Adjuvant Chemoradiotherapy or Radiotherapy Alone for Early Squamous Cervical Cancer with a Single Surgical-Pathological High-Risk Factor
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. Outcomes and Definitions
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
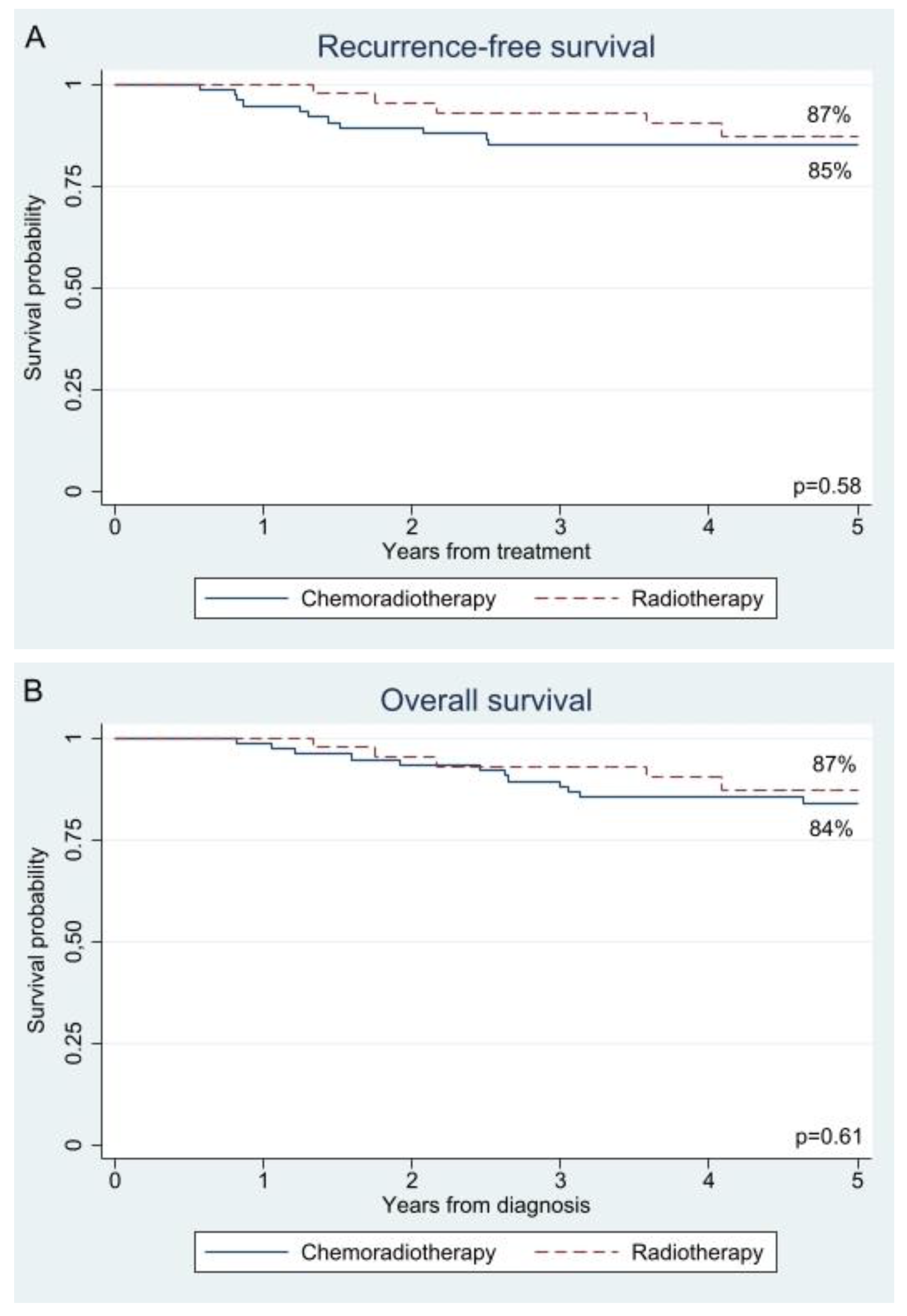
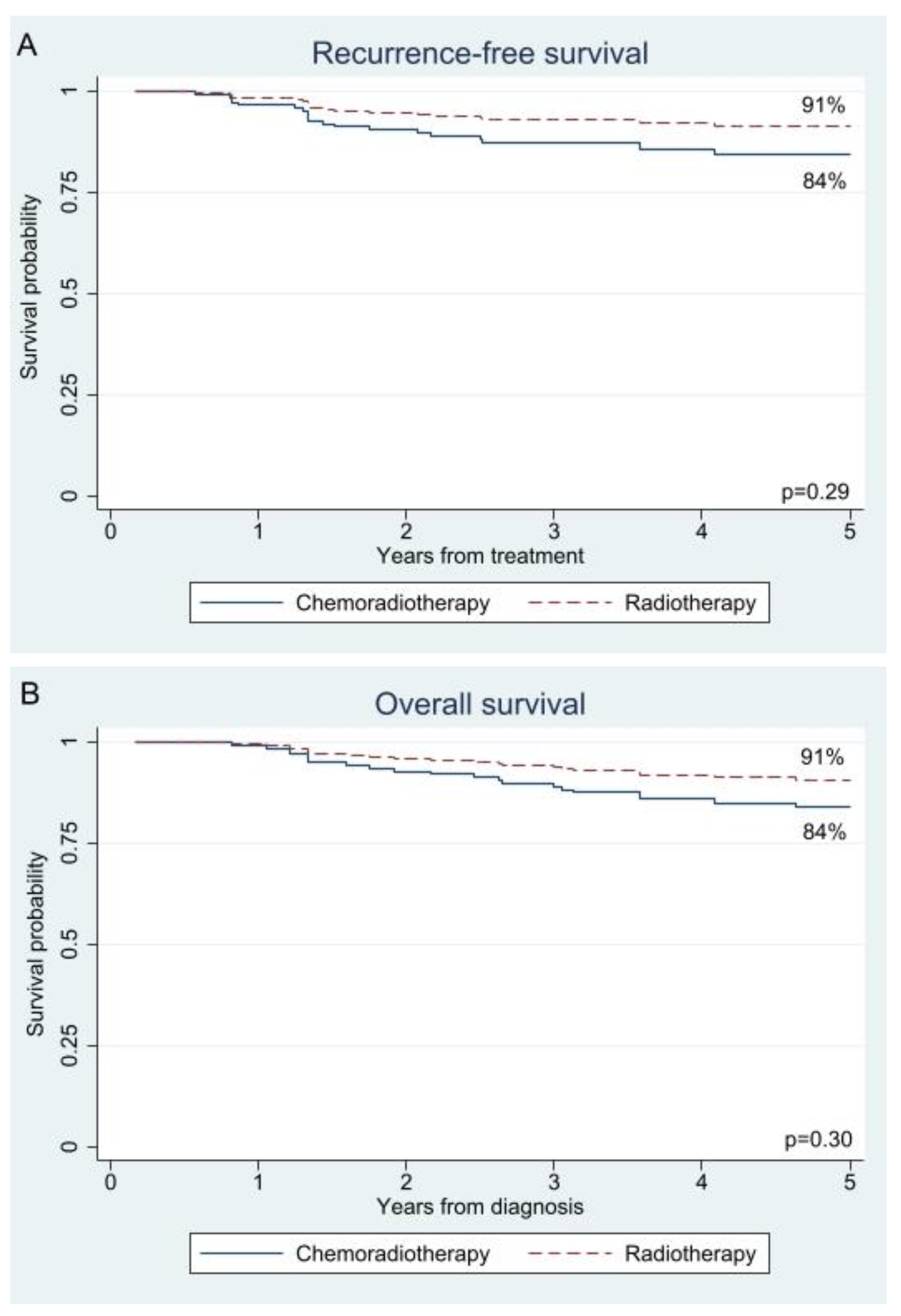
3.2. Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cibula, D.; Pötter, R.; Planchamp, F.; Avall-Lundqvist, E.; Fischerova, D.; Haie Meder, C.; Köhler, C.; Landoni, F.; Lax, S.; Lindegaard, J.C.; et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology guidelines for the management of patients with cervical cancer. Radiother. Oncol. 2018, 127, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Specialisten, F.M. Richtlijn Cervixcarcinoom. Available online: https://richtlijnendatabase.nl/richtlijn/cervixcarcinoom/startpagina_-_cervixcarcinoom.html (accessed on 24 April 2023).
- Bhatla, N.; Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the cervix uteri. Int. J. Gynaecol. Obstet. 2018, 143 (Suppl. S2), 22–36. [Google Scholar] [CrossRef] [PubMed]
- Peters, W.A., 3rd; Liu, P.Y.; Barrett, R.J., 2nd; Stock, R.J.; Monk, B.J.; Berek, J.S.; Souhami, L.; Grigsby, P.; Gordon, W., Jr.; Alberts, D.S. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2000, 18, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- Monk, B.J.; Wang, J.; Im, S.; Stock, R.J.; Peters, W.A., 3rd; Liu, P.Y.; Barrett, R.J., 2nd; Berek, J.S.; Souhami, L.; Grigsby, P.W.; et al. Rethinking the use of radiation and chemotherapy after radical hysterectomy: A clinical-pathologic analysis of a Gynecologic Oncology Group/Southwest Oncology Group/Radiation Therapy Oncology Group trial. Gynecol. Oncol. 2005, 96, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Samlal, R.A.; van der Velden, J.; Schilthuis, M.S.; González González, D.; Ten Kate, F.J.; Hart, A.A.; Lammes, F.B. Identification of high-risk groups among node-positive patients with stage IB and IIA cervical carcinoma. Gynecol. Oncol. 1997, 64, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Lee, S.H.; Lee, K.B.; Park, C.Y. The influence of number of high risk factors on clinical outcomes in patients with early-stage cervical cancer after radical hysterectomy and adjuvant chemoradiation. Obs. Gynecol Sci 2016, 59, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Garrido, M.M.; Kelley, A.S.; Paris, J.; Roza, K.; Meier, D.E.; Morrison, R.S.; Aldridge, M.D. Methods for constructing and assessing propensity scores. Health Serv. Res. 2014, 49, 1701–1720. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, P.T.; Frumovitz, M.; Pareja, R.; Lopez, A.; Vieira, M.; Ribeiro, R.; Buda, A.; Yan, X.; Shuzhong, Y.; Chetty, N.; et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N. Engl. J. Med. 2018, 379, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.; Siu, S.S.; Luesley, D.; Bryant, A.; Dickinson, H.O. Radiotherapy and chemoradiation after surgery for early cervical cancer. Cochrane Database Syst. Rev. 2012, 5, Cd007583. [Google Scholar] [CrossRef] [PubMed]
- Querleu, D.; Cibula, D.; Abu-Rustum, N.R. 2017 Update on the Querleu-Morrow Classification of Radical Hysterectomy. Ann. Surg. Oncol. 2017, 24, 3406–3412. [Google Scholar] [CrossRef] [PubMed]
- Derks, M.; van Lonkhuijzen, L.R.; Bakker, R.M.; Stiggelbout, A.M.; de Kroon, C.D.; Westerveld, H.; Roovers, J.P.; Kenter, G.G.; Ter Kuile, M.M. Long-Term Morbidity and Quality of Life in Cervical Cancer Survivors: A Multicenter Comparison Between Surgery and Radiotherapy as Primary Treatment. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2017, 27, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Shimada, M.; Aoki, Y.; Sakamoto, M.; Takeshima, N.; Fujiwara, H.; Matsumoto, T.; Mikami, M.; Sugiyama, T. Comparison of adjuvant therapy for node-positive clinical stage IB-IIB cervical cancer: Systemic chemotherapy versus pelvic irradiation. Int. J. Cancer 2017, 141, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Nusbaum, D.J.; Machida, H.; Huang, Y.; Khetan, V.; Matsuzaki, S.; Klar, M.; Grubbs, B.H.; Roman, L.D.; Wright, J.D. Populational trends and outcomes of postoperative radiotherapy for high-risk early-stage cervical cancer with lymph node metastasis: Concurrent chemo-radiotherapy versus radiotherapy alone. Am. J. Obstet. Gynecol. 2020, 222, e481–e484. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Feng, Y.L.; Wan, T.; Zhang, Y.N.; Cao, X.P.; Huang, Y.W.; Xiong, Y.; Huang, X.; Zheng, M.; Li, Y.F.; et al. Effectiveness of Sequential Chemoradiation vs Concurrent Chemoradiation or Radiation Alone in Adjuvant Treatment After Hysterectomy for Cervical Cancer: The STARS Phase 3 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, A.; Takekuma, M.; Mori, K.; Usami, T.; Kondo, E.; Nishio, S.; Nishino, K.; Miyamoto, Y.; Yoshimura, R.; Watanabe, M.; et al. A randomized phase III trial of adjuvant chemotherapy versus concurrent chemoradiotherapy for postoperative cervical cancer: Japanese Gynecologic Oncology Group study (JGOG1082). Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2021, 31, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Nusbaum, D.J.; Matsuzaki, S.; Klar, M.; Shimada, M.; Takekuma, M.; Roman, L.D. Utilization and outcomes of adjuvant systemic chemotherapy alone in high risk, early stage cervical cancer in the United States. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2021, 31, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Falcetta, F.S.; Medeiros, L.R.; Edelweiss, M.I.; Pohlmann, P.R.; Stein, A.T.; Rosa, D.D. Adjuvant platinum-based chemotherapy for early stage cervical cancer. Cochrane Database Syst. Rev. 2016, 11, Cd005342. [Google Scholar] [CrossRef]
- Liang, J.; He, T.; Li, H.; Guo, X.; Zhang, Z. Improve individual treatment by comparing treatment benefits: Cancer artificial intelligence survival analysis system for cervical carcinoma. J. Transl. Med. 2022, 20, 293. [Google Scholar] [CrossRef] [PubMed]
- Gülseren, V.; Kocaer, M.; Çakır, İ.; Özdemir, İ.A.; Sancı, M.; Güngördük, K. Postoperative nomogram for the prediction of disease-free survival in lymph node-negative stage I-IIA cervical cancer patients treated with radical hysterectomy. J Obs. Gynaecol 2020, 40, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Kim, D.Y.; Lee, S.W.; Park, J.Y.; Suh, D.S.; Kim, J.H.; Kim, Y.M.; Kim, Y.T.; Nam, J.H. A postoperative scoring system for distant recurrence in node-positive cervical cancer patients after radical hysterectomy and pelvic lymph node dissection with para-aortic lymph node sampling or dissection. Gynecol. Oncol. 2017, 144, 536–540. [Google Scholar] [CrossRef]
| Characteristics | Missing | Chemoradiotherapy | Radiotherapy | p-Value |
|---|---|---|---|---|
| N | 0 (0%) | 76 (62%) | 46 (38%) | - |
| Median age (range) | 0 (0%) | 42 (28–74) | 45 (26–71) | 0.26 |
| Year of diagnosis (range) | 0 (0%) | 2012 (2009–2017) | 2010 (2001–2018) | 0.008 § |
| FIGO 2018 * | 0 (0%) | 0.06 | ||
| IB | 3 (4%) | 1 (2%) | ||
| IIA | 0 (0%) | 1 (2%) | ||
| IIB | 5 (7%) | 9 (20%) | ||
| IIIC1 | 68 (89%) | 35 (76%) | ||
| Mean tumour diameter in mm (standard deviation) | 1 (1%) | 29 (12.6) | 35 (16.8) | 0.021 § |
| Lymphovascular space invasion, yes | 2 (2%) | 59 (80%) | 30 (65%) | 0.09 |
| Tumour grade | 11 (9%) | <0.001 § | ||
| 1 | 1 (2%) | 0 (0%) | ||
| 2 | 33 (50%) | 8 (18%) | ||
| 3 | 32 (48%) | 37 (82%) | ||
| Pathological parametrial invasion | 0 (0%) | 5 (7%) | 9 (20%) | 0.040 § |
| Positive resection margin | 0 (0%) | 3 (4%) | 2 (4%) | 1.00 |
| Lymph node metastasis | 0 (0%) | 68 (89%) | 35 (76%) | 0.07 |
| Median number of lymph node metastasis (range) | 0 (0%) | 1 (1–5) | 1 (1–4) | 0.025 § |
| Mean number of lymph node metastasis (standard deviation) † | 0 (0%) | 1.82 (1.17) | 1.34 (0.73) | |
| Surgical approach | 0 (0%) | <0.001 § | ||
| Open | 60 (79%) | 46 (100%) | ||
| Minimally invasive | 16 (21%) | 0 (0%) | ||
| Oncological outcome | ||||
| Median follow-up time for recurrences in years (range) | 0 (0%) | 9 (0–14) | 5 (0–17) | <0.001 § |
| Mean follow-up time for overall survival in years (standard deviation) | 0 (0%) | 8 (3.6) | 6 (3.7) | <0.001 § |
| Vital status, death | 0 (0%) | 15 (20%) | 5 (11%) | 0.31 |
| Recurrence, yes | 0 (0%) | 13 (17%) | 5 (11%) | 0.44 |
| Recurrence site ‡ | 0 (0%) | 0.71 | ||
| Locoregionaal | 5 (38%) | 1 (20%) | ||
| Para-aortic | 1 (8%) | 0 (0%) | ||
| Distant | 7 (54%) | 4 (80%) |
| Analysis Type | Recurrence-Free Survival | Overall Survival | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | ||
| Univariable | CRT | 1.00 | Reference | 1.00 | Reference | ||
| RT | 0.59 | 0.21–1.65 | 0.31 | 0.68 | 0.24–1.88 | 0.46 | |
| IPTW | CRT | 1.00 | Reference | 1.00 | Reference | ||
| RT | 0.54 | 0.17–1.71 | 0.29 | 0.56 | 0.19–1.67 | 0.30 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olthof, E.P.; Wenzel, H.H.B.; van der Velden, J.; Stalpers, L.J.A.; van der Aa, M.A.; Mom, C.H. Adjuvant Chemoradiotherapy or Radiotherapy Alone for Early Squamous Cervical Cancer with a Single Surgical-Pathological High-Risk Factor. Cancers 2025, 17, 2041. https://doi.org/10.3390/cancers17122041
Olthof EP, Wenzel HHB, van der Velden J, Stalpers LJA, van der Aa MA, Mom CH. Adjuvant Chemoradiotherapy or Radiotherapy Alone for Early Squamous Cervical Cancer with a Single Surgical-Pathological High-Risk Factor. Cancers. 2025; 17(12):2041. https://doi.org/10.3390/cancers17122041
Chicago/Turabian StyleOlthof, Ester P., Hans H. B. Wenzel, Jacobus van der Velden, Lukas J. A. Stalpers, Maaike A. van der Aa, and Constantijne H. Mom. 2025. "Adjuvant Chemoradiotherapy or Radiotherapy Alone for Early Squamous Cervical Cancer with a Single Surgical-Pathological High-Risk Factor" Cancers 17, no. 12: 2041. https://doi.org/10.3390/cancers17122041
APA StyleOlthof, E. P., Wenzel, H. H. B., van der Velden, J., Stalpers, L. J. A., van der Aa, M. A., & Mom, C. H. (2025). Adjuvant Chemoradiotherapy or Radiotherapy Alone for Early Squamous Cervical Cancer with a Single Surgical-Pathological High-Risk Factor. Cancers, 17(12), 2041. https://doi.org/10.3390/cancers17122041








