Redefining the Use of Regorafenib and Trifluridine/Tipiracil Without Bevacizumab in Refractory Metastatic Colorectal Cancer: Findings from the ReTrITA Study
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Outcome Parameters
2.2. Drug Administration
2.3. Statistical Analysis
3. Results
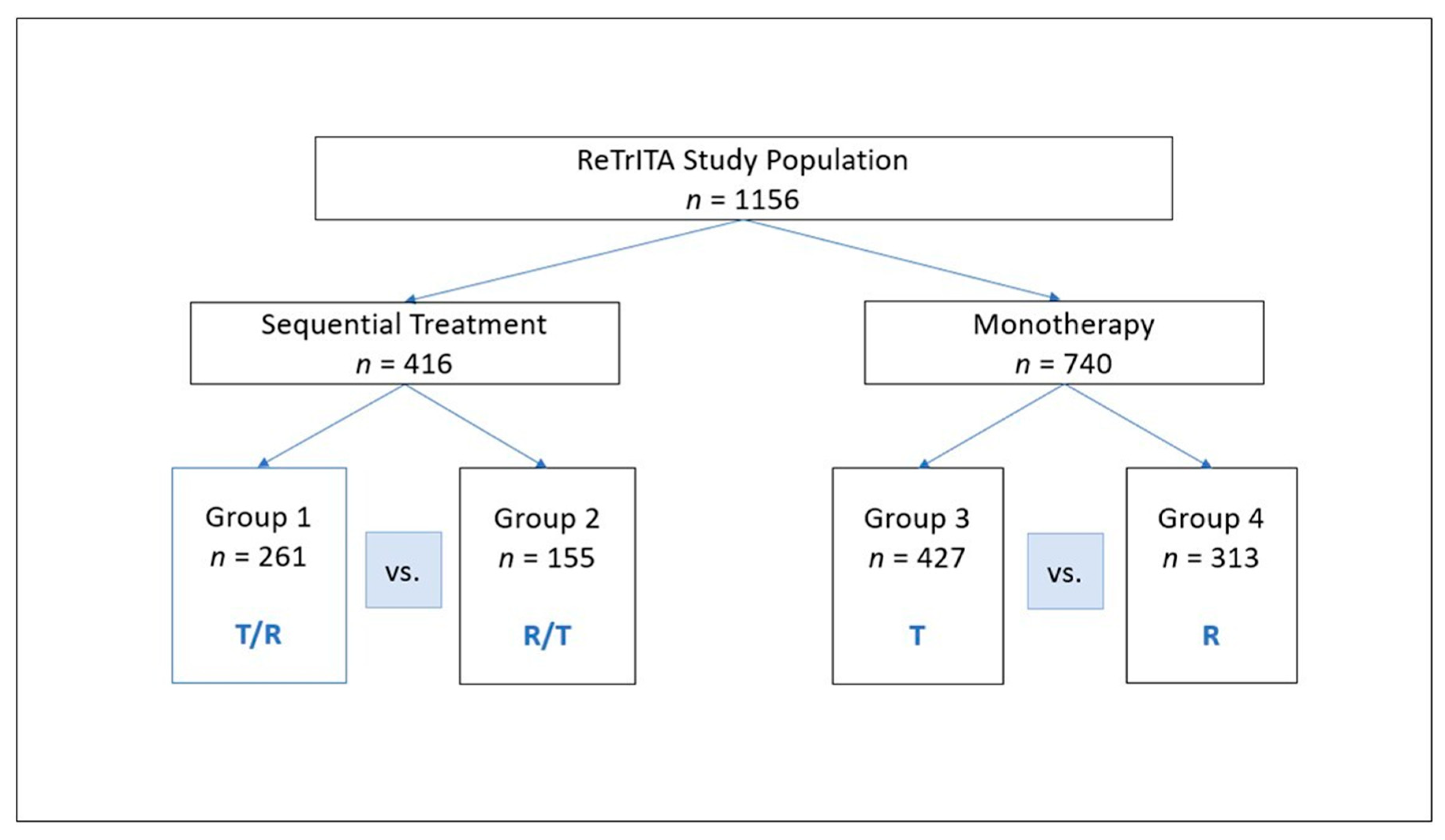
3.1. Patient Characteristics
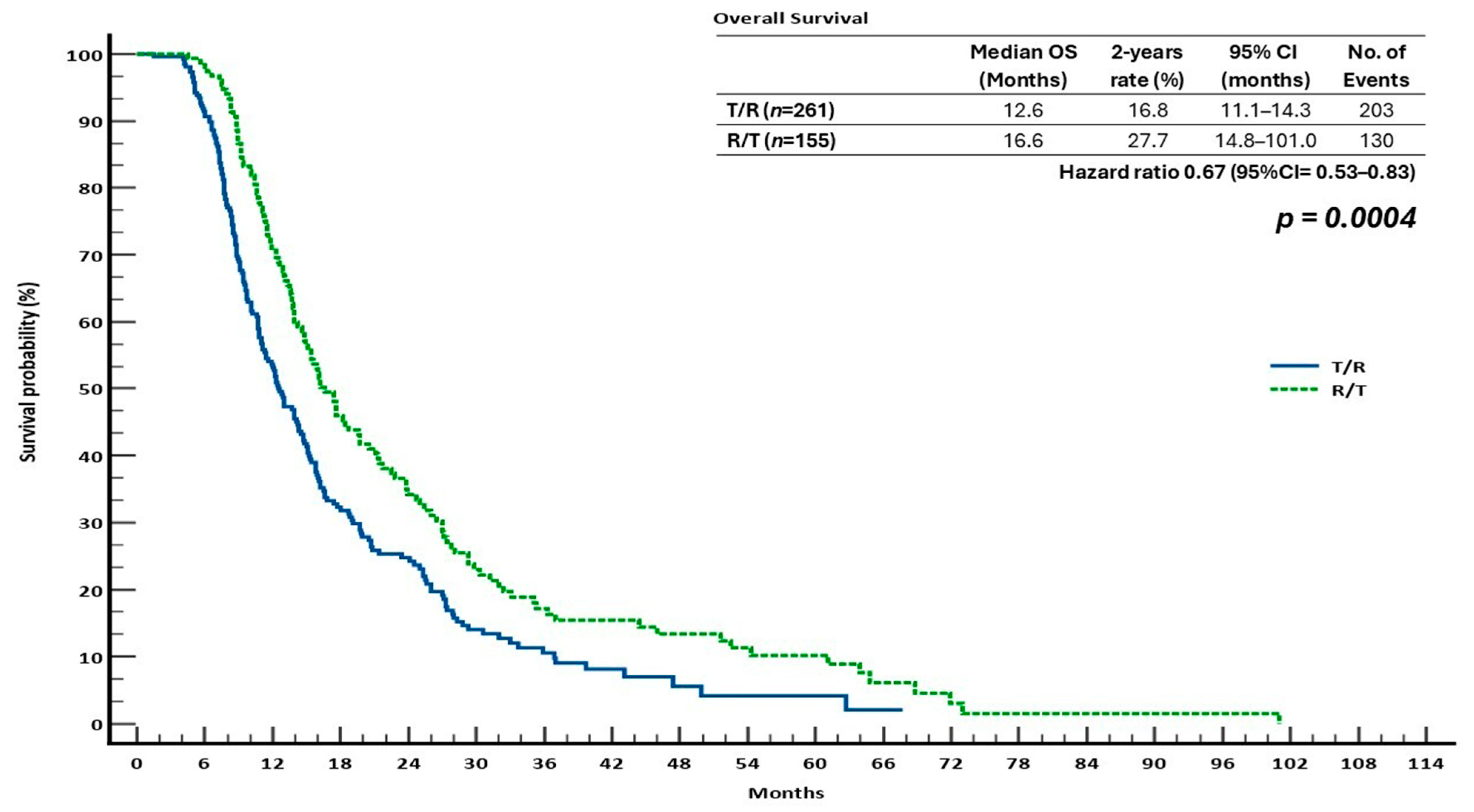
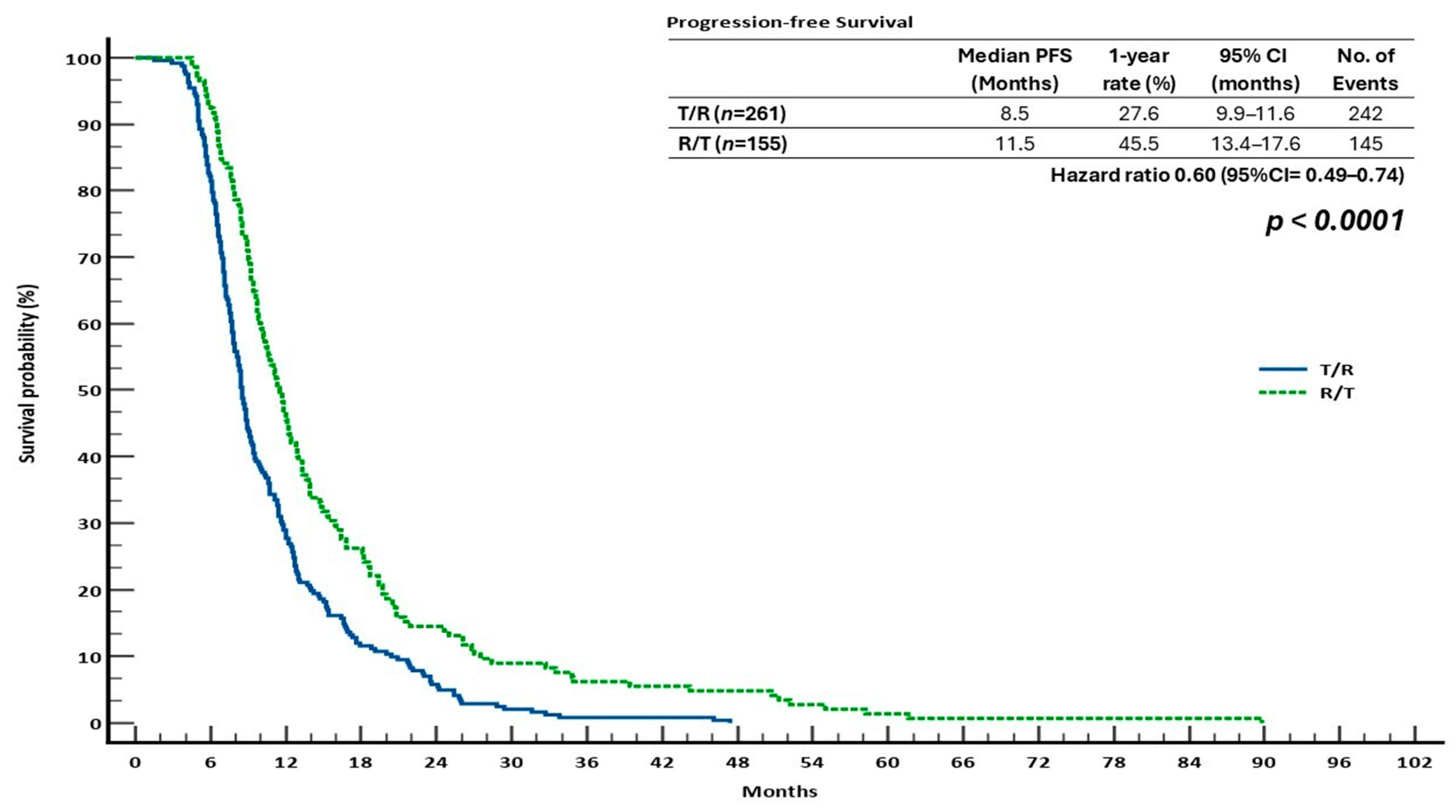
3.2. Survival Outcomes in the Sequential Treatment Groups
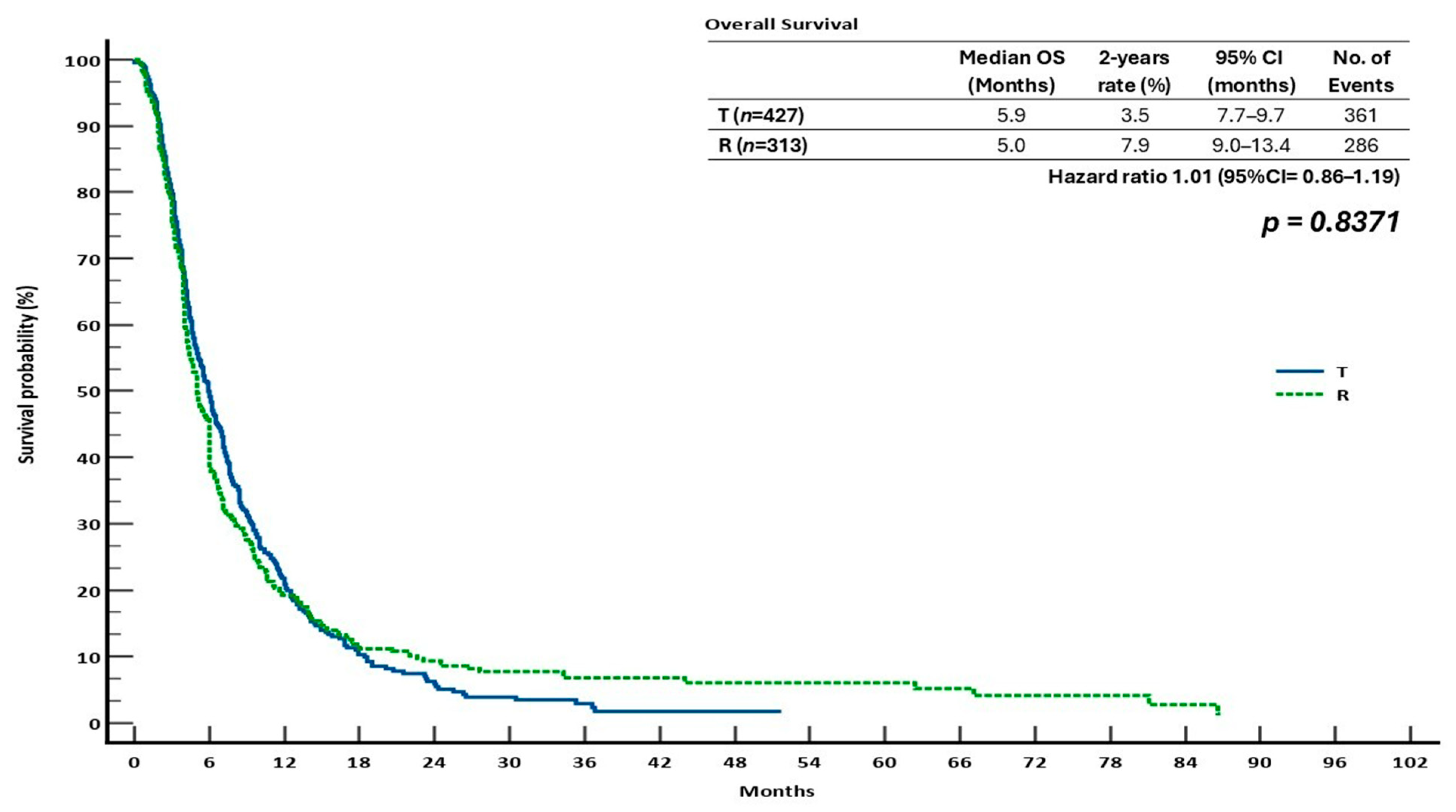
3.3. Survival Outcomes in the Monotherapy Groups
3.4. Summary of Efficacy Outcomes
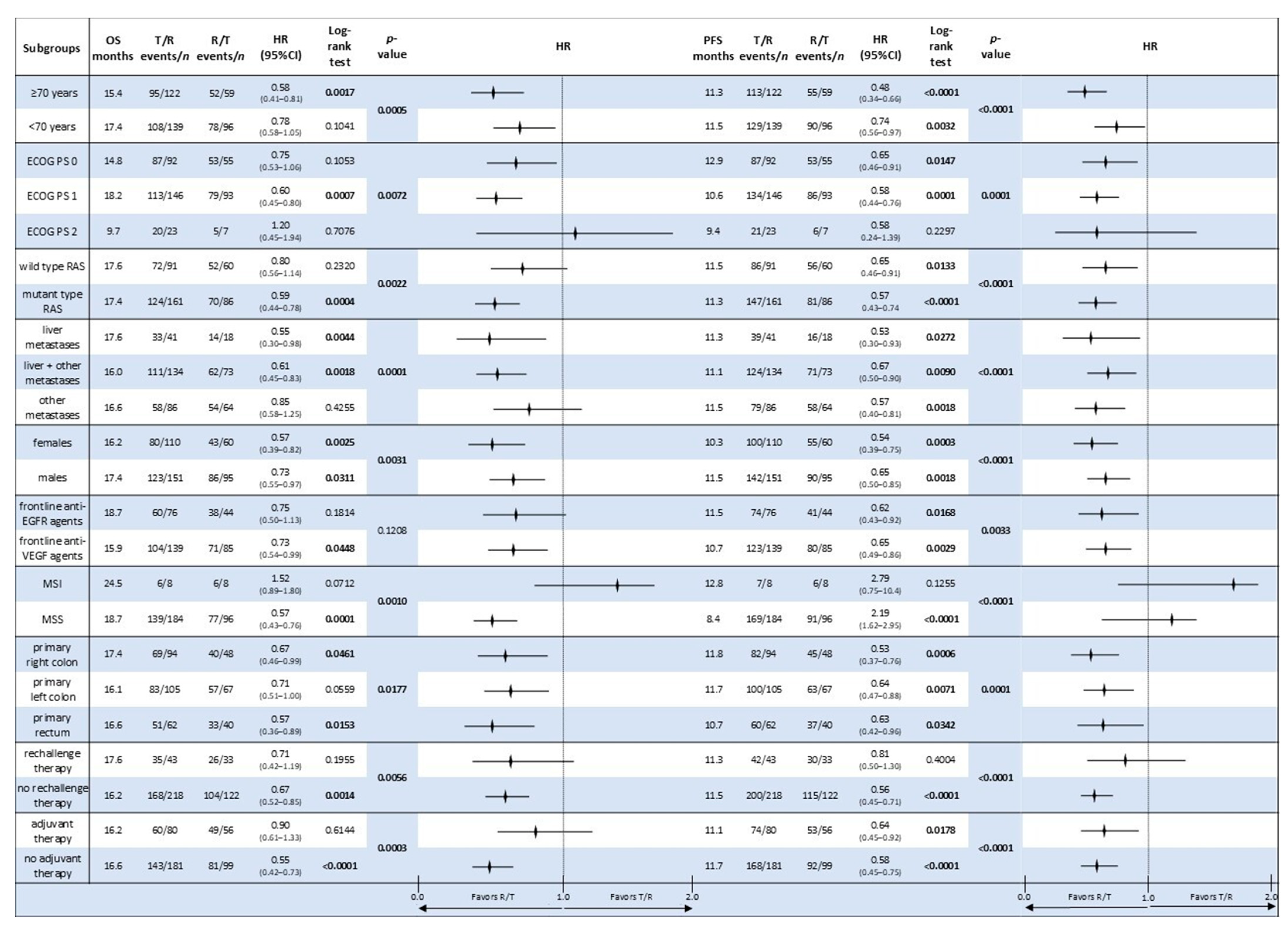
3.5. Comparative Analysis of Subgroup Survival in the Sequential Treatment Groups
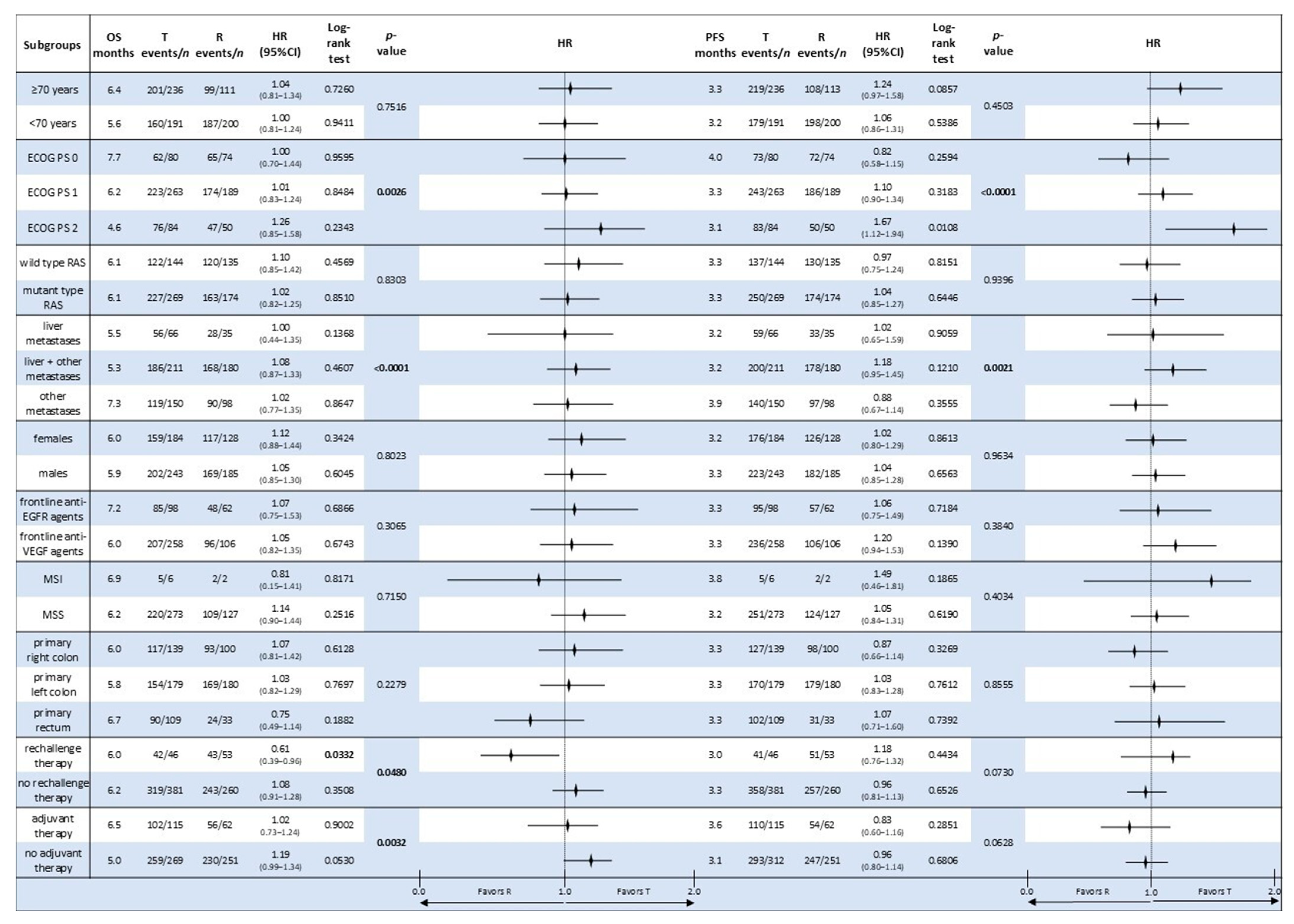
3.6. Comparative Analysis of Subgroup Survival in the Monotherapy Groups
4. Safety
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Colon Cancer Version 3.2025—24 April 2025. Available online: www.nccn.org/professionals/physician_gls/pdf/colon.pdf (accessed on 10 May 2025).
- National Cancer Institute. Cancer Stat Facts: Colorectal Cancer. Available online: https://seer.cancer.gov/statfacts/html/colorect.html (accessed on 10 May 2025).
- Van Cutsem, E.; Nordlinger, B.; Adam, R.; Köhne, C.H.; Pozzo, C.; Poston, G.; Ychou, M.; Rougier, P.; European Colorectal Metastases Treatment Group. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur. J. Cancer 2006, 42, 2212–2221. [Google Scholar] [CrossRef] [PubMed]
- Yoo, P.S.; Lopez-Soler, R.I.; Longo, W.E.; Cha, C.H. Liver resection for metastatic colorectal cancer in the age of neoadjuvant chemotherapy and bevacizumab. Clin. Color. Cancer 2006, 6, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Kemeny, N. Management of liver metastases from colorectal cancer. Oncology 2006, 20, 1161–1176, 1179, discussion 1179–1180, 1185–1186. [Google Scholar] [PubMed]
- Muratore, A.; Zorzi, D.; Bouzari, H.; Amisano, M.; Massucco, P.; Sperti, E.; Capussotti, L. Asymptomatic colorectal cancer with un-resectable liver metastases: Immediate colorectal resection or up-front systemic chemotherapy? Ann. Surg. Oncol. 2007, 14, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Inoue, Y.; Komeda, K.; Shimizu, T.; Asakuma, M.; Hirokawa, F.; Miyamoto, Y.; Okuda, J.; Takeshita, A.; Shibayama, Y.; et al. Clinicopathological analysis of recurrence patterns and prognostic factors for survival after hepatectomy for colorectal liver metastasis. BMC Surg. 2010, 10, 27. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis; treatment and follow-up. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Kim, T.W.; Van Cutsem, E.; Geva, R.; Jäger, D.; Hara, H.; Burge, M.; O’Neil, B.; Kavan, P.; Yoshino, T.; et al. Phase II Open-Label Study of Pembrolizumab in Treatment-Refractory, Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: KEYNOTE-164. J. Clin. Oncol. 2020, 38, 11–19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- André, T.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- Cremolini, C.; Rossini, D.; Dell’Aquila, E.; Lonardi, S.; Conca, E.; Del Re, M.; Busico, A.; Pietrantonio, F.; Danesi, R.; Aprile, G.; et al. Rechallenge for Patients with RAS and BRAF Wild-Type Metastatic Colorectal Cancer with Acquired Resistance to Firstline Cetuximab and Irinotecan: A Phase 2 Single-Arm Clinical Trial. JAMA Oncol. 2019, 5, 343–350. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mauri, G.; Pizzutilo, E.G.; Amatu, A.; Bencardino, K.; Palmeri, L.; Bonazzina, E.F.; Tosi, F.; Stella, G.C.; Burrafato, G.; Scaglione, F.; et al. Retreatment with anti-EGFR monoclonal antibodies in metastatic colorectal cancer: Systematic review of different strategies. Cancer Treat. Rev. 2019, 73, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Calegari, M.; Zurlo, I.; Dell’Aquila, E.; Basso, M.; Orlandi, A.; Bensi, M.; Camarda, F.; Anghelone, A.; Pozzo, C.; Sperduti, I.; et al. Chemotherapy Rechallenge or Reintroduction Compared to Regorafenib or Trifluridine/Tipiracil for Pretreated Metastatic Colorectal Cancer Patients: A Propensity Score Analysis of Treatment Beyond Second Line (Proserpyna Study). Clin. Color. Cancer 2024, 24, 1–10.e4. [Google Scholar] [CrossRef] [PubMed]
- Bazarbashi, S.; Alkhatib, R.; Aseafan, M.; Tuleimat, Y.; Abdel-Aziz, N.; Mahrous, M.; Elsamany, S.; Elhassan, T.; Alghamdi, M. Efficacy of Chemotherapy Rechallenge Versus Regorafenib or Trifluridine/Tipiracil in Third-Line Setting of Metastatic Colorectal Cancer: A Multicenter Retrospective Comparative Study. JCO Glob. Oncol. 2024, 10, e2300461. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Signorelli, C.; Calegari, M.A.; Anghelone, A.; Passardi, A.; Frassineti, G.L.; Bittoni, A.; Lucchetti, J.; Angotti, L.; Di Giacomo, E.; Zurlo, I.V.; et al. Survival Outcomes with Regorafenib and/or Trifluridine/Tipiracil Sequencing to Rechallenge with Third-Line Regimens in Metastatic Colorectal Cancer: A Multicenter Retrospective Real-World Subgroup Comparison from the ReTrITA Study. Curr. Oncol. 2024, 31, 7793–7808. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Siena, S.; Di Bartolomeo, M.; Raghav, K.; Masuishi, T.; Loupakis, F.; Kawakami, H.; Yamaguchi, K.; Nishina, T.; Fakih, M.; Elez, E.; et al. Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): A multicentre, open-label, phase 2 trial. Lancet Oncol. 2021, 22, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Cercek, A.; Siena, S.; André, T.; Ng, K.; Van Cutsem, E.; Wu, C.; Fountzilas, C.; Kardosh, A.; Lenz, H.J.; Elez, E.; et al. Tucatinib plus trastuzumab for chemotherapy-refractory, HER2-positive, RAS wild-type unresectable or metastatic colorectal cancer (MOUNTAINEER): A multicentre, open-label, phase 2 study. Lancet Oncol. 2023, 24, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Sartore-Bianchi, A.; Trusolino, L.; Martino, C.; Bencardino, K.; Lonardi, S.; Bergamo, F.; Zagonel, V.; Leone, F.; Depetris, I.; Martinelli, E.; et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wildtype, HER2-positive metastatic colorectal cancer (HERACLES): A proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 738–746, Erratum in Lancet Oncol. 2016, 17, e420. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Hurwitz, H.; Raghav, K.P.S.; McWilliams, R.R.; Fakih, M.; VanderWalde, A.; Swanton, C.; Kurzrock, R.; Burris, H.; Sweeney, C.; et al. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): An updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2019, 20, 518–530. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Theik, N.W.Y.; Muminovic, M.; Alvarez-Pinzon, A.M.; Shoreibah, A.; Hussein, A.M.; Raez, L.E. NTRK Therapy among Different Types of Cancers, Review and Future Perspectives. Int. J. Mol. Sci. 2024, 25, 2366. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Di Nicolantonio, F.; Schrock, A.B.; Lee, J.; Morano, F.; Fucà, G.; Nikolinakos, P.; Drilon, A.; Hechtman, J.F.; Christiansen, J.; et al. RET fusions in a small subset of advanced colorectal cancers at risk of being neglected. Ann. Oncol. 2018, 29, 1394–1401. [Google Scholar] [CrossRef] [PubMed]
- Grothey, A.; Van Cutsem, E.; Sobrero, A.; Siena, S.; Falcone, A.; Ychou, M.; Humblet, Y.; Bouché, O.; Mineur, L.; Barone, C.; et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013, 381, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Mayer, R.J.; Van Cutsem, E.; Falcone, A.; Yoshino, T.; Garcia-Carbonero, R.; Mizunuma, N.; Yamazaki, K.; Shimada, Y.; Tabernero, J.; Komatsu, Y.; et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N. Engl. J. Med. 2015, 372, 1909–1919. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, T.; Wu, Z.; Li, Y.; Ma, H.; Wang, L.; Lei, S.; Chen, W. Fruquintinib in combination with sintilimab or TAS-102 as third-line or above treatment in patients with metastatic colorectal cancer: A real-world study. Transl. Cancer Res. 2023, 12, 3034–3044. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dasari, A.; Lonardi, S.; Garcia-Carbonero, R.; Elez, E.; Yoshino, T.; Sobrero, A.; Yao, J.; García-Alfonso, P.; Kocsis, J.; Cubillo Gracian, A.; et al. Fruquintinib versus placebo in patients with refractory metastatic colorectal cancer (FRESCO-2): An international, multicentre, randomised, double-blind, phase 3 study. Lancet 2023, 402, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Prager, G.W.; Taieb, J.; Fakih, M.; Ciardiello, F.; Van Cutsem, E.; Elez, E.; Cruz, F.M.; Wyrwicz, L.; Stroyakovskiy, D.; Pápai, Z.; et al. Trifluridine-Tipiracil and Bevacizumab in Refractory Metastatic Colorectal Cancer. N. Engl. J. Med. 2023, 388, 1657–1667. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, C.; Calegari, M.A.; Basso, M.; Anghelone, A.; Lucchetti, J.; Minelli, A.; Angotti, L.; Zurlo, I.V.; Schirripa, M.; Chilelli, M.G.; et al. Treatment Settings and Outcomes with Regorafenib and Trifluridine/Tipiracil at Third-Line Treatment and beyond in Metastatic Colorectal Cancer: A Real-World Multicenter Retrospective Study. Curr. Oncol. 2023, 30, 5456–5469. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bekaii-Saab, T.S.; Ou, F.S.; Ahn, D.H.; Boland, P.M.; Ciombor, K.K.; Heying, E.N.; Dockter, T.J.; Jacobs, N.L.; Pasche, B.C.; Cleary, J.M.; et al. Regorafenib dose-optimisation in patients with refractory metastatic colorectal cancer (ReDOS): A randomised, multicentre, open-label, phase 2 study. Lancet Oncol. 2019, 20, 1070–1082. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bekaii-Saab, T.S.; Cosgrove, D.; Sruti, I.; Shi, J.; Dai, W.; Patton, G.A.; Appukkuttan, S.; Hocum, B.T.; Katta, A.; Earl, J.; et al. Real-world (RW) treatment patterns and clinical outcomes of patients treated with sequential regorafenib and trifluridine/tipiracil ± bevacizumab (BEV) for metastatic colorectal cancer (mCRC) in the United States (US) community oncology setting: The SEQRT2 study. J. Clin. Oncol. 2025, 43 (Suppl. 4), 62. [Google Scholar] [CrossRef]
- Ahn, D.H.; Bekaii-Saab, T.S.; Yuan, C.; Kurtinecz, M.; Pan, X.; Vassilev, Z.; Pisa, F.; Ostojic, H. Sequential Treatment with Regorafenib and Trifluridine/Tipiracil ± Bevacizumab in Refractory Metastatic Colorectal Cancer in Community Clinical Practice in the USA. Cancers 2025, 17, 969. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chida, A.; Kawakami, T.; Hamano, T.; Masuishi, T.; Mitani, S.; Yasui, H.; Ishiguro, A.; Ando, T.; Sawada, K.; Harada, K.; et al. Prospective observational study investigating the impact of treatment sequence using regorafenib and FTD/TPI for metastatic colorectal cancer on overall survival (OSERO study). J. Clin. Oncol. 2024, 42 (Suppl. S3), 103. [Google Scholar] [CrossRef]
- Ducreux, M.; Ben Abdelghani, M.; Tougeron, D.; Bouche, O.; Viaud, J.; Akouz, F.K.; Martin-Babau, J.; Bachet, J.B.; Coutzac, C.; Castanie, H.; et al. 3O PRODIGE 68—UCGI 38—SOREGATT: A randomized phase II study comparing the sequences of regorafenib (reg) and trifluridine/tipiracil (t/t) after failure of standard therapies in patients (pts) with metastatic colorectal cancer (mCRC). Ann. Oncol. 2024, 35 (Suppl. S1), S1–S74. [Google Scholar] [CrossRef]
- Cherri, S.; Libertini, M.; Noventa, S.; Oneda, E.; Meriggi, F.; Zaniboni, A. What Is Next for Refractory Colorectal Cancer CRC? Looking Beyond SUNLIGHT, FRESCO2, RECOURSE and CORRECT. Int. J. Mol. Sci. 2025, 26, 2522. [Google Scholar] [CrossRef] [PubMed]
| T/R | R/T | p-Value | T | R | p-Value | |
|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | |||
| Total | 261 (100) | 155 (100) | <0.0001 | 427 (100) | 313 (100) | <0.0001 |
| Age Median (min–max) | 69 (86–34) | 67 (85–44) | 0.8638 | 71 (89–40) | 65 (33–86) | 0.6069 |
| Age | 0.0847 | <0.0001 | ||||
| ≥70 yrs | 122 (46.7) | 59 (38.1) | 236 (55.3) | 113 (36.1) | ||
| <70 yrs | 139 (53.3) | 96 (61.9) | 191 (44.7) | 200 (63.9) | ||
| Sex | 0.4912 | 0.5502 | ||||
| Female | 110 (42.1) | 60 (38.7) | 184 (43.1) | 128 (40.9) | ||
| Male | 151 (57.9) | 95 (61.3) | 243 (56.9) | 185 (59.1) | ||
| RAS status | 0.3205 | 0.0119 | ||||
| Wild type | 91 (34.9) | 60 (38.7) | 144 (33.7) | 135 (43.1) | ||
| Mutant type | 161 (61.7) | 86 (55.5) | 269 (63.1) | 174 (55.6) | ||
| Unknown | 9 (3.4) | 9 (5.8) | 14 (3.2) | 4 (1.3) | ||
| Primary tumour location | 0.5758 | <0.0001 | ||||
| Right side | 94 (36.0) | 48 (31.0) | 139 (32.5) | 100 (32.0) | ||
| Left side | 105 (40.2) | 67 (43.2) | 179 (42.0) | 180 (57.5) | ||
| Rectum | 62 (23.8) | 40 (25.8) | 109 (25.5) | 33 (10.5) | ||
| MMR | 0.1670 | <0.0001 | ||||
| MSI | 8 (3.1) | 8 (5.2) | 6 (1.4) | 2 (0.6) | ||
| MMR | 184 (70.5) | 96 (61.9) | 273 (63.9) | 127 (40.6) | ||
| Unknown | 69 (26.4) | 51 (32.9) | 148 (34.7) | 184 (58.8) | ||
| PS ECOG | 0.2504 | 0.1741 | ||||
| 0 | 92 (35.2) | 55 (35.5) | 80 (18.7) | 74 (23.6) | ||
| 1 | 146 (55.9) | 93 (60.0) | 263 (61.6) | 189 60.4) | ||
| 2 | 23 (8.9) | 7 (4.5) | 84 (19.7) | 50 (16.0) | ||
| Prior adjuvant therapy | 0.2501 | 0.0022 | ||||
| Yes | 80 (30.7) | 56 (36.1) | 115 (29.9) | 62 (19.8%) | ||
| No | 181 (69.3) | 99 (63.9) | 269 (70.1) | 251 (80.2) | ||
| Metastatic disease sites | 0.1847 | 0.0658 | ||||
| Liver only | 41 (15.7) | 18 (11.6) | 66 (15.5) | 35 (11.2) | ||
| Liver + other | 134 (51.3) | 73 (47.1) | 211 (49.4) | 180 (57.5) | ||
| Others | 86 (33.0) | 64 (41.3) | 150 (35.1) | 98 (31.3) | ||
| CT: 1° line regimen | 0.4942 | <0.0001 | ||||
| Monochemotherapy | 18 (6.9) | 6 (3.9) | 9 (2.1) | 1 (0.3) | ||
| Doublet chemotherapy | 215 (82.4) | 128 (82.6) | 344 (80.6) | 168 (53.7) | ||
| Triplet chemotherapy | 19 7.3) | 13 (8.4) | 37 (8.7) | 16 (5.1) | ||
| Unknown | 9 (3.4) | 8 (5.2) | 37 8.7) | 128 (40.9) | ||
| CT: 2° line regimen | 0.9257 | <0.0001 | ||||
| Monochemotherapy | 24 (9.2) | 13 (8.4) | 48 (11.2) | 17 (5.4) | ||
| Doublet chemotherapy | 201 77.0) | 123 (79.4) | 323 (75.6) | 151 (48.2) | ||
| Triplet chemotherapy | 6 2.3) | 4 (2.6) | 8 (1.9) | 7 (2.2) | ||
| Unknown | 30 (11.5) | 15 (9.7) | 48 (11.2) | 138 (44.1) | ||
| Rechallenge therapy | 0.2197 | 0.0151 | ||||
| yes | 43 (16.5) | 33 (21.3) | 46 (10.8) | 53 (16.9) | ||
| no | 218 (83.5) | 122 (78.7) | 381 (89.2) | 260 (83.1) | ||
| Biological agents: 1°line | 0.9494 | <0.0001 | ||||
| Anti-EGFR use | 76 (29.1) | 44 (28.4) | 98 (23.0) | 62 (19.8) | ||
| Anti-VEGF use | 139 (53.3) | 85 (54.8) | 258 (60.4) | 106 (33.9) | ||
| None | 46 (17.6) | 26 (16.8) | 71 (16.6) | 145 (46.3) | ||
| Biological agents: 2°line | 0.1836 | <0.0001 | ||||
| Anti-EGFR use | 16 (6.1) | 10 (6.5) | 27 (6.3) | 14 (4.5) | ||
| Anti-VEGF use | 157 (60.2) | 106 (68.4) | 249 (58.3) | 120 (38.3) | ||
| None | 88 (33.7) | 39 (25.2) | 151 (35.4) | 179 (57.2) |
| OS | PFS | ORR | DCR | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mOS (mos) | 2y-OS (%) | 3y-OS (%) | HR (95% CI) | p-Value | mPFS (mos) | 1y-PFS | 2y-PFS | HR (95% CI) | p-Value | PR + CR (%) | p-Value | PR + CR +SD (%) | p-Value | |
| T/R | 12.6 | 16.8 | 5.3 | 0.67 (0.53–0.83) | 0.0004 | 8.5 | 27.6 | 5.7 | 0.60 (0.49–0.74) | <0.0001 | 3.4 | 1.0 | 32.9 | 0.9564 |
| R/T | 16.6 | 27.7 | 12.9 | 11.5 | 45.5 | 14.4 | 5.5 | 52.1 | ||||||
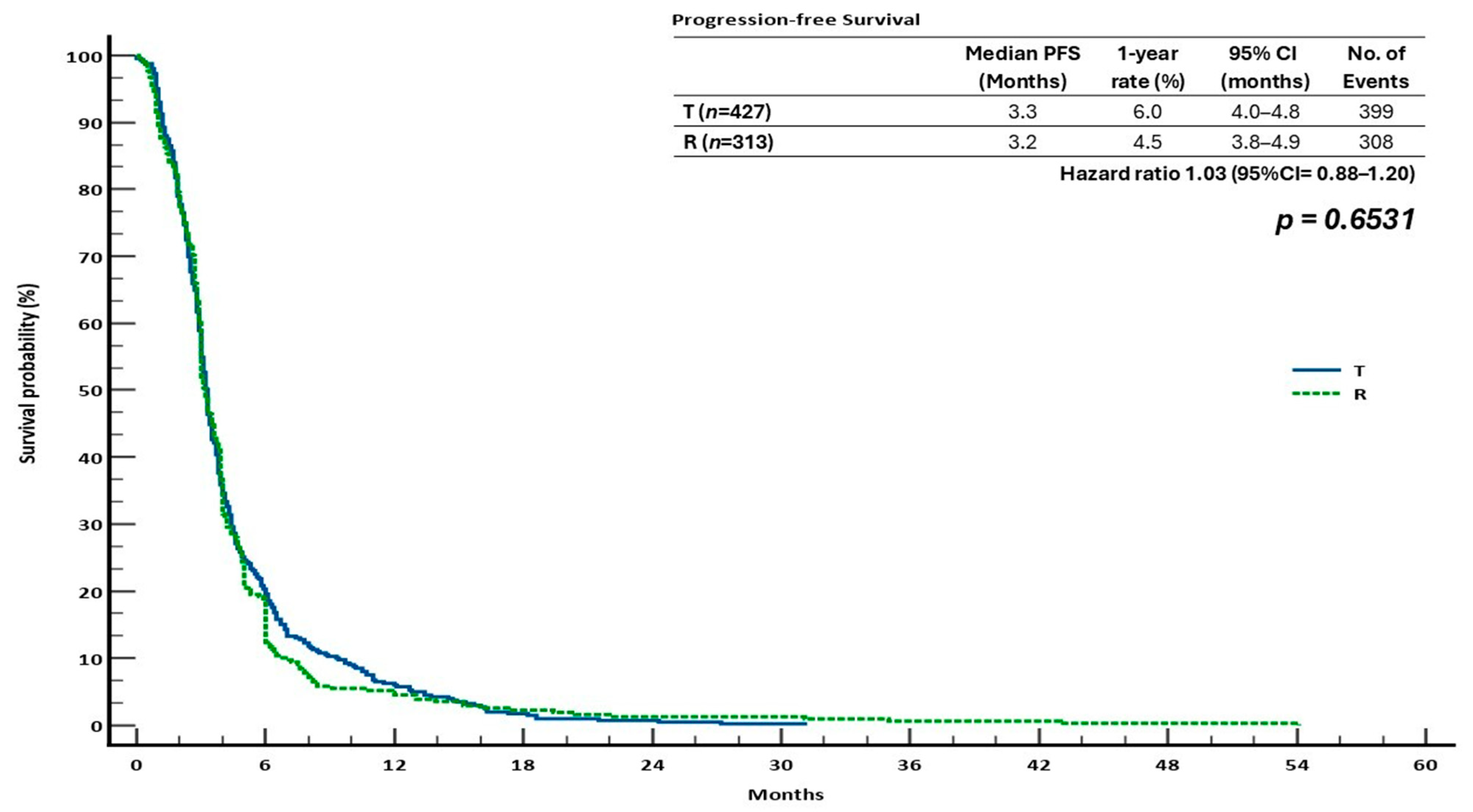
| T | 5.9 | 3.5 | 1.1 | 1.01 (0.86–1.19) | 0.8371 | 3.3 | 6.0 | 0.7 | 1.03 (0.88–1.20) | 0.6531 | 2.8 | 0.2100 | 23.5 | <0.0001 |
| R | 5.0 | 7.9 | 4.4 | 3.2 | 4.5 | 1.2 | 2.1 | 22.9 | ||||||
| T/R | R/T | T | R | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | p-Value | n | % | n | % | p-Value | |
| All G3/G4 events | 242 | 100 | 184 | 100 | 0.0050 | 234 | 100 | 155 | 100 | 0.0001 |
| All patients who experienced G3/G4 toxicities | 145 | 55.5 | 107 | 69.0 | 0.0167 | 177 | 41.4 | 117 | 37.3 | 0.0005 |
| All haematologic G3/G4 events | 124 | 51.2 | 85 | 46.2 | 0.0070 | 182 | 77.8 | 13 | 8.4 | <0.0001 |
| All non-haematologic G3/G4 events | 118 | 48.8 | 99 | 53.8 | 0.1971 | 52 | 22.2 | 142 | 91.6 | <0.0001 |
| Most common haematologic G3/G4 toxicities | 0.9385 | 0.0336 | ||||||||
| Neutropenia | 90 | 72.6 | 59 | 69.4 | 127 | 69.8 | 4 | 30.8 | ||
| Febrile neutropenia | 2 | 1.6 | 1 | 1.2 | 1 | 0.5 | 0 | 0.0 | ||
| Thrombocytopenia | 7 | 5.6 | 5 | 5.9 | 6 | 3.3 | 1 | 7.7 | ||
| Anaemia | 25 | 20.2 | 20 | 23.5 | 48 | 26.4 | 8 | 61.5 | ||
| Most common non-haematologic G3/G4 toxicities | 0.0057 | <0.0001 | ||||||||
| Fatigue | 47 | 39.8 | 31 | 31.3 | 28 | 53.8 | 33 | 23.2 | ||
| Hand–foot skin reaction | 11 | 9.3 | 26 | 26.3 | 0 | 0.0 | 27 | 19.0 | ||
| Hypertension | 6 | 5.1 | 11 | 11.1 | 1 | 1.9 | 14 | 9.9 | ||
| Liver dysfunctions | 4 | 3.4 | 4 | 4.0 | 1 | 1.9 | 5 | 3.5 | ||
| Diarrhea | 17 | 14.4 | 6 | 6.1 | 10 | 19.2 | 13 | 9.2 | ||
| Skin disorders | 2 | 1.7 | 3 | 3.0 | 0 | 0.0 | 11 | 7.7 | ||
| Others | 31 | 26.3 | 18 | 18.2 | 12 | 23.1 | 39 | 27.5 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Signorelli, C.; Calegari, M.A.; Anghelone, A.; Passardi, A.; Gallio, C.; Bittoni, A.; Lucchetti, J.; Angotti, L.; Di Giacomo, E.; Zurlo, I.V.; et al. Redefining the Use of Regorafenib and Trifluridine/Tipiracil Without Bevacizumab in Refractory Metastatic Colorectal Cancer: Findings from the ReTrITA Study. Cancers 2025, 17, 2037. https://doi.org/10.3390/cancers17122037
Signorelli C, Calegari MA, Anghelone A, Passardi A, Gallio C, Bittoni A, Lucchetti J, Angotti L, Di Giacomo E, Zurlo IV, et al. Redefining the Use of Regorafenib and Trifluridine/Tipiracil Without Bevacizumab in Refractory Metastatic Colorectal Cancer: Findings from the ReTrITA Study. Cancers. 2025; 17(12):2037. https://doi.org/10.3390/cancers17122037
Chicago/Turabian StyleSignorelli, Carlo, Maria Alessandra Calegari, Annunziato Anghelone, Alessandro Passardi, Chiara Gallio, Alessandro Bittoni, Jessica Lucchetti, Lorenzo Angotti, Emanuela Di Giacomo, Ina Valeria Zurlo, and et al. 2025. "Redefining the Use of Regorafenib and Trifluridine/Tipiracil Without Bevacizumab in Refractory Metastatic Colorectal Cancer: Findings from the ReTrITA Study" Cancers 17, no. 12: 2037. https://doi.org/10.3390/cancers17122037
APA StyleSignorelli, C., Calegari, M. A., Anghelone, A., Passardi, A., Gallio, C., Bittoni, A., Lucchetti, J., Angotti, L., Di Giacomo, E., Zurlo, I. V., Morelli, C., Dell’Aquila, E., Artemi, A., Gemma, D., Emiliani, A., Ribelli, M., Corsi, D. C., Arrivi, G., Mazzuca, F., ... Basso, M. (2025). Redefining the Use of Regorafenib and Trifluridine/Tipiracil Without Bevacizumab in Refractory Metastatic Colorectal Cancer: Findings from the ReTrITA Study. Cancers, 17(12), 2037. https://doi.org/10.3390/cancers17122037







