Validation of the Updated Porto Proposal in Papillary Thyroid Microtumors: Analysis of Cases at a University Hospital in Catalonia, Spain
Simple Summary
Abstract
1. Introduction
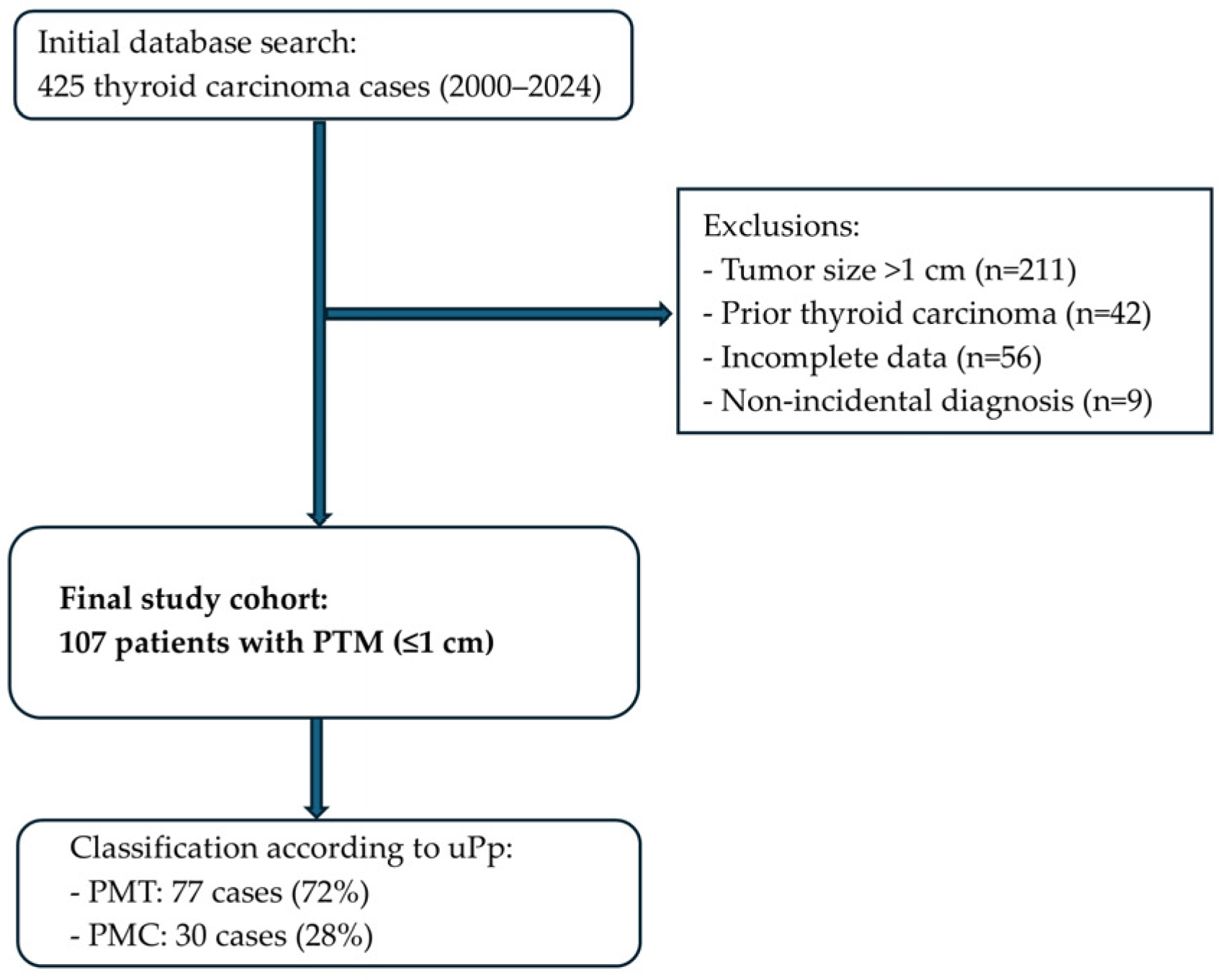
2. Materials and Methods
2.1. Study Design
2.2. Patient Selection
- (a)
- patients ≥18 years;
- (b)
- without a known family history of thyroid cancer;
- (c)
- for tumors detected incidentally;
- (d)
- measuring less than 1 cm (in the case of multiple PTMs the sum of their diameters does not exceed 1 cm);
- (e)
- lacking worrisome features.
2.3. Patient Selection Process
2.4. Data Collection
2.5. Statistical Analysis
2.6. Ethical Considerations
3. Results
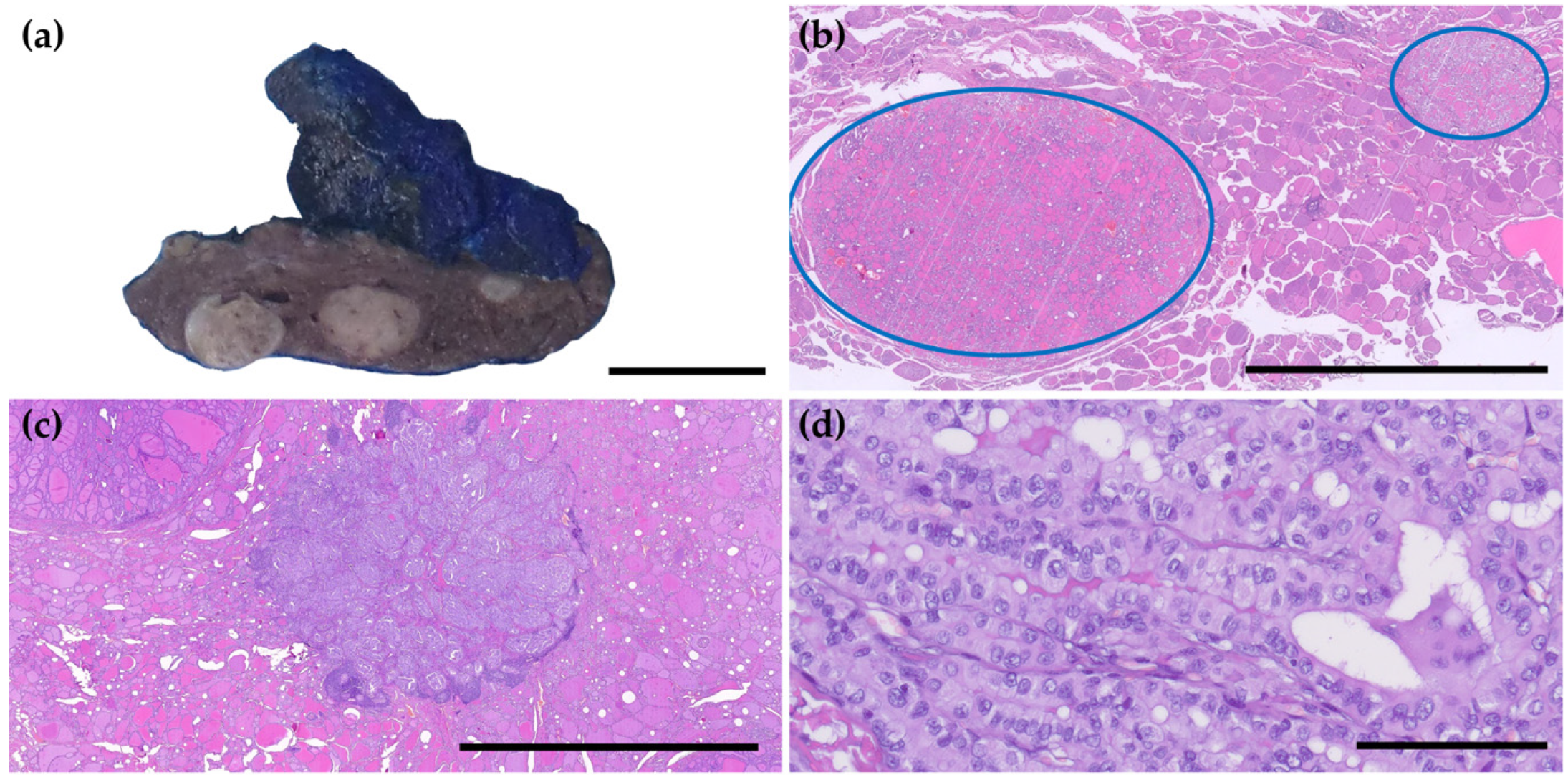
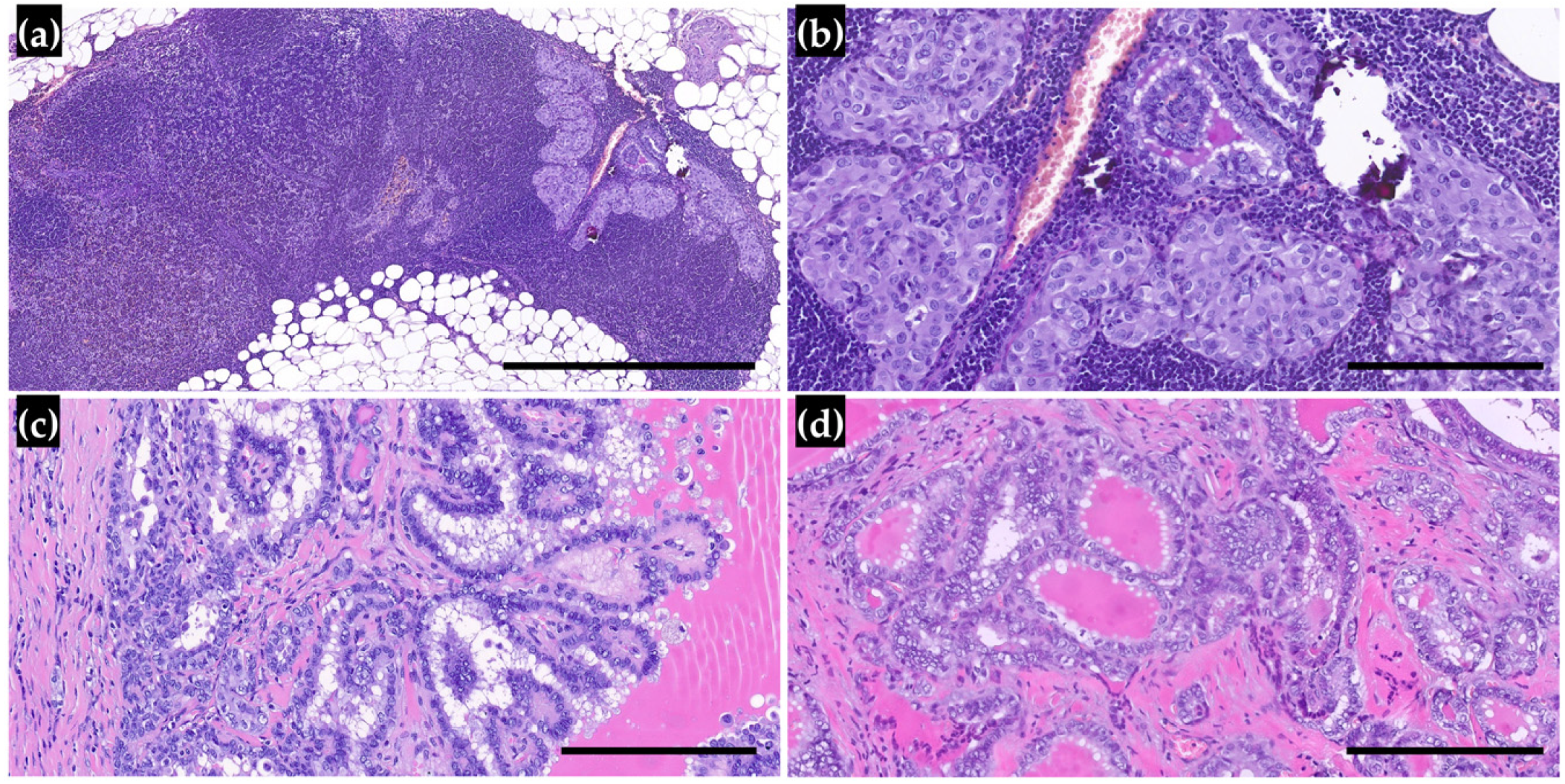
3.1. Clinicopathological Characteristics of PTMs and Reclassification
3.2. Lymph Node Metastasis and Outcomes in Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PTM | Papillary Thyroid Microcarcinoma |
| PMT | Papillary Microtumor |
| PMC | Papillary Microcarcinoma |
References
- Baloch, Z.W.; Asa, S.L.; Barletta, J.A.; Ghossein, R.A.; Juhlin, C.C.; Jung, C.K.; LiVolsi, V.A.; Papotti, M.G.; Sobrinho-Simões, M.; Tallini, G.; et al. Overview of the 2022 WHO Classification of Thyroid Neoplasms. Endocr. Pathol. 2022, 33, 27–63. [Google Scholar] [CrossRef] [PubMed]
- La Vecchia, C.; Malvezzi, M.; Bosetti, C.; Garavello, W.; Bertuccio, P.; Levi, F.; Negri, E. Thyroid Cancer Mortality and Incidence: A Global Overview. Int. J. Cancer 2015, 136, 2187–2195. [Google Scholar] [CrossRef] [PubMed]
- Kyrilli, A.; Schoinochoriti, R.; Chatzopoulos, V.; Bahar, N.; Bouziotis, J.; D’Haene, N.; Salmon, I.; Ruiz, M.; Corvilain, B. Thyrotropin (TSH) and Thyroid Autoimmunity Are Predictive Factors for the Incidental Discovery of Papillary Thyroid Microcarcinoma during Thyroidectomy. Endocrine 2024, 86, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wang, X.; Rui, Z.; Wang, Y.; Meng, Z.; Wang, R. Clinical Features and Therapeutic Outcomes of Patients with Papillary Thyroid Microcarcinomas and Larger Tumors. Nucl. Med. Commun. 2019, 40, 477–483. [Google Scholar] [CrossRef]
- Sugitani, I. Active Surveillance of Low-Risk Papillary Thyroid Microcarcinoma. Best Pract. Res. Clin. Endocrinol. Metab. 2023, 37, 101630. [Google Scholar] [CrossRef]
- Liang, Y.; Fan, E.X.; Zhang, J.; Xu, T.; Song, J.; Huang, F.; Wang, D. Construction and Validation of a Diagnostic Model for High-Risk Papillary Thyroid Microcarcinoma. Front. Endocrinol. 2024, 15, 1431584. [Google Scholar] [CrossRef]
- Rosai, J.; LiVolsi, V.A.; Sobrinho-Simoes, M.; Williams, E.D. Renaming Papillary Microcarcinoma of the Thyroid Gland: The Porto Proposal. Int. J. Surg. Pathol. 2003, 11, 249–251. [Google Scholar] [CrossRef]
- Aliyev, E.; Ladra-González, M.J.; Sánchez-Ares, M.; Abdulkader-Nallib, I.; Piso-Neira, M.; Rodríguez-Carnero, G.; Vieiro-Balo, P.; Pérez-Becerra, R.; Gude-Sampedro, F.; Barreiro-Morandeira, F.; et al. Thyroid Papillary Microtumor Validation of the (Updated) Porto Proposal Assessing Sex Hormone Receptor Expression and Mutational BRAF Gene Status. Am. J. Surg. Pathol. 2020, 44, 1161–1172. [Google Scholar] [CrossRef]
- Sutherland, R.; Tsang, V.; Clifton-Bligh, R.J.; Gild, M.L. Papillary Thyroid Microcarcinoma: Is Active Surveillance Always Enough? Clin. Endocrinol. 2021, 95, 811–817. [Google Scholar] [CrossRef]
- Bernstein, J.; Virk, R.K.; Hui, P.; Prasad, A.; Westra, W.H.; Tallini, G.; Adeniran, A.J.; Udelsman, R.; Sasaki, C.T.; Roman, S.A.; et al. Tall Cell Variant of Papillary Thyroid Microcarcinoma: Clinicopathologic Features with BRAFV600E Mutational Analysis. Thyroid 2013, 23, 1525–1531. [Google Scholar] [CrossRef]
- Rodolico, V.; Cabibi, D.; Pizzolanti, G.; Richiusa, P.; Gebbia, N.; Martorana, A.; Russo, A.; Amato, M.C.; Galluzzo, A.; Giordano, C. BRAFV600E Mutation and P27kip1 Expression in Papillary Carcinomas of the Thyroid ≤1 Cm and Their Paired Lymph Node Metastases. Cancer 2007, 110, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chen, G.; Sheng, C.; Gusdon, A.M.; Huang, Y.; Lv, Z.; Xu, H.; Xing, M.; Qu, S. BRAFV600E Mutation in Papillary Thyroid Microcarcinoma: A Meta-Analysis. Endocr. Relat. Cancer 2015, 22, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sadow, P.M.; Suh, H.; Lee, K.E.; Choi, J.Y.; Suh, Y.J.; Wang, T.S.; Lubitz, C.C. BRAF(V600E) Is Correlated with Recurrence of Papillary Thyroid Microcarcinoma: A Systematic Review, Multi-Institutional Primary Data Analysis, and Meta-Analysis. Thyroid Off. J. Am. Thyroid Assoc. 2016, 26, 248–255. [Google Scholar] [CrossRef]
- Vaccarella, S.; Dal Maso, L.; Laversanne, M.; Bray, F.; Plummer, M.; Franceschi, S. The Impact of Diagnostic Changes on the Rise in Thyroid Cancer Incidence: A Population-Based Study in Selected High-Resource Countries. Thyroid 2015, 25, 1127–1136. [Google Scholar] [CrossRef]
- Slijepcevic, N.; Zivaljevic, V.; Marinkovic, J.; Sipetic, S.; Diklic, A.; Paunovic, I. Retrospective Evaluation of the Incidental Finding of 403 Papillary Thyroid Microcarcinomas in 2466 Patients Undergoing Thyroid Surgery for Presumed Benign Thyroid Disease. BMC Cancer 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Lucandri, G.; Fiori, G.; Falbo, F.; Pende, V.; Farina, M.; Mazzocchi, P.; Santonati, A.; Bosco, D.; Spada, A.; Santoro, E. Papillary Thyroid Microcarcinoma: Differences between Lesions in Incidental and Nonincidental Settings—Considerations on These Clinical Entities and Personal Experience. Curr. Oncol. 2024, 31, 941–951. [Google Scholar] [CrossRef]
- Walgama, E.; Sacks, W.L.; Ho, A.S. Papillary Thyroid Microcarcinoma: Optimal Management versus Overtreatment. Curr. Opin. Oncol. 2020, 32, 1–6. [Google Scholar] [CrossRef]
- Spaziani, E.; Di Filippo, A.R.; Di Cristofano, C.; Tamagnini, G.T.; Spaziani, M.; Caruso, G.; Salina, G.; Valle, G.; Picchio, M.; De Cesare, A. Incidental Papillary Thyroid Microcarcinoma in Consecutive Patients Undergoing Thyroid Surgery for Benign Disease. A Single Center Experience. Ann. Ital. Chir. 2023, 94, 142–146. [Google Scholar]
- Mehanna, H.; Al-Maqbili, T.; Carter, B.; Martin, E.; Campain, N.; Watkinson, J.; McCabe, C.; Boelaert, K.; Franklyn, J.A. Differences in the Recurrence and Mortality Outcomes Rates of Incidental and Nonincidental Papillary Thyroid Microcarcinoma: A Systematic Review and Meta-Analysis of 21 329 Person-Years of Follow-Up. J. Clin. Endocrinol. Metab. 2014, 99, 2834–2843. [Google Scholar] [CrossRef]
- Jiwang, L.; Yahong, L.; Kai, L.; Bo, H.; Yuejiao, Z.; Haotian, W.; Tao, Y. Clinicopathologic Factors and Preoperative Ultrasonographic Characteristics for Predicting Central Lymph Node Metastasis in Papillary Thyroid Microcarcinoma: A Single Center Retrospective Study. Braz. J. Otorhinolaryngol. 2022, 88, 36–45. [Google Scholar] [CrossRef]
- Alqaryan, S.; Almousa, H.; Almutairi, R.; Altuwaijri, A.; Doubi, A.; Alqahtani, Z.; Almayouf, M.; Albarrak, M.; Alessa, M.; Aldhahri, S.; et al. Papillary Thyroid Microcarcinoma with and without Nodal Metastasis A Comparative Analysis. Saudi Med. J. 2024, 45, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Dirikoc, A.; Tam, A.A.; Ince, N.; Ozdemir, D.; Topaloglu, O.; Alkan, A.; Yazgan, A.K.; Ersoy, R.; Cakir, B. Papillary Thyroid Microcarcinomas That Metastasize to Lymph Nodes. Am. J. Otolaryngol. Head Neck Med. Surg. 2021, 42, 103023. [Google Scholar] [CrossRef]
- Shi, C.; Guo, Y.; Lv, Y.; Nanding, A.; Shi, T.; Qin, H.; He, J. Clinicopathological Features and Prognosis of Papillary Thyroid Microcarcinoma for Surgery and Relationships with the BRAFV600E Mutational Status and Expression of Angiogenic Factors. PLoS ONE 2016, 11, e0167414. [Google Scholar] [CrossRef] [PubMed]
- Cupisti, K.; Lehwald, N.; Anlauf, M.; Riemer, J.; Werner, T.A.; Krieg, A.; Witte, J.; Chanab, A.; Baldus, S.E.; Krausch, M.; et al. Encapsulation Status of Papillary Thyroid Microcarcinomas Is Associated with the Risk of Lymph Node Metastases and Tumor Multifocality. Horm. Metab. Res. 2014, 46, 138–144. [Google Scholar] [CrossRef]
- Amendola, S.; Piticchio, T.; Scappaticcio, L.; Sellasie, S.W.; Volpe, S.; Le Moli, R.; Coppola, L.; Guidobaldi, L.; Pedicini, F.; Carbone, C.; et al. Papillary Thyroid Carcinoma: ≤ 10 Mm Does Not Always Mean PN0. A Multicentric Real-World Study. Updates Surg. 2024, 76, 1055–1061. [Google Scholar] [CrossRef]
- Brito, J.P.; Hay, I.D. Management of Papillary Thyroid Microcarcinoma. Endocrinol. Metab. Clin. N. Am. 2019, 48, 199–213. [Google Scholar] [CrossRef]
- Tuttle, R.M.; Fagin, J.A.; Minkowitz, G.; Wong, R.J.; Roman, B.; Patel, S.; Untch, B.; Ganly, I.; Shaha, A.R.; Shah, J.P.; et al. Natural History and Tumor Volume Kinetics of Papillary Thyroid Cancers During Active Surveillance. JAMA Otolaryngol. Head Neck Surg. 2017, 143, 1015–1020. [Google Scholar] [CrossRef]
- Sugitani, I.; Ito, Y.; Takeuchi, D.; Nakayama, H.; Masaki, C.; Shindo, H.; Teshima, M.; Horiguchi, K.; Yoshida, Y.; Kanai, T.; et al. Indications and Strategy for Active Surveillance of Adult Low-Risk Papillary Thyroid Microcarcinoma: Consensus Statements from the Japan Association of Endocrine Surgery Task Force on Management for Papillary Thyroid Microcarcinoma. Thyroid 2021, 31, 183–192. [Google Scholar] [CrossRef]
- Mauri, G.; Hegedüs, L.; Bandula, S.; Cazzato, R.L.; Czarniecka, A.; Dudeck, O.; Fugazzola, L.; Netea-Maier, R.; Russ, G.; Wallin, G.; et al. European Thyroid Association and Cardiovascular and Interventional Radiological Society of Europe 2021 Clinical Practice Guideline for the Use of Minimally Invasive Treatments in Malignant Thyroid Lesions. Eur. Thyroid J. 2021, 10, 185–197. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Faro, F.N.; Bertelli, A.A.T.; Scalissi, N.M.; Cury, A.N.; Padovani, R.D.P.; Ferraz, C. Active Surveillance versus Immediate Surgery in the Management of Low-Risk Papillary Thyroid Microcarcinoma: Comparison of Long-Term Costs in Brazil. Arch. Endocrinol. Metab. 2024, 68. [Google Scholar] [CrossRef] [PubMed]
- Moten, A.S.; Zhao, H.; Willis, A.I. The Overuse of Radioactive Iodine in Low-Risk Papillary Thyroid Cancer Patients. Surg. Oncol. 2019, 29, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Jang, H.W.; Ahn, H.S.; Ahn, S.; Park, S.Y.; Oh, Y.L.; Hahn, S.Y.; Shin, J.H.; Kim, J.H.; Kim, J.S.; et al. High Serum TSH Level Is Associated with Progression of Papillary Thyroid Microcarcinoma during Active Surveillance. J. Clin. Endocrinol. Metab. 2018, 103, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Scherer, H.C.; Fernandes, P.M.; Scheffel, R.S.; Zanella, A.B.; Maia, A.L.; Dora, J.M. Papillary Thyroid Microcarcinoma: Insights from a Cohort of 257 Thyroidectomized Patients. Horm. Metab. Res. 2023, 55, 161–168. [Google Scholar] [CrossRef]
| PMC N (%) | PMT N (%) | p-Value | ||
|---|---|---|---|---|
| Sex | Male | 9 (30) | 21 (70) | 0.7779 |
| Female | 21 (27.3) | 56 (72.7) | ||
| Location | Right lobe | 15 (31.9) | 32 (68.1) | 0.2079 |
| Left lobe | 9 (20.5) | 35 (79.5) | ||
| Isthmus | 2 (25) | 6 (75) | ||
| Both lobes | 4 (57.1) | 3 (42.9) | ||
| Focality | Unifocal | 18 (21.7) | 65 (78.3) | 0.0041 |
| Multifocal | 12 (52.2) | 11 (47.8) | ||
| Subtype | Classic | 10 (35.7) | 18 (64.3) | <0.0001 |
| Follicular | 10 (15.4) | 55 (84.6) | ||
| Tall cell | 7 (100) | 0 (0) | ||
| Oncocytic | 1 (25) | 3 (75) | ||
| Solid | 1 (50) | 1 (50) | ||
| Warthin-like | 1 (100) | 0 (0) | ||
| Treatment | Right HT | 5 (29.4) | 12 (70.6) | 0.5813 |
| Left HT | 3 (17.6) | 14 (82.4) | ||
| TT | 22 (30.1) | 51 (69.9) | ||
| Mean | Mean | |||
| Age at diagnosis | 50.23 | 56.12 | 0.0811 | |
| Size (mm) | 4.53 | 3.26 | 0.0147 | |
| LNM N (%) | No LNM N (%) | p-Value | ||
|---|---|---|---|---|
| PMC/PMT | PMC | 9 (30) | 21 (70) | 0.0004 |
| PMT | 4 (5.2) | 73 (94.8) | ||
| Sex | Male | 3 (10) | 27 (90) | 0.671 |
| Female | 10 (13) | 67 (87) | ||
| Location | Right lobe | 4 (8.5) | 43 (91.5) | 0.0027 |
| Left lobe | 4 (9.1) | 40 (90.9) | ||
| Isthmus | 1 (12.5) | 7 (87.5) | ||
| Both lobes | 4 (57.1) | 3 (42.9) | ||
| Focality | Unifocal | 5 (6) | 78 (94) | 0.0002 |
| Multifocal | 8 (34.8) | 15 (65.2) | ||
| Incidental finding | Yes | 3 (4.2) | 68 (95.8) | 0.0004 |
| No | 10 (27.8) | 26 (72.2) | ||
| Subtype | Classic | 7 (25) | 21 (75) | 0.0465 |
| Follicular | 3 (4.6) | 62 (95.4) | ||
| Tall cell | 1 (14.3) | 6 (85.7) | ||
| Oncocytic | 1 (25) | 3 (75) | ||
| Solid | 1 (50) | 1 (50) | ||
| Warthin-like | 0 (0) | 1 (100) | ||
| Treatment | Right HT | 1 (5.9) | 16 (94.1) | 0.3997 |
| Left HT | 1 (5.9) | 16 (94.1) | ||
| TT | 11 (15.1) | 62 (84.9) | ||
| Mean | Mean | |||
| Age at diagnosis | 47 | 55.5 | 0.0807 | |
| Size (mm) | 4.62 | 3.48 | 0.1188 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saez de Gordoa, K.; Tasso, E.; Rei, A.; Ramonda, M.; Salinas, B.; Cobo-Lopez, S.; Orois, A.; Cobo, A.; Manyalich-Blasi, M.; Ramón y Cajal, T.; et al. Validation of the Updated Porto Proposal in Papillary Thyroid Microtumors: Analysis of Cases at a University Hospital in Catalonia, Spain. Cancers 2025, 17, 2021. https://doi.org/10.3390/cancers17122021
Saez de Gordoa K, Tasso E, Rei A, Ramonda M, Salinas B, Cobo-Lopez S, Orois A, Cobo A, Manyalich-Blasi M, Ramón y Cajal T, et al. Validation of the Updated Porto Proposal in Papillary Thyroid Microtumors: Analysis of Cases at a University Hospital in Catalonia, Spain. Cancers. 2025; 17(12):2021. https://doi.org/10.3390/cancers17122021
Chicago/Turabian StyleSaez de Gordoa, Karmele, Elias Tasso, Alexandre Rei, Martin Ramonda, Belinda Salinas, Sandra Cobo-Lopez, Aida Orois, Amparo Cobo, Marti Manyalich-Blasi, Teresa Ramón y Cajal, and et al. 2025. "Validation of the Updated Porto Proposal in Papillary Thyroid Microtumors: Analysis of Cases at a University Hospital in Catalonia, Spain" Cancers 17, no. 12: 2021. https://doi.org/10.3390/cancers17122021
APA StyleSaez de Gordoa, K., Tasso, E., Rei, A., Ramonda, M., Salinas, B., Cobo-Lopez, S., Orois, A., Cobo, A., Manyalich-Blasi, M., Ramón y Cajal, T., Mora, M., Hanzu, F., Vidal Pérez, O., & Rodrigo-Calvo, M. T. (2025). Validation of the Updated Porto Proposal in Papillary Thyroid Microtumors: Analysis of Cases at a University Hospital in Catalonia, Spain. Cancers, 17(12), 2021. https://doi.org/10.3390/cancers17122021







