; Matros, E. A paradigm shift in U.S. breast reconstruction: Increasing implant rates. Plast. Reconstr. Surg. 2013, 131, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Esparham, A.; Shoar, S.; Whittington, J.; Shafaee, Z. National trends and in-hospital outcomes for immediate implant-based versus autologous-based breast reconstruction: A propensity score-matched analysis. Ann. Surg. Oncol. 2025, 32, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Amro, C.; Sorenson, T.J.; Boyd, C.J.; Al-Hilli, Z.; Haddock, N.T.; Haddock, M.G.; Teotia, S.S.; Haddock, N.L.; Haddock, S.N.; Haddock, M.N. The Evolution of Implant-Based Breast Reconstruction: Innovations, Trends, and Future Directions. J. Clin. Med. 2024, 13, 7407. [Google Scholar] [CrossRef]
- Mauri, D.; Pavlidis, N.; Ioannidis, J.P. Neoadjuvant versus adjuvant systemic treatment in breast cancer: A meta-analysis. J. Natl. Cancer Inst. 2005, 97, 188–194. [Google Scholar] [CrossRef]
- Deptuła, M.; Zieliński, J.; Wardowska, A.; Pikuła, M. Wound healing complications in oncological patients: Perspectives for cellular therapy. Adv. Dermatol. Allergol. 2019, 36, 139–146. [Google Scholar] [CrossRef]
- Grant, D.S.; Williams, T.L.; Zahid, M.; Cerretti, D.P.; Broxmeyer, H.E.; Kleinman, H.K. Comparison of antiangiogenic activities using paclitaxel (Taxol) and docetaxel (Taxotere). Int. J. Cancer 2003, 104, 121–129. [Google Scholar] [CrossRef]
- Cappetta, D.; De Angelis, A.; Sapio, L.; Prezioso, L.; Illiano, M.; Quaini, F.; Rossi, F.; Berrino, L.; Naviglio, S.; Urbanek, K.; et al. Oxidative stress and cellular response to doxorubicin: A common factor in the complex milieu of anthracycline cardiotoxicity. Oxidative Med. Cell. Longev. 2017, 2, 1521020. [Google Scholar] [CrossRef]
- Hart, S.E.; Brown, D.L.; Kim, H.M.; Qi, J.; Hamill, J.B.; Wilkins, E.G. Association of Clinical Complications of Chemotherapy and Patient-Reported Outcomes After Immediate Breast Reconstruction. JAMA Surg. 2021, 156, 847–855. [Google Scholar] [CrossRef]
- Peled, A.W.; Itakura, K.; Foster, R.D.; Hamolsky, D.; Tanaka, J.; Ewing, C.; Alvarado, M.; Esserman, L.J.; Hwang, E.S. Impact of chemotherapy on postoperative complications after mastectomy and immediate breast reconstruction. Arch. Surg. 2010, 145, 880–885. [Google Scholar] [CrossRef]
- Dolen, U.C.; Schmidt, A.C.; Um, G.T.; Sharma, K.; Naughton, M.; Zoberi, I.; Margenthaler, J.M.; Myckatyn, T.M. Impact of Neoadjuvant and Adjuvant Chemotherapy on Immediate Tissue Expander Breast Reconstruction. Ann. Surg. Oncol. 2016, 23, 2357–2366. [Google Scholar] [CrossRef]
- El-Sabawi, B.; Sosin, M.; Carey, J.N.; Nahabedian, M.Y.; Patel, K.M. Breast reconstruction and adjuvant therapy: A systematic review of surgical outcomes. J. Surg. Oncol. 2015, 112, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Elixhauser, A.; Steiner, C.; Harris, D.R.; Coffey, R.M. Comorbidity measures for use with administrative data. Med. Care 1998, 36, 8–27. [Google Scholar] [CrossRef]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.R.; Kuo, W.L.; Yu, C.C.; Chen, S.C.; Huang, J.J. Reconstructive outcome analysis of the impact of neoadjuvant chemotherapy on immediate breast reconstruction: A retrospective cross-sectional study. BMC Cancer 2021, 21, 522. [Google Scholar] [CrossRef]
- Donker, M.; Hage, J.J.; Woerdeman, L.A.E.; Rutgers, E.J.T.; Sonke, G.S.; Vrancken Peeters, M.T.F.D. Surgical complications of skin sparing mastectomy and immediate prosthetic reconstruction after neoadjuvant chemotherapy for invasive breast cancer. Eur. J. Surg. Oncol. 2012, 38, 25–30. [Google Scholar] [CrossRef]
- Varghese, J.; Gohari, S.S.; Rizki, H.; Faheem, M.; Langridge, B.; Kümmel, S.; Johnson, L.; Schmid, P. A systematic review and meta-analysis on the effect of neoadjuvant chemotherapy on complications following immediate breast reconstruction. Breast 2021, 2, 55–62. [Google Scholar] [CrossRef]
- Mitchem, J.; Herrmann, D.; Margenthaler, J.A.; Aft, R.L. Impact of neoadjuvant chemotherapy on rate of tissue expander/implant loss and progression to successful breast reconstruction following mastectomy. Am. J. Surg. 2008, 196, 519–522. [Google Scholar] [CrossRef]
- Mehrara, B.J.; Santoro, T.D.; Arcilla, E.; Watson, J.P.; Shaw, W.W.; Da Lio, A.L. Complications after microvascular breast reconstruction: Experience with 1195 flaps. Plast. Reconstr. Surg. 2006, 118, 1100–1109. [Google Scholar] [CrossRef]
- Deutsch, M.F.; Smith, M.; Wang, B.; Ainsle, N.; Schusterman, M.A. Immediate breast reconstruction with the TRAM flap after neoadjuvant therapy. Ann. Plast. Surg. 1999, 42, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Chattha, A.S.; Cohen, J.B.; Bucknor, A.; Chen, A.D.; Tobias, A.M.; Lee, B.T.; Lin, S.J. Surgical site infection in immediate breast reconstruction: Does chemotherapy timing make a difference? J. Surg. Oncol. 2018, 117, 1440–1446. [Google Scholar] [CrossRef]
- Forouhi, P.; Dixon, J.M.; Leonard, R.C.; Chetty, U. Prospective randomized study of surgical morbidity following primary systemic therapy for breast cancer. Br. J. Surg. 1995, 82, 79–82. [Google Scholar] [CrossRef]
- Abt, N.B.; Flores, J.M.; Baltodano, P.A.; Sarhane, K.A.; Abreu, F.M.; Cooney, C.M.; Manahan, M.A.; Stearns, V.; Makary, M.A.; Rosson, G.D. Neoadjuvant chemotherapy and short-term morbidity in patients undergoing mastectomy with and without breast reconstruction. JAMA Surg. 2014, 149, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Donker, M.; van Tienhoven, G.; Straver, M.E.; Meijnen, P.; van de Velde, C.J.H.; Mansel, R.E.; Bogaerts, J.; Duez, N.; Cataliotti, L.; Westenberg, A.H. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981–22023 AMAROS): A randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014, 15, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Gardner, K.J.; Salas, E.; Sun, Q.; Yang, C.; Bunte, R.; Chen, Y.; Lee, M.J.; Dentchev, T.; Nguyen, T.; et al. Development and characterization of an in vitro model for radiation-induced fibrosis. Radiat. Res. 2018, 189, 326–336. [Google Scholar] [CrossRef]
- Straub, J.M.; New, J.; Hamilton, C.D.; Lominska, C.; Shnayder, Y.; Thomas, S.M.; Tarnawski, R.; Henke, L.E.; Robinson, R.A.; Kimple, R.J.; et al. Radiation-induced fibrosis: Mechanisms and implications for therapy. J. Cancer Res. Clin. Oncol. 2015, 141, 1985–1994. [Google Scholar] [CrossRef]
- Yoshida, S.; Koshima, I.; Hamada, Y.; Sasaki, A.; Fujioka, Y.; Nagamatsu, S.; Yokota, K.; Harima, M.; Yamashita, S. Lymphovenous anastomosis aids wound healing in lymphedema: Relationship between lymphedema and delayed wound healing from a view of immune mechanisms. Adv. Wound Care 2019, 8, 263–269. [Google Scholar] [CrossRef]
- Gousopoulos, E.; Proulx, S.T.; Scholl, J.; Uecker, M.; Detmar, M. Prominent lymphatic vessel hyperplasia with progressive dysfunction and distinct immune cell infiltration in lymphedema. Am. J. Pathol. 2016, 186, 2193–2203. [Google Scholar] [CrossRef]
- Owsley, J.Q., Jr.; Peterson, R.A. (Eds.) Augmentation mammaplasty. In Symposium on Aesthetic Surgery of the Breast; Mosby: Maryland Heights, MO, USA, 1978; pp. 256–263. [Google Scholar]
| Before Matching | After Matching | |||||
|---|---|---|---|---|---|---|
| Patients Receiving Neoadjuvant Chemotherapy, n = 796 (%) | Patients Receiving Adjuvant Chemotherapy, n = 1287 (%) | p Value | Patients Receiving Neoadjuvant Chemotherapy, n = 718 (%) | Patients Receiving Adjuvant Chemotherapy, n = 718 (%) | p Value | |
| Capsulectomy only | 0.088 | 0.192 | ||||
| Not performed | 706 (88.7) | 1171 (91.0) | 637 (88.7) | 652 (90.8) | ||
| Performed | 90 (11.3) | 116 (9.0) | 81 (11.3) | 66 (9.2) | ||
| Age (year) | <0.001 | 0.976 | ||||
| 20–29 | 22 (2.8) | 18 (1.4) | 12 (1.7) | 13 (1.8) | ||
| 30–39 | 184 (23.1) | 217 (16.9) | 148 (20.6) | 140 (19.5) | ||
| 40–49 | 331 (41.6) | 591 (45.9) | 315 (43.9) | 316 (44.0) | ||
| 50–59 | 216 (27.1) | 358 (27.8) | 200 (27.9) | 209 (29.1) | ||
| 60–69 | 40 (5.0) | 96 (7.5) | 40 (5.6) | 36 (5.0) | ||
| 70–79 | 3 (0.4) | 7 (0.5) | 3 (0.4) | 4 (0.6) | ||
| CCI (Weight number, mean ± SD) | 4.14 ± 2.61 | 3.67 ± 2.27 | <0.001 | 3.90 ± 2.44 | 3.85 ± 2.46 | 0.683 |
| Endocrine therapy | 0.005 | 0.465 | ||||
| Not performed | 226 (28.4) | 294 (22.8) | 186 (25.9) | 172 (24.2) | ||
| Performed | 570 (71.6) | 993 (77.2) | 532 (74.1) | 544 (75.8) | ||
| HER2-target therapy | 0.570 | 0.689 | ||||
| Not performed | 541 (68.0) | 890 (69.2) | 493 (68.7) | 500 (69.6) | ||
| Performed | 255 (32.0) | 397 (30.8) | 225 (31.3) | 218 (30.4) | ||
| Radiotherapy | <0.001 | 0.914 | ||||
| Not performed | 429 (53.9) | 902 (70.1) | 428 (59.6) | 426 (59.3) | ||
| Performed | 367 (46.1) | 385 (29.9) | 290 (40.4) | 292 (40.7) | ||
| Lymphedema | 0.005 | 0.866 | ||||
| No | 694 (87.2) | 1172 (91.1) | 638 (88.9) | 640 (89.1) | ||
| Yes | 102 (12.8) | 115 (8.9) | 80 (11.1) | 78 (10.9) | ||
| Axillary surgery | <0.001 | 0.196 | ||||
| SLNB only | 261 (32.8) | 549 (42.7) | 246 (34.3) | 223 (31.1) | ||
| ALND | 535 (67.2) | 738 (57.3) | 472 (65.7) | 495 (68.9) | ||
| Diabetes | 0.556 | 0.423 | ||||
| No | 748 (94.0) | 1201 (93.3) | 674 (93.9) | 861 (94.9) | ||
| Yes | 48 (6.0) | 86 (6.7) | 44 (6.1) | 37 (5.1) | ||
| Dyslipidemia | 0.936 | 0.418 | ||||
| No | 595 (74.8) | 960 (64.6) | 543 (75.6) | 556 (77.4) | ||
| Yes | 201 (25.2) | 327 (25.4) | 175 (24.4) | 162 (22.6) | ||
| Autoimmune disease * | 0.856 | 0.739 | ||||
| No | 698 (87.7) | 1132 (88.0) | 635 (88.4) | 639 (89.0) | ||
| Yes | 98 (12.3) | 155 (12.0) | 83 (11.6) | 79 (11.0) | ||
| Rheumatoid arthritis | 0.537 | 0.889 | ||||
| No | 762 (95.7) | 1239 (96.3) | 691 (96.2) | 692 (96.4) | ||
| Yes | 34 (4.3) | 48 (3.7) | 27 (3.8) | 26 (3.6) | ||
| Lupus erythematous | 0.639 | 0.500 | ||||
| No | 794 (99.8) | 1285 (99.8) | 716 (99.7) | 718 (100.0) | ||
| Yes | 2 (0.2) | 2 (0.2) | 2 (0.3) | 0 (0.0) | ||
| Systemic sclerosis | - | - | ||||
| No | 796 (100.0) | 1287 (100.0) | 718 (100.0) | 718 (0.0) | ||
| Yes | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Sicca syndrome | >0.999 | >0.999 | ||||
| No | 796 (100.0) | 1286 (99.9) | 718 (100.0) | 717 (99.9) | ||
| Yes | 0 (0.0) | 1 (0.1) | 0 (0.0) | 1 (0.1) | ||
| Psoriasis | 0.724 | 0.488 | ||||
| No | 784 (98.5) | 1270 (98.7) | 707 (98.5) | 710 (98.9) | ||
| Yes | 12 (1.5) | 17 (1.3) | 11 (1.5) | 8 (1.1) | ||
| Behcet’s disease | >0.999 | >0.999 | ||||
| No | 796 (100.0) | 1286 (99.9) | 718 (100.0) | 717 (99.9) | ||
| Yes | 0 (0.0) | 1 (0.1) | 0 (0.0) | 1 (0.1) | ||
| Autoimmune hepatitis | - | - | ||||
| No | 796 (100.0) | 1287 (100.0) | 718 (100.0) | 718 (0.0) | ||
| Yes | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Autoimmune thyroiditis | 0.506 | 0.807 | ||||
| No | 787 (98.9) | 1268 (98.5) | 709 (98.8) | 710 (98.9) | ||
| Yes | 9 (1.1) | 19 (1.5) | 9 (1.2) | 8 (1.1) | ||
| Autoimmune adrenalitis | - | - | ||||
| No | 796 (100.0) | 1287 (100.0) | 718 (100.0) | 718 (0.0) | ||
| Yes | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Systemic connective tissue disorder | 0.382 | >0.999 | ||||
| No | 795 (99.9) | 1287 (100.0) | 717 (99.9) | 718 (100.0) | ||
| Yes | 1 (0.1) | 0 (0.0) | 1 (0.1) | 0 (0.0) | ||
| Atopic dermatitis | 0.263 | 0.280 | ||||
| No | 757 (95.1) | 1209 (93.9) | 686 (95.5) | 677 (94.3) | ||
| Yes | 39 (4.9) | 78 (6.1) | 32 (4.5) | 41 (5.7) | ||
| Vitiligo | >0.999 | 0.625 | ||||
| No | 793 (99.6) | 1283 (99.7) | 715 (99.6) | 717 (99.9) | ||
| Yes | 3 (0.4) | 4 (0.3) | 3 (0.4) | 1 (0.1) | ||
| Steroid medication | 0.185 | 0.519 | ||||
| No | 757 (95.1) | 1206 (93.7) | 684 (95.3) | 689 (96.0) | ||
| Yes | 39 (4.9) | 81 (6.3) | 34 (4.7) | 29 (4.0) | ||
| Before Matching | After Matching | |||||
|---|---|---|---|---|---|---|
| Patients Receiving Neoadjuvant Chemotherapy, n = 921 (%) | Patients Receiving Adjuvant Chemotherapy, n = 1299 (%) | p Value | Patients Receiving Neoadjuvant Chemotherapy, n = 767 (%) | Patients Receiving Adjuvant Chemotherapy, n = 767 (%) | p Value | |
| Capsulectomy only | 0.088 | 0.441 | ||||
| Not performed | 817 (88.7) | 1181 (90.9) | 687 (89.6) | 696 (90.7) | ||
| Performed | 104 (11.3) | 118 (9.1) | 80 (10.4) | 71 (9.3) | ||
| Both capsulectomy and implant change | <0.001 | - | ||||
| Not performed | 902 (97.9) | 1299 (100) | 767 (100) | 767 (100) | ||
| Performed | 19 (2.1) | 0 | 0 | 0 | ||
| Age (year) | <0.001 | 0.945 | ||||
| 20–29 | 31 (3.4) | 18 (1.4) | 11 (1.4) | 15 (2.0) | ||
| 30–39 | 229 (24.9) | 220 (16.9) | 155 (20.2) | 158 (20.6) | ||
| 40–49 | 383 (41.6) | 596 (45.9) | 345 (45.4) | 351 (45.8) | ||
| 50–59 | 233 (25.3) | 362 (27.9) | 209 (27.3) | 198 (25.8) | ||
| 60–69 | 42 (4.6) | 96 (7.4) | 41 (5.4) | 43 (5.6) | ||
| 70–79 | 3 (0.3) | 7 (0.5) | 3 (0.4) | 2 (0.3) | ||
| CCI (Weight number, mean ± SD) | 4.29 ± 2.73 | 3.66 ± 2.27 | <0.001 | 3.98 ± 2.51 | 3.92 ± 2.470 | 0.644 |
| Endocrine therapy | 0.001 | 0.408 | ||||
| Not performed | 268 (29.1) | 300 (23.1) | 197 (25.7) | 183 (23.9) | ||
| Performed | 653 (70.9) | 999 (76.9) | 570 (74.3) | 584 (76.1) | ||
| HER2-target therapy | 0.479 | 0.779 | ||||
| Not performed | 651 (70.7) | 900 (69.3) | 544 (70.9) | 539 (70.3) | ||
| Performed | 270 (29.3) | 399 (30.7) | 223 (29.1) | 228 (29.7) | ||
| Radiotherapy | <0.001 | 0.958 | ||||
| Not performed | 542 (58.9) | 912 (70.2) | 493 (64.3) | 492 (64.2) | ||
| Performed | 379 (41.1) | 387 (29.8) | 274 (35.7) | 275 (35.9) | ||
| Lymphedema | 0.046 | 0.799 | ||||
| No | 815 (88.5) | 1183 (91.1) | 691 (90.1) | 688 (89.7) | ||
| Yes | 106 (11.5) | 116 (8.9) | 76 (9.9) | 79 (10.3) | ||
| Axillary surgery | 0.393 | 0.172 | ||||
| SLNB only | 381 (41.4) | 561 (43.2) | 308 (40.2) | 282 (36.8) | ||
| ALND | 540 (58.6) | 738 (56.8) | 459 (59.8) | 485 (63.2) | ||
| Diabetes | 0.707 | 0.172 | ||||
| No | 863 (93.7) | 1212 (93.3) | 718 (93.6) | 722 (94.1) | ||
| Yes | 58 (6.3) | 87 (6.7) | 49 (6.4) | 45 (5.9) | ||
| Dyslipidemia | 0.829 | 0.906 | ||||
| No | 684 (74.3) | 970 (74.7) | 577 (75.2) | 579 (75.5) | ||
| Yes | 237 (25.7) | 329 (25.3) | 190 (24.8) | 188 (24.5) | ||
| Autoimmune disease * | 0.202 | 0.580 | ||||
| No | 792 (86.0) | 1141 (87.8) | 680 (88.7) | 673 (87.7) | ||
| Yes | 129 (14.0) | 158 (12.2) | 87 (11.3) | 94 (12.3) | ||
| Rheumatoid arthritis | 0.499 | 0.444 | ||||
| No | 881 (95.7) | 1250 (96.2) | 738 (96.2) | 732 (95.4) | ||
| Yes | 40 (4.3) | 49 (3.8) | 29 (3.8) | 35 (4.6) | ||
| Lupus erythematous | 0.24 | - | ||||
| No | 917 (99.6) | 1297 (99.9) | 764 (99.6) | 767 (100.0) | ||
| Yes | 4 (0.4) | 2 (0.1) | 3 (0.4) | 0 (0.0) | ||
| Systemic sclerosis | - | - | ||||
| No | 921 (100.0) | 1299 (100.0) | 767 (100.0) | 767 (100.0) | ||
| Yes | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Sicca syndrome | >0.999 | |||||
| No | 921 (100.0) | 1298 (99.9) | 767 (100.0) | 767 (100.0) | ||
| Yes | 0 (0.0) | 1 (0.1) | 0 (0.0) | 0 (0.0) | ||
| Psoriasis | 0.676 | 0.132 | ||||
| No | 907 (98.5) | 1282 (96.7) | 756 (98.6) | 762 (99.4) | ||
| Yes | 14 (1.5) | 17 (1.3) | 11 (1.4) | 5 (0.6) | ||
| Behcet’s disease | >0.999 | >0.999 | ||||
| No | 921 (100.0) | 1298 (99.9) | 767 (100.0) | 766 (99.9) | ||
| Yes | 0 (0.0) | 1 (0.1) | 0 (0.0) | 1 (0.1) | ||
| Autoimmune hepatitis | - | |||||
| No | 921 (100.0) | 1299 (100.0) | 767 (100.0) | 767 (100.0) | ||
| Yes | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Autoimmune thyroiditis | 0.698 | 0.668 | ||||
| No | 908 (98.6) | 1278 (98.4) | 757 (98.7) | 755 (98.4) | ||
| Yes | 13 (1.4) | 21 (1.6) | 10 (1.3) | 12 (1.6) | ||
| Autoimmune adrenalitis | - | - | ||||
| No | 921 (100.0) | 1299 (100.0) | 767 (100.0) | 767 (100.0) | ||
| Yes | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Systemic connective tissue disorder | 0.415 | >0.999 | ||||
| No | 920 (99.9) | 1299 (100.0) | 766 (99.9) | 767 (100.0) | ||
| Yes | 1 (0.1) | 0 (0.0) | 1 (0.1) | 0 (0.0) | ||
| Atopic dermatitis | 0.777 | 0.108 | ||||
| No | 863 (93.7) | 1221 (94.0) | 734 (95.7) | 720 (93.9) | ||
| Yes | 58 (6.3) | 78 (6.0) | 33 (4.3) | 47 (6.1) | ||
| Vitiligo | >0.999 | 0.250 | ||||
| No | 918 (99.7) | 1295 (99.7) | 764 (99.6) | 767 (100.0) | ||
| Yes | 3 (0.3) | 4 (0.3) | 3 (0.4) | 0 (0.0) | ||
| Steroid medication | 0.059 | 0.580 | ||||
| No | 880 (95.6) | 1217 (93.7) | 729 (95.1) | 734 (95.7) | ||
| Yes | 41 (4.4) | 82 (6.3) | 38 (4.9) | 33 (4.3) | ||
| Before Matching | After Matching | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis Model 1 * | Multivariate Analysis Model 2 ** | Univariate Analysis | Multivariate Analysis Model 1 * | Multivariate Analysis Model 2 ** | |||||||
| HR (95% CIs) | p Value | HR (95% CIs) | p Value | HR (95% CIs) | p Value | HR (95% CIs) | p Value | HR (95% CIs) | p Value | HR (95% CIs) | p Value | |
| Age (per 10 years) | 1.239 (1.067–1.440) | 0.005 | 1.253 (1.068–1.469) | 0.006 | 1.254 (1.070–1.470) | 0.005 | 1.146 (0.955–1.374) | 0.143 | 1.157 (0.952–1.406) | 0.142 | 1.163 (0.958–1.412) | 0.127 |
| CCI (Weight number) | 1.033 (0.979–1.090) | 0.004 | 1.004 (0.947–1.065) | 0.886 | 1.007 (0.949–1.067) | 0.824 | 0.999 (0.934–1.067) | 0.967 | 0.973 (0.906–1.046) | 0.460 | 0.976 (0.909–1.048) | 0.511 |
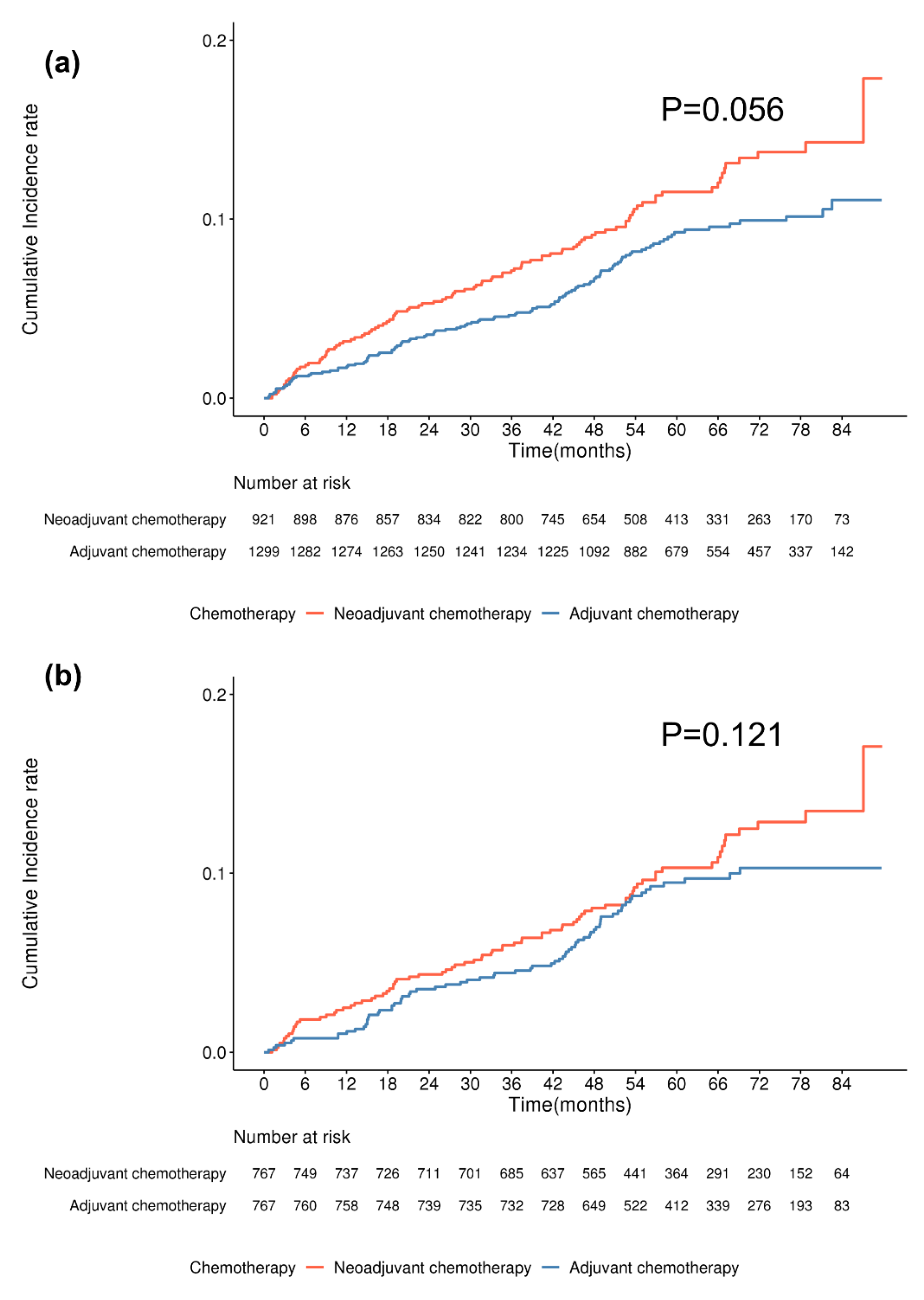
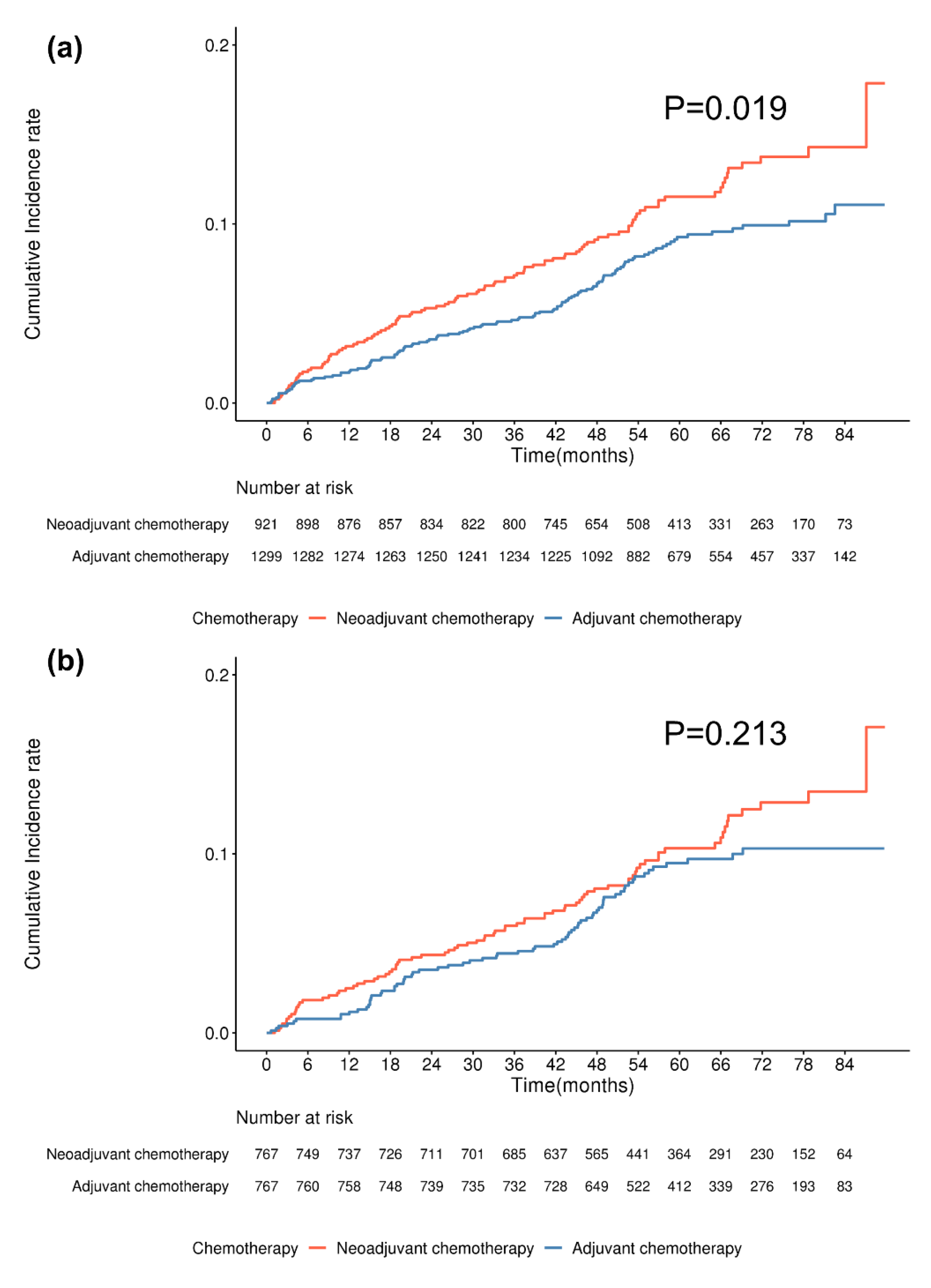
| Chemotherapy type | 0.056 | 0.071 | 0.068 | 0.122 | 0.133 | 0.118 | ||||||
| Neoadjuvant | reference | reference | Reference | reference | reference | reference | ||||||
| Adjuvant | 0.765 (0.581–1.007) | 0.768 (0.577–1.023) | 0.766 (0.575–1.020) | 0.774 (0.559–1.071) | 0.778 (0.561–1.079) | 0.771 (0.556–1.068) | ||||||
| Endocrine therapy | 0.813 | 0.679 | 0.717 | 0.854 | 0.961 | 0.994 | ||||||
| Not performed | reference | reference | reference | reference | reference | reference | ||||||
| Performed | 0.963 (0.703–1.318) | 1.072 (0.772–1.489) | 1.063 (0.765–1.476) | 0.966 (0.665–1.402) | 1.010 (0.682–1.495) | 0.998 (0.675–1.477) | ||||||
| HER2-target therapy | 0.259 | 0.390 | 0.381 | 0.854 | 0.771 | 0.830 | ||||||
| Not performed | reference | reference | reference | reference | reference | reference | ||||||
| Performed | 1.180 (0.885–1.573) | 1.139 (0.846–1.533) | 1.141 (0.849–1.534) | 1.033 (0.729–1.464) | 1.055 (0.734–1.516) | 1.041 (0.725–1.494) | ||||||
| Radiotherapy | 0.004 | 0.006 | 0.006 | 0.011 | 0.008 | 0.008 | ||||||
| Not performed | reference | reference | reference | reference | reference | reference | ||||||
| Performed | 1.504 (1.143–1.978) | 1.505 (1.128–2.008) | 1.505 (1.128–2.008) | 1.526 (1.104–2.108) | 1.569 (1.123–2.191) | 1.566 (1.122–2.188) | ||||||
| Lymphedema | <0.001 | 0.003 | 0.002 | 0.005 | 0.007 | 0.006 | ||||||
| No | reference | reference | reference | reference | reference | reference | ||||||
| Yes | 1.907 (1.336–2.722) | 1.778 (1.221–2.588) | 1.800 (1.238–2.617) | 1.812 (1.194–2.752) | 1.840 (1.186–2.855) | 1.842 (1.189–2.855) | ||||||
| Axillary surgery | 0.141 | 0.016 | 0.016 | 0.051 | 0.007 | 0.008 | ||||||
| SLNB | reference | reference | reference | reference | reference | reference | ||||||
| ALND | 0.809 (0.610–1.073) | 0.693 (0.515–0.933) | 0.695 (0.517–0.935) | 0.713 (0.507–1.002) | 0.615 (0.431–0.876) | 0.622 (0.437–0.885) | ||||||
| Diabetes | 0.192 | 0.546 | 0.558 | 0.291 | 0.280 | 0.302 | ||||||
| No | reference | reference | reference | reference | reference | reference | ||||||
| Yes | 1.392 (0.847–2.286) | 1.181 (0.689–2.023) | 1.174 (0.686–2.012) | 1.392 (0.753–2.574) | 1.454 (0.737–2.870) | 1.431 (0.725–2.823) | ||||||
| Dyslipidemia | 0.397 | 0.821 | 0.822 | 0.910 | 0.441 | 0.434 | ||||||
| No | reference | reference | reference | reference | reference | reference | ||||||
| Yes | 1.142 (0.840–1.552) | 0.963 (0.692–1.338) | 0.963 (0.693–1.338) | 0.978 (0.664–1.441) | 0.849 (0.559–1.288) | 0.847 (0.558–1.285) | ||||||
| Autoimmune disease | 0.345 | 0.619 | 0.740 | 0.914 | ||||||||
| No | reference | reference | reference | reference | ||||||||
| Yes | 1.212 (0.813–1.806) | 1.109 (0.738–1.666) | 1.090 (0.657–1.807) | 1.029 (0.614–1.723) | ||||||||
| Rheumatoid arthritis | 0.212 | 0.482 | 0.722 | 0.910 | ||||||||
| No | reference | reference | reference | reference | ||||||||
| Yes | 1.472 (0.802–2.703) | 1.252 (0.669–2.345) | 1.160 (0.512–2.627) | 0.949 (0.382–2.354) | ||||||||
| Lupus erythematous | 0.384 | 0.501 | 0.132 | 0.116 | ||||||||
| No | reference | reference | reference | reference | ||||||||
| Yes | 2.394 (0.335–17.087) | 1.994 (0.267–14.908) | 4.550 (0.635–32.607) | 5.836 (0.648–52.568) | ||||||||
| Systemic connective tissue disorder | 0.262 | 0.278 | ||||||||||
| No | reference | reference | ||||||||||
| Yes | 4.918 (0.304–79.530) | 4.672 (0.288–75.884) | ||||||||||
| Sicca syndrome | 0.308 | 0.327 | ||||||||||
| No | reference | reference | ||||||||||
| Yes | 4.248 (0.263–68.651) | 4.027 (0.248–65.384) | ||||||||||
| Psoriasis | 0.290 | 0.315 | 0.512 | 0.615 | ||||||||
| No | reference | reference | reference | reference | ||||||||
| Yes | 0.346 (0.049–2.464) | 0.365 (0.051–2.605) | 0.518 (0.072–3.700) | 0.603 (0.084–4.334) | ||||||||
| Behcet’s disease | 0.308 | 0.327 | ||||||||||
| No | reference | reference | ||||||||||
| Yes | 4.248 (0.263–68.651) | 4.027 (0.248–65.384) | ||||||||||
| Autoimmune thyroiditis | 0.735 | 0.537 | 0.635 | 0.596 | ||||||||
| No | reference | reference | reference | reference | ||||||||
| Yes | 0.786 (0.195–3.164) | 0.642 (0.158–2.616) | 0.621 (0.087–4.440) | 0.586 (0.081–4.224) | ||||||||
| Atopic dermatitis | 0.186 | 0.291 | 0.435 | 0.582 | ||||||||
| No | reference | reference | reference | reference | ||||||||
| Yes | 1.426 (0.843–2.413) | 1.332 (0.782–2.266) | 1.309 (0.667–2.569) | 1.211 (0.612–2.398) | ||||||||
| Vitiligo | 0.860 | 0.827 | ||||||||||
| No | reference | reference | ||||||||||
| Yes | 0.778 (0.048–12.573) | 1.365 (0.084–22.126) | ||||||||||
| Steroid medication | 0.536 | 0.603 | 0.545 | 0.874 | 0.943 | 0.913 | ||||||
| No | reference | reference | reference | reference | reference | reference | ||||||
| Yes | 1.187 (0.690–2.042) | 1.157 (0.668–2.004) | 1.184 (0.685–2.047) | 1.063 (0.498–2.272) | 0.972 (0.452–2.091) | 1.958 (0.446–2.060) | ||||||
| Before Matching | After Matching | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis Model 1 * | Multivariate Analysis Model 2 ** | Univariate Analysis | Multivariate Analysis Model 1 * | Multivariate Analysis Model 2 ** | |||||||
| HR (95% CIs) | p Value | HR (95% CIs) | p Value | HR (95% CIs) | p Value | HR (95% CIs) | p Value | HR (95% CIs) | p Value | HR (95% CIs) | p Value | |
| Age (per 10 years) | 1.219 (1.055–1.407) | 0.007 | 1.242 (1.066–1.447) | 0.006 | 1.242 (1.066–1.446) | 0.005 | 1.179 (0.985–1.412) | 0.073 | 1.190 (0.977–1.449) | 0.084 | 1.193 (0.980–1.453) | 0.079 |
| CCI (Weight number) | 1.041 (0.990–1.095) | 0.118 | 1.012 (0.958–1.069) | 0.675 | 1.014 (0.960–1.071) | 0.623 | 1.007 (0.945–1.074) | 0.825 | 0.982 (0.916–1.052) | 0.598 | 0.984 (0.918–1.054) | 0.639 |
| Chemotherapy type | 0.020 | 0.02 | 0.025 | 0.213 | 0.214 | 0.212 | ||||||
| Neoadjuvant | reference | reference | reference | reference | reference | reference | ||||||
| Adjuvant | 0.730 (0561–0.951) | 0.728 (0.557–0.951) | 0.731 (0.556–0.961) | 0.816 (0.593–1.124) | 0.816 (0.592–1.124) | 0.815 (0.592–1.123) | ||||||
| Endocrine therapy | 0.760 | 0.742 | 0.785 | 0.498 | 0.365 | 0.366 | ||||||
| Not performed | reference | reference | reference | reference | reference | reference | ||||||
| Performed | 0.954 (0.706–1.290) | 1.054 (0.770–1.442) | 1.044 (0.764–1.429) | 1.143 (0.777–1.681) | 1.206 (0.805–1.806) | 1.205 (0.804–1.805) | ||||||
| HER2-target therapy | 0.496 | 0.639 | 0.624 | 0.485 | 0.558 | 0.566 | ||||||
| Not done | reference | reference | reference | reference | reference | reference | ||||||
| Done | 1.103 (0.832–1.460) | 1.072 (0.802–1.432) | 1.075 (0.805–1.435) | 0.880 (0.615–1.260) | 0.895 (0.618–1.296) | 0.898 (0.620–1.298) | ||||||
| Radiotherapy | 0.003 | 0.005 | 0.004 | 0.028 | 0.012 | 0.013 | ||||||
| Not performed | reference | reference | reference | reference | reference | reference | ||||||
| Performed | 1.491 (1.144–1.943) | 1.497 (1.134–1.978) | 1.498 (1.134–1.979) | 1.432 (1.039–1.974) | 1.531 (1.098–2.134) | 1.527 (1.095–2.129) | ||||||
| Lymphedema | <0.001 | 0.002 | 0.002 | 0.026 | 0.023 | 0.021 | ||||||
| No | reference | reference | reference | reference | reference | reference | ||||||
| Yes | 1.887 (1.336–2.667) | 1.775 (1.233–2.557) | 1.801 (1.252–2.590) | 1.639 (1.060–2.536) | 1.703 (1.078–2.691) | 1.710 (1.083–2.701) | ||||||
| Axillary surgery | 0.046 | 0.002 | 0.002 | 0.003 | <0.001 | <0.001 | ||||||
| SLNB | reference | reference | reference | reference | reference | reference | ||||||
| ALND | 0.761 (0.581–0.996) | 0.643 (0.485–0.853) | 0.645 (0.487–0.855) | 0.604 (0.436–0.837) | 0.523 (0.373–0.734) | 0.516 (0.368–0.725) | ||||||
| Diabetes | 0.123 | 0.454 | 0.461 | 0.065 | 0.104 | 0.100 | ||||||
| No | reference | reference | reference | reference | reference | reference | ||||||
| Yes | 1.447 (0.904–2.316) | 1.215 (0.730–2.023) | 1.212 (0.728–2.018) | 1.678 (0.968–2.908) | 1.660 (0.900–3.062) | 1.672 (0.907–3.083) | ||||||
| Dyslipidemia | 0.405 | 0.785 | 0.792 | 0.837 | 0.525 | 0.555 | ||||||
| No | reference | reference | reference | reference | reference | reference | ||||||
| Yes | 1.134 (0.843–1.525) | 0.957 (0.696–1.315) | 0.958 (0.697–1.317) | 1.040 (0.717–1.507) | 0.877 (0.585–1.315) | 0.885 (0.590–1.327) | ||||||
| Autoimmune disease | 0.343 | 0.657 | 0.354 | 0.227 | ||||||||
| No | reference | reference | reference | reference | ||||||||
| Yes | 1.202 (0.822–1.757) | 1.092 (0.741–1.610) | 0.764 (0.433–1.350) | 0.699 (0.391–1.249) | ||||||||
| Rheumatoid arthritis | 0.201 | 0.503 | 0.970 | 0.792 | ||||||||
| No | reference | reference | reference | reference | ||||||||
| Yes | 1.462 (0.817–2.617) | 1.228 (0.673–2.240) | 0.984 (0.435–2.228) | 0.894 (0.390–2.052) | ||||||||
| Lupus erythematous | 0.483 | 0.610 | 0.513 | |||||||||
| No | reference | reference | reference | |||||||||
| Yes | 2.021 (0.284–14.405) | 1.686 (0.227–12.535) | 2.547 (0.155–41.858) | |||||||||
| Systemic connective tissue disorder | 0.275 | |||||||||||
| No | reference | |||||||||||
| Yes | 4.717 (0.292–76.216) | |||||||||||
| Sicca syndrome | 0.320 | |||||||||||
| No | reference | |||||||||||
| Yes | 4.103 (0.254–66.270) | |||||||||||
| Psoriasis | 0.251 | 0.272 | 0.411 | |||||||||
| No | reference | reference | reference | |||||||||
| Yes | 0.317 (0.045–2.255) | 0.333 (0.047–2.371) | 0.312 (0.019–5.030) | |||||||||
| Behcet’s disease | 0.320 | |||||||||||
| No | reference | |||||||||||
| Yes | 4.103 (0.254–66.270) | |||||||||||
| Autoimmune thyroiditis | 0.582 | 0.421 | 0.354 | |||||||||
| No | reference | reference | reference | |||||||||
| Yes | 0.677 (0.168–2.720) | 0.563 (0.139–2.284) | 0.268 (0.017–4.347) | |||||||||
| Atopic dermatitis | 0.149 | 0.241 | 0.970 | 0.831 | ||||||||
| No | reference | reference | reference | reference | ||||||||
| Yes | 1.440 (0.878–2.363) | 1.349 (0.818–2.224) | 0.985 (0.461–2.105) | 0.920 (0.427–1.981) | ||||||||
| Vitiligo | 0.837 | 0.680 | ||||||||||
| No | reference | reference | ||||||||||
| Yes | 0.746 (0.046–12.041) | 1.798 (0.111–29.162) | ||||||||||
| Steroid medication | 0.666 | 0.703 | 0.659 | 0.793 | 0.955 | 0.925 | ||||||
| No | reference | reference | reference | reference | reference | reference | ||||||
| Yes | 1.127 (0.656–1.936) | 1.112 (0.644–1.921) | 1.131 (0.655–1.951) | 1.100 (0.540–2.242) | 1.021 (0.497–2.098) | 1.035 (0.504–2.126) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.A.; Lee, H.S.; Jeon, S.; Kim, D.; Lee, Y.J.; Bae, S.Y.; Park, W.-C.; Yoon, C.I.; Choi, J. Impact of Chemotherapy on Implant-Based Breast Reconstruction in Breast Cancer Patients: A Nationwide, Retrospective, Cohort Study. Cancers 2025, 17, 2053. https://doi.org/10.3390/cancers17122053
Lee JA, Lee HS, Jeon S, Kim D, Lee YJ, Bae SY, Park W-C, Yoon CI, Choi J. Impact of Chemotherapy on Implant-Based Breast Reconstruction in Breast Cancer Patients: A Nationwide, Retrospective, Cohort Study. Cancers. 2025; 17(12):2053. https://doi.org/10.3390/cancers17122053
Chicago/Turabian StyleLee, Jin Ah, Hye Sun Lee, Soyoung Jeon, Dooreh Kim, Young Joo Lee, Soo Youn Bae, Woo-Chan Park, Chang Ik Yoon, and Jangyoun Choi. 2025. "Impact of Chemotherapy on Implant-Based Breast Reconstruction in Breast Cancer Patients: A Nationwide, Retrospective, Cohort Study" Cancers 17, no. 12: 2053. https://doi.org/10.3390/cancers17122053
APA StyleLee, J. A., Lee, H. S., Jeon, S., Kim, D., Lee, Y. J., Bae, S. Y., Park, W.-C., Yoon, C. I., & Choi, J. (2025). Impact of Chemotherapy on Implant-Based Breast Reconstruction in Breast Cancer Patients: A Nationwide, Retrospective, Cohort Study. Cancers, 17(12), 2053. https://doi.org/10.3390/cancers17122053






