Fluorescence Guidance in Glioma Surgery: A Narrative Review of Current Evidence and the Drive Towards Objective Margin Differentiation
Simple Summary
Abstract
1. Introduction
2. 5-Aminolevulinic Acid
2.1. Use Case
2.1.1. Patient Population
2.1.2. Dose, Administration and Timing
2.1.3. Side Effects and Safety
2.2. Equipment
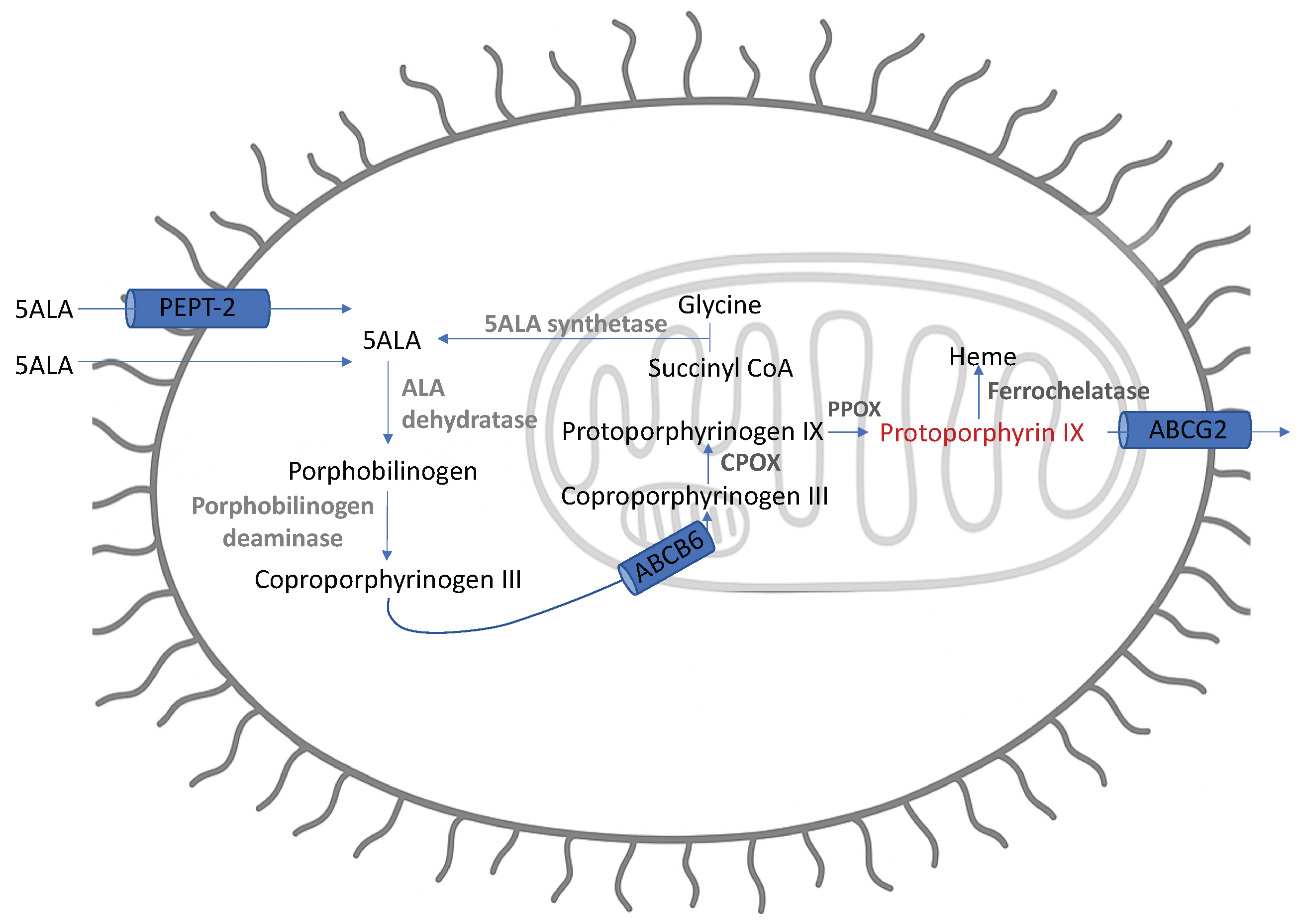
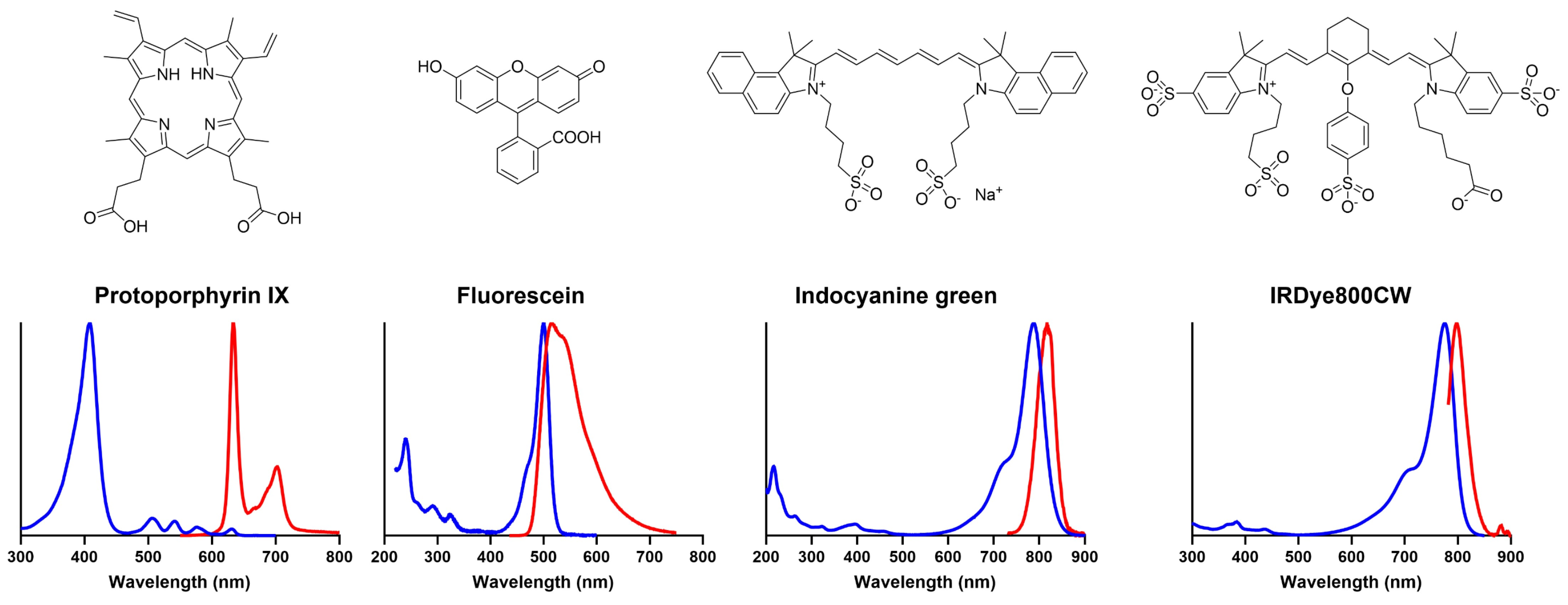
2.3. Mechanism of Action
2.3.1. Increased Intracellular Synthesis and Retention
2.3.2. Immune Synthesis
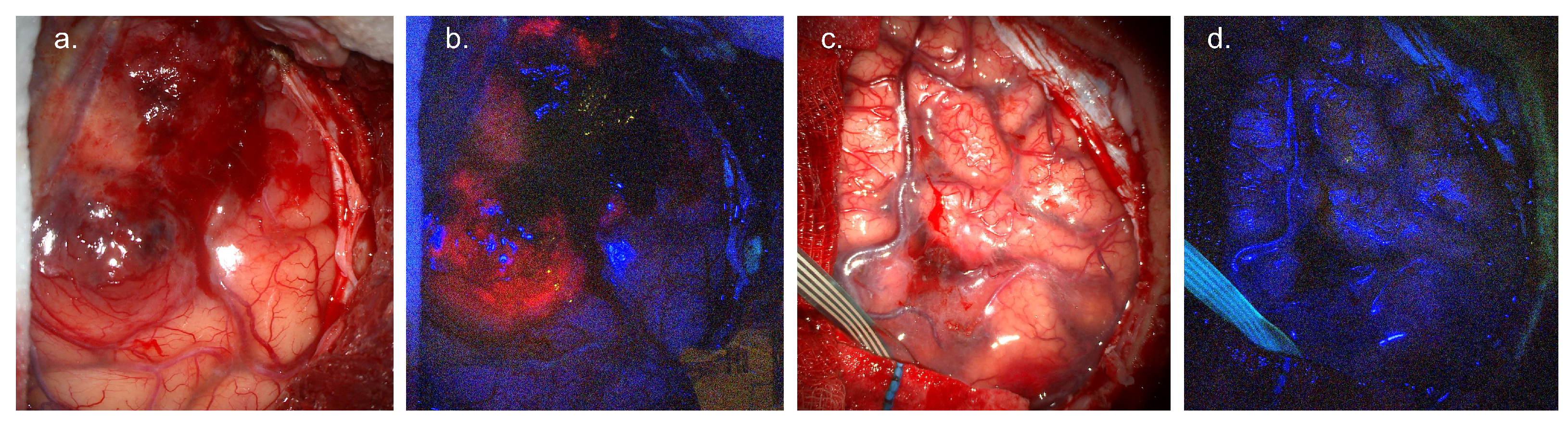
2.4. Evidence Base
2.4.1. Diagnostic Accuracy
2.4.2. Clinical Efficacy
| Study | Study Type | n | EOR/GTR | OS | PFS | Complications | Notes |
|---|---|---|---|---|---|---|---|
| Picart 2023 [31] | Phase 3 RCT 5-ALA vs. WL | 147 5-ALA: 67 WL: 69 | GTR 5-ALA: 79% WL: 47.8% | 24m 5-ALA: 30.1% WL: 37.7% | 6m 5-ALA: 70.2% WL: 68.4% | Deficit at 3m 5-ALA: 13.2% WL: 12.9% | All SOC neuronav used. Post-op protocol RT + CTh (Stupp). |
| Stummer 2006 [8] | Phase 3 RCT 5-ALA vs. WL | 270 5-ALA: 139 WL: 131 | GTR 5-ALA: 65% WL: 36% | 5-ALA: 15.2m WL: 13.5m | 6m 5-ALA: 41% WL: 21% Median 5-ALA: 5.1m WL: 3.6m | NR | Neuro-navigation precluded. Post-op protocol recommended RT only. Industry sponsored. |
| Eljamel 2008 [65] | Prospective single-centre RCT | 27 5-ALA: 13 WL: 14 | GTR 5-ALA: 77% WL: 29% | NR | 6m 5-ALA: 80% WL: 70% Mean 5-ALA: 52.8w WL: 24.2w | Neurology NR | Combination of FGS and Photofin (R) used in 5-ALA group. Post-op protocol for RT alone. n = 7 also received CTh. |
2.5. Limitations
2.6. Regulatory Issues
2.7. Emerging Use Cases
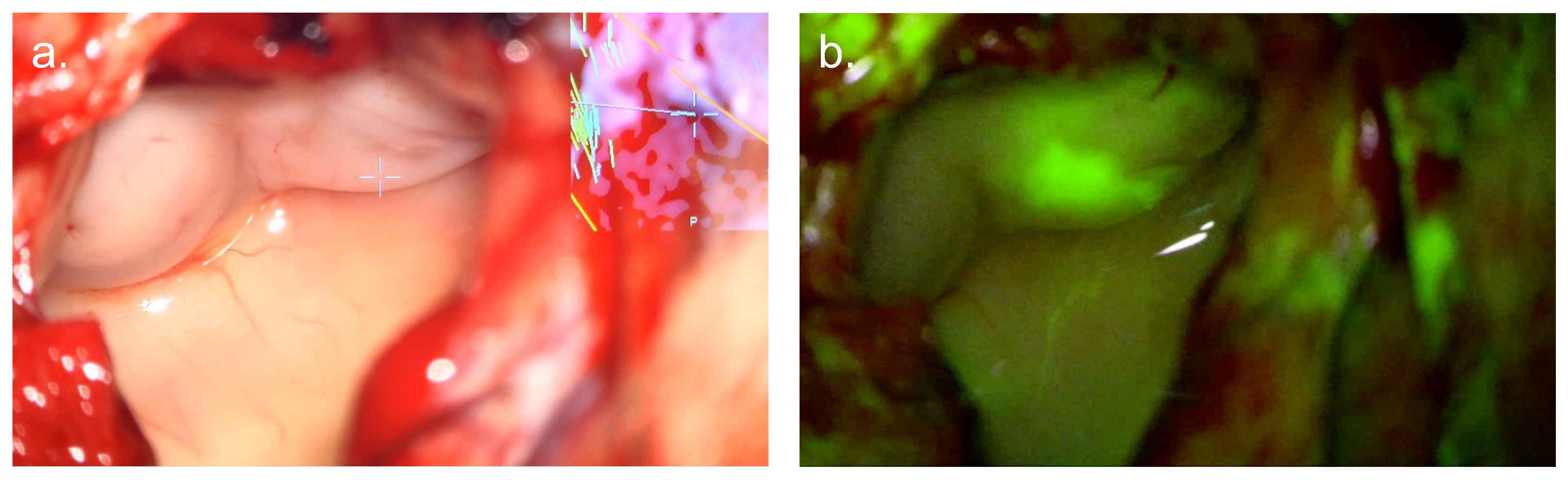
3. Fluorescein
3.1. Use Case
3.1.1. Patient Population
3.1.2. Dose, Administration and Timing
3.1.3. Side Effects and Safety
3.2. Equipment
3.3. Mechanism of Action
3.4. Evidence Base
3.4.1. Diagnostic Accuracy
3.4.2. Clinical Efficacy
3.5. Limitations
3.6. Regulatory Issues
| Study | Study Type | n | EOR/GTR | OS | PFS | Complications | Notes |
|---|---|---|---|---|---|---|---|
| Ling 2024 [107] | Prospective non-randomised | 90 FS LD: 30 FS StD: 30 Cont: 30 | GTR% FS LD: 90 FS StD: 86.7 Cont: 66.3 | — | 6m% FS LD: 90 FS StD: 86.7 Cont: 66.3 | Dependent 6m: FS LD: 10% FS StD: 13.3% Cont: 36.7% | LD: 1 mg/kg StdD: 5 mg/kg Adm: Post-intubation Historic single-centre control |
| Falco 2019/2023 [128,129] | Prospective non-randomised | 279 HGG: 128 GBM: 93 LGG: 11 | GTR (%) HGG: 74.2 GBM: 82.8 | Median (m) GBM: 16 | Median (m) GBM: 12 | No adverse reactions | 5 mg/kg Adm: After induction No LGG fluorescence Retrospective survival analysis |
| Acerbi 2018 [106] | Prospective, multicentric phase II, FLUOGLIO | 46 | GTR FS: 82.6 5-ALA: 32 WL: 36 | Median (m) 12 | Median (m) 7 6m: 56.6 12m: 15.2 | No FS related AE KPS returned to baseline 3m | 5–10 mg/kg Adm: After induction 30 adjacent to eloquent areas STUPP [2] completed in 20% |
| Chen 2012 [130] | Prospective non-randomised | 22 FS: 10 Control: 12 | GTR (%) FS: 80 Control: 33.3 | — | Median (m) FS: 7.2 Control: 4.8 | No significant difference in KPS between groups | 15–20 mg/kg WL guided Adm: Following dural opening HGG: 11 LGG: 11 |
| Koc 2008 [131] | Prospective non-randomised | 80 FS: 47 Control: 33 | GTR (%) FS: 83 WL: 55 | Median (w) FS: 44 WL: 42 | - | No significant difference in KPS between groups | 20 mg/kg WL guided Adm: Prior to dural opening |
4. ICG
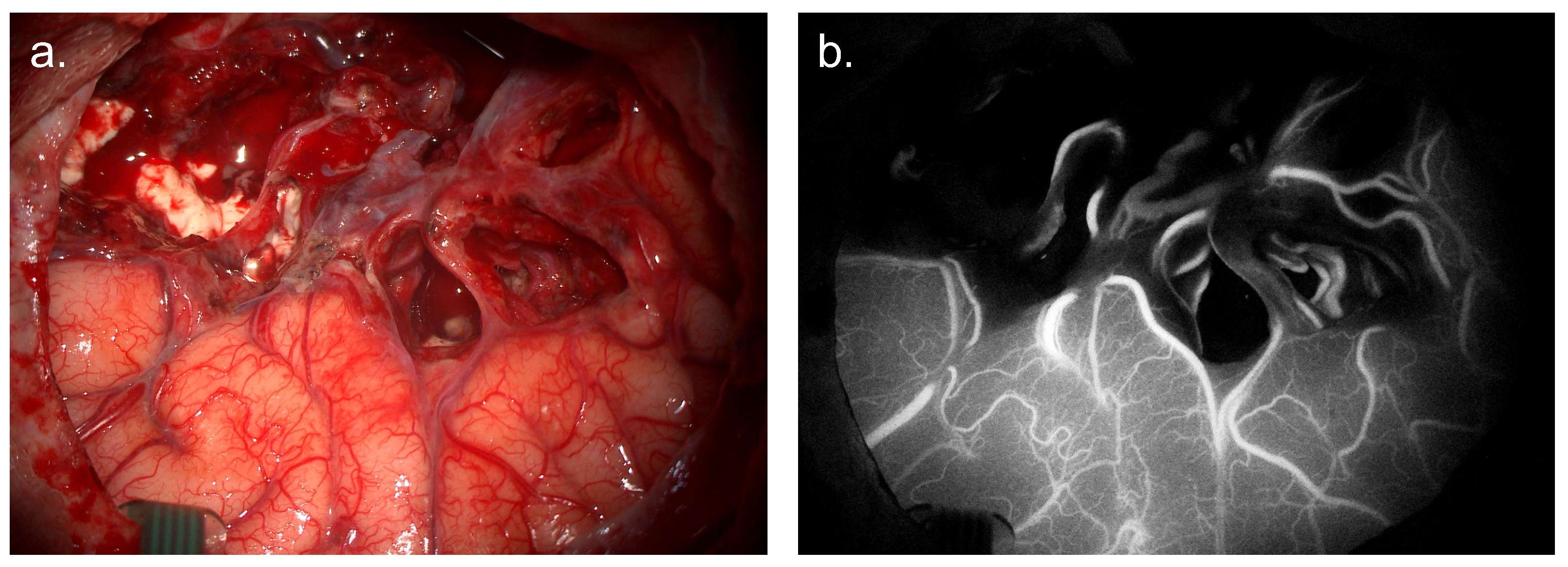
4.1. ICG Angiography
4.1.1. Dose, Administration and Timing
4.1.2. Mechanism of Action
4.1.3. Evidence Base
4.2. ICG Second-Window Tumour Differentiation
4.2.1. Mechanism of Action
4.2.2. Dose, Administration, and Timing
4.2.3. Evidence Base
4.3. Side Effects and Safety
4.4. Equipment
4.5. Limitations
4.6. Regulatory Issues
5. Towards Objective Intra-Operative Fluorescence
5.1. Steady-State Fluorescence Spectroscopy
5.2. Quantitative Fluorescence
5.2.1. Optical Distortion Correction
5.2.2. Fluorescence Unmixing
5.2.3. Emission Form
5.3. Time-Resolved Fluorescence Decay
5.4. Confocal Laser Endomicroscopy
5.5. Future Prospects
6. Novel Fluorophore Development
6.1. Tumour Targeting
6.2. Enhanced Fluorescence
6.3. Barriers to Novel Fluorophores
6.4. Future Prospects
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GTR | gross total resection |
| HGG | high-grade glioma |
| LGG | low-grade glioma |
| CNS | central nervous system |
| IM | infiltrative margin |
| FGS | fluorescence-guided surgery |
| EOR | extent of resection |
| 5-ALA | 5-aminoleuvulinic acid |
| FS | fluorescein sodium |
| ICG | indocyanine green |
| PpIX | protoporphyrin IX |
| EMA | European Medicines Agency |
| FDA | Federal Drugs Agency |
| WHO | World Health Organisation |
| NICE | National Institute for Health and Care Excellence |
| ABCG2 | ATP-binding cassette G2 |
| ABCB6 | ATP-binding cassette B6 |
| CPOX | coproporphyrinogen III oxidase |
| PPOX | protoporphyrinogen oxidase |
| MRNA | messenger RNA |
| FECH | ferrochelatase |
| PPV | positive predictive value |
| NPV | negative predictive value |
| Sens | sensitivity |
| Spec | specificity |
| GBM | glioblastoma multiforme |
| RCT | randomised control trial |
| PFS | progression-free survival |
| OS | overall survival |
| iMRI | intraoperative magnetic resonance imaging |
| FLAIR | fluid attenuated inversion recovery |
| WL | white light |
| SOC | standard of care |
| RT | radiotherapy |
| CTh | chemotherapy |
| DVT | deep-vein thrombosis |
| QALY | quality-adjusted life year |
| Da | Daltons |
| LD | low dose |
| StD | standard dose |
| D | dose |
| Adm | administration |
| CE | contrast enhancing |
| NCE | non-contrast enhancing |
| Lymph | lymphoma |
| Met | metastases |
| CRET | complete resection of enhancing tumour |
| KPS | Karnofsky performance score |
| NANO | neurologic assessment in neuro-oncology |
| AE | adverse event |
| m | months |
| w | weeks |
| NIR | near-infrared |
| SWIR | short-wave infrared |
| FS | frozen section |
| EPR | enhanced permeability and retention |
| QF | quantitative fluorescence |
| HSI | hyperspectral Imaging |
| EGFR | epidermal growth factor receptor |
Appendix A
| Publication | Pathology | n | Sens (%) | Spec (%) | PPV(%) | NPV (%) | Notes |
|---|---|---|---|---|---|---|---|
| Schupper 2021 [30] | HGG | 65 | 98.5 | 29.4 | 95.4 | 35.7 | New + Recurrent |
| Coburger 2017 [52] | GBM | 33 | 84 | 100 | - | - | 99 biopsies in 33 patients New diagnoses |
| Hauser 2016 [51] | GBM | 12 | 91 | 43 | 96 | 12.5 | New diagnoses |
| Lau 2016 [50] | HGG | 59 | 81.7 84.2: GBM | 64.5 62.1: GBM | 93 91.8: GBM | 37.7 43.9: GBM | New + Recurrent |
| Yamada 2015 [49] | HGG | 97 | 95 99: Core | 53 | 92 | 69 | New + Recurrent |
| Coburger 2014 [48] | HGG | 34 | 91 | 80 | 99 | 22 | New + Recurrent |
| Stummer 2014 [47] | GBM | 22 | - | - | 96 100: Strong 95: Vague | 39.5 | New diagnoses Results from visible fluorescence |
| Panciani 2012 [204] | GBM | 23 | 91 | 89 | 89 | 91 | New diagnoses |
| Ewelt 2011 [17] | HGG | 17 | 70.6 | 92.3 | - | - | New diagnoses HGG subgroup analysis |
| Diez Valle 2011 [205] | GBM | 36 | - | - | 100: Strong 97: Vague | 66 | New + Recurrent |
| Roberts 2011 [206] | GBM | 11 | 75 | 71 | 95 | 26 | New diagnoses |
| Nabavi 2009 [207] | HGG | 36 | 82 | 97 | 96.6 98.2: Strong 95.3: Weak | - | Recurrent Biopsy based analysis |
| Hefti 2008 [208] | HGG | 57 | 87 100: Strong 76: Weak | 85 98: Strong 85: Weak | - | - | New diagnoses |
| Stummer 2000 [25] | GBM | 52 | 89 | 96 | 99 | 50 | New diagnoses |
| Stummer 1998 [55] | HGG | 9 | 85 | 100 | - | - | New diagnoses 10mg/kg dose |
| Publication | Pathology | n | Sens (%) | Spec (%) | PPV(%) | NPV (%) | Notes |
|---|---|---|---|---|---|---|---|
| Ling 2024 [107] | HGG LGG | 23 7 | LD: 93.5 StD: 91.7 LD: 64.3 StD: 66.47 | LD: 82.6 StD 81 LD: 57.1 StD: 61.1 | - | - | LD: 1 mg/kg StD: 5 mg/kg Adm: After induction of anaesthesia HGG: 138 biopsies LGG 42 biopsies Filtered microscope |
| Sweeney 2022 [123] | HGG | 34 | 62 | 100 | 100 | 81 | D: 5 mg/kg Adm: After induction of anaesthesia Subjective cavity assessment with MRI 560 nm customised filter |
| Hong 2019 [209] | HGG | 42 | 90.8 | 93.3 | - | - | D: 1.5–2 mg/kg Adm: 90 min prior to dural opening 87 biopsies Filtered microscope |
| Chen 2019 [210] | HGG | 49 | 91.7 | 90 | - | - | D: 5 mg/kg Adm: Immediately prior to anaesthetic 98 biopsies at boundary only Filtered microscope |
| Acerbi 2018 [106] | HGG | 13 | 80.8 | 79.1 | 80.8 | 79.1 | D: 5–10 mg/kg Adm: After induction of anaesthesia 50 biopsies Filtered microscope |
| Neira 2017 [110] | GBM | 32 | 75.6 CE: 87.9 NCE: 69.4 | 75 CE: - NCE: 66.7 | 96 CE: 98.6 NCE: 96.2 | - | D: 3 mg/kg Adm: After induction of anaesthesia 90 biopsies Filtered microscope |
| Catapano 2017 [211] | HGG | 23 | 84.61 | 95 | - | - | D: 5 mg/kg Adm: At induction of anaesthesia Biopsy no not reported Filtered microscope |
| Diaz 2015 [127] | HGG | 12 | 82.8 | 90.9 | - | - | D: 3 mg/kg Adm: After induction of anaesthesia 67 biopsies at boundary only Filtered microscope |
| Murray 1982 [212] | HGG LGG Lypmh Met | 14 2 3 3 | 85 | 95 | - | - | D: 10–20 mL 10% Adm: At induction of anaesthesia 186 biopsies WL only |
| Publication | Pathology | n | Sens (%) | Spec (%) | PPV(%) | NPV (%) | Notes |
|---|---|---|---|---|---|---|---|
| Lee 2016 [140] | HGG LGG | 11 4 | 98 | 45 | 82 | 90 | D: 5 mg/kg Adm: 24 h pre-op Calculation from fluorescent tumours only (n = 12) False + ve in gliosis, choroid plexus, scar tissue |
| Zeh 2017 [147] | GBM | 10 | 85.7 | 25 | 85.7 | 25 | D: 5 mg/kg Adm: 24 h pre-op High sensitivity, low specificity |
| Cho 2020 [144] | HGG | 36 | 97 | 56 | 94 | 71 | D: 2.5–5 mg/kg Adm: 24 h pre-op 78 biopsies Dose reduced at mid point following dose-reduction study [143] |
| Shen 2021 [145] | HGG | 23 | 93.8 | 82.2 | 91 | 87.2 | D: 1 mg/kg Adm: 48 h pre-op 1874 biopsies NIR II Fluorescence Use of CNN for classification 70% train 30% test |
| Shi 2022 [146] | HGG | 15 | 100 | 91.36 | 95.65 | 100 | D: 1 mg/kg Adm: 48 h pre-op 235 biopsies NIR II fluorescence Ex-vivo biopsy analysis |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Chaichana, K.L.; Jusue-Torres, I.; Navarro-Ramirez, R.; Raza, S.M.; Pascual-Gallego, M.; Ibrahim, A.; Hernandez-Hermann, M.; Gomez, L.; Ye, X.; Weingart, J.D.; et al. Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro-Oncol. 2014, 16, 113–122. [Google Scholar] [CrossRef]
- Hervey-Jumper, S.L.; Berger, M.S. Role of surgical resection in low- and high-grade gliomas. Curr. Treat. Options Neurol. 2014, 16, 284. [Google Scholar] [CrossRef]
- Plaha, P.; Camp, S.; Cook, J.; McCulloch, P.; Voets, N.; Ma, R.; Taphoorn, M.J.; Dirven, L.; Grech-Sollars, M.; Watts, C.; et al. FUTURE-GB: Functional and ultrasound-guided resection of glioblastoma—A two-stage randomised control trial. BMJ Open 2022, 12, e064823. [Google Scholar] [CrossRef] [PubMed]
- Jusue-Torres, I.; Lee, J.; Germanwala, A.V.; Burns, T.C.; Parney, I.F. Effect of Extent of Resection on Survival of Patients with Glioblastoma, IDH–Wild-Type, WHO Grade 4 (WHO 2021): Systematic Review and Meta-Analysis. World Neurosurg. 2023, 171. [Google Scholar] [CrossRef]
- Moore, G.E. Fluorescein as an Agent in the Differentiation of Normal and Malignant Tissues. Science 1947, 106, 130–131. [Google Scholar] [CrossRef]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.J.; Group, A.L.G.S. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Schupper, A.J.; Rao, M.; Mohammadi, N.; Baron, R.; Lee, J.Y.K.; Acerbi, F.; Hadjipanayis, C.G. Fluorescence-Guided Surgery: A Review on Timing and Use in Brain Tumor Surgery. Front. Neurol. 2021, 12, 682151. [Google Scholar] [CrossRef]
- Orillac, C.; Stummer, W.; Orringer, D.A. Fluorescence Guidance and Intraoperative Adjuvants to Maximize Extent of Resection. Neurosurgery 2021, 89, 727–736. [Google Scholar] [CrossRef]
- Picart, T.; Gautheron, A.; Caredda, C.; Ray, C.; Mahieu-Williame, L.; Montcel, B.; Guyotat, J. Fluorescence-Guided Surgical Techniques in Adult Diffuse Low-Grade Gliomas: State-of-the-Art and Emerging Techniques: A Systematic Review. Cancers 2024, 16, 2698. [Google Scholar] [CrossRef] [PubMed]
- Sachar, M.; Anderson, K.E.; Ma, X. Protoporphyrin IX: The good, the bad, and the ugly. J. Pharmacol. Exp. Ther. 2016, 356, 267–275. [Google Scholar] [CrossRef]
- Stummer, W.; Stocker, S.; Novotny, A.; Heimann, A.; Sauer, O.; Kempski, O.; Plesnila, N.; Wietzorrek, J.; Reulen, H.J. In vitro and in vivo porphyrin accumulation by C6 glioma cells after exposure to 5-aminolevulinic acid. J. Photochem. Photobiol. B Biol. 1998, 45, 160–169. [Google Scholar] [CrossRef]
- Hadjipanayis, C.G.; Stummer, W. 5-ALA and FDA approval for glioma surgery. J. Neurooncol. 2019, 141, 479–486. [Google Scholar] [CrossRef]
- NICE. Brain Tumours (Primary) and Brain Metastases in Over 16s NICE Guideline. In NICE Guideline; National Institute of Health and Care Excellence: London, UK, 2018. [Google Scholar]
- Widhalm, G.; Wolfsberger, S.; Minchev, G.; Woehrer, A.; Krssak, M.; Czech, T.; Prayer, D.; Asenbaum, S.; Hainfellner, J.A.; Knosp, E. 5-Aminolevulinic acid is a promising marker for detection of anaplastic foci in diffusely infiltrating gliomas with nonsignificant contrast enhancement. Cancer 2010, 116, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Ewelt, C.; Floeth, F.W.; Felsberg, J.; Steiger, H.J.; Sabel, M.; Langen, K.J.; Stoffels, G.; Stummer, W. Finding the anaplastic focus in diffuse gliomas: The value of Gd-DTPA enhanced MRI, FET-PET, and intraoperative, ALA-derived tissue fluorescence. Clin. Neurol. Neurosurg. 2011, 113, 541–547. [Google Scholar] [CrossRef]
- Widhalm, G.; Kiesel, B.; Woehrer, A.; Traub-Weidinger, T.; Preusser, M.; Marosi, C.; Prayer, D.; Hainfellner, J.A.; Knosp, E.; Wolfsberger, S. 5-Aminolevulinic acid induced fluorescence is a powerful intraoperative marker for precise histopathological grading of gliomas with non-significant contrast-enhancement. PLoS ONE 2013, 8, e76988. [Google Scholar] [CrossRef] [PubMed]
- Jaber, M.; Wolfer, J.; Ewelt, C.; Holling, M.; Hasselblatt, M.; Niederstadt, T.; Zoubi, T.; Weckesser, M.; Stummer, W. The Value of 5-Aminolevulinic Acid in Low-grade Gliomas and High-grade Gliomas Lacking Glioblastoma Imaging Features: An Analysis Based on Fluorescence, Magnetic Resonance Imaging, 18F-Fluoroethyl Tyrosine Positron Emission Tomography, and Tumor Molecular Factors. Neurosurgery 2016, 78, 401–411. [Google Scholar] [CrossRef]
- Kiesel, B.; Freund, J.; Reichert, D.; Wadiura, L.; Erkkilae, M.T.; Woehrer, A.; Hervey-Jumper, S.; Berger, M.S.; Widhalm, G. 5-ALA in Suspected Low-Grade Gliomas: Current Role, Limitations, and New Approaches. Front. Oncol. 2021, 11, 699301. [Google Scholar] [CrossRef]
- Bianconi, A.; Bonada, M.; Zeppa, P.; Colonna, S.; Tartara, F.; Melcarne, A.; Garbossa, D.; Cofano, F. How Reliable Is Fluorescence-Guided Surgery in Low-Grade Gliomas? A Systematic Review Concerning Different Fluorophores. Cancers 2023, 15, 4130. [Google Scholar] [CrossRef]
- Jaber, M.; Ewelt, C.; Wolfer, J.; Brokinkel, B.; Thomas, C.; Hasselblatt, M.; Grauer, O.; Stummer, W. Is Visible Aminolevulinic Acid-Induced Fluorescence an Independent Biomarker for Prognosis in Histologically Confirmed (World Health Organization 2016) Low-Grade Gliomas? Neurosurgery 2019, 84, 1214–1224. [Google Scholar] [CrossRef] [PubMed]
- medac GmbH. Gliolan 30 mg/mL Powder for Oral Solution SmPC; medac GmbH: Wedel, Germany, 2023. [Google Scholar]
- Giakoumettis, D.; Kritis, A.; Foroglou, N. C6 cell line: The gold standard in glioma research. Hippokratia 2018, 22, 105–112. [Google Scholar]
- Stummer, W.; Novotny, A.; Stepp, H.; Goetz, C.; Bise, K.; Reulen, H.J. Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: A prospective study in 52 consecutive patients. J. Neurosurg. 2000, 93, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Suero Molina, E. Fluorescence Imaging/Agents in Tumor Resection. Neurosurg. Clin. N. Am. 2017, 28, 569–583. [Google Scholar] [CrossRef]
- Molina, E.S.; Black, D.; Kaneko, S.; Müther, M.; Stummer, W. Double dose of 5-aminolevulinic acid and its effect on protoporphyrin IX accumulation in low-grade glioma. J. Neurosurg. 2022, 137, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, S.; Suero Molina, E.; Ewelt, C.; Warneke, N.; Stummer, W. Fluorescence-Based Measurement of Real-Time Kinetics of Protoporphyrin IX After 5-Aminolevulinic Acid Administration in Human In Situ Malignant Gliomas. Neurosurgery 2019, 85, E739–E746. [Google Scholar] [CrossRef]
- Kaneko, S.; Suero Molina, E.; Sporns, P.; Schipmann, S.; Black, D.; Stummer, W. Fluorescence real-time kinetics of protoporphyrin IX after 5-ALA administration in low-grade glioma. J. Neurosurg. 2022, 136, 9–15. [Google Scholar] [CrossRef]
- Schupper, A.J.; Baron, R.; Cheung, W.; Rodriguez, J.; Kalkanis, S.N.; Chohan, M.; Nahed, B.V.; Zacharia, B.E.; Jensen, R.L.; Olsen, J.; et al. 213 5-Aminolevulinic Acid for Enhanced Surgical Visualization of High-Grade Gliomas: A Prospective, Multicenter Study. Neurosurgery 2022, 68, 65. [Google Scholar] [CrossRef]
- Picart, T.; Pallud, J.; Berthiller, J.; Dumot, C.; Berhouma, M.; Ducray, F.; Armoiry, X.; Margier, J.; Guerre, P.; Varlet, P.; et al. Use of 5-ALA fluorescence-guided surgery versus white-light conventional microsurgery for the resection of newly diagnosed glioblastomas (RESECT study): A French multicenter randomized phase III study. J. Neurosurg. 2024, 140, 987–1000. [Google Scholar] [CrossRef]
- Stepp, H.; Stummer, W. 5-ALA in the management of malignant glioma. Lasers Surg. Med. 2018, 50, 399–419. [Google Scholar] [CrossRef]
- Stepp, H.; Stummer, W. Delineating normal from diseased brain by aminolevulinic acid-induced fluorescence. In Optical Methods and Instrumentation in Brain Imaging and Therapy; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Colditz, M.J.; Leyen, K.V.; Jeffree, R.L. Aminolevulinic acid (ALA)-protoporphyrin IX fluorescence guided tumour resection. Part 2: Theoretical, biochemical and practical aspects. J. Clin. Neurosci. 2012, 19, 1611–1616. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, I.U.; Jahn, M.; Jahn, D. The biochemistry of heme biosynthesis. Arch. Biochem. Biophys. 2008, 474, 238–251. [Google Scholar] [CrossRef]
- Novotny, A.; Stummer, W. 5-Aminolevulinic acid and the blood-brain barrier—A review. Med. Laser Appl. 2003, 18, 36–40. [Google Scholar] [CrossRef]
- Mischkulnig, M.; Roetzer-Pejrimovsky, T.; Lötsch-Gojo, D.; Kastner, N.; Bruckner, K.; Prihoda, R.; Lang, A.; Martinez-Moreno, M.; Furtner, J.; Berghoff, A.; et al. Heme Biosynthesis Factors and 5-ALA Induced Fluorescence: Analysis of mRNA and Protein Expression in Fluorescing and Non-fluorescing Gliomas. Front. Med. 2022, 9, 907442. [Google Scholar] [CrossRef]
- Pustogarov, N.; Panteleev, D.; Goryaynov, S.A.; Ryabova, A.V.; Rybalkina, E.Y.; Revishchin, A.; Potapov, A.A.; Pavlova, G. Hiding in the Shadows: CPOX Expression and 5-ALA Induced Fluorescence in Human Glioma Cells. Mol. Neurobiol. 2017, 54, 5699–5708. [Google Scholar] [CrossRef]
- Mischkulnig, M.; Kiesel, B.; Lötsch, D.; Roetzer, T.; Borkovec, M.; Wadiura, L.I.; Mercea, P.A.; Jaklin, F.J.; Hervey-Jumper, S.; Roessler, K.; et al. TCGA mrna expression analysis of the heme biosynthesis pathway in diffusely infiltrating gliomas: A comparison of typically 5-ala fluorescent and non-fluorescent gliomas. Cancers 2020, 12, 2043. [Google Scholar] [CrossRef] [PubMed]
- Teng, L.; Nakada, M.; Zhao, S.G.; Endo, Y.; Furuyama, N.; Nambu, E.; Pyko, I.V.; Hayashi, Y.; Hamada, J.I. Silencing of ferrochelatase enhances 5-aminolevulinic acid-based fluorescence and photodynamic therapy efficacy. Br. J. Cancer 2011, 104, 798–807. [Google Scholar] [CrossRef]
- Nasir-Moin, M.; Wadiura, L.I.; Sacalean, V.; Juros, D.; Movahed-Ezazi, M.; Lock, E.K.; Smith, A.; Lee, M.; Weiss, H.; Müther, M.; et al. Localization of protoporphyrin IX during glioma-resection surgery via paired stimulated Raman histology and fluorescence microscopy. Nat. Biomed. Eng. 2024, 8, 672–688. [Google Scholar] [CrossRef]
- Liu, Z.; Mela, A.; Argenziano, M.G.; Banu, M.A.; Furnari, J.; Kotidis, C.; Sperring, C.P.; Humala, N.; Mahajan, A.; Bruce, J.N.; et al. Single-cell analysis of 5-aminolevulinic acid intraoperative labeling specificity for glioblastoma. J. Neurosurg. 2024, 140, 968–978. [Google Scholar] [CrossRef]
- Lang, A.; Jeron, R.L.; Lontzek, B.; Kiesel, B.; Mischkulnig, M.; Berghoff, A.S.; Ricken, G.; Wöhrer, A.; Rössler, K.; Lötsch-Gojo, D.; et al. Mapping high-grade glioma immune infiltration to 5-ALA fluorescence levels: TCGA data computation, classical histology, and digital image analysis. J. Neuro-Oncol. 2023, 164, 211–220. [Google Scholar] [CrossRef]
- Pinton, L.; Masetto, E.; Vettore, M.; Solito, S.; Magri, S.; D’Andolfi, M.; Del Bianco, P.; Lollo, G.; Benoit, J.P.; Okada, H.; et al. The immune suppressive microenvironment of human gliomas depends on the accumulation of bone marrow-derived macrophages in the center of the lesion. J. ImmunoTher. Cancer 2019, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Dietze, A.; Berg, K. ALA-induced porphyrin formation and fluorescence in synovitis tissue: In-vitro and in vivo studies. Photodiagn. Photodyn. Ther. 2005, 2, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, D.; Yi, B.; Cai, H.; Xi, Z.; Lou, X.; Li, Z. Comprehensive Analysis of CD163 as a Prognostic Biomarker and Associated with Immune Infiltration in Glioblastoma Multiforme. BioMed Res. Int. 2021, 2021, 8357585. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Tonn, J.C.; Goetz, C.; Ullrich, W.; Stepp, H.; Bink, A.; Pietsch, T.; Pichlmeier, U. 5-Aminolevulinic acid-derived tumor fluorescence: The diagnostic accuracy of visible fluorescence qualities as corroborated by spectrometry and histology and postoperative imaging. Neurosurgery 2014, 74, 310–319. [Google Scholar] [CrossRef]
- Coburger, J.; Engelke, J.; Scheuerle, A.; Thal, D.R.; Hlavac, M.; Wirtz, C.R.; König, R. Tumor detection with 5-aminolevulinic acid fluorescence and Gd-DTPA-enhanced intraoperative MRI at the border of contrast-enhancing lesions: A prospective study based on histopathological assessment. Neurosurg. Focus FOC 2014, 36, E3. [Google Scholar] [CrossRef]
- Yamada, S.; Muragaki, Y.; Maruyama, T.; Komori, T.; Okada, Y. Role of neurochemical navigation with 5-aminolevulinic acid during intraoperative MRI-guided resection of intracranial malignant gliomas. Clin. Neurol. Neurosurg. 2015, 130, 134–139. [Google Scholar] [CrossRef]
- Lau, D.; Hervey-Jumper, S.L.; Chang, S.; Molinaro, A.M.; McDermott, M.W.; Phillips, J.J.; Berger, M.S. A prospective Phase II clinical trial of 5-aminolevulinic acid to assess the correlation of intraoperative fluorescence intensity and degree of histologic cellularity during resection of high-grade gliomas. J. Neurosurg. 2016, 124, 1300–1309. [Google Scholar] [CrossRef]
- Hauser, S.B.; Kockro, R.A.; Actor, B.; Sarnthein, J.; Bernays, R.L. Combining 5-aminolevulinic acid fluorescence and intraoperative magnetic resonance imaging in glioblastoma surgery: A histology-based evaluation. Neurosurgery 2016, 78, 475–483. [Google Scholar] [CrossRef]
- Coburger, J.; Scheuerle, A.; Pala, A.; Thal, D.; Wirtz, C.R.; König, R. Histopathological Insights on Imaging Results of Intraoperative Magnetic Resonance Imaging, 5-Aminolevulinic Acid, and Intraoperative Ultrasound in Glioblastoma Surgery. Neurosurgery 2017, 81, 165–174. [Google Scholar] [CrossRef]
- Stummer, W.; Koch, R.; Valle, R.D.; Roberts, D.W.; Sanai, N.; Kalkanis, S.; Hadjipanayis, C.G.; Suero Molina, E. Intraoperative fluorescence diagnosis in the brain: A systematic review and suggestions for future standards on reporting diagnostic accuracy and clinical utility. Acta Neurochir. 2019, 161, 2083–2098. [Google Scholar] [CrossRef]
- Omoto, K.; Matsuda, R.; Nakagawa, I.; Motoyama, Y.; Nakase, H. False-positive inflammatory change mimicking glioblastoma multiforme under 5-aminolevulinic acid-guided surgery: A case report. Surg. Neurol. Int. 2018, 9, 49. [Google Scholar] [CrossRef]
- Stummer, W.; Stocker, S.; Wagner, S.; Stepp, H.; Fritsch, C.; Goetz, C.; Goetz, A.E.; Kiefmann, R.; Reulen, H.J. Intraoperative detection of malignant gliomas by 5-aminolevulinic acid-induced porphyrin fluorescence. Neurosurgery 1998, 42, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, R.; Katayama, Y.; Watanabe, T.; Yoshino, A.; Fukushima, T.; Sakatani, K. Quantitative spectroscopic analysis of 5-aminolevulinic acid-induced protoporphyrin IX fluorescence intensity in diffusely infiltrating astrocytomas. Neurol. Med. Chir. 2007, 47, 53–57. [Google Scholar] [CrossRef]
- Tsugu, A.; Ishizaka, H.; Mizokami, Y.; Osada, T.; Baba, T.; Yoshiyama, M.; Nishiyama, J.; Matsumae, M. Impact of the combination of 5-aminolevulinic acid-induced fluorescence with intraoperative magnetic resonance imaging-guided surgery for glioma. World Neurosurg. 2011, 76, 120–127. [Google Scholar] [CrossRef]
- Saito, K.; Hirai, T.; Takeshima, H.; Kadota, Y.; Yamashita, S.; Ivanova, A.; Yokogami, K. Genetic Factors Affecting Intraoperative 5-aminolevulinic Acid-induced Fluorescence of Diffuse Gliomas. Radiol. Oncol. 2017, 51, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Goryaynov, S.A.; Widhalm, G.; Goldberg, M.F.; Chelushkin, D.; Spallone, A.; Chernyshov, K.A.; Ryzhova, M.; Pavlova, G.; Revischin, A.; Shishkina, L.; et al. The Role of 5-ALA in Low-Grade Gliomas and the Influence of Antiepileptic Drugs on Intraoperative Fluorescence. Front. Oncol. 2019, 9, 423. [Google Scholar] [CrossRef] [PubMed]
- Cordova, J.S.; Gurbani, S.S.; Holder, C.A.; Olson, J.J.; Schreibmann, E.; Shi, R.; Guo, Y.; Shu, H.K.G.; Shim, H.; Hadjipanayis, C.G. Semi-Automated Volumetric and Morphological Assessment of Glioblastoma Resection with Fluorescence-Guided Surgery. Mol. Imaging Biol. 2016, 18, 454–462. [Google Scholar] [CrossRef]
- Coburger, J.; Hagel, V.; Wirtz, C.R.; König, R. Surgery for glioblastoma: Impact of the combined use of 5-aminolevulinic acid and intraoperative MRI on extent of resection and survival. PLoS ONE 2015, 10, 0131872. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro-Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Eatz, T.A.; Eichberg, D.G.; Lu, V.M.; Di, L.; Komotar, R.J.; Ivan, M.E. Intraoperative 5-ALA fluorescence-guided resection of high-grade glioma leads to greater extent of resection with better outcomes: A systematic review. J. Neurooncol. 2022, 156, 233–256. [Google Scholar] [CrossRef]
- Gandhi, S.; Tayebi Meybodi, A.; Belykh, E.; Cavallo, C.; Zhao, X.; Syed, M.P.; Borba Moreira, L.; Lawton, M.T.; Nakaji, P.; Preul, M.C. Survival Outcomes Among Patients With High-Grade Glioma Treated With 5-Aminolevulinic Acid-Guided Surgery: A Systematic Review and Meta-Analysis. Front. Oncol. 2019, 9, 620. [Google Scholar] [CrossRef] [PubMed]
- Eljamel, M.S.; Goodman, C.; Moseley, H. ALA and Photofrin fluorescence-guided resection and repetitive PDT in glioblastoma multiforme: A single centre Phase III randomised controlled trial. Lasers Med. Sci. 2008, 23, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Hosmann, A.; Millesi, M.; Wadiura, L.I.; Kiesel, B.; Mercea, P.A.; Mischkulnig, M.; Borkovec, M.; Furtner, J.; Roetzer, T.; Wolfsberger, S.; et al. 5-ala fluorescence is a powerful prognostic marker during surgery of low-grade gliomas (Who grade ii)—experience at two specialized centers. Cancers 2021, 13, 2540. [Google Scholar] [CrossRef]
- Johansson, A.; Palte, G.; Schnell, O.; Tonn, J.C.; Herms, J.; Stepp, H. 5-Aminolevulinic acid-induced protoporphyrin IX levels in tissue of human malignant brain tumors. Photochem. Photobiol. 2010, 86, 1373–1378. [Google Scholar] [CrossRef] [PubMed]
- Jungk, C.; Warta, R.; Mock, A.; Friauf, S.; Hug, B.; Capper, D.; Abdollahi, A.; Debus, J.; Bendszus, M.; von Deimling, A.; et al. Location-dependent patient outcome and recurrence patterns in IDH1-wildtype glioblastoma. Cancers 2019, 11, 122. [Google Scholar] [CrossRef]
- Kirkpatrick, J.P.; Laack, N.N.; Shih, H.A.; Gondi, V. Management of GBM: A problem of local recurrence. J. Neuro-Oncol. 2017, 134, 487–493. [Google Scholar] [CrossRef]
- Tonn, J.C.; Stummer, W. Fluorescence-guided resection of malignant gliomas using 5-aminolevulinic acid: Practical use, risks, and pitfalls. Clin. Neurosurg. 2008, 55, 20–26. [Google Scholar]
- Schucht, P.; Knittel, S.; Slotboom, J.; Seidel, K.; Murek, M.; Jilch, A.; Raabe, A.; Beck, J. 5-ALA complete resections go beyond MR contrast enhancement: Shift corrected volumetric analysis of the extent of resection in surgery for glioblastoma. Acta Neurochir. 2014, 156, 305–312. [Google Scholar] [CrossRef]
- Haddad, A.F.; Young, J.S.; Morshed, R.A.; Berger, M.S. FLAIRectomy: Resecting beyond the Contrast Margin for Glioblastoma. Brain Sci. 2022, 12, 544. [Google Scholar] [CrossRef]
- Wach, J.; Vychopen, M.; Kühnapfel, A.; Seidel, C.; Güresir, E. A Systematic Review and Meta-Analysis of Supramarginal Resection versus Gross Total Resection in Glioblastoma: Can We Enhance Progression-Free Survival Time and Preserve Postoperative Safety? Cancers 2023, 15, 1772. [Google Scholar] [CrossRef]
- Certo, F.; Stummer, W.; Farah, J.O.; Freyschlag, C.; Visocchi, M.; Morrone, A.; Altieri, R.; Toccaceli, G.; Peschillo, S.; Thomè, C.; et al. Supramarginal resection of glioblastoma: 5-ALA fluorescence, combined intraoperative strategies and correlation with survival. J. Neurosurg. Sci. 2019, 63, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanayis, C.G.; Widhalm, G.; Stummer, W. What is the Surgical Benefit of Utilizing 5-Aminolevulinic Acid for Fluorescence-Guided Surgery of Malignant Gliomas? Neurosurgery 2015, 77, 663–673. [Google Scholar] [CrossRef]
- Masubuchi, T.; Kajimoto, Y.; Kawabata, S.; Nonoguchi, N.; Fujishiro, T.; Miyatake, S.I.; Kuroiwa, T. Experimental study to understand nonspecific protoporphyrin ix fluorescence in brain tissues near tumors after 5-aminolevulinic acid administration. Photomed. Laser Surg. 2013, 31, 428–433. [Google Scholar] [CrossRef]
- La Rocca, G.; Della Pepa, G.M.; Menna, G.; Altieri, R.; Ius, T.; Rapisarda, A.; Olivi, A.; Sabatino, G. State of the art of fluorescence guided techniques in neurosurgery. J. Neurosurg. Sci. 2019, 63, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Utsuki, S.; Oka, H.; Sato, S.; Shimizu, S.; Suzuki, S.; Tanizaki, Y.; Kondo, K.; Miyajima, Y.; Fujii, K. Histological examination of false positive tissue resection using 5-aminolevulinic acid-induced fluorescence guidance. Neurol. Med.-Chir. 2007, 47, 210–214. [Google Scholar] [CrossRef]
- Slof, J.; Díez Valle, R.; Galván, J. Análisis coste-efectividad de la cirugía del glioma maligno guiada por fluorescencia con ácido 5-aminolevulínico. Neurologia 2015, 30, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Warsi, N.M.; Zewude, R.; Karmur, B.; Pirouzmand, N.; Hachem, L.; Mansouri, A. The cost-effectiveness of 5-ALA in high-grade glioma surgery: A quality-based systematic review. Can. J. Neurol. Sci. 2020, 47, 793–799. [Google Scholar] [CrossRef]
- Tummers, W.S.; Warram, J.M.; Tipirneni, K.E.; Fengler, J.; Jacobs, P.; Shankar, L.; Henderson, L.; Ballard, B.; Pogue, B.W.; Weichert, J.P.; et al. Regulatory aspects of optical methods and exogenous targets for cancer detection. Cancer Res. 2017, 77, 2197–2206. [Google Scholar] [CrossRef]
- European Medicines Agency. EMA Scientific Discussion: 5-ALA; European Medicines Agency: Amsterdam, The Netherlands, 2007.
- Centre for Drug Evaluation and Research. Centre for Drug Evaluation and Research: Clinical Review—5-ALA; Centre for Drug Evaluation and Research: Silver Spring, MD, USA, 2015.
- Beck, T.J.; Kreth, F.W.; Beyer, W.; Mehrkens, J.H.; Obermeier, A.; Stepp, H.; Stummer, W.; Baumgartner, R. Interstitial photodynamic therapy of nonresectable malignant glioma recurrences using 5-aminolevulinic acid induced protoporphyrin IX. Lasers Surg. Med. 2007, 39, 386–393. [Google Scholar] [CrossRef]
- Stummer, W.; Beck, T.; Beyer, W.; Mehrkens, J.H.; Obermeier, A.; Etminan, N.; Stepp, H.; Tonn, J.C.; Baumgartner, R.; Herms, J.; et al. Long-sustaining response in a patient with non-resectable, distant recurrence of glioblastoma multiforme treated by interstitial photodynamic therapy using 5-ALA: Case report. J. Neurooncol. 2008, 87, 103–109. [Google Scholar] [CrossRef]
- Dupont, C.; Vermandel, M.; Leroy, H.A.; Quidet, M.; Lecomte, F.; Delhem, N.; Mordon, S.; Reyns, N. INtraoperative photoDYnamic Therapy for GliOblastomas (INDYGO): Study Protocol for a Phase i Clinical Trial. Clin. Neurosurg. 2019, 84, E414–E419. [Google Scholar] [CrossRef] [PubMed]
- Peciu-Florianu, I.; Vannod-Michel, Q.; Vauleon, E.; Bonneterre, M.E.; Reyns, N. Long term follow-up of patients with newly diagnosed glioblastoma treated by intraoperative photodynamic therapy: An update from the INDYGO trial (NCT03048240). J. Neuro-Oncol. 2024, 168, 495–505. [Google Scholar] [CrossRef]
- Montcel, B.; Mahieu-Williame, L.; Armoiry, X.; Meyronet, D.; Guyotat, J. Two-peaked 5-ALA-induced PpIX fluorescence emission spectrum distinguishes glioblastomas from low grade gliomas and infiltrative component of glioblastomas. Biomed. Opt. Express 2013, 4, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Alston, L.; Mahieu-Williame, L.; Hebert, M.; Kantapareddy, P.; Meyronet, D.; Rousseau, D.; Guyotat, J.; Montcel, B. Spectral complexity of 5-ALA induced PpIX fluorescence in guided surgery: A clinical study towards the discrimination of healthy tissue and margin boundaries in high and low grade gliomas. Biomed. Opt. Express 2019, 10, 2478–2492. [Google Scholar] [CrossRef]
- Gautheron, A.; Bernstock, J.D.; Picart, T.; Guyotat, J.; Valdés, P.A.; Montcel, B. 5-ALA induced PpIX fluorescence spectroscopy in neurosurgery: A review. Front. Neurosci. 2024, 18, 1310282. [Google Scholar] [CrossRef] [PubMed]
- Black, D.; Kaneko, S.; Walke, A.; Konig, S.; Stummer, W.; Suero Molina, E. Characterization of autofluorescence and quantitative protoporphyrin IX biomarkers for optical spectroscopy-guided glioma surgery. Sci. Rep. 2021, 11, 20009. [Google Scholar] [CrossRef]
- Elliot, M.M.; Segaud, D.S.; MacCormac, M.O.; Patel, M.S.; Reisz, D.Z.; Lavrador, M.J.; Vergani, M.F.; Bhangoo, M.R.; Ashkan, P.K.; Al-Sarraj, P.S.; et al. An Ex-Vivo Spectral and Lifetime-Decay Analysis of 5-ALA Derived PPIX fluorescence: Objective fluorescence Mapping in Glioma Surgery. Neuro-Oncol. 2024, 26, vii8–vii9. [Google Scholar] [CrossRef]
- Suero Molina, E.; Black, D.; Walke, A.; Azemi, G.; D’Alessandro, F.; König, S.; Stummer, W. Unraveling the blue shift in porphyrin fluorescence in glioma: The 620 nm peak and its potential significance in tumor biology. Front. Neurosci. 2023, 17, 1261679. [Google Scholar] [CrossRef]
- Mowforth, O.D.; Brannigan, J.; El Khoury, M.; Sarathi, C.I.P.; Bestwick, H.; Bhatti, F.; Mair, R. Personalised therapeutic approaches to glioblastoma: A systematic review. Front. Med. 2023, 10, 1166104. [Google Scholar] [CrossRef]
- Duffau, H. Surgery for malignant brain gliomas: Fluorescence-guided resection or functional-based resection? Front. Surg. 2019, 6, 21. [Google Scholar] [CrossRef]
- Schebesch, K.M.; Hoehne, J.; Hohenberger, C.; Proescholdt, M.; Riemenschneider, M.J.; Wendl, C.; Brawanski, A. Fluorescein sodium-guided resection of cerebral metastases-experience with the first 30 patients. Acta Neurochir. 2015, 157, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Teich, W.; Frenzel, F.; Hoffmann, K.; Radke, J.; Rösler, J.; Faust, K.; Blank, A.; Brandenburg, S.; Misch, M.; et al. Optical Characterization of Sodium Fluorescein In Vitro and Ex Vivo. Front. Oncol. 2021, 11, 654300. [Google Scholar] [CrossRef] [PubMed]
- Sjöback, R.; Nygren, J.; Kubista, M. Absorption and fluorescence properties of fluorescein. Spectrochim. Acta Part A Mol. Spectrosc. 1995, 51, L7–L21. [Google Scholar] [CrossRef]
- Suero Molina, E.; Schipmann, S.; Stummer, W. Maximizing safe resections: The roles of 5-aminolevulinic acid and intraoperative MR imaging in glioma surgery—Review of the literature. Neurosurg. Rev. 2019, 42, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Zhang, J.; Liu, L. Fluorescence properties of twenty fluorescein derivatives: Lifetime, quantum yield, absorption and emission spectra. J. Fluoresc. 2014, 24, 819–826. [Google Scholar] [CrossRef]
- Belykh, E.; Shaffer, K.V.; Lin, C.; Byvaltsev, V.A.; Preul, M.C.; Chen, L. Blood-Brain Barrier, Blood-Brain Tumor Barrier, and Fluorescence-Guided Neurosurgical Oncology: Delivering Optical Labels to Brain Tumors. Front. Oncol. 2020, 10, 739. [Google Scholar] [CrossRef]
- Smith, E.J.; Gohil, K.; Thompson, C.M.; Naik, A.; Hassaneen, W. Fluorescein-Guided Resection of High Grade Gliomas: A Meta-Analysis. World Neurosurg. 2021, 155, 181–188. [Google Scholar] [CrossRef]
- Cavallo, C.; De Laurentis, C.; Vetrano, I.G.; Falco, J.; Broggi, M.; Schiariti, M.; Ferroli, P.; Acerbi, F. The utilization of fluorescein in brain tumor surgery: A systematic review. J. Neurosurg. Sci. 2018, 62, 690–703. [Google Scholar] [CrossRef]
- Okuda, T.; Yoshioka, H.; Kato, A. Fluorescence-guided surgery for glioblastoma multiforme using high-dose fluorescein sodium with excitation and barrier filters. J. Clin. Neurosci. 2012, 19, 1719–1722. [Google Scholar] [CrossRef]
- Shinoda, J.; Yano, H.; Yoshimura, S.I.; Okumura, A.; Kaku, Y.; Iwama, T.; Sakai, N. Fluorescence-guided resection of glioblastoma multiforme by using high-dose fluorescein: Technical note. sodium. J. Neurosurg. 2003, 99, 597–603. [Google Scholar] [CrossRef]
- Acerbi, F.; Broggi, M.; Schebesch, K.M.; Hohne, J.; Cavallo, C.; De Laurentis, C.; Eoli, M.; Anghileri, E.; Servida, M.; Boffano, C.; et al. Fluorescein-Guided Surgery for Resection of High-Grade Gliomas: A Multicentric Prospective Phase II Study (FLUOGLIO). Clin. Cancer Res. 2018, 24, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Ling, G.; Guo, T.; Guo, F.; Piao, H. Effectiveness and Safety of Ultra-low-dose Fluorescein Sodium-Guided Resection of Malignant Glioma. World Neurosurg. 2024, 185, e774–e785. [Google Scholar] [CrossRef]
- Schebesch, K.M.; Höhne, J.; Rosengarth, K.; Noeva, E.; Schmidt, N.O.; Proescholdt, M. Fluorescein-guided resection of newly diagnosed high-grade glioma: Impact on extent of resection and outcome. Brain Spine 2022, 2, 101690. [Google Scholar] [CrossRef]
- Folaron, M.; Strawbridge, R.; Samkoe, K.S.; Filan, C.; Roberts, D.W.; Davis, S.C. Elucidating the kinetics of sodium fluorescein for fluorescence-guided surgery of glioma. J. Neurosurg. 2019, 131, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Neira, J.A.; Ung, T.H.; Sims, J.S.; Malone, H.R.; Chow, D.S.; Samanamud, J.L.; Zanazzi, G.J.; Guo, X.; Bowden, S.G.; Zhao, B.; et al. Aggressive resection at the infiltrative margins of glioblastoma facilitated by intraoperative fluorescein guidance. J. Neurosurg. 2017, 127, 111–122. [Google Scholar] [CrossRef]
- Kuroiwa, T.; Kajimoto, Y.; Ohta, T. Development of a fluorescein operative microscope for use during malignant glioma surgery a technical note and prelminary report. Surg. Neurol. 1998, 50, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Acerbi, F.; Broggi, M.; Broggi, G.; Ferroli, P. What is the best timing for fluorescein injection during surgical removal of high-grade gliomas? Acta Neurochir. 2015, 157, 1377–1378. [Google Scholar] [CrossRef]
- Xu, K.; Tzankova, V.; Li, C.; Sharma, S. Intravenous fluorescein angiography–associated adverse reactions. Can. J. Ophthalmol. 2016, 51, 321–325. [Google Scholar] [CrossRef]
- Restelli, F.; Bonomo, G.; Monti, E.; Broggi, G.; Acerbi, F.; Broggi, M. Safeness of sodium fluorescein administration in neurosurgery: Case-report of an erroneous very high-dose administration and review of the literature. Brain Spine 2022, 2, 101703. [Google Scholar] [CrossRef]
- Fan, C.; Jiang, Y.; Liu, R.; Wu, G.; Wu, G.; Xu, K.; Miao, Z. Safety and feasibility of low-dose fluorescein-guided resection of glioblastoma. Clin. Neurol. Neurosurg. 2018, 175, 57–60. [Google Scholar] [CrossRef]
- Maugeri, R.; Villa, A.; Pino, M.; Imperato, A.; Giammalva, G.R.; Costantino, G.; Graziano, F.; Gulì, C.; Meli, F.; Francaviglia, N.; et al. With a little help from my friends: The role of intraoperative fluorescent dyes in the surgical management of high-grade gliomas. Brain Sci. 2018, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Acerbi, F.; Broggi, M.; Eoli, M.; Anghileri, E.; Cuppini, L.; Pollo, B.; Schiariti, M.; Visintini, S.; Orsi, C.; Franzini, A.; et al. Fluorescein-guided surgery for grade IV gliomas with a dedicated filter on the surgical microscope: Preliminary results in 12 cases. Acta Neurochir. 2013, 155, 1277–1286. [Google Scholar] [CrossRef]
- Zhang, X.; Habib, A.; Jaman, E.; Mallela, A.N.; Amankulor, N.M.; Zinn, P.O. Headlight and loupe-based fluorescein detection system in brain tumor surgery: A first-in-human experience. J. Neurosurg. Sci. 2023, 67, 374–379. [Google Scholar] [CrossRef]
- Blair, N.P.; Evans, M.A.; Lesar, T.S.; Zeimer, R.C. Fluorescein and fluorescein glucuronide pharmacokinetics after intravenous injection. Investig. Ophthalmol. Vis. Sci. 1986, 27, 1107–1114. [Google Scholar]
- Ichioka, T.; Miyatake, S.I.; Asai, N.; Kajimoto, Y.; Nakagawa, T.; Hayashi, H.; Kuroiwa, T. Enhanced detection of malignant glioma xenograft by fluorescein-human serum albumin conjugate. J. Neuro-Oncol. 2004, 67, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W. Poor man’s fluorescence? Acta Neurochir. 2015, 157, 1379–1381. [Google Scholar] [CrossRef]
- Molina, E.S.; Brokinkel, B. Sodium fluorescein versus 5-aminolevulinic acid to visualize high-grade gliomas. J. Neurosurg. 2020, 133, 1627–1630. [Google Scholar] [CrossRef]
- Sweeney, J.F.; Rosoklija, G.; Sheldon, B.L.; Bondoc, M.; Bandlamuri, S.; Adamo, M.A. Comparison of sodium fluorescein and intraoperative ultrasonography in brain tumor resection. J. Clin. Neurosci. 2022, 106, 141–144. [Google Scholar] [CrossRef]
- Luzzi, S.; Lucifero, A.G.; Martinelli, A.; Maestro, M.D.; Savioli, G.; Simoncelli, A.; Lafe, E.; Preda, L.; Galzio, R. Supratentorial high-grade gliomas: Maximal safe anatomical resection guided by augmented reality high-definition fiber tractography and fluorescein. Neurosurg. Focus 2021, 51, E5. [Google Scholar] [CrossRef]
- Hansen, R.W.; Pedersen, C.B.; Halle, B.; Korshoej, A.R.; Schulz, M.K.; Kristensen, B.W.; Poulsen, F.R. Comparison of 5-aminolevulinic acid and sodium fluorescein for intraoperative tumor visualization in patients with high-grade gliomas: A single-center retrospective study. J. Neurosurg. 2019, 133, 1324–1331. [Google Scholar] [CrossRef]
- Katsevman, G.A.; Turner, R.C.; Urhie, O.; Voelker, J.L.; Bhatia, S. Utility of sodium fluorescein for achieving resection targets in glioblastoma: Increased gross- Or near-total resections and prolonged survival. J. Neurosurg. 2020, 132, 914–920. [Google Scholar] [CrossRef]
- Diaz, R.J.; Dios, R.R.; Hattab, E.M.; Burrell, K.; Rakopoulos, P.; Sabha, N.; Hawkins, C.; Zadeh, G.; Rutka, J.T.; Cohen-Gadol, A.A. Study of the biodistribution of fluorescein in glioma-infiltrated mouse brain and histopathological correlation of intraoperative findings in high-grade gliomas resected under fluorescein fluorescence guidance. J. Neurosurg. 2015, 122, 1360–1369. [Google Scholar] [CrossRef]
- Falco, J.; Cavallo, C.; Vetrano, I.G.; de Laurentis, C.; Siozos, L.; Schiariti, M.; Broggi, M.; Ferroli, P.; Acerbi, F. Fluorescein Application in Cranial and Spinal Tumors Enhancing at Preoperative MRI and Operated with a Dedicated Filter on the Surgical Microscope: Preliminary Results in 279 Patients Enrolled in the FLUOCERTUM Prospective Study. Front. Surg. 2019, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Falco, J.; Rubiu, E.; Broggi, M.; Farinotti, M.; Vetrano, I.G.; Schiariti, M.; Anghileri, E.; Eoli, M.; Pollo, B.; Moscatelli, M.; et al. Towards an Established Intraoperative Oncological Favorable Tool: Results of Fluorescein-Guided Resection from a Monocentric, Prospective Series of 93 Primary Glioblastoma Patients. J. Clin. Med. 2023, 12, 178. [Google Scholar] [CrossRef]
- Chen, B.; Wang, H.; Ge, P.; Zhao, J.; Li, W.; Gu, H.; Wang, G.; Luo, Y.; Chen, D. Gross total resection of glioma with the intraoperative fluorescence-Guidance of fluorescein sodium. Int. J. Med Sci. 2012, 9, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Koc, K.; Anik, I.; Cabuk, B.; Ceylan, S. Fluorescein sodium-guided surgery in glioblastoma multiforme: A prospective evaluation. Br. J. Neurosurg. 2008, 22, 99–103. [Google Scholar] [CrossRef]
- Teng, C.W.; Huang, V.; Arguelles, G.R.; Zhou, C.; Cho, S.S.; Harmsen, S.; Lee, J.Y.K. Applications of indocyanine green in brain tumor surgery: Review of clinical evidence and emerging technologies. Neurosurg. Focus 2021, 50, E4. [Google Scholar] [CrossRef] [PubMed]
- Raabe, A.; Beck, J.; Gerlach, R.; Zimmermann, M.; Seifert, V.; Macdonald, R.L.; Meyer, B.; Selman, W.R. Near-infrared indocyanine green video angiography: A new method for intraoperative assessment of vascular flow. Neurosurgery 2003, 52, 132–139. [Google Scholar] [CrossRef]
- Scerrati, A.; Della Pepa, G.M.; Conforti, G.; Sabatino, G.; Puca, A.; Albanese, A.; Maira, G.; Marchese, E.; Esposito, G. Indocyanine green video-angiography in neurosurgery: A glance beyond vascular applications. Clin. Neurol. Neurosurg. 2014, 124, 106–113. [Google Scholar] [CrossRef]
- Haglund, M.M.; Berger, M.S.; Hochman, D.W. Enhanced optical imaging of human gliomas and tumor margins. Neurosurgery 1996, 38, 308–317. [Google Scholar] [CrossRef]
- Kim, E.H.; Cho, J.M.; Chang, J.H.; Kim, S.H.; Lee, K.S. Application of intraoperative indocyanine green videoangiography to brain tumor surgery. Acta Neurochir. 2011, 153, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Ferroli, P.; Acerbi, F.; Albanese, E.; Tringali, G.; Broggi, M.; Franzini, A.; Broggi, G. Application of intraoperative indocyanine green angiography for CNS tumors: Results on the first 100 cases. In Acta Neurochirurgica Supplementum; Springer Nature: Berlin/Heidelberg, Germany, 2011; Volume 109. [Google Scholar] [CrossRef]
- Acerbi, F.; Vetrano, I.G.; Sattin, T.; Falco, J.; de Laurentis, C.; Zattra, C.M.; Bosio, L.; Rossini, Z.; Broggi, M.; Schiariti, M.; et al. Use of ICG videoangiography and FLOW 800 analysis to identify the patient-specific venous circulation and predict the effect of venous sacrifice: A retrospective study of 172 patients. Neurosurg. Focus 2018, 45, E7. [Google Scholar] [CrossRef] [PubMed]
- Eyupoglu, I.Y.; Hore, N.; Fan, Z.; Buslei, R.; Merkel, A.; Buchfelder, M.; Savaskan, N.E. Intraoperative vascular DIVA surgery reveals angiogenic hotspots in tumor zones of malignant gliomas. Sci. Rep. 2015, 5, 7958. [Google Scholar] [CrossRef]
- Lee, J.Y.; Thawani, J.P.; Pierce, J.; Zeh, R.; Martinez-Lage, M.; Chanin, M.; Venegas, O.; Nims, S.; Learned, K.; Keating, J.; et al. Intraoperative Near-Infrared Optical Imaging Can Localize Gadolinium-Enhancing Gliomas During Surgery. Neurosurgery 2016, 79, 856–871. [Google Scholar] [CrossRef]
- Maeda, H.; Sawa, T.; Konno, T. Mechanism of tumor-targeted delivery of macromolecular drugs, including the EPR effect in solid tumor and clinical overview of the prototype polymeric drug SMANCS. J. Control. Release 2001, 74, 47–61. [Google Scholar] [CrossRef]
- Jiang, J.X.; Keating, J.J.; Jesus, E.M.D.; Judy, R.P.; Madajewski, B.; Venegas, O.; Okusanya, O.T.; Singhal, S. Optimization of the enhanced permeability and retention effect for near-infrared imaging of solid tumors with indocyanine green. Am. J. Nucl. Med. Mol. Imaging 2015, 5, 390–400. [Google Scholar] [PubMed]
- Newton, A.D.; Predina, J.D.; Corbett, C.J.; Frenzel-Sulyok, L.G.; Xia, L.; Petersson, E.J.; Tsourkas, A.; Nie, S.; Delikatny, E.J.; Singhal, S. Optimization of Second Window Indocyanine Green for Intraoperative Near-Infrared Imaging of Thoracic Malignancy. J. Am. Coll. Surg. 2019, 228, 188–197. [Google Scholar] [CrossRef]
- Cho, S.S.; Salinas, R.; De Ravin, E.; Teng, C.W.; Li, C.; Abdullah, K.G.; Buch, L.; Hussain, J.; Ahmed, F.; Dorsey, J.; et al. Near-Infrared Imaging with Second-Window Indocyanine Green in Newly Diagnosed High-Grade Gliomas Predicts Gadolinium Enhancement on Postoperative Magnetic Resonance Imaging. Mol. Imaging Biol. 2020, 22, 1427–1437. [Google Scholar] [CrossRef]
- Shen, B.; Zhang, Z.; Shi, X.; Cao, C.; Zhang, Z.; Hu, Z.; Ji, N.; Tian, J. Real-time intraoperative glioma diagnosis using fluorescence imaging and deep convolutional neural networks. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3482–3492. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, Z.; Zhang, Z.; Cao, C.; Cheng, Z.; Hu, Z.; Tian, J.; Ji, N. Near-Infrared Window II Fluorescence Image-Guided Surgery of High-Grade Gliomas Prolongs the Progression-Free Survival of Patients. IEEE Trans. Biomed. Eng. 2022, 69, 1889–1900. [Google Scholar] [CrossRef]
- Zeh, R.; Sheikh, S.; Xia, L.; Pierce, J.; Newton, A.; Predina, J.; Cho, S.; Nasrallah, M.; Singhal, S.; Dorsey, J.; et al. The second window ICG technique demonstrates a broad plateau period for near infrared fluorescence tumor contrast in glioblastoma. PLoS ONE 2017, 12, 0182034. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.S.; Salinas, R.; Lee, J.Y.K. Indocyanine-Green for Fluorescence-Guided Surgery of Brain Tumors: Evidence, Techniques, and Practical Experience. Front. Surg. 2019, 6, 11. [Google Scholar] [CrossRef]
- Meira, J.; Marques, M.L.; Falcão-Reis, F.; Gomes, E.R.; Carneiro, A. Immediate reactions to fluorescein and indocyanine green in retinal angiography: Review of literature and proposal for patient’s evaluation. Clin. Ophthalmol. 2020, 2020, 171–178. [Google Scholar] [CrossRef]
- Pogue, B.W.; Gibbs-Strauss, S.; Valdes, P.A.; Samkoe, K.; Roberts, D.W.; Paulsen, K.D. Review of Neurosurgical Fluorescence Imaging Methodologies. IEEE J. Sel. Top. Quantum Electron. 2010, 16, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Lavrador, J.P.; Marchi, F.; Elhag, A.; Kalyal, N.; Mthunzi, E.; Awan, M.; Wroe-Wright, O.; Díaz-Baamonde, A.; Mirallave-Pescador, A.; Reisz, Z.; et al. In Situ Light-Source Delivery During 5-Aminulevulinic Acid-Guided High-Grade Glioma Resection: Spatial, Functional and Oncological Informed Surgery. Biomedicines 2024, 12, 2748. [Google Scholar] [CrossRef]
- Roque, D.; Kalyal, N.; Chowdhury, Y.A.; Elhag, A.; Elliot, M.; Ashkan, K.; Vergani, F.; Bhangoo, R.; Lavrador, J.P. Letter to Editor: Fluorescence-guided surgery for high-grade gliomas. Brain Spine 2024, 4, 104147. [Google Scholar] [CrossRef]
- Della Pepa, G.M.; Mattogno, P.; Menna, G.; Agostini, L.; Olivi, A.; Doglietto, F. A Comparative Analysis with Exoscope and Optical Microscope for Intraoperative Visualization and Surgical Workflow in 5-Aminolevulinic Acid–Guided Resection of High-Grade Gliomas. World Neurosurg. 2023, 170, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Suero Molina, E.; Hellwig, S.J.; Walke, A.; Jeibmann, A.; Stepp, H.; Stummer, W. Development and validation of a triple-LED surgical loupe device for fluorescence-guided resections with 5-ALA. J. Neurosurg. 2021, 137, 582–590. [Google Scholar] [CrossRef]
- Giantini-Larsen, A.M.; Parker, W.E.; Cho, S.S.; Goldberg, J.L.; Carnevale, J.A.; Michael, A.P.; Teng, C.W.; De Ravin, E.; Brennan, C.W.; Lee, J.Y.; et al. The Evolution of 5-Aminolevulinic Acid Fluorescence Visualization: Time for a Headlamp/Loupe Combination. World Neurosurg. 2022, 159, 136–143. [Google Scholar] [CrossRef]
- Croce, A.C.; Fiorani, S.; Locatelli, D.; Nano, R.; Ceroni, M.; Tancioni, F.; Giombelli, E.; Benericetti, E.; Bottiroli, G. Diagnostic Potential of Autofluorescence for an Assisted Intraoperative Delineation of Glioblastoma Resection Margins. Photochem. Photobiol. 2003, 77, 309–318. [Google Scholar] [CrossRef]
- Toms, S.A.; Lin, W.C.; Weil, R.J.; Johnson, M.D.; Jansen, E.D.; Mahadevan-Jansen, A. Intraoperative optical spectroscopy identifies infiltrating glioma margins with high sensitivity. Neurosurgery 2005, 57, 382–391. [Google Scholar] [CrossRef]
- Vasefi, F.; MacKinnon, N.; Farkas, D.L.; Kateb, B. Review of the potential of optical technologies for cancer diagnosis in neurosurgery: A step toward intraoperative neurophotonics. Neurophotonics 2016, 4, 011010. [Google Scholar] [CrossRef]
- Valdes, P.A.; Juvekar, P.; Agar, N.Y.; Gioux, S.; Golby, A.J. Quantitative wide-field imaging techniques for fluorescence guided neurosurgery. Front. Surg. 2019, 6, 31. [Google Scholar] [CrossRef]
- Shapey, J.; Xie, Y.; Nabavi, E.; Ebner, M.; Saeed, S.R.; Kitchen, N.; Dorward, N.; Grieve, J.; McEvoy, A.W.; Miserocchi, A.; et al. Optical properties of human brain and tumour tissue: An ex vivo study spanning the visible range to beyond the second near-infrared window. J. Biophotonics 2022, 15, e202100072. [Google Scholar] [CrossRef]
- Kim, A.; Khurana, M.; Moriyama, Y.; Wilson, B.C. Quantification of in vivo fluorescence decoupled from the effects of tissue optical properties using fiber-optic spectroscopy measurements. J. Biomed. Opt. 2010, 15, 67006. [Google Scholar] [CrossRef] [PubMed]
- Valdes, P.A.; Kim, A.; Brantsch, M.; Niu, C.; Moses, Z.B.; Tosteson, T.D.; Wilson, B.C.; Paulsen, K.D.; Roberts, D.W.; Harris, B.T. delta-aminolevulinic acid-induced protoporphyrin IX concentration correlates with histopathologic markers of malignancy in human gliomas: The need for quantitative fluorescence-guided resection to identify regions of increasing malignancy. Neuro Oncol. 2011, 13, 846–856. [Google Scholar] [CrossRef]
- Haj-Hosseini, N.; Richter, J.; Andersson-Engels, S.; Wardell, K. Optical touch pointer for fluorescence guided glioblastoma resection using 5-aminolevulinic acid. Lasers Surg. Med. 2010, 42, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Valdes, P.A.; Leblond, F.; Kim, A.; Wilson, B.C.; Paulsen, K.D.; Roberts, D.W. A spectrally constrained dual-band normalization technique for protoporphyrin IX quantification in fluorescence-guided surgery. Opt. Lett. 2012, 37, 1817–1819. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Rodrigues, F.; Schucht, P.; Preuss, M.; Wiewrodt, D.; Nestler, U.; Stein, M.; Artero, J.M.; Platania, N.; Skjoth-Rasmussen, J.; et al. Predicting the “usefulness” of 5-ALA-derived tumor fluorescence for fluorescence-guided resections in pediatric brain tumors: A European survey. Acta Neurochir. 2014, 156, 2315–2324. [Google Scholar] [CrossRef]
- Valdes, P.A.; Angelo, J.P.; Choi, H.S.; Gioux, S. qF-SSOP: Real-time optical property corrected fluorescence imaging. Biomed. Opt. Express 2017, 8, 3597–3605. [Google Scholar] [CrossRef]
- Xie, Y.; Thom, M.; Ebner, M.; Wykes, V.; Desjardins, A.; Miserocchi, A.; Ourselin, S.; McEvoy, A.W.; Vercauteren, T. Wide-field spectrally resolved quantitative fluorescence imaging system: Toward neurosurgical guidance in glioma resection. J. Biomed. Opt. 2017, 22, 1–14. [Google Scholar] [CrossRef] [PubMed]
- MacCormac, O.; Noonan, P.; Janatka, M.; Horgan, C.C.; Bahl, A.; Qiu, J.; Elliot, M.; Trotouin, T.; Jacobs, J.; Patel, S.; et al. Lightfield hyperspectral imaging in neuro-oncology surgery: An IDEAL 0 and 1 study. Front. Neurosci. 2023, 17, 1239764. [Google Scholar] [CrossRef]
- Lozovaya, G.I.; Masinovsky, Z.; Sivash, A.A. Protoporphyrin ix as a possible ancient photosensitizer: Spectral and photochemical studies. Orig. Life Evol. Biosph. 1990, 20, 321–330. [Google Scholar] [CrossRef]
- Marcu, L.; Hartl, B.A. Fluorescence Lifetime Spectroscopy and Imaging in Neurosurgery. IEEE J. Sel. Top. Quantum Electron. 2012, 18, 1465–1477. [Google Scholar] [CrossRef]
- Erkkila, M.T.; Bauer, B.; Hecker-Denschlag, N.; Madera Medina, M.J.; Leitgeb, R.A.; Unterhuber, A.; Gesperger, J.; Roetzer, T.; Hauger, C.; Drexler, W.; et al. Widefield fluorescence lifetime imaging of protoporphyrin IX for fluorescence-guided neurosurgery: An ex vivo feasibility study. J. Biophotonics 2019, 12, e201800378. [Google Scholar] [CrossRef] [PubMed]
- Erkkila, M.T.; Reichert, D.; Gesperger, J.; Kiesel, B.; Roetzer, T.; Mercea, P.A.; Drexler, W.; Unterhuber, A.; Leitgeb, R.A.; Woehrer, A.; et al. Macroscopic fluorescence-lifetime imaging of NADH and protoporphyrin IX improves the detection and grading of 5-aminolevulinic acid-stained brain tumors. Sci. Rep. 2020, 10, 20492. [Google Scholar] [CrossRef] [PubMed]
- Reichert, D.; Erkkilae, M.T.; Gesperger, J.; Wadiura, L.I.; Lang, A.; Roetzer, T.; Woehrer, A.; Andreana, M.; Unterhuber, A.; Wilzbach, M.; et al. Fluorescence Lifetime Imaging and Spectroscopic Co-Validation for Protoporphyrin IX-Guided Tumor Visualization in Neurosurgery. Front. Oncol. 2021, 11, 741303. [Google Scholar] [CrossRef]
- Russell, J.A.; Diamond, K.R.; Collins, T.J.; Tiedje, H.F.; Hayward, J.E.; Farrell, T.J.; Patterson, M.S.; Fang, Q. Characterization of fluorescence lifetime of photofrin and delta-aminolevulinic acid induced protoporphyrin IX in living cells using single- and two-photon excitation. IEEE J. Sel. Top. Quantum Electron. 2008, 14, 158–166. [Google Scholar] [CrossRef]
- Kantelhardt, S.R.; Diddens, H.; Leppert, J.; Rohde, V.; Hüttmann, G.; Giese, A. Multiphoton excitation fluorescence microscopy of 5-aminolevulinic acid induced fluorescence in experimental gliomas. Lasers Surg. Med. 2008, 40, 273–281. [Google Scholar] [CrossRef]
- Restelli, F.; Mathis, A.M.; Höhne, J.; Mazzapicchi, E.; Acerbi, F.; Pollo, B.; Quint, K. Confocal laser imaging in neurosurgery: A comprehensive review of sodium fluorescein-based CONVIVO preclinical and clinical applications. Front. Oncol. 2022, 12, 998384. [Google Scholar] [CrossRef]
- Wagner, A.; Brielmaier, M.C.; Kampf, C.; Baumgart, L.; Aftahy, A.K.; Meyer, H.S.; Kehl, V.; Höhne, J.; Schebesch, K.M.; Schmidt, N.O.; et al. Fluorescein-stained confocal laser endomicroscopy versus conventional frozen section for intraoperative histopathological assessment of intracranial tumors. Neuro-Oncol. 2024, 26, 922–932. [Google Scholar] [CrossRef]
- Kotwal, A.; Saragadam, V.; Bernstock, J.D.; Sandoval, A.; Veeraraghavan, A.; Valdés, P.A. Hyperspectral imaging in neurosurgery: A review of systems, computational methods, and clinical applications. J. Biomed. Opt. 2024, 30, 023512. [Google Scholar] [CrossRef] [PubMed]
- Valdés, P.A.; Leblond, F.; Anthony, K.; Harris, B.T.; Wilson, B.C.; Fan, X.; Tosteson, T.D.; Hartov, A.; Ji, S.; Erkmen, K.; et al. Quantitative fluorescence in intracranial tumor: Implications for ALA-induced PpIX as an intraoperative biomarker—Clinical article. J. Neurosurg. 2011, 115, 11–17. [Google Scholar] [CrossRef]
- Fabelo, H.; Ortega, S.; Kabwama, S.; Callico, G.M.; Bulters, D.; Szolna, A.; Pineiro, J.F.; Sarmiento, R. HELICoiD project: A new use of hyperspectral imaging for brain cancer detection in real-time during neurosurgical operations. In Proceedings of the Hyperspectral Imaging Sensors: Innovative Applications and Sensor Standards 2016, Baltimore, MD, USA, 17–21 April 2016; Volume 9860. [Google Scholar] [CrossRef]
- Kravchenko, Y.; Sikora, K.; Wireko, A.A.; Lyndin, M. Fluorescence visualization for cancer DETECTION: EXPERIENCE and perspectives. Heliyon 2024, 10, e2439. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, J.; Zhang, Y.; Zhuang, J.; Ge, M.; Shi, B.; Li, J.; Xu, G.; Xu, S.; Fan, C.; et al. Visualizing glioma margins by real-time tracking of γ-glutamyltranspeptidase activity. Biomaterials 2018, 173, 1–10. [Google Scholar] [CrossRef]
- Urano, Y.; Asanuma, D.; Hama, Y.; Koyama, Y.; Barrett, T.; Kamiya, M.; Nagano, T.; Watanabe, T.; Hasegawa, A.; Choyke, P.L.; et al. Selective molecular imaging of viable cancer cells with pH-activatable fluorescence probes. Nat. Med. 2009, 15, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.E.; Tummers, W.S.; Teraphongphom, N.; van den Berg, N.S.; Hasan, A.; Ertsey, R.D.; Nagpal, S.; Recht, L.D.; Plowey, E.D.; Vogel, H.; et al. First-in-human intraoperative near-infrared fluorescence imaging of glioblastoma using cetuximab-IRDye800. J. Neurooncol. 2018, 139, 135–143. [Google Scholar] [CrossRef]
- Sexton, K.; Tichauer, K.; Samkoe, K.S.; Gunn, J.; Hoopes, P.J.; Pogue, B.W. Fluorescent Affibody Peptide Penetration in Glioma Margin Is Superior to Full Antibody. PLoS ONE 2013, 8, 0060390. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; van den Berg, N.S.; Rosenthal, E.L.; Iv, M.; Zhang, M.; Vega Leonel, J.C.M.; Walters, S.; Nishio, N.; Granucci, M.; Raymundo, R.; et al. EGFR-targeted intraoperative fluorescence imaging detects high-grade glioma with panitumumab-IRDye800 in a phase 1 clinical trial. Theranostics 2021, 11, 7130–7143. [Google Scholar] [CrossRef]
- Xie, R.; Wu, Z.; Zeng, F.; Cai, H.; Wang, D.; Gu, L.; Zhu, H.; Lui, S.; Guo, G.; Song, B.; et al. Retro-enantio isomer of angiopep-2 assists nanoprobes across the blood-brain barrier for targeted magnetic resonance/fluorescence imaging of glioblastoma. Signal Transduct. Target. Ther. 2021, 6, 309. [Google Scholar] [CrossRef]
- Taniguchi, M.; Lindsey, J.S. Database of Absorption and Fluorescence Spectra of >300 Common Compounds for use in PhotochemCAD. Photochem. Photobiol. 2018, 94, 290–327. [Google Scholar] [CrossRef] [PubMed]
- Patil, C.G.; Walker, D.G.; Miller, D.M.; Butte, P.; Morrison, B.; Kittle, D.S.; Hansen, S.J.; Nufer, K.L.; Byrnes-Blake, K.A.; Yamada, M.; et al. Phase 1 Safety, Pharmacokinetics, and Fluorescence Imaging Study of Tozuleristide (BLZ-100) in Adults with Newly Diagnosed or Recurrent Gliomas. Clin. Neurosurg. 2019, 85, 439–440. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.M.; Lee, A.; Ojemann, J.; Cole, B.; Poliachik, S.; Ishak, L.; Hansen, S.; Novak, J.; Leary, S. Phase 1 dose escalation and expansion safety study of BLZ-100 in pediatric subjects with primary central nervous system tumors. J. Clin. Oncol. 2016, 34, 15. [Google Scholar] [CrossRef]
- Li, D.; Zhang, J.; Chi, C.; Xiao, X.; Wang, J.; Lang, L.; Ali, I.; Niu, G.; Zhang, L.; Tian, J.; et al. First-in-human study of PET and optical dual-modality image-guided surgery in glioblastoma using 68Ga-IRDye800CW-BBN. Theranostics 2018, 8, 2508–2520. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, J.; Chi, C.; Che, W.; Dong, G.; Wang, J.; Du, Y.; Wang, R.; Zhu, Z.; Tian, J.; et al. Lower-grade gliomas surgery guided by GRPR-targeting PET/NIR dual-modality image probe: A prospective and single-arm clinical trial. Theranostics 2024, 14, 819–829. [Google Scholar] [CrossRef]
- Gil, H.M.; Price, T.W.; Chelani, K.; Bouillard, J.G.; Calaminus, S.D.J.; Stasiuk, G.J. NIR-quantum dots in biomedical imaging and their future. iScience 2021, 24, 102189. [Google Scholar] [CrossRef]
- Singh, A.; Kim, W.; Kim, Y.; Jeong, K.; Kang, C.S.; Kim, Y.S.; Koh, J.; Mahajan, S.D.; Prasad, P.N.; Kim, S. Multifunctional Photonics Nanoparticles for Crossing the Blood–Brain Barrier and Effecting Optically Trackable Brain Theranostics. Adv. Funct. Mater. 2016, 26, 7057–7066. [Google Scholar] [CrossRef]
- Roller, B.T.; Munson, J.M.; Brahma, B.; Santangelo, P.J.; Pai, S.B.; Bellamkonda, R.V. Evans blue nanocarriers visually demarcate margins of invasive gliomas. Drug Deliv. Transl. Res. 2015, 5, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Roque, D.; Cruz, N.; Ferreira, H.A.; Reis, C.P.; Matela, N.; Herculano-Carvalho, M.; Cascão, R.; Faria, C.C. Nanoparticle-Based Treatment in Glioblastoma. J. Pers. Med. 2023, 13, 1328. [Google Scholar] [CrossRef]
- Nunn, A.D. The cost of developing imaging agents for routine clinical use. Investig. Radiol. 2006, 41, 205. [Google Scholar] [CrossRef]
- Nicholson, J.G.; Fine, H.A. Diffuse glioma heterogeneity and its therapeutic implications. Cancer Discov. 2021, 11, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Oliva, R.; Domínguez-García, S.; Carrascal, L.; Abalos-Martínez, J.; Pardillo-Díaz, R.; Verástegui, C.; Castro, C.; Nunez-Abades, P.; Geribaldi-Doldán, N. Evolution of Experimental Models in the Study of Glioblastoma: Toward Finding Efficient Treatments. Front. Oncol. 2021, 10, 614295. [Google Scholar] [CrossRef]
- Purshouse, K.; Bulbeck, H.J.; Rooney, A.G.; Noble, K.E.; Carruthers, R.D.; Thompson, G.; Hamerlik, P.; Yap, C.; Kurian, K.M.; Jefferies, S.J.; et al. Adult brain tumour research in 2024: Status, challenges and recommendations. Neuropathol. Appl. Neurobiol. 2024, 50, e12979. [Google Scholar] [CrossRef]
- Rezai, A.R.; D’Haese, P.F.; Finomore, V.; Carpenter, J.; Ranjan, M.; Wilhelmsen, K.; Mehta, R.I.; Wang, P.; Najib, U.; Vieira Ligo Teixeira, C.; et al. Ultrasound Blood–Brain Barrier Opening and Aducanumab in Alzheimer’s Disease. N. Engl. J. Med. 2024, 390, 55–62. [Google Scholar] [CrossRef]
- Pogue, B.W.; Zhu, T.C.; Ntziachristos, V.; Wilson, B.C.; Paulsen, K.D.; Gioux, S.; Nordstrom, R.; Pfefer, T.J.; Tromberg, B.J.; Wabnitz, H.; et al. AAPM Task Group Report 311: Guidance for performance evaluation of fluorescence-guided surgery systems. Med. Phys. 2024, 51, 740–771. [Google Scholar] [CrossRef] [PubMed]
- Dirven, L.; Armstrong, T.S.; Blakeley, J.O.; Brown, P.D.; Grant, R.; Jalali, R.; Leeper, H.; Mendoza, T.; Nayak, L.; Reijneveld, J.C.; et al. Working plan for the use of patient-reported outcome measures in adults with brain tumours: A Response Assessment in Neuro-Oncology (RANO) initiative. Lancet Oncol. 2018, 19, e173–e180. [Google Scholar] [CrossRef]
- Panciani, P.P.; Fontanella, M.; Schatlo, B.; Garbossa, D.; Agnoletti, A.; Ducati, A.; Lanotte, M. Fluorescence and image guided resection in high grade glioma. Clin. Neurol. Neurosurg. 2012, 114, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Diez Valle, R.; Tejada Solis, S.; Idoate Gastearena, M.A.; Garcia de Eulate, R.; Dominguez Echavarri, P.; Aristu Mendiroz, J. Surgery guided by 5-aminolevulinic fluorescence in glioblastoma: Volumetric analysis of extent of resection in single-center experience. J. Neurooncol. 2011, 102, 105–113. [Google Scholar] [CrossRef]
- Roberts, D.W.; Valdés, P.A.; Harris, B.T.; Fontaine, K.M.; Hartov, A.; Fan, X.; Ji, S.; Lollis, S.S.; Pogue, B.W.; Leblond, F.; et al. Coregistered fluorescence-enhanced tumor resection of malignant glioma: Relationships between δ-aminolevulinic acid-induced protoporphyrin IX fluorescence, magnetic resonance imaging enhancement, and neuropathological parameters: Clinical article. J. Neurosurg. 2011, 114, 595–603. [Google Scholar] [CrossRef]
- Nabavi, A.; Thurm, H.; Zountsas, B.; Pietsch, T.; Lanfermann, H.; Pichlmeier, U.; Mehdorn, M. Five-aminolevulinic acid for fluorescence-guided resection of recurrent malignant gliomas: A phase II study. Neurosurgery 2009, 65, 1070–1077. [Google Scholar] [CrossRef]
- Hefti, M.; von Campe, G.; Moschopulos, M.; Siegner, A.; Looser, H.; Landolt, H. 5-aminolevulinic acid induced protoporphyrin IX fluorescence in high-grade glioma surgery: A one-year experience at a single institutuion. Swiss. Med. Wkly. 2008, 138, 180–185. [Google Scholar] [PubMed]
- Hong, J.; Chen, B.; Yao, X.; Yang, Y. Outcome comparisons of high-grade glioma resection with or without fluorescein sodium-guidance. Curr. Probl. Cancer 2019, 43, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, X.; Zhu, X.; Wu, L.; Ma, S.; Yan, J.; Yan, D. Diffusion Tensor Imaging with Fluorescein Sodium Staining in the Resection of High-Grade Gliomas in Functional Brain Areas. World Neurosurg. 2019, 124, e595–e603. [Google Scholar] [CrossRef] [PubMed]
- Catapano, G.; Sgulò, F.G.; Seneca, V.; Lepore, G.; Columbano, L.; di Nuzzo, G. Fluorescein-Guided Surgery for High-Grade Glioma Resection: An Intraoperative “Contrast-Enhancer”. World Neurosurg. 2017, 104, 239–247. [Google Scholar] [CrossRef]
- Murray, K.J. Improved surgical resection of human brain tumors: Part 1. A preliminary study. Surg. Neurol. 1982, 17, 316–319. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elliot, M.; Ségaud, S.; Lavrador, J.P.; Vergani, F.; Bhangoo, R.; Ashkan, K.; Xie, Y.; Stasiuk, G.J.; Vercauteren, T.; Shapey, J. Fluorescence Guidance in Glioma Surgery: A Narrative Review of Current Evidence and the Drive Towards Objective Margin Differentiation. Cancers 2025, 17, 2019. https://doi.org/10.3390/cancers17122019
Elliot M, Ségaud S, Lavrador JP, Vergani F, Bhangoo R, Ashkan K, Xie Y, Stasiuk GJ, Vercauteren T, Shapey J. Fluorescence Guidance in Glioma Surgery: A Narrative Review of Current Evidence and the Drive Towards Objective Margin Differentiation. Cancers. 2025; 17(12):2019. https://doi.org/10.3390/cancers17122019
Chicago/Turabian StyleElliot, Matthew, Silvère Ségaud, Jose Pedro Lavrador, Francesco Vergani, Ranjeev Bhangoo, Keyoumars Ashkan, Yijing Xie, Graeme J. Stasiuk, Tom Vercauteren, and Jonathan Shapey. 2025. "Fluorescence Guidance in Glioma Surgery: A Narrative Review of Current Evidence and the Drive Towards Objective Margin Differentiation" Cancers 17, no. 12: 2019. https://doi.org/10.3390/cancers17122019
APA StyleElliot, M., Ségaud, S., Lavrador, J. P., Vergani, F., Bhangoo, R., Ashkan, K., Xie, Y., Stasiuk, G. J., Vercauteren, T., & Shapey, J. (2025). Fluorescence Guidance in Glioma Surgery: A Narrative Review of Current Evidence and the Drive Towards Objective Margin Differentiation. Cancers, 17(12), 2019. https://doi.org/10.3390/cancers17122019