Simple Summary
Brain tumours are difficult to remove. In part, this is because it is difficult for surgeons to accurately identify the boundaries between these tumours and the surrounding brain. Medications that cause tumours to fluoresce (emit light) during surgery were pioneered in brain tumours to help surgeons identify such tumour boundaries, and are now in regular use. We describe the evidence for the three medications currently used for fluorescence guided brain tumour surgery. We discuss how they work, how surgeons use them, the evidence for their accuracy and their effect on the outcomes of surgery. We highlight their limitations, and areas where further research could improve their use. We also explore how new work is aiming to expand the information that surgeons can collect from brain tumour fluorescence whilst also improving its accuracy. This includes the development of new systems to accurately measure the amount of emitted light (termed quantitative fluorescence), and the development of new fluorescent medications that are designed to overcome the limitations of current methods.
Abstract
Fluorescence-guided surgery (FGS) was pioneered for glioma and is now established as the standard of care. Gliomas are infiltrative tumours with diffuse margins. FGS provides improved intra-operative identification of tumour margins based on tumour-specific emission visible to the operating surgeon, resulting in increased rates of gross total resection. Multiple fluorescence agents may be used including 5-ALA, fluorescein sodium, and indocyanine green (ICG). This review details the indication, required equipment, mechanism of action, evidence base, limitations, and regulatory issues for each fluorophore as utilised in current clinical practice. FGS for glioma is limited by a reliance on subjective interpretation of visible fluorescence, which is often not present in low-grade glioma (LGG) or at the infiltrative tumour margin. Consequently, there has been a drive to develop enhanced, objective FGS techniques utilising both quantitative fluorescence (QF) imaging systems and novel fluorophores. This review provides an overview of emerging QF imaging systems for FGS. The pipeline for novel fluorophore development is also summarised.
Keywords:
fluorescence; fluorescence-guided surgery; glioma; 5-ALA; PpIX; fluorescein; ICG; quantitative fluorescence 1. Introduction
Gliomas are heterogeneous, diffuse, infiltrative tumours that represent the most common and fatal group of primary brain tumours worldwide [1]. Since the introduction of the Stupp protocol of adjuvant radiotherapy and chemotherapy [2], gross total resection (GTR) has become the most significant modifiable determinant of prognosis for high-grade glioma (HGG) [3,4]. This must be balanced with the preservation of neurological function, emphasised by the increasing use of “deterioration-free survival” as a patient-centred outcome measure [5]. However, achieving a maximal, functional resection remains challenging, and despite modern surgical adjuncts, GTR is only achieved in between 15 and 67% of HGG resections [6].
The use of fluorescence for margin differentiation in glioma was first pioneered in 1948 [7] and, following a landmark Phase 3 trial in 2006 [8], has taken on an increasingly prominent role in neurosurgical oncology. Fluorescence-guided surgery (FGS) utilises the properties of molecules termed fluorophores to absorb electromagnetic radiation (excitation) and then emit light (emission) at a different wavelength. Ideal fluorophores must demonstrate a robust safety profile, have sufficiently long half-lives to allow intra-operative detection, and integrate into the surgical workflow. Crucially, they must selectively accumulate in pathological tissue and demonstrate sufficient diagnostic accuracy to guide resection. 5-Aminolevulinic acid (5-ALA) and fluorescein sodium (FS) are now routinely used for FGS in glioma. Indocyanine green (ICG), pioneered in vascular neurosurgery, has also demonstrated increasing utility with the development of novel “second-window” imaging techniques.
This review explores the evidence for these fluorophores as used in current practice, including the regulatory frameworks that govern their use. Emerging theories of immune-mediated action for common clinical fluorophores are discussed, along with the implications that fluorescence imaging may be able to provide real-time intraoperative insights into the tumour micro-environment, provided it can be imaged with sufficient spatial and spectral resolution. Whilst other recent reviews have focussed on technologies currently available in the clinic [9,10] or for specific subtypes of glioma [11], this review provides a more forward-looking perspective towards emerging FGS technologies, discussing these in the context of current surgical adjuncts, highlighting their potential, current limitations, and areas for further development.
FGS for glioma surgery is constrained by a reliance on subjective emission, i.e., the operating surgeon’s perception of fluorescence light. Commercially available equipment to mitigate this is detailed, including microscope-embedded excitation and emission filters. Other novel research techniques being developed for objective and quantitative fluorescence detection in glioma surgery are also explored, highlighting recent work to increase the sensitivity and specificity of FGS using quantitative fluorescence and derive novel information on tumour behaviour using its spectral signature. Finally, we review emerging development pipelines for novel fluorophores, including rationally designed molecules aimed at optimising both tumour targeting and signal-to-noise ratio. Significantly, we highlight the disappointing early results from in vivo trials of novel fluorophores within the context of the prolonged pathway to regulatory approval.
2. 5-Aminolevulinic Acid
5-ALA (, Medac, Wedel, Germany) is a non-fluorescent molecule involved in the heme biosynthesis pathway. It undergoes an intra-cellular, multi-step conversion to the fluorescent protoporphyrin IX (PpIX) before a rate-limited conversion to heme [12]. PpIX absorbs light in the blue range, with a maxima at 405 nm before emitting fluorescence with an emission maxima at 635 nm that can be visualised as red/pink fluorescence [13] (Figure 1).
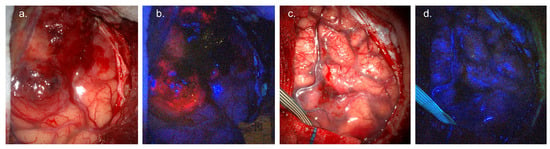
Figure 1.
5-ALA fluorescence-guided surgery. (a) White-light visualisation of glioblastoma involving cortical surface. (b) Surgical field under blue (405 nm) light, demonstrating avid red/pink tumour fluorescence. (c) White-light visualisation of WHO Grade 2 oligodendroglioma involving cortical surface. (d) Blue-light imaging, demonstrating lack of visible tumour fluorescence. Images courtesy of Neuro PPEye study team, King’s College London, UK (NCT05397574).
2.1. Use Case
2.1.1. Patient Population
Following a landmark Phase 3 trial [8], 5-ALA FGS was approved for HGG in Europe in 2007 and in 2017 in the USA [14]. Whilst widely used in HGG [15], off-label use in LGG has progressively increased, following emerging evidence that PpIX fluorescence in LGG can indicate areas of malignant transformation and act as a prognostic biomarker [16,17,18,19,20,21,22].
2.1.2. Dose, Administration and Timing
Manufacturer guidance for 5-ALA recommends an oral dose of 20 mg/kg to be given 2–4 h prior to the induction of anaesthesia [23]. Dose guidelines were initially based on preclinical studies in rats [13,24] and human case series [25] before its use in the landmark Phase 3 trial [8]. A subsequent 2017 Phase 1/2 trial of twenty-one patients designed to test the efficacy of alternative dose regimes confirmed that maximal measurable fluorescence with minimal side effects was obtained using 20 mg/kg in HGG [26].
Limited evidence for optimal dosing has been published in LGG. Whilst the majority of trials use the established 20 mg/kg, a trial of 23 patients gave 10 LGG patients 40 mg/mL and showed a significant increase in PpIX concentration using this increased dose [27]. Whilst there was no increase in visible fluorescence, the need for further research into dose escalation in LGG is highlighted.
5-ALA is taken orally as a liquid suspension. Preclinical HGG studies in rats demonstrated peak fluorescence 6 h after oral administration [13]. Current recommendations are for administration 3–4 h prior to theatre to allow for transfer time, induction of anaesthesia, positioning, and surgical approach. It is recommended that an additional dose is given if surgery is delayed by 12 h or more. More recently, attempts have been made to systematically assess the temporal changes in PpIX fluorescence over time in humans, utilising pseudo-quantitative hyperspectral imaging [9,28,29]. In 68 HGG and 25 LGG patients, a later fluorescence intensity peak was observed between 7 and 8 h from 5-ALA administration, particularly at tumour margins where fluorescence can provide the most pertinent information for margin differentiation. Consequently, the authors recommended earlier dosing at 4–5 h prior to surgery.
2.1.3. Side Effects and Safety
Reported side effects in 5-ALA trials have been limited to mild, transient liver enzyme elevation, photosensitivity, and nausea [8,30,31]. In the UK, a 24 h period of protection from sunlight is recommended given the risk of transient photosensitivity [15].
2.2. Equipment
5-ALA FGS requires an excitation light source incorporating the Soret band absorption of PpIX at 405 nm. A long-pass band filter at 440 nm is therefore used to filter reflected excitation light whilst allowing the passage of low-intensity 450 nm background light and PpIX emission at 635 nm. Commercial systems typically use proprietary algorithms to scale the reflected blue light to overcome background autofluorescence and non-specific “red” signal whilst highlighting PpIX emission [32]. As such, normal brain appears blue, whilst PpIX fluorescence appears as a solid red at the tumour core, fading to pink towards the margin [33] Figure 1. Several manufacturers, including Zeiss (Göttingen, Germany), Leica (Wetzlar, Germany), and Möller-Wedel (Wedel, Germany), provide comprehensive 5-ALA packages integrated into surgical microscopes [34]. More recently, groups have begun to develop surgical loupes optimised for 5-ALA fluorescence, along with advanced spectroscopic detection systems, discussed in more detail below (5. Towards objective margin differentiation).
2.3. Mechanism of Action
5-ALA is metabolised to PpIX during the mitochondrial phase of the heme biosynthesis pathway before a final and rate-limited chelation with iron forms heme [12]. Two models have been proposed for PpIX accumulation in glioma.
2.3.1. Increased Intracellular Synthesis and Retention
Classic models for 5-ALA induced tumour fluorescence are based on increased intra-tumoural synthesis and reduced efflux of PpIX [35] along with tumour-driven breakdown of the blood–brain barrier (BBB) [34,36], as illustrated in Figure 2.

Figure 2.
Simplified schematic of PpIX synthesis in heme biosynthetic pathway. ABCG2: ATP-binding cassette G2; ABCB6: ATP-binding cassette B6; CPOX: coproporphyrinogen oxidase; PPOX: protoporphyrinogen oxidase.
Robust identification of the steps within the heme biosynthesis pathway responsible for tumour-specific PpIX accumulation has been challenging. Studies of protein expression in highly fluorescent tumour samples show an upregulation of CPOX and PPOX driving increased PpIX synthesis, which is then coupled with downregulation of the ABCG2 membrane transporter, reducing PpIX efflux [37,38]. Analysis of mRNA expression has shown inconsistent results, including an increase [39], no significant difference [38,39], and downregulation [38,40] of enzymes involved in PpIX synthesis. Reasons for the apparent decoupling of mRNA and protein expression remain unclear; however, the correlation of CPOX, PPOX, and ABCG2 expression with fluorescence provides a model for increased PpIX accumulation consistent with the well-characterised metabolic heme pathway.
2.3.2. Immune Synthesis
An alternative model of PpIX accumulation highlights the role of the glioma immune micro-environment in driving PpIX fluorescence. Using paired Raman histology and two-photon excitation fluorescence microscopy in ex vivo samples from 115 patients, PpIX was demonstrated to accumulate preferentially in cells of myeloid origin, consistent with tumour-associated macrophages [41]. This builds on work using single-cell sequencing in glioma samples, showing significant PpIX fluorescence in infiltrating myeloid cells [42] and correlating with analysis showing stronger PpIX fluorescence in tumours with intense immune infiltration [43] and strong macrophage uptake of PpIX [44,45]. Such work points to a possible future role of PpIX in the investigation of the glioma immune micro-environment, linking to overall prognosis [46].
2.4. Evidence Base
2.4.1. Diagnostic Accuracy
The core function of FGS is to facilitate reliable and accurate differentiation of tumours from surrounding parenchyma under operative conditions. This has been well studied for 5-ALA in HGG (Table A1). Studies utilising modern surgical microscope filters show sensitivity and specificity ranging from 84 to 98.5% and 29.4 to 100% [30,47,48,49,50,51,52], respectively. Given the documented challenges in calculating objective sensitivity and specificity from brain tumours, where truly random sampling is not possible [53], diagnostic accuracy is more precisely expressed as positive predictive value (PPV) and negative predictive value (NPV)—reported as 92–96% and 12.5–69%, respectively. It is important to note that 5-ALA fluorescence has been reported in non-pathological tissue, including abnormal brain [50] and inflammatory tissue [54]. Care must be taken to correlate fluorescence with anatomy, pre-operative imaging, and complementary surgical adjuncts such as neurophysiological monitoring.
As use of 5-ALA FGS increased in Europe, users began to explore the potential to display areas of intra-tumoural malignant transformation in patients with suspected LGG [55]. Case series exploring 5-ALA in radiologically suspected LGG have replicated the observation that focal areas of positive fluorescence, in an otherwise non-fluorescing tumour, are highly predictive of malignant transformation [17], with reported PPV ranging from 85% [16] to 100% [18]. These findings underpin the increasing use of 5-ALA off-label in radiologically suspected LGG, particularly when there is suspicion of malignant transformation.
Studies of 5-ALA in histologically confirmed LGG have shown more equivocal results. Rates of fluorescence for WHO Grade 2 glioma range from 0% in small cases series [16,56,57] to 25% and [58] 35% [59] in larger series. In the largest case series to date, including 82 WHO Grade 2 gliomas, 16% showed visible fluorescence using contemporary operating microscopes with fluorescence filter sets [19]. Interestingly, whilst these tumours were still classified as histologically WHO Grade 2, they showed significantly increased proliferation compared to non-fluorescent LGGs. A follow-up series of 79 patients showed visible fluorescence in 22% [22]. Such low rates of visible fluorescence have driven efforts to develop novel technologies for the visualisation of 5-ALA in LGG (see Section 5).
2.4.2. Clinical Efficacy
Two Phase 3 randomised control trials have demonstrated that 5-ALA FGS improves extent of resection (EOR) in HGG (Table 1). The landmark 2006 RCT [8] showed an increase in HGG GTR from 36% using white light to 65% in 270 patients. This led to the regulatory approval and widespread uptake of 5-ALA in Europe. To address concerns about the lack of modern surgical adjuncts such as neuronavigation that were not available for use in the Stummer trial, a further RCT was conducted [31]. This, again, confirmed an increase in GTR for HGG, from 47.8% using white light to 79% using 5-ALA, in 147 patients. Other case series report significant variations in rates of GTR, ranging from 30 [60] to 100% [61]. These are explained to some extent by variations in the definition of GTR, the variable use of additional surgical adjuncts, tumour location (eloquent vs. non-eloquent), and the surgical learning curve. This presents an ongoing challenge to pooled analysis in FGS.
This heterogeneity, along with differences in adjuvant treatment regimens (particularly the uptake of the Stupp [2] protocol from 2005 onwards), rates of redo surgery, salvage chemotherapy, and patient selection, also leads to significant limitations in discerning current survival rates for patients with HGG. Whilst not powered as survival studies, neither Phase 3 RCT reached statistical significance for OS, whilst PFS at 6 months did significantly improve in the 2006 trial [8]. Changes to the histological diagnosis of HGG between 1998 and 2024, including four updates to the WHO CNS tumour classification system [62], also limit comparison between studies and may account for the wide range of reported survival. Notwithstanding these limitations, recent meta-analyses do seem to demonstrate that 5-ALA does improve both progression-free survival (PFS) and overall survival (OS). A recent meta-analysis of 1984 patients found that 5-ALA increased PFS vs. WL for 88.4% of patients and OS in 67.5% [63], adding to a previous meta-analysis suggesting a 1-month increase in PFS and a 3-month increase in OS [64].

Table 1.
Randomised control trials assessing the effect of 5-ALA on clinical outcome.
Table 1.
Randomised control trials assessing the effect of 5-ALA on clinical outcome.
| Study | Study Type | n | EOR/GTR | OS | PFS | Complications | Notes |
|---|---|---|---|---|---|---|---|
| Picart 2023 [31] | Phase 3 RCT 5-ALA vs. WL | 147 5-ALA: 67 WL: 69 | GTR 5-ALA: 79% WL: 47.8% | 24m 5-ALA: 30.1% WL: 37.7% | 6m 5-ALA: 70.2% WL: 68.4% | Deficit at 3m 5-ALA: 13.2% WL: 12.9% | All SOC neuronav used. Post-op protocol RT + CTh (Stupp). |
| Stummer 2006 [8] | Phase 3 RCT 5-ALA vs. WL | 270 5-ALA: 139 WL: 131 | GTR 5-ALA: 65% WL: 36% | 5-ALA: 15.2m WL: 13.5m | 6m 5-ALA: 41% WL: 21% Median 5-ALA: 5.1m WL: 3.6m | NR | Neuro-navigation precluded. Post-op protocol recommended RT only. Industry sponsored. |
| Eljamel 2008 [65] | Prospective single-centre RCT | 27 5-ALA: 13 WL: 14 | GTR 5-ALA: 77% WL: 29% | NR | 6m 5-ALA: 80% WL: 70% Mean 5-ALA: 52.8w WL: 24.2w | Neurology NR | Combination of FGS and Photofin (R) used in 5-ALA group. Post-op protocol for RT alone. n = 7 also received CTh. |
Abbreviations: EOR: extent of resection; GTR: gross total resection; OS: overall survival; PFS: progression-free survival; WL: white light; SOC: standard of care; RT: radiotherapy; CTh: chemotherapy.
Despite limited visible fluorescence, 5-ALA may also act as a prognostic biomarker in LGG. An analysis of survival in LGG found shorter PFS in tumours demonstrating intra-operative fluorescence [22,66], correlating with the evidence suggesting visible fluorescence correlates with increased proliferation [19].
2.5. Limitations
Use of 5-ALA for margin detection is limited by imaging geometry, high cost, reliance on subjective interpretation, and low emission in LGG. Efforts to address these limitations through the development of quantitative fluorescence detection systems are detailed in (5. Towards objective margin differentiation).
The infiltrative nature of glioma means low levels of fluorescence are typically seen at the tumour margin [67] and biopsies from the non-fluorescent boundaries often contain neoplastic cells [51]. Recurrence typically occurs at resection cavity margins, even with complete resection of visible fluorescence [68,69]. A reliance on subjective interpretation of low levels of visible fluorescence at tumour boundaries contributes to the low NPV of 5-ALA fluorescence in HGG, introduces inter-observer variability to 5-ALA FGS [70], and limits the use of 5-ALA in LGG. Despite fluorescence highlighting tumour beyond the margins of MRI enhancement [71], correlation of visible 5ALA fluorescence with peri-tumoural fluid attenuated inversion recovery (FLAIR) signal change in HGG is currently unclear [72] As evidence emerges on the use of supra-marginal resection in glioma [73], an ongoing prospective evaluation of the value added by FGS is essential [74]. Surgeons must also be alert to the small but clinically significant rates of false-positive 5-ALA fluorescence, typically found in areas of high macrophage infiltration [75,76,77,78]. These issues have underpinned a drive in recent years to develop systems able to quantify fluorescence emission in FGS.
5-ALA FGS also imposes a significant cost burden on neurosurgical units. Proprietary microscope FGS systems typically incur significant upfront costs, and use of 5-ALA has been estimated to cost EUR 9021 per quality-adjusted life year (QALY) in Spain [79] and up to USD 12,817 per QALY in the USA [80]. In the UK, the cost of using 5-ALA is estimated at up to GBP 1,000,000 per neurosurgical unit per year [15].
2.6. Regulatory Issues
5-ALA’s use as an optical imaging agent in HGG was approved by the European Medicines Agency in 2007 and a decade later by the FDA in 2017. It remains the only fluorescent agent licenced for use in glioma surgery. Off-label use in LGG is common throughout Europe, Japan, Korea, and North America [14]. Delayed approval for 5-ALA use in the USA was a result of the FDA classifying 5-ALA as a cross-over therapeutic and imaging agent. This requires evidence that the agent not only results in increased rates of GTR but also confers increased survival benefits [81]. However, both FDA and EMA scientific reviews note a lack of definitive evidence for the selection of dose, timing of administration, and definition of “border” in HGG [82,83].
2.7. Emerging Use Cases
5-ALA was pioneered for tumour differentiation, but research groups have since explored its photo-sensitising potential, complex spectral behaviour, and localisation of PpIX in the glioma micro-environment. Potential novel use cases have emerged including use in photodynamic therapy, real-time intra-operative tumour classification, and prognostication.
PpIX exposed to adequate light reacts to produce cytotoxic single oxygen radicals. Case reports using PpIX photodynamic therapy for the treatment of small glioma lesions have shown early promise [84,85]. A Phase 1 clinical trial of 10 patients was commenced in 2017 and has completed recruitment—Intraoperarive Photodynamic Therapy for Gliobastomas (INDYGO) [86]. A recent 5-year report noted a median follow-up of 23 months, with Kaplan–Meir estimated median overall survival of 23.4 months, a 12-month OS of 80%, and 12-month PFS of 60% [87].
The potential for 5-ALA to provide insight into glioma pathology and the molecular profile of tumours was noted during early investigations in LGG [16]. As a deeper understanding of the spectral complexity of PpIX fluorescence in glioma has developed [88,89], along with technologies for intra-operative spectral characterisation of PpIX [90], the possibility of extracting neuropathological information from the PpIX emission spectra has emerged. Distinct, environmentally driven photochemical states of PpIX emitting at 620 nm and 635 nm have been unmixed from glioma fluorescence, and the ratio is hypothesised to correlate with markers of malignancy [89,91,92]. However, despite this promising evidence, it remains unclear to what extent the 620 nm peak represents PpIX versus other 5-ALA metabolism intermediaries [93]. Improved, spectrally unmixed datasets paired with gold-standard molecular histology from human glioma are required to understand the information on tumour biology contained in 5-ALA-derived fluorescence emission. This work has the potential to dovetail with emerging evidence of mechanisms of PpIX accumulation to provide an insight into the tumour immune micro-environment and molecular pathology [41]. As evidence emerges for the use of personalised oncological treatment in HGG [94], this holds the potential to provide critical insights into the behaviour of heterogenous tumour tissue left at function resection boundaries [95].
3. Fluorescein
Fluorescein is a fluorescent small molecule available in 1 g vials costing approximately 5 Euros (EUR 5.70, GBP 4.30) per vial [96]. Following intravenous administration, it weakly binds to plasma proteins such as albumin and accumulates extracellularly in areas of BBB breakdown. Fluorescein absorbs light in the range of 465–490 nm with a maxima at 480 nm, before emitting a yellow/green fluorescence at 520–690 nm with a maxima at 525 nm [97,98,99,100] as illustrated in Figure 3.

Figure 3.
Fluorescein fluorescence guided surgery (a) White-light visualisation of infiltrated gyrus. (b) Surgical field under yellow (560 nm) light, demonstrating green fluorescence at areas of tumour infiltration. Images courtesy of Mr Ryan Mathew, University of Leeds, UK.
3.1. Use Case
3.1.1. Patient Population
In 1948, Moore described the utility of fluorescein for the differentiation of “intra-cranial malignancies”—ranging from meningioma to glioblastoma [7]. In glioma surgery, use of fluorescein has typically been confined to HGG, where breakdown of the BBB is expected [101]. A 2021 meta-analysis of 336 HGG patients [102] concluded that fluorescein-guided surgery led to comparable rates of GTR vs. 5-ALA, with a significant increase vs. white light alone. Whilst there has been an increasing appetite to explore the role of FGS in LGG, a 2023 systematic review found limited evidence of the use of fluorescein in LGG [21]. Indeed, only 13 WHO Grade 2 patients had undergone surgery using fluorescein, of which 38% showed visible fluorescence [21].
3.1.2. Dose, Administration and Timing
Fluorescein dose for glioma surgery remains unstandardised [103]. Whilst Moore used a fixed, high dose of 1 g, studies published prior to the advent of selective fluorescence microscopy typically report effective doses of 15–20 mg/kg [102,104,105]. The development of improved visualization techniques including the microscope filters described below have led to a progressive reduction in the apparent effective dose, with the largest phase II study to date utilising 5–10 mg/kg [106]. A 2024 prospective study of 60 HGG patients included 30 treated with 1 mg/kg—and concluded such “ultra-low” doses may provide comparable tumour discrimination to 5 mg/kg with reduced side effect profile [107].
Fluorescein is water soluble and given intravenously, but there is a lack of data on the optimal timing administration for use in glioma surgery [9]. Most authors report administration at the time of anaesthetic induction, approximately 60–90 min prior to tumour resection, to allow the washout of the unbound form and clearance from non-tumour tissue [106,107,108]. Investigation of fluorescein pharmacokinetics [109] has led to calls for administration to be pushed back further—at 2–4 h prior to tumour resection, to ensure washout of non-specific fluorescence resulting from unbound fluorescein [9,101]. Other authors, however, advocate administration around the time of dural opening (i.e. within 10 min of commencing tumour resection) [104,110]. Whilst this mirrors the use of fluorescein in retinal angiography, it has been criticised because unbound fluorescein may cross the BBB in the acute administration phase. There is also a risk of “contrast leak” from vessels damaged during surgery [111]. Both of these phenomena introduce the possibility of non-specific fluorescence and reduced signal-to-noise ratio [112].
3.1.3. Side Effects and Safety
Fluorescein has been widely studied in ophthalmology and is considered to have an excellent safety profile. Adverse events are reported in 3.3% of patients [113,114]. Allergic reactions can include respiratory, cardiac, anaphylaxis, and allergic skin testing before surgery is recommended [115]. Care must be taken to avoid extravasation during injection. Yellow discolouration of the skin and sclera is common and typically resolves in 24 h. Contraindications are the use of beta blockers along with renal or hepatic insufficiency.
3.2. Equipment
When used at 20 mg/kg, fluorescein in HGG is visible to the naked eye when excited with blue light [102]. A number of proprietary microscope filters have been developed for use with fluorescein, all of which are tuned to provide excitation light in the 460–500 nm range and for observation in the 540–590 nm range. The improved visualisation provided by these filters has driven work to reduce the dose administered as described above and contributed significantly to increased use of fluorescein in HGG [116]. These systems also allow “mixing” of red and blue light using proprietary algorithms to improve visualisation of non-fluorescent tissue, allowing non-pathological structures to be identified and preserved whilst operating in fluorescence mode [117]. Given the high cost of these systems, early work has begun in developing headlight and loupe fluorescein detection kits for use in environments where resources are constrained [118].
3.3. Mechanism of Action
Upon administration, fluorescein undergoes dose-dependent binding to serum proteins and distributes through the circulation and interstitial spaces [119]. Unbound fluorescein crosses the BBB in mouse models, and high concentrations are found throughout the brain following human equivalent dosing [109]. Non-specific fluorescence from this unbound component peaks 15–30 min following administration and progressively decreases thereafter. The bound fraction shows minimal transport across the intact BBB but crosses readily into the brain at areas of BBB disruption and thereby accumulates in the extracellular space [106,120], accentuated by the enhanced permeability and retention effect in tumour tissue [101]. Areas lacking an intact BBB, such as dura and choroid, are intensely fluorescent.
3.4. Evidence Base
3.4.1. Diagnostic Accuracy
Fluorescein’s mechanism of action and variability in dose, equipment, and timing has led to concerns about its diagnostic accuracy that have hindered uptake in some centres [121,122]. Using current systems, biopsy-based sensitivities in small, non-randomised prospective studies range from 75.6 to 93.5%, with specificity ranging from 75 to 90.9% (Table A2). When biopsies are limited to contrast-enhancing tumour, the lower range increases to 82.8% and 82.6% for sensitivity and specificity, respectively [110]. Sensitivity and specificity do not appear to correlate with dose, and indeed lower doses with adequate filtering may reduce non-specific fluorescence that can lower diagnostic accuracy [107]. Relatively few studies have calculated PPV and NPV for fluorescein in glioma surgery. FLUOGLIO utilised a subset of 50 biopsies in 13 patients to calculate a PPV of 80.8 and NPV of 79.1. Other groups calculated PPV and NPV using inspection of the surgical cavity compared with post-op MRI imaging in 43 HGG patients. These results suggesting a PPV of 100 and NPV of 81 are encouraging; however, they are limited by constraints of post-operative MRI in the detection of tumours [123].
Whilst published values for the diagnostic accuracy of using fluorescein in glioma surgery are comparable with 5-ALA, the quality and volume of evidence underpinning these calculations are more circumspect. This is particularly significant given the low number of studies that report the clinically relevant parameters of PPV and NPV. Diagnostic accuracy data in LGG is limited and appears significantly lower than for HGG. Ling et al. [107] reported that 42 biopsies taken from 7 patients undergoing fluorescein-guided LGG surgery showed a sensitivity of 64.3–66.47 and specificity 57.1–61.1% [107]. Although potentially useful for highlighting areas of contrast enhancement, the evidence in LGG currently does not support its use outside of a research setting.
3.4.2. Clinical Efficacy
Fluorescein is well established as safe in glioma surgery; however, to date, no Phase 3 RCTs have been conducted to evaluate its impact on rates of glioma resection and survival. Despite this, logistical and cost advantages of fluorescein have led to a sustained attempt to establish its efficacy. A meta-analysis [102] pooled data from 331 patients across 21 studies to demonstrate a GTR of 81%, comparable with that seen in the 5-ALA Phase 3 trials. The authors note, however, that 11 studies do not include a control group, and there is significant heterogeneity in the definitions of GTR, timing, and dose of fluorescein and in the grading of HGG. Furthermore, a significant number are non-randomised, retrospective studies. Only 7 of the 21 included data on overall survival, and 2 included data on PFS. None reported mean overall survival time.
Five prospective studies reporting survival data show GTR ranging from 74 to 90% (Table 2). Median PFS in three studies ranges from 7 to 12 months, with overall survival from 12 to 16 months. Other retrospective datasets have recorded GTR rates of 81.1–97.4% [105,124,125,126]. Median PFS was 9.2 months in the largest reporting dataset to date [125].
It is important to note the significant limitations to this evidence base. Whilst in recent years protocols have become more standardized, there remains variability in the dose and timing of administration that hinders data pooling. A large proportion of the literature does not include case-matched controls or relies on historic control data with the inherent risk of bias. As with all surgical studies in glioma, comparison of survival over time is hindered by changes to the postoperative treatment available to patients, and a significant number of published fluorescein studies do not report survival.
3.5. Limitations
Fluorescein is limited by subjective interpretation, a low signal-to-noise ratio at tumour margins—worsened by the potential for surrounding oedema [122]—and low emission in tumours such as LGG where BBB breakdown is limited [21]. An inherent limitation of fluorescein lies in its non-specific mechanism of action, showing extracellular accumulation in areas of BBB breakdown rather than being specifically targeted to tumour tissue [127].
Care must also be taken with timing the administration of fluorescein given the propensity of the unbound component to freely cross the BBB in the early period following injection.
3.6. Regulatory Issues
Fluorescein is approved by both the FDA and the National Institute for Health and Care Excellence (NICE) for ocular angiography. The Italian drug agency has approved fluorescein as a fluorescence tracer for aggressive CNS tumours [128]; however, this is not the case elsewhere in Europe or the USA. All use in low-grade gliomas remains off-label.

Table 2.
Prospective trials assessing the effect of FS on clinical outcome.
Table 2.
Prospective trials assessing the effect of FS on clinical outcome.
| Study | Study Type | n | EOR/GTR | OS | PFS | Complications | Notes |
|---|---|---|---|---|---|---|---|
| Ling 2024 [107] | Prospective non-randomised | 90 FS LD: 30 FS StD: 30 Cont: 30 | GTR% FS LD: 90 FS StD: 86.7 Cont: 66.3 | — | 6m% FS LD: 90 FS StD: 86.7 Cont: 66.3 | Dependent 6m: FS LD: 10% FS StD: 13.3% Cont: 36.7% | LD: 1 mg/kg StdD: 5 mg/kg Adm: Post-intubation Historic single-centre control |
| Falco 2019/2023 [128,129] | Prospective non-randomised | 279 HGG: 128 GBM: 93 LGG: 11 | GTR (%) HGG: 74.2 GBM: 82.8 | Median (m) GBM: 16 | Median (m) GBM: 12 | No adverse reactions | 5 mg/kg Adm: After induction No LGG fluorescence Retrospective survival analysis |
| Acerbi 2018 [106] | Prospective, multicentric phase II, FLUOGLIO | 46 | GTR FS: 82.6 5-ALA: 32 WL: 36 | Median (m) 12 | Median (m) 7 6m: 56.6 12m: 15.2 | No FS related AE KPS returned to baseline 3m | 5–10 mg/kg Adm: After induction 30 adjacent to eloquent areas STUPP [2] completed in 20% |
| Chen 2012 [130] | Prospective non-randomised | 22 FS: 10 Control: 12 | GTR (%) FS: 80 Control: 33.3 | — | Median (m) FS: 7.2 Control: 4.8 | No significant difference in KPS between groups | 15–20 mg/kg WL guided Adm: Following dural opening HGG: 11 LGG: 11 |
| Koc 2008 [131] | Prospective non-randomised | 80 FS: 47 Control: 33 | GTR (%) FS: 83 WL: 55 | Median (w) FS: 44 WL: 42 | - | No significant difference in KPS between groups | 20 mg/kg WL guided Adm: Prior to dural opening |
Abbreviations: EOR: extent of resection; GTR: gross total resection; OS: overall survival; PFS: progression-free survival; M: months; W: weeks; LD: low dose; StdD: standard dose; Cont: control; Adm: administration; KPS: Karnofsky score; RCT: randomized controlled trial; WL: White light.
4. ICG
Indocyanine green (ICG) is an amphiphilic tricarbocyanine molecule that absorbs light at 778–806 nm before emitting in the near-infrared (NIR) window at 835 nm, with a secondary peak in the second NIR (NIRII) or shortwave infrared (SWIR) window from 1000 to 1700 nm [132]. This allows improved tissue penetration, simultaneous white light, and fluorescence imaging and significant improvements in signal-to-noise ratio. In glioma surgery, ICG has been used for angiography, as discussed in Section 4.1, and tumour differentiation using the “second window” effect, as discussed in Section 4.2.
4.1. ICG Angiography
ICG is established in intracerebral angiography. A 2003 landmark study demonstrated a tight correlation between intraoperative fluorescence and postoperative angiography in aneurysm and dural arteriovenous fistulas, and this technique is now routinely used in vascular neurosurgery [133]. Following this success, groups have exploited differences in the vascular structure of gliomas and the surrounding parenchyma with the aim of reducing postoperative complications resulting from vascular injury and improving tumour differentiation Figure 4.

Figure 4.
ICG angiography during glioma surgery. (a) White-light visualisation of resection cavity showing skeletonized cortical and tumour associated vessels. (b) IR imaging of surgical field with 800 nm excitation light, demonstrating abnormal tumour vessels and preserved flow in overlying cortical vessels. Images courtesy of Neuro PPEye study team, King’s College London, UK (NCT05397574).
4.1.1. Dose, Administration and Timing
When used for angiography, ICG is administered as a standard intravenous bolus dose of 0.2–0.5 mg/kg, immediately prior to imaging in the NIR window [134].
4.1.2. Mechanism of Action
ICG rapidly binds to plasma proteins and is confined to the vascular compartment. This allows high-fidelity fluorescence imaging of the intracranial vessels during surgery. This “first-window” fluorescence is rapidly cleared by the liver and excreted in bile—with a half-life of 10–15 min, allowing multiple imaging runs during a single procedure.
4.1.3. Evidence Base
ICG angiography was first performed for glioma in 1996 [135]. Doses of 2 mg/kg were used in 9 glioma patients to demonstrate a dynamic difference in perfusion between tumour tissue and parenchyma. In 2011, two groups used the technique to demonstrate tumour-specific vascular patterns, identify adjacent vessels for preservation, and highlight patients with a high post-operative risk of ischaemia [136,137]). A 2018 retrospective review of 10 gliomas allowed the identification of arterialised and thrombosed veins in 3 cases, allowing disconnection of the main venous collector to restore normal venous flow [138].
A 2015 study expanded the technique from the protection of vessels to tumour differentiation [139]. ICG was used with 5-ALA to demonstrate differing vascular architecture in three distinct “zones”, comprising tumour core, transition zone, and surrounding parenchyma. As noted by the authors, this indirect technique introduces the significant risk of resection of functional tissue and has not been evaluated using standard measures of diagnostic accuracy or validated in larger patient cohorts.
4.2. ICG Second-Window Tumour Differentiation
Prolonged ICG fluorescence and reduced tumour clearance [135] was used for the first time in humans for tissue differentiation based on the “second window effect” in 2016 [140]. This is a distinct traditional first-window use of ICG, where fluorescence imaging is performed immediately following injection when the fluorophore is confined to the vascular compartment. Instead, ICG is administered at higher doses up to 24–48 h prior to imaging, which is performed when the fluorophore has circulated throughout the body and accumulated in pathological tissue, as discussed below.
4.2.1. Mechanism of Action
Second-window ICG is underpinned by the enhanced permeability and retention (EPR) hypothesis. The combined increase in vascular permeability and impairment of lymphatic drainage allows the accumulation of ICG in tumours such that it can be visualised 24–48 h after administration [141].
4.2.2. Dose, Administration, and Timing
Second-window ICG requires doses higher than the FDA maximum recommended human dose [132]. Initial recommendations of 5 mg/kg [140] were based on pre-clinical studies using murine models, backed by first-in-human trials for thoracic carcinoma showing at doses below a 5 mg/kg tumour-to-background fluorescence ratio, and perceived fluorescence was low [142]. Doses up to 10 mg/kg did not improve subjective fluorescence perception. A subsequent dose-escalation study in 45 patients with thoracic malignancy using doses of 1–5 mg/kg and 2–3 mg/kg was found to give acceptable signal across all subtypes tested [143]. This led to a dose decrease, initially to 2.5 mg/kg [144] and further to 1 mg/kg as detection camera systems have improved [145,146]. To date, a dose-escalation/optimization study has not been performed for human glioma surgery using second-window ICG.
Preclinical studies demonstrate a peak tumour-to-background ratio at 24 h from administration [142], with mouse GBM models showing a broad plateau up to 48 h [147]. Current evidence indicates second window fluorescence is not significantly time dependent from 6 to 48 h; however this is yet to be robustly tested in humans.
4.2.3. Evidence Base
No large studies have investigated the use of second-window ICG on survival in glioma. A small study of 15 GBM patients undergoing FGS vs. 18 controls suggested an improved PFS of 9 months vs. 7 months (p = 0.0002) [146]. Studies of diagnostic accuracy have typically been small, have not included control arms, and are limited by the constraints of tumour sampling in vivo. These indicate a high sensitivity with low specificity. This is improving as more sophisticated detection systems, development of AI post-processing algorithms, and lower dose protocols are developed (Table A3). Published sensitivity ranges from 85.7 to 100% [140,145,146,147,148]. Specificity ranges from 25 to 56% in early studies using 5 mg/kg [140,147,148], up to 91.36% in more recent, small studies [146]. Due to this limited evidence base, the use of second-window ICG in tumour surgery remains confined to the research setting.
4.3. Side Effects and Safety
Side effects from ICG are rare, and its use is well established in hepatic, ophthalmic, and neurovascular imaging. Nausea, vomiting, and skin rash are reported in 0.2% of cases, with hypotension, arrhythmia, and anaphylactic shock in 0.05% [149].
4.4. Equipment
ICG fluorescence is emitted in the infrared spectrum and is not visible to the naked eye. Early studies used stand-alone, exoscope-based IR imaging systems that were validated for use in the operating theatre (Visionsense Iridium, Visionsense, Philadelphia, Pennsylvania) [140,147,148]. More recently, commercial IR imaging systems integrated into neurosurgical microscopes have become well established for imaging ICG fluorescence in neurovascular surgery, including Infrared800 and Flow800 (Zeiss, Göttingen, Germany) and Glow800 (Leica, Wetzlar, Germany), all of which can be adapted to utilise second-window imaging of tumours. More recent work evaluating imaging in both the SWIR and NIR II windows utilise either multispectral imaging systems [146] or specialised, filtered CCD cameras with laser excitation [145], which remain limited to the research setting.
4.5. Limitations
ICG in glioma remains a research tool. Promising work identifying tumour vessels has not been expanded to show a robust impact on either resection, complication rates, or survival. As novel outcome measures such as deterioration-free survival take increasing prominence in the neurosurgical literature [5], this type of technique aimed at reducing complications warrants further study.
Second-window ICG imaging is limited by its non-specific mechanism of action and low specificity. Progress has been made in this regard through the use of improved imaging modalities, including NIR II fluorescence and the use of convoluted neural networks for imaging analysis [145,146]. Work evaluating these systems in larger, randomised patient groups has the potential to significantly expand the neurosurgical armamentarium.
4.6. Regulatory Issues
FDA approval was granted in 1956 for the use of ICG in cardio-circulatory and hepatic investigation. This was expanded to ophthalmic angiography in 1975. The Leica FL800 system received FDA approval for ICG-guided cerebral angiography in 2006, and the first Zeiss system was approved in 2008 (K100468) [150]. It is important to note that use of cerebral angiography for tumour differentiation remains off-label, and FDA approvals remains device specific. Second-window ICG imaging remains off-label and limited to a research context.
5. Towards Objective Intra-Operative Fluorescence
FGS systems in clinical use provide a qualitative fluorescence image using the operative microscope, which is interpreted by the operating surgeon. The emitted light is passed through wavelength-specific filters to the surgical optics, and surgeons use this visible light to make judgments on the tissue under interrogation. Reliance on visible fluorescence generates positive predictive values of >90%, however is limited by low emission at infiltrative margins, and in LGG. As such, negative predictive values are as low as 35% [9]. Attempts to improve sensitivity through increasing fluorophore dose have been trailed using 5-ALA in LGG, with rates of visible fluorescence remaining limited to 60% [27].
A number of novel imaging systems have been released with the aim of improving the sensitivity and specificity of FGS by improving imaging geometry. Systems such as the Nico Myriad Spectra (Nico Corporation, Indianapolis, IN, USA) allow the introduction of excitation light immediately adjacent to the tissue under interrogation, with early experience within our group suggesting this approach can be particularly useful during minimally invasive approaches [151,152]. This remains to be validated in a prospective, multicentre trial. Similarly, a small retrospective study of 10 patients with HGG has suggested use of an exoscope may allow for improved fluorescence visualization versus an operative microscope [153]. A number of groups have developed surgical loupes modified for FGS with promising initial results, suggesting visible fluorescence intensities up to 9.9 times higher than when using the operative microscope [118,154,155].
These techniques remain constrained by subjective analysis of visible fluorescence. This limitation underpins the ongoing drive to develop objective, sensitive and specific imaging systems for the evaluation of glioma infiltration utilising established fluorophores.
5.1. Steady-State Fluorescence Spectroscopy
Fluorescence spectroscopy involves the collection of spectrally (i.e., wavelength-specific) resolved information either intra-operatively or ex vivo. This tissue-specific “optical fingerprint” can be used for tissue differentiation and non-invasive interrogation of tumour metabolism. This approach can either be based on the measurement of emitted photons, so-called “intensity” and “steady-state” spectroscopy, or the measurement of fluorescence lifetime “time-resolved fluorescence spectroscopy”. Time-resolved fluorescence spectroscopy measures the time between absorption of an excitation photon and emission of a fluorescence photon and is discussed in Section 5.3.
Early steady-state spectroscopy studies used point probes encompassing laser excitation with spatially paired detectors without exogenous fluorescence labels. These demonstrated that when excited at 360 nm, the emission from endogenous fluorophores such as NADH, FAD, and Lipofuscin was significantly different in glioma compared to non-infiltrated brain tissue samples, both ex vivo and in vivo [156]. This technique, described as label-free spectroscopy, has been trialled in pilot studies involving 39 patients, with reported sensitivities of 100% and specificities of 76%. Subsequent works report sensitivities and specificities of up to 94% and 93%, respectively [157]. These techniques also allow the identification of endogenous fluorophores involved in metabolism, with the potential of generating real-time insights into tumour metabolic pathways [158].
Label-based spectroscopic fluorescence for glioma surgery is underpinned by the hypothesis that fluorophores accumulate in LGG and the IM at levels too low for visual detection but in quantities significantly higher than non-neoplastic tissue. Point probe systems detecting “raw” fluorescence emission of PpIX has demonstrated a PPV of 96.2%, with an NPV of 39.5% [47].
5.2. Quantitative Fluorescence
Whilst an improvement on basic visual interpretation, spectroscopy utilising raw fluorescence emission remains qualitative in nature. Emission in complex biological systems such as glioma tissue is affected by a combination of optically distorting effects, the presence of overlapping endogenous fluorophores, and the photochemical state and quantum efficiency of the measured fluorophore. Objective, quantitative fluorescence (QF) spectroscopy requires the correction of each of these factors. The majority of QF work has been performed using 5-ALA, and a brief summary of these techniques is given below. The interested reader is referred to Valdes et al.’s [159] dedicated review article for further reading [159] on this topic.
5.2.1. Optical Distortion Correction
Fluorescence excitation and emission in tissue undergo a non-linear distortion as light is absorbed and scattered [159]. These properties are highly variable in glioma [160] and must be corrected to allow quantitative fluorescence. One method for this involves probe-based spectroscopy capable of measuring diffuse reflectance, coupled with computational models of light propagation alongside fluorescence emission [161,162,163,164,165]. Wide-field methods utilise highly selective cameras with tuneable spectral filters or hyperspectral cameras. These allow for spectral scanning and reconstruction of spatially and spectrally resolved data with optical correction based on diffuse reflectance [159,166,167]. Both are limited by a lack of validation in tightly paired optical and fluorescence readings and a reliance on diffuse reflectance for correction. Probe measurements are further constrained by a narrow detection area. Conversely, wide-field scanning techniques are affected by delays in image acquisition. More recent hyperspectral “snapshot” and “light-field” systems can overcome the issue of imaging delay by capturing spectral and spatial information simultaneously in a mosaic pattern at the cost of spatial and spectral resolution [168].
5.2.2. Fluorescence Unmixing
Following optical distortion correction, the calculated spectra comprise “tumour” fluorophore emission coupled with overlapping endogenous fluorophores such as free NADH, oxidised flavins, and lipofuscin. Attempts to isolate tumour-specific fluorescence have been performed with 5-ALA, using published datasets for PpIX spectra, whilst empirically calculating the spectra of auto-fluorescence constituents using known emission peaks [91]. The pre-determined constituents are then fitted to the measured spectra to extract PpIX-specific fluorescence. This represents a significant step forward; however, the method remains limited by the semi-empirical nature of auto-fluorescence characterisation and the need for high spectral resolution to be able to implement this technique in vivo.
5.2.3. Emission Form
QF requires an understanding of fluorophore behaviour in the glioma micro-environment. PpIX exhibits two emission forms in vitro, characterised by primary emission peaks at 620 nm (PpIX620) and 635 nm (PpIX635). These forms differ in quantum efficiency and are thought to represent different aggregations [169]. The presence of both forms has been demonstrated in glioma tissue, with the ratio (PpIX635/620) hypothesised to provide an insight into malignancy [88,89]. It is uncertain whether other tetrapyrrole intermediaries within the heme biosynthesis pathway, such as coporphyrinogen III or uroporphyrinogen III, contribute to the 620 nm peak observed in vivo [93], further hindering accurate quantification. To date, hardware constraints have hindered the collection of data with sufficient spatial and spectral resolution for unmixing in vivo without introducing unacceptable delays in the surgical workflow; however, this remains a promising area for development.
5.3. Time-Resolved Fluorescence Decay
An alternative to spectral fluorophore characterisation is the use of fluorescence lifetime. This is a photophysical parameter that represents the energy relaxation dynamics of individual fluorophores. On a molecular level, fluorescence lifetime represents the average time a molecule spends in an excited state after absorption of excitation light before the emission of fluorescence. These measurements are largely independent of excitation intensity and allow unmixing of fluorophores in the temporal dimension. Lifetime has been explored in neuro-oncology to investigate tumour metabolic status [170], and more recently PpIX content [171,172,173] and differentiation of PpIX [174] from endogenous fluorophores [175]. Acquiring lifetime measurements is challenging to translate into the operating theatre, requiring a high-frequency pulsed laser for fluorophore excitation, a high precision, gated detector with high sensitivity to interference, and an extended acquisition time. Despite its limitations, attempts have been made to engineer systems that integrate into the surgical workflow [173] by pairing readings with raw fluorescence emission, albeit limited by a lack of optical distortion correction and limited integration.
5.4. Confocal Laser Endomicroscopy
Confocal laser endomicroscopy (CLE) uses sodium fluorescein as a contrast agent to generate intraoperative histological images in near real time—thus avoiding the challenges relating to non-specific targeting and complex fluorophore/tissue interactions as detailed above. Commercial CLE systems such as the Zeiss CONVIVO have recently been approved by the FDA.
To date CLE in glioma has only been used as a diagnostic tool [176]. The first prospective, multi-centre phase II non-inferiority study investigated the diagnostic accuracy of CLE vs. frozen section (FS) in 210 patients—86 of which were glioma [177]. This reported a CLE accuracy of 87% vs. 91% FS, below the threshold for non-inferiority (p = 0.367)—however a significant learning curve for CLE and higher diagnostic accuracy for tumour specimens was apparent. Ongoing improvement in diagnostic accuracy with wider uptake of the technique remains possible. However, the processing time of CLE was significantly faster than FS—with a median processing time of 3 min and 27 min, respectively. A similar study remains recruiting at the time of writing (Bern, Switzerland, CLEBT, NCT04280952 accessed 16 January 2025). Whilst a use of CLE in guiding marginal tumour resection has been hypothesised, to date there are no studies the authors are aware of assessing the impact of CLE on extent of resection or survival in glioma and this exciting use case remains to be fully explored.
5.5. Future Prospects
As discussed above, the development of novel imaging technologies has made objective fluorescence mapping in glioma FGS a viable possibility over the last 10–15 years. At present, these techniques are confined by the inherent trade-off between spatial resolution, spectral resolution, and acquisition time [178,179]. As technologies such as hyperspectral imaging rapidly improve, these hardware constraints are being progressively overcome. This is eloquently highlighted by the astounding progression from large, bulky spectral scanning systems with acquisition times in minutes [180] to stand-alone and microscope-integrated systems that acquire hypercube images in less than a second [168]. This hardware development, combined with the novel signal processing techniques described above holds the potential not only to improve the positive and negative predictive value of FGS at the tumour margins, but to unlock critical prognostic data present in the detailed spectral signature of current fluorophores to inform surgical decision making in real time.
6. Novel Fluorophore Development
Current fluorophores are limited by non-specific mechanisms of action, overlapping endogenous autofluorescence, depth of tissue penetration, emission/excitation wavelengths that preclude simultaneous white-light and fluorescence imaging, and distortion by commonly present compounds such as haemoglobin. To overcome these limitations, a number of novel fluorescence agents are currently in development. These have the dual aim of improving the intensity and visibility of tumour-associated fluorescence and improving specificity through improved tumour targeting. In 2024 approximately 40 fluorescence agents were under investigation in 85 clinical trials across all tumours in the United States alone [181]. The development of novel fluorophores focuses on two key aspects of FGS: tumour targeting to improve specificity and emission detection to improve sensitivity.
6.1. Tumour Targeting
Improving fluorescence targeting can utilise both “activatable” fluorophores, where an aspect of the tumour environment acts either to remove a fluorescence quenching moiety [182] or induce an activating structural change [183] alongside the targeting of receptors overexpressed in glioma. This has been attempted in Phase 1 clinical studies using humanised, monoclonal antibodies [184]; smaller antibody fragments aimed at improving penetration of the BBB [185,186]; and rationally designed small molecules [187] conjugated to existing fluorophores (Figure 5).
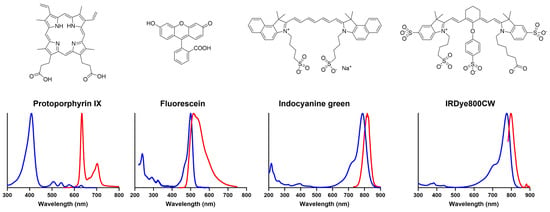
Figure 5.
Chemical structures and optical emission spectra of key fluorophores used in, and under investigation for, glioma FGS [188]. Excitation spectra are shown in blue, and emission spectra are shown in red. IRDye800CW spectra courtesy of Mr Jean-Romain Lotthe, Kings College London.
Use of a modified chlorotoxin peptide conjugated to ICG for tumour targeting (Tozuleristide, BLZ-100, Blaze Bioscience, Seattle, WA, USA) showed promise in a Phase 1 study [189], with optimal window dose showing a sensitivity of 82% and specificity of 89%. This remains the only novel fluorophore to complete a Phase 2/3 clinical in glioma, with equivocal early results posted in December 2024. In 105 subjects, sensitivity was 79.8%; however, specificity for equivocal tissue was 34.7% (NCT03579602 accessed 16 January 2025). A full breakdown of the study is yet to be published in the peer-reviewed literature.
Two Phase 2/3 trials investigating Panitumumab for EGFR targeting are ongoing (NCT04085887, not yet recruiting, accessed 16 January 2025, NCT03510208 estimated completion 25 December, accessed 16 January 2025). Seven Phase 1 trials have been completed investigating targeted fluorophores, including BLZ-100 [189,190], the GRPR-targeted 8Ga-IRDye800CW-BBN [191,192], the gamma-glutamyltranspeptidase-activated Ga-IRDye800 [182], and EGFR-targeting Cetuximab-IRDye800CW [184]. The EGFR-targeted ABY-029 recently posted results (NCT02901925 accessed 16 January 2025) but is yet to appear in the literature.
6.2. Enhanced Fluorescence
Efforts to improve sensitivity have focused on the development of fluorophores, which emit in the NIR and NIR II spectrum [184,189] to improve tissue penetration and reduce autofluorescence. Improving penetration of the BBB has also been shown to improve sensitivity through increased fluorescence levels [187].
Nanoparticles such as quantum dots are also being explored [193]. These can be engineered to include surface coatings and negative surface charge to prevent serum protein binding, improving both half-life and accumulation via EPR at areas of the BBB breakdown. Despite the lack of specific targeting, this technique has shown early promise in glioblastoma, albeit with some concerns around the sensitivity at tumour margins [194,195]. At present nanotracers remain limited to preclinical studies for FGS but have commenced clinical studies as drug delivery and treatment adjuncts [196].
6.3. Barriers to Novel Fluorophores
The challenges in development of novel fluorophores include high research and development costs [197], molecular heterogeneity [198], the paucity of preclinical disease models [199], safety concerns [193] and the relative lack of findings in glioma [200]. FDA approval also typically requires overall survival to be assessed, which is problematic for FGS agents given the heterogeneity of treatment and outcomes based on tumour location, size, molecular characteristics, and fitness for post-operative systemic therapies. The 5-ALA pathway is instructive for the development and clinical translation of novel fluorophores and highlights the significant evidence and time required for approval [14]. To date, the few phase II clinical trials of novel fluorophores have been either lacking or equivocal.
6.4. Future Prospects
The development of novel fluorophores for glioma FGS has been dealt a blow by recent early phase trial results (NCT03579602 accessed 16 January 2025). Nevertheless, the need for agents that allow the operating field to be safely visualised whilst simultaneously labelling pathological tissue with high sensitivity and specificity remains pressing. The recent award of the Nobel Prize in chemistry for the discovery and development of quantum dots in 2023 highlights the huge potential for nano-molecules in this field. This is synergistic with developing techniques such as ultrasound [201] for focal blood–brain barrier disruption, which is a formidable obstacle to the delivery of new agents to the surgical field. Beyond challenges of safety and specificity described above, clinicians keen to explore novel agents in their practice, translational researchers, and glioma funders should be conscious of the formidable regulatory issues and time required to bring these agents into routine use.
7. Conclusions
Fluorescence guidance has become an established and widely accepted adjunct in glioma surgery, with demonstrable benefits to the extent of resection.
Of the fluorophores in current clinical use, 5-ALA is alone in receiving regulatory approval for HGG surgery in the USA and Europe, backed by RCTs showing increased extent of resection. However, FGS in LGG is limited by low levels of visible fluorescence. All FGS techniques remain constrained by the challenges of detection.
Pharmacokinetic studies highlight the potential for FGS techniques and fluorophores to be optimised for administration timing and dose. The development of pathology-specific, standardized protocols for dose, administration timing, and detection techniques is essential, particularly in the case of fluorescein, where significant variation in practice can be seen across the literature, significantly undermining the evidence bases for its use. With increasing emphasis on molecular markers in glioma diagnosis in the updated 2021 World Health Organisation (WHO) classification of tumours of the central nervous system, the possibility for fluorescence to be studied in molecular rather than histologically defined subgroups is an exciting ongoing area of study.
Current methods of FGS are impacted by subjective interpretation, the inability to safely operate under excitation light, non-linear tissue optical distortions, overlapping fluorescence, and, in particular subgroups, low emission. Exciting work is underway to overcome these limitations, with promising integrated quantitative fluorescence systems for glioma surgery. This work has been spearheaded using point probes in conjunction with 5-ALA and is expanding to include wide-field techniques that can more seamlessly integrate with surgical workflows. Synergistic developments in detection hardware, including hyperspectral imaging cameras with novel AI-driven analyses hold the potential to quantify specific fluorophores and understand the relevance of their changing spectral signatures within the heterogeneous glioma micro-environment. Confocal microscopy gives the potential for in vivo virtual biopsies integrated with wide-field infiltrative margin detection.
All established fluorophores covered in this review are limited either by their inherent molecular properties or mechanism of action. The development of novel, tumour-targeted fluorophores with tailored fluorescence characteristics holds great promise, but clinicians should be aware of the significant timelines involved in development, validation, and regulatory approval and disappointing Phase 1 results.
As fluorescence-guided surgery develops, the development of consensus guidelines for evaluation is crucial. These are beginning to emerge in the technical [202] and clinical [53] literature. Researchers and clinicians should understand the distinction between PPV, NPV, sensitivity, and specificity. The development of measurable, relevant, and accepted outcome measures in clinical trials remains a challenge across the glioma literature, and patient-specific measures such as deficit-free survival are increasingly considered essential [203].
Author Contributions
Conceptualization, M.E. and J.S.; methodology, M.E., J.S. and T.V.; data curation, M.E., S.S., G.J.S., Y.X. and J.S.; writing—original draft preparation, M.E.; writing—review and editing, S.S., J.P.L., F.V., R.B., K.A., Y.X., G.J.S., T.V. and J.S.; visualization, Y.X.; supervision, J.S. and T.V. All authors have read and agreed to the published version of the manuscript.
Funding
M.E. and S.S. were funded by Wellcome Trust grant number WT223880/X/21/Z, J.S. and T.V. received funding from the National Institute for Health Research (NIHR) under its Invention for Innovation (i4i) Programme (Grant Reference Number NIHR202114). T.V. was supported by a Medtronic/RAEng Research Chair (RSCRF1819/7/34). M.E., S.S., Y.X., T.V. and J.S. received funding and support from the Wellcome EPSRC Centre for Medical Engineering. Y.X. was supported by the RAEng Research Fellowship (RF2122-21-354). For the purpose of open access, the authors have applied a CC BY public copyright license to any Author-Accepted Manuscript version arising from this submission.
Data Availability Statement
No new data were created or analysed in this study. Data sharing is not applicable to this article.
Acknowledgments
We are grateful to Ryan Mathew for providing images of fluorescein-guided FGS and to Jean-Romain Lotthe for providing spectra for IRDye 800CW.
Conflicts of Interest
J.S. and T.V. are shareholders and co-founders of Hypervision Surgical Ltd., a King’s College London spinout company developing hyperspectral technology for surgical use. Hypervision surgical had no role in the review design, interpretation of data, or writing of this manuscript.
Abbreviations
The following abbreviations are used in this manuscript:
| GTR | gross total resection |
| HGG | high-grade glioma |
| LGG | low-grade glioma |
| CNS | central nervous system |
| IM | infiltrative margin |
| FGS | fluorescence-guided surgery |
| EOR | extent of resection |
| 5-ALA | 5-aminoleuvulinic acid |
| FS | fluorescein sodium |
| ICG | indocyanine green |
| PpIX | protoporphyrin IX |
| EMA | European Medicines Agency |
| FDA | Federal Drugs Agency |
| WHO | World Health Organisation |
| NICE | National Institute for Health and Care Excellence |
| ABCG2 | ATP-binding cassette G2 |
| ABCB6 | ATP-binding cassette B6 |
| CPOX | coproporphyrinogen III oxidase |
| PPOX | protoporphyrinogen oxidase |
| MRNA | messenger RNA |
| FECH | ferrochelatase |
| PPV | positive predictive value |
| NPV | negative predictive value |
| Sens | sensitivity |
| Spec | specificity |
| GBM | glioblastoma multiforme |
| RCT | randomised control trial |
| PFS | progression-free survival |
| OS | overall survival |
| iMRI | intraoperative magnetic resonance imaging |
| FLAIR | fluid attenuated inversion recovery |
| WL | white light |
| SOC | standard of care |
| RT | radiotherapy |
| CTh | chemotherapy |
| DVT | deep-vein thrombosis |
| QALY | quality-adjusted life year |
| Da | Daltons |
| LD | low dose |
| StD | standard dose |
| D | dose |
| Adm | administration |
| CE | contrast enhancing |
| NCE | non-contrast enhancing |
| Lymph | lymphoma |
| Met | metastases |
| CRET | complete resection of enhancing tumour |
| KPS | Karnofsky performance score |
| NANO | neurologic assessment in neuro-oncology |
| AE | adverse event |
| m | months |
| w | weeks |
| NIR | near-infrared |
| SWIR | short-wave infrared |
| FS | frozen section |
| EPR | enhanced permeability and retention |
| QF | quantitative fluorescence |
| HSI | hyperspectral Imaging |
| EGFR | epidermal growth factor receptor |
Appendix A

Table A1.
Key prospective studies of 5-ALA diagnostic accuracy. Abbreviations: Sens: sensitivity; Spec: specificity; PPV: positive predictive value; NPV: negative predictive value; Core: tumour core; GBM: glioblastoma multiforme.
Table A1.
Key prospective studies of 5-ALA diagnostic accuracy. Abbreviations: Sens: sensitivity; Spec: specificity; PPV: positive predictive value; NPV: negative predictive value; Core: tumour core; GBM: glioblastoma multiforme.
| Publication | Pathology | n | Sens (%) | Spec (%) | PPV(%) | NPV (%) | Notes |
|---|---|---|---|---|---|---|---|
| Schupper 2021 [30] | HGG | 65 | 98.5 | 29.4 | 95.4 | 35.7 | New + Recurrent |
| Coburger 2017 [52] | GBM | 33 | 84 | 100 | - | - | 99 biopsies in 33 patients New diagnoses |
| Hauser 2016 [51] | GBM | 12 | 91 | 43 | 96 | 12.5 | New diagnoses |
| Lau 2016 [50] | HGG | 59 | 81.7 84.2: GBM | 64.5 62.1: GBM | 93 91.8: GBM | 37.7 43.9: GBM | New + Recurrent |
| Yamada 2015 [49] | HGG | 97 | 95 99: Core | 53 | 92 | 69 | New + Recurrent |
| Coburger 2014 [48] | HGG | 34 | 91 | 80 | 99 | 22 | New + Recurrent |
| Stummer 2014 [47] | GBM | 22 | - | - | 96 100: Strong 95: Vague | 39.5 | New diagnoses Results from visible fluorescence |
| Panciani 2012 [204] | GBM | 23 | 91 | 89 | 89 | 91 | New diagnoses |
| Ewelt 2011 [17] | HGG | 17 | 70.6 | 92.3 | - | - | New diagnoses HGG subgroup analysis |
| Diez Valle 2011 [205] | GBM | 36 | - | - | 100: Strong 97: Vague | 66 | New + Recurrent |
| Roberts 2011 [206] | GBM | 11 | 75 | 71 | 95 | 26 | New diagnoses |
| Nabavi 2009 [207] | HGG | 36 | 82 | 97 | 96.6 98.2: Strong 95.3: Weak | - | Recurrent Biopsy based analysis |
| Hefti 2008 [208] | HGG | 57 | 87 100: Strong 76: Weak | 85 98: Strong 85: Weak | - | - | New diagnoses |
| Stummer 2000 [25] | GBM | 52 | 89 | 96 | 99 | 50 | New diagnoses |
| Stummer 1998 [55] | HGG | 9 | 85 | 100 | - | - | New diagnoses 10mg/kg dose |

Table A2.
Key studies of fluorescein diagnostic accuracy. Abbreviations: LD: low dose; SD: standard dose; D: dose; Adm: administration protocol; Sens: sensitivity; Spec: specificity; PPV: positive predictive value; NPV: negative predictive value; CE: contrast enhancing; NCE: non-contrast enhancing; WL: white light; Lymph: lymphoma; Met: metastases; GBM: glioblastoma multiforme.
Table A2.
Key studies of fluorescein diagnostic accuracy. Abbreviations: LD: low dose; SD: standard dose; D: dose; Adm: administration protocol; Sens: sensitivity; Spec: specificity; PPV: positive predictive value; NPV: negative predictive value; CE: contrast enhancing; NCE: non-contrast enhancing; WL: white light; Lymph: lymphoma; Met: metastases; GBM: glioblastoma multiforme.
| Publication | Pathology | n | Sens (%) | Spec (%) | PPV(%) | NPV (%) | Notes |
|---|---|---|---|---|---|---|---|
| Ling 2024 [107] | HGG LGG | 23 7 | LD: 93.5 StD: 91.7 LD: 64.3 StD: 66.47 | LD: 82.6 StD 81 LD: 57.1 StD: 61.1 | - | - | LD: 1 mg/kg StD: 5 mg/kg Adm: After induction of anaesthesia HGG: 138 biopsies LGG 42 biopsies Filtered microscope |
| Sweeney 2022 [123] | HGG | 34 | 62 | 100 | 100 | 81 | D: 5 mg/kg Adm: After induction of anaesthesia Subjective cavity assessment with MRI 560 nm customised filter |
| Hong 2019 [209] | HGG | 42 | 90.8 | 93.3 | - | - | D: 1.5–2 mg/kg Adm: 90 min prior to dural opening 87 biopsies Filtered microscope |
| Chen 2019 [210] | HGG | 49 | 91.7 | 90 | - | - | D: 5 mg/kg Adm: Immediately prior to anaesthetic 98 biopsies at boundary only Filtered microscope |
| Acerbi 2018 [106] | HGG | 13 | 80.8 | 79.1 | 80.8 | 79.1 | D: 5–10 mg/kg Adm: After induction of anaesthesia 50 biopsies Filtered microscope |
| Neira 2017 [110] | GBM | 32 | 75.6 CE: 87.9 NCE: 69.4 | 75 CE: - NCE: 66.7 | 96 CE: 98.6 NCE: 96.2 | - | D: 3 mg/kg Adm: After induction of anaesthesia 90 biopsies Filtered microscope |
| Catapano 2017 [211] | HGG | 23 | 84.61 | 95 | - | - | D: 5 mg/kg Adm: At induction of anaesthesia Biopsy no not reported Filtered microscope |
| Diaz 2015 [127] | HGG | 12 | 82.8 | 90.9 | - | - | D: 3 mg/kg Adm: After induction of anaesthesia 67 biopsies at boundary only Filtered microscope |
| Murray 1982 [212] | HGG LGG Lypmh Met | 14 2 3 3 | 85 | 95 | - | - | D: 10–20 mL 10% Adm: At induction of anaesthesia 186 biopsies WL only |

Table A3.
Key studies of second-window ICG diagnostic accuracy. Abbreviations: Sens: sensitivity; Spec: specificity; PPV: positive predictive value; NPV: negative predictive value; D: dose; Adm: administration; NIR: near-infrared; CNN: convoluted neural network.
Table A3.
Key studies of second-window ICG diagnostic accuracy. Abbreviations: Sens: sensitivity; Spec: specificity; PPV: positive predictive value; NPV: negative predictive value; D: dose; Adm: administration; NIR: near-infrared; CNN: convoluted neural network.
| Publication | Pathology | n | Sens (%) | Spec (%) | PPV(%) | NPV (%) | Notes |
|---|---|---|---|---|---|---|---|
| Lee 2016 [140] | HGG LGG | 11 4 | 98 | 45 | 82 | 90 | D: 5 mg/kg Adm: 24 h pre-op Calculation from fluorescent tumours only (n = 12) False + ve in gliosis, choroid plexus, scar tissue |
| Zeh 2017 [147] | GBM | 10 | 85.7 | 25 | 85.7 | 25 | D: 5 mg/kg Adm: 24 h pre-op High sensitivity, low specificity |
| Cho 2020 [144] | HGG | 36 | 97 | 56 | 94 | 71 | D: 2.5–5 mg/kg Adm: 24 h pre-op 78 biopsies Dose reduced at mid point following dose-reduction study [143] |
| Shen 2021 [145] | HGG | 23 | 93.8 | 82.2 | 91 | 87.2 | D: 1 mg/kg Adm: 48 h pre-op 1874 biopsies NIR II Fluorescence Use of CNN for classification 70% train 30% test |
| Shi 2022 [146] | HGG | 15 | 100 | 91.36 | 95.65 | 100 | D: 1 mg/kg Adm: 48 h pre-op 235 biopsies NIR II fluorescence Ex-vivo biopsy analysis |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Chaichana, K.L.; Jusue-Torres, I.; Navarro-Ramirez, R.; Raza, S.M.; Pascual-Gallego, M.; Ibrahim, A.; Hernandez-Hermann, M.; Gomez, L.; Ye, X.; Weingart, J.D.; et al. Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro-Oncol. 2014, 16, 113–122. [Google Scholar] [CrossRef]
- Hervey-Jumper, S.L.; Berger, M.S. Role of surgical resection in low- and high-grade gliomas. Curr. Treat. Options Neurol. 2014, 16, 284. [Google Scholar] [CrossRef]
- Plaha, P.; Camp, S.; Cook, J.; McCulloch, P.; Voets, N.; Ma, R.; Taphoorn, M.J.; Dirven, L.; Grech-Sollars, M.; Watts, C.; et al. FUTURE-GB: Functional and ultrasound-guided resection of glioblastoma—A two-stage randomised control trial. BMJ Open 2022, 12, e064823. [Google Scholar] [CrossRef] [PubMed]
- Jusue-Torres, I.; Lee, J.; Germanwala, A.V.; Burns, T.C.; Parney, I.F. Effect of Extent of Resection on Survival of Patients with Glioblastoma, IDH–Wild-Type, WHO Grade 4 (WHO 2021): Systematic Review and Meta-Analysis. World Neurosurg. 2023, 171. [Google Scholar] [CrossRef]
- Moore, G.E. Fluorescein as an Agent in the Differentiation of Normal and Malignant Tissues. Science 1947, 106, 130–131. [Google Scholar] [CrossRef]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.J.; Group, A.L.G.S. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Schupper, A.J.; Rao, M.; Mohammadi, N.; Baron, R.; Lee, J.Y.K.; Acerbi, F.; Hadjipanayis, C.G. Fluorescence-Guided Surgery: A Review on Timing and Use in Brain Tumor Surgery. Front. Neurol. 2021, 12, 682151. [Google Scholar] [CrossRef]
- Orillac, C.; Stummer, W.; Orringer, D.A. Fluorescence Guidance and Intraoperative Adjuvants to Maximize Extent of Resection. Neurosurgery 2021, 89, 727–736. [Google Scholar] [CrossRef]
- Picart, T.; Gautheron, A.; Caredda, C.; Ray, C.; Mahieu-Williame, L.; Montcel, B.; Guyotat, J. Fluorescence-Guided Surgical Techniques in Adult Diffuse Low-Grade Gliomas: State-of-the-Art and Emerging Techniques: A Systematic Review. Cancers 2024, 16, 2698. [Google Scholar] [CrossRef] [PubMed]
- Sachar, M.; Anderson, K.E.; Ma, X. Protoporphyrin IX: The good, the bad, and the ugly. J. Pharmacol. Exp. Ther. 2016, 356, 267–275. [Google Scholar] [CrossRef]
- Stummer, W.; Stocker, S.; Novotny, A.; Heimann, A.; Sauer, O.; Kempski, O.; Plesnila, N.; Wietzorrek, J.; Reulen, H.J. In vitro and in vivo porphyrin accumulation by C6 glioma cells after exposure to 5-aminolevulinic acid. J. Photochem. Photobiol. B Biol. 1998, 45, 160–169. [Google Scholar] [CrossRef]
- Hadjipanayis, C.G.; Stummer, W. 5-ALA and FDA approval for glioma surgery. J. Neurooncol. 2019, 141, 479–486. [Google Scholar] [CrossRef]
- NICE. Brain Tumours (Primary) and Brain Metastases in Over 16s NICE Guideline. In NICE Guideline; National Institute of Health and Care Excellence: London, UK, 2018. [Google Scholar]
- Widhalm, G.; Wolfsberger, S.; Minchev, G.; Woehrer, A.; Krssak, M.; Czech, T.; Prayer, D.; Asenbaum, S.; Hainfellner, J.A.; Knosp, E. 5-Aminolevulinic acid is a promising marker for detection of anaplastic foci in diffusely infiltrating gliomas with nonsignificant contrast enhancement. Cancer 2010, 116, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Ewelt, C.; Floeth, F.W.; Felsberg, J.; Steiger, H.J.; Sabel, M.; Langen, K.J.; Stoffels, G.; Stummer, W. Finding the anaplastic focus in diffuse gliomas: The value of Gd-DTPA enhanced MRI, FET-PET, and intraoperative, ALA-derived tissue fluorescence. Clin. Neurol. Neurosurg. 2011, 113, 541–547. [Google Scholar] [CrossRef]
- Widhalm, G.; Kiesel, B.; Woehrer, A.; Traub-Weidinger, T.; Preusser, M.; Marosi, C.; Prayer, D.; Hainfellner, J.A.; Knosp, E.; Wolfsberger, S. 5-Aminolevulinic acid induced fluorescence is a powerful intraoperative marker for precise histopathological grading of gliomas with non-significant contrast-enhancement. PLoS ONE 2013, 8, e76988. [Google Scholar] [CrossRef] [PubMed]
- Jaber, M.; Wolfer, J.; Ewelt, C.; Holling, M.; Hasselblatt, M.; Niederstadt, T.; Zoubi, T.; Weckesser, M.; Stummer, W. The Value of 5-Aminolevulinic Acid in Low-grade Gliomas and High-grade Gliomas Lacking Glioblastoma Imaging Features: An Analysis Based on Fluorescence, Magnetic Resonance Imaging, 18F-Fluoroethyl Tyrosine Positron Emission Tomography, and Tumor Molecular Factors. Neurosurgery 2016, 78, 401–411. [Google Scholar] [CrossRef]
- Kiesel, B.; Freund, J.; Reichert, D.; Wadiura, L.; Erkkilae, M.T.; Woehrer, A.; Hervey-Jumper, S.; Berger, M.S.; Widhalm, G. 5-ALA in Suspected Low-Grade Gliomas: Current Role, Limitations, and New Approaches. Front. Oncol. 2021, 11, 699301. [Google Scholar] [CrossRef]
- Bianconi, A.; Bonada, M.; Zeppa, P.; Colonna, S.; Tartara, F.; Melcarne, A.; Garbossa, D.; Cofano, F. How Reliable Is Fluorescence-Guided Surgery in Low-Grade Gliomas? A Systematic Review Concerning Different Fluorophores. Cancers 2023, 15, 4130. [Google Scholar] [CrossRef]
- Jaber, M.; Ewelt, C.; Wolfer, J.; Brokinkel, B.; Thomas, C.; Hasselblatt, M.; Grauer, O.; Stummer, W. Is Visible Aminolevulinic Acid-Induced Fluorescence an Independent Biomarker for Prognosis in Histologically Confirmed (World Health Organization 2016) Low-Grade Gliomas? Neurosurgery 2019, 84, 1214–1224. [Google Scholar] [CrossRef] [PubMed]
- medac GmbH. Gliolan 30 mg/mL Powder for Oral Solution SmPC; medac GmbH: Wedel, Germany, 2023. [Google Scholar]
- Giakoumettis, D.; Kritis, A.; Foroglou, N. C6 cell line: The gold standard in glioma research. Hippokratia 2018, 22, 105–112. [Google Scholar]
- Stummer, W.; Novotny, A.; Stepp, H.; Goetz, C.; Bise, K.; Reulen, H.J. Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: A prospective study in 52 consecutive patients. J. Neurosurg. 2000, 93, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Suero Molina, E. Fluorescence Imaging/Agents in Tumor Resection. Neurosurg. Clin. N. Am. 2017, 28, 569–583. [Google Scholar] [CrossRef]
- Molina, E.S.; Black, D.; Kaneko, S.; Müther, M.; Stummer, W. Double dose of 5-aminolevulinic acid and its effect on protoporphyrin IX accumulation in low-grade glioma. J. Neurosurg. 2022, 137, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, S.; Suero Molina, E.; Ewelt, C.; Warneke, N.; Stummer, W. Fluorescence-Based Measurement of Real-Time Kinetics of Protoporphyrin IX After 5-Aminolevulinic Acid Administration in Human In Situ Malignant Gliomas. Neurosurgery 2019, 85, E739–E746. [Google Scholar] [CrossRef]
- Kaneko, S.; Suero Molina, E.; Sporns, P.; Schipmann, S.; Black, D.; Stummer, W. Fluorescence real-time kinetics of protoporphyrin IX after 5-ALA administration in low-grade glioma. J. Neurosurg. 2022, 136, 9–15. [Google Scholar] [CrossRef]
- Schupper, A.J.; Baron, R.; Cheung, W.; Rodriguez, J.; Kalkanis, S.N.; Chohan, M.; Nahed, B.V.; Zacharia, B.E.; Jensen, R.L.; Olsen, J.; et al. 213 5-Aminolevulinic Acid for Enhanced Surgical Visualization of High-Grade Gliomas: A Prospective, Multicenter Study. Neurosurgery 2022, 68, 65. [Google Scholar] [CrossRef]
- Picart, T.; Pallud, J.; Berthiller, J.; Dumot, C.; Berhouma, M.; Ducray, F.; Armoiry, X.; Margier, J.; Guerre, P.; Varlet, P.; et al. Use of 5-ALA fluorescence-guided surgery versus white-light conventional microsurgery for the resection of newly diagnosed glioblastomas (RESECT study): A French multicenter randomized phase III study. J. Neurosurg. 2024, 140, 987–1000. [Google Scholar] [CrossRef]
- Stepp, H.; Stummer, W. 5-ALA in the management of malignant glioma. Lasers Surg. Med. 2018, 50, 399–419. [Google Scholar] [CrossRef]
- Stepp, H.; Stummer, W. Delineating normal from diseased brain by aminolevulinic acid-induced fluorescence. In Optical Methods and Instrumentation in Brain Imaging and Therapy; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Colditz, M.J.; Leyen, K.V.; Jeffree, R.L. Aminolevulinic acid (ALA)-protoporphyrin IX fluorescence guided tumour resection. Part 2: Theoretical, biochemical and practical aspects. J. Clin. Neurosci. 2012, 19, 1611–1616. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, I.U.; Jahn, M.; Jahn, D. The biochemistry of heme biosynthesis. Arch. Biochem. Biophys. 2008, 474, 238–251. [Google Scholar] [CrossRef]
- Novotny, A.; Stummer, W. 5-Aminolevulinic acid and the blood-brain barrier—A review. Med. Laser Appl. 2003, 18, 36–40. [Google Scholar] [CrossRef]
- Mischkulnig, M.; Roetzer-Pejrimovsky, T.; Lötsch-Gojo, D.; Kastner, N.; Bruckner, K.; Prihoda, R.; Lang, A.; Martinez-Moreno, M.; Furtner, J.; Berghoff, A.; et al. Heme Biosynthesis Factors and 5-ALA Induced Fluorescence: Analysis of mRNA and Protein Expression in Fluorescing and Non-fluorescing Gliomas. Front. Med. 2022, 9, 907442. [Google Scholar] [CrossRef]
- Pustogarov, N.; Panteleev, D.; Goryaynov, S.A.; Ryabova, A.V.; Rybalkina, E.Y.; Revishchin, A.; Potapov, A.A.; Pavlova, G. Hiding in the Shadows: CPOX Expression and 5-ALA Induced Fluorescence in Human Glioma Cells. Mol. Neurobiol. 2017, 54, 5699–5708. [Google Scholar] [CrossRef]
- Mischkulnig, M.; Kiesel, B.; Lötsch, D.; Roetzer, T.; Borkovec, M.; Wadiura, L.I.; Mercea, P.A.; Jaklin, F.J.; Hervey-Jumper, S.; Roessler, K.; et al. TCGA mrna expression analysis of the heme biosynthesis pathway in diffusely infiltrating gliomas: A comparison of typically 5-ala fluorescent and non-fluorescent gliomas. Cancers 2020, 12, 2043. [Google Scholar] [CrossRef] [PubMed]
- Teng, L.; Nakada, M.; Zhao, S.G.; Endo, Y.; Furuyama, N.; Nambu, E.; Pyko, I.V.; Hayashi, Y.; Hamada, J.I. Silencing of ferrochelatase enhances 5-aminolevulinic acid-based fluorescence and photodynamic therapy efficacy. Br. J. Cancer 2011, 104, 798–807. [Google Scholar] [CrossRef]
- Nasir-Moin, M.; Wadiura, L.I.; Sacalean, V.; Juros, D.; Movahed-Ezazi, M.; Lock, E.K.; Smith, A.; Lee, M.; Weiss, H.; Müther, M.; et al. Localization of protoporphyrin IX during glioma-resection surgery via paired stimulated Raman histology and fluorescence microscopy. Nat. Biomed. Eng. 2024, 8, 672–688. [Google Scholar] [CrossRef]
- Liu, Z.; Mela, A.; Argenziano, M.G.; Banu, M.A.; Furnari, J.; Kotidis, C.; Sperring, C.P.; Humala, N.; Mahajan, A.; Bruce, J.N.; et al. Single-cell analysis of 5-aminolevulinic acid intraoperative labeling specificity for glioblastoma. J. Neurosurg. 2024, 140, 968–978. [Google Scholar] [CrossRef]
- Lang, A.; Jeron, R.L.; Lontzek, B.; Kiesel, B.; Mischkulnig, M.; Berghoff, A.S.; Ricken, G.; Wöhrer, A.; Rössler, K.; Lötsch-Gojo, D.; et al. Mapping high-grade glioma immune infiltration to 5-ALA fluorescence levels: TCGA data computation, classical histology, and digital image analysis. J. Neuro-Oncol. 2023, 164, 211–220. [Google Scholar] [CrossRef]
- Pinton, L.; Masetto, E.; Vettore, M.; Solito, S.; Magri, S.; D’Andolfi, M.; Del Bianco, P.; Lollo, G.; Benoit, J.P.; Okada, H.; et al. The immune suppressive microenvironment of human gliomas depends on the accumulation of bone marrow-derived macrophages in the center of the lesion. J. ImmunoTher. Cancer 2019, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Dietze, A.; Berg, K. ALA-induced porphyrin formation and fluorescence in synovitis tissue: In-vitro and in vivo studies. Photodiagn. Photodyn. Ther. 2005, 2, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, D.; Yi, B.; Cai, H.; Xi, Z.; Lou, X.; Li, Z. Comprehensive Analysis of CD163 as a Prognostic Biomarker and Associated with Immune Infiltration in Glioblastoma Multiforme. BioMed Res. Int. 2021, 2021, 8357585. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Tonn, J.C.; Goetz, C.; Ullrich, W.; Stepp, H.; Bink, A.; Pietsch, T.; Pichlmeier, U. 5-Aminolevulinic acid-derived tumor fluorescence: The diagnostic accuracy of visible fluorescence qualities as corroborated by spectrometry and histology and postoperative imaging. Neurosurgery 2014, 74, 310–319. [Google Scholar] [CrossRef]
- Coburger, J.; Engelke, J.; Scheuerle, A.; Thal, D.R.; Hlavac, M.; Wirtz, C.R.; König, R. Tumor detection with 5-aminolevulinic acid fluorescence and Gd-DTPA-enhanced intraoperative MRI at the border of contrast-enhancing lesions: A prospective study based on histopathological assessment. Neurosurg. Focus FOC 2014, 36, E3. [Google Scholar] [CrossRef]
- Yamada, S.; Muragaki, Y.; Maruyama, T.; Komori, T.; Okada, Y. Role of neurochemical navigation with 5-aminolevulinic acid during intraoperative MRI-guided resection of intracranial malignant gliomas. Clin. Neurol. Neurosurg. 2015, 130, 134–139. [Google Scholar] [CrossRef]
- Lau, D.; Hervey-Jumper, S.L.; Chang, S.; Molinaro, A.M.; McDermott, M.W.; Phillips, J.J.; Berger, M.S. A prospective Phase II clinical trial of 5-aminolevulinic acid to assess the correlation of intraoperative fluorescence intensity and degree of histologic cellularity during resection of high-grade gliomas. J. Neurosurg. 2016, 124, 1300–1309. [Google Scholar] [CrossRef]
- Hauser, S.B.; Kockro, R.A.; Actor, B.; Sarnthein, J.; Bernays, R.L. Combining 5-aminolevulinic acid fluorescence and intraoperative magnetic resonance imaging in glioblastoma surgery: A histology-based evaluation. Neurosurgery 2016, 78, 475–483. [Google Scholar] [CrossRef]
- Coburger, J.; Scheuerle, A.; Pala, A.; Thal, D.; Wirtz, C.R.; König, R. Histopathological Insights on Imaging Results of Intraoperative Magnetic Resonance Imaging, 5-Aminolevulinic Acid, and Intraoperative Ultrasound in Glioblastoma Surgery. Neurosurgery 2017, 81, 165–174. [Google Scholar] [CrossRef]
- Stummer, W.; Koch, R.; Valle, R.D.; Roberts, D.W.; Sanai, N.; Kalkanis, S.; Hadjipanayis, C.G.; Suero Molina, E. Intraoperative fluorescence diagnosis in the brain: A systematic review and suggestions for future standards on reporting diagnostic accuracy and clinical utility. Acta Neurochir. 2019, 161, 2083–2098. [Google Scholar] [CrossRef]
- Omoto, K.; Matsuda, R.; Nakagawa, I.; Motoyama, Y.; Nakase, H. False-positive inflammatory change mimicking glioblastoma multiforme under 5-aminolevulinic acid-guided surgery: A case report. Surg. Neurol. Int. 2018, 9, 49. [Google Scholar] [CrossRef]
- Stummer, W.; Stocker, S.; Wagner, S.; Stepp, H.; Fritsch, C.; Goetz, C.; Goetz, A.E.; Kiefmann, R.; Reulen, H.J. Intraoperative detection of malignant gliomas by 5-aminolevulinic acid-induced porphyrin fluorescence. Neurosurgery 1998, 42, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, R.; Katayama, Y.; Watanabe, T.; Yoshino, A.; Fukushima, T.; Sakatani, K. Quantitative spectroscopic analysis of 5-aminolevulinic acid-induced protoporphyrin IX fluorescence intensity in diffusely infiltrating astrocytomas. Neurol. Med. Chir. 2007, 47, 53–57. [Google Scholar] [CrossRef]
- Tsugu, A.; Ishizaka, H.; Mizokami, Y.; Osada, T.; Baba, T.; Yoshiyama, M.; Nishiyama, J.; Matsumae, M. Impact of the combination of 5-aminolevulinic acid-induced fluorescence with intraoperative magnetic resonance imaging-guided surgery for glioma. World Neurosurg. 2011, 76, 120–127. [Google Scholar] [CrossRef]
- Saito, K.; Hirai, T.; Takeshima, H.; Kadota, Y.; Yamashita, S.; Ivanova, A.; Yokogami, K. Genetic Factors Affecting Intraoperative 5-aminolevulinic Acid-induced Fluorescence of Diffuse Gliomas. Radiol. Oncol. 2017, 51, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Goryaynov, S.A.; Widhalm, G.; Goldberg, M.F.; Chelushkin, D.; Spallone, A.; Chernyshov, K.A.; Ryzhova, M.; Pavlova, G.; Revischin, A.; Shishkina, L.; et al. The Role of 5-ALA in Low-Grade Gliomas and the Influence of Antiepileptic Drugs on Intraoperative Fluorescence. Front. Oncol. 2019, 9, 423. [Google Scholar] [CrossRef] [PubMed]
- Cordova, J.S.; Gurbani, S.S.; Holder, C.A.; Olson, J.J.; Schreibmann, E.; Shi, R.; Guo, Y.; Shu, H.K.G.; Shim, H.; Hadjipanayis, C.G. Semi-Automated Volumetric and Morphological Assessment of Glioblastoma Resection with Fluorescence-Guided Surgery. Mol. Imaging Biol. 2016, 18, 454–462. [Google Scholar] [CrossRef]
- Coburger, J.; Hagel, V.; Wirtz, C.R.; König, R. Surgery for glioblastoma: Impact of the combined use of 5-aminolevulinic acid and intraoperative MRI on extent of resection and survival. PLoS ONE 2015, 10, 0131872. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro-Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Eatz, T.A.; Eichberg, D.G.; Lu, V.M.; Di, L.; Komotar, R.J.; Ivan, M.E. Intraoperative 5-ALA fluorescence-guided resection of high-grade glioma leads to greater extent of resection with better outcomes: A systematic review. J. Neurooncol. 2022, 156, 233–256. [Google Scholar] [CrossRef]
- Gandhi, S.; Tayebi Meybodi, A.; Belykh, E.; Cavallo, C.; Zhao, X.; Syed, M.P.; Borba Moreira, L.; Lawton, M.T.; Nakaji, P.; Preul, M.C. Survival Outcomes Among Patients With High-Grade Glioma Treated With 5-Aminolevulinic Acid-Guided Surgery: A Systematic Review and Meta-Analysis. Front. Oncol. 2019, 9, 620. [Google Scholar] [CrossRef] [PubMed]
- Eljamel, M.S.; Goodman, C.; Moseley, H. ALA and Photofrin fluorescence-guided resection and repetitive PDT in glioblastoma multiforme: A single centre Phase III randomised controlled trial. Lasers Med. Sci. 2008, 23, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Hosmann, A.; Millesi, M.; Wadiura, L.I.; Kiesel, B.; Mercea, P.A.; Mischkulnig, M.; Borkovec, M.; Furtner, J.; Roetzer, T.; Wolfsberger, S.; et al. 5-ala fluorescence is a powerful prognostic marker during surgery of low-grade gliomas (Who grade ii)—experience at two specialized centers. Cancers 2021, 13, 2540. [Google Scholar] [CrossRef]
- Johansson, A.; Palte, G.; Schnell, O.; Tonn, J.C.; Herms, J.; Stepp, H. 5-Aminolevulinic acid-induced protoporphyrin IX levels in tissue of human malignant brain tumors. Photochem. Photobiol. 2010, 86, 1373–1378. [Google Scholar] [CrossRef] [PubMed]
- Jungk, C.; Warta, R.; Mock, A.; Friauf, S.; Hug, B.; Capper, D.; Abdollahi, A.; Debus, J.; Bendszus, M.; von Deimling, A.; et al. Location-dependent patient outcome and recurrence patterns in IDH1-wildtype glioblastoma. Cancers 2019, 11, 122. [Google Scholar] [CrossRef]
- Kirkpatrick, J.P.; Laack, N.N.; Shih, H.A.; Gondi, V. Management of GBM: A problem of local recurrence. J. Neuro-Oncol. 2017, 134, 487–493. [Google Scholar] [CrossRef]
- Tonn, J.C.; Stummer, W. Fluorescence-guided resection of malignant gliomas using 5-aminolevulinic acid: Practical use, risks, and pitfalls. Clin. Neurosurg. 2008, 55, 20–26. [Google Scholar]
- Schucht, P.; Knittel, S.; Slotboom, J.; Seidel, K.; Murek, M.; Jilch, A.; Raabe, A.; Beck, J. 5-ALA complete resections go beyond MR contrast enhancement: Shift corrected volumetric analysis of the extent of resection in surgery for glioblastoma. Acta Neurochir. 2014, 156, 305–312. [Google Scholar] [CrossRef]
- Haddad, A.F.; Young, J.S.; Morshed, R.A.; Berger, M.S. FLAIRectomy: Resecting beyond the Contrast Margin for Glioblastoma. Brain Sci. 2022, 12, 544. [Google Scholar] [CrossRef]
- Wach, J.; Vychopen, M.; Kühnapfel, A.; Seidel, C.; Güresir, E. A Systematic Review and Meta-Analysis of Supramarginal Resection versus Gross Total Resection in Glioblastoma: Can We Enhance Progression-Free Survival Time and Preserve Postoperative Safety? Cancers 2023, 15, 1772. [Google Scholar] [CrossRef]
- Certo, F.; Stummer, W.; Farah, J.O.; Freyschlag, C.; Visocchi, M.; Morrone, A.; Altieri, R.; Toccaceli, G.; Peschillo, S.; Thomè, C.; et al. Supramarginal resection of glioblastoma: 5-ALA fluorescence, combined intraoperative strategies and correlation with survival. J. Neurosurg. Sci. 2019, 63, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanayis, C.G.; Widhalm, G.; Stummer, W. What is the Surgical Benefit of Utilizing 5-Aminolevulinic Acid for Fluorescence-Guided Surgery of Malignant Gliomas? Neurosurgery 2015, 77, 663–673. [Google Scholar] [CrossRef]
- Masubuchi, T.; Kajimoto, Y.; Kawabata, S.; Nonoguchi, N.; Fujishiro, T.; Miyatake, S.I.; Kuroiwa, T. Experimental study to understand nonspecific protoporphyrin ix fluorescence in brain tissues near tumors after 5-aminolevulinic acid administration. Photomed. Laser Surg. 2013, 31, 428–433. [Google Scholar] [CrossRef]
- La Rocca, G.; Della Pepa, G.M.; Menna, G.; Altieri, R.; Ius, T.; Rapisarda, A.; Olivi, A.; Sabatino, G. State of the art of fluorescence guided techniques in neurosurgery. J. Neurosurg. Sci. 2019, 63, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Utsuki, S.; Oka, H.; Sato, S.; Shimizu, S.; Suzuki, S.; Tanizaki, Y.; Kondo, K.; Miyajima, Y.; Fujii, K. Histological examination of false positive tissue resection using 5-aminolevulinic acid-induced fluorescence guidance. Neurol. Med.-Chir. 2007, 47, 210–214. [Google Scholar] [CrossRef]
- Slof, J.; Díez Valle, R.; Galván, J. Análisis coste-efectividad de la cirugía del glioma maligno guiada por fluorescencia con ácido 5-aminolevulínico. Neurologia 2015, 30, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Warsi, N.M.; Zewude, R.; Karmur, B.; Pirouzmand, N.; Hachem, L.; Mansouri, A. The cost-effectiveness of 5-ALA in high-grade glioma surgery: A quality-based systematic review. Can. J. Neurol. Sci. 2020, 47, 793–799. [Google Scholar] [CrossRef]
- Tummers, W.S.; Warram, J.M.; Tipirneni, K.E.; Fengler, J.; Jacobs, P.; Shankar, L.; Henderson, L.; Ballard, B.; Pogue, B.W.; Weichert, J.P.; et al. Regulatory aspects of optical methods and exogenous targets for cancer detection. Cancer Res. 2017, 77, 2197–2206. [Google Scholar] [CrossRef]
- European Medicines Agency. EMA Scientific Discussion: 5-ALA; European Medicines Agency: Amsterdam, The Netherlands, 2007.
- Centre for Drug Evaluation and Research. Centre for Drug Evaluation and Research: Clinical Review—5-ALA; Centre for Drug Evaluation and Research: Silver Spring, MD, USA, 2015.
- Beck, T.J.; Kreth, F.W.; Beyer, W.; Mehrkens, J.H.; Obermeier, A.; Stepp, H.; Stummer, W.; Baumgartner, R. Interstitial photodynamic therapy of nonresectable malignant glioma recurrences using 5-aminolevulinic acid induced protoporphyrin IX. Lasers Surg. Med. 2007, 39, 386–393. [Google Scholar] [CrossRef]
- Stummer, W.; Beck, T.; Beyer, W.; Mehrkens, J.H.; Obermeier, A.; Etminan, N.; Stepp, H.; Tonn, J.C.; Baumgartner, R.; Herms, J.; et al. Long-sustaining response in a patient with non-resectable, distant recurrence of glioblastoma multiforme treated by interstitial photodynamic therapy using 5-ALA: Case report. J. Neurooncol. 2008, 87, 103–109. [Google Scholar] [CrossRef]
- Dupont, C.; Vermandel, M.; Leroy, H.A.; Quidet, M.; Lecomte, F.; Delhem, N.; Mordon, S.; Reyns, N. INtraoperative photoDYnamic Therapy for GliOblastomas (INDYGO): Study Protocol for a Phase i Clinical Trial. Clin. Neurosurg. 2019, 84, E414–E419. [Google Scholar] [CrossRef] [PubMed]
- Peciu-Florianu, I.; Vannod-Michel, Q.; Vauleon, E.; Bonneterre, M.E.; Reyns, N. Long term follow-up of patients with newly diagnosed glioblastoma treated by intraoperative photodynamic therapy: An update from the INDYGO trial (NCT03048240). J. Neuro-Oncol. 2024, 168, 495–505. [Google Scholar] [CrossRef]
- Montcel, B.; Mahieu-Williame, L.; Armoiry, X.; Meyronet, D.; Guyotat, J. Two-peaked 5-ALA-induced PpIX fluorescence emission spectrum distinguishes glioblastomas from low grade gliomas and infiltrative component of glioblastomas. Biomed. Opt. Express 2013, 4, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Alston, L.; Mahieu-Williame, L.; Hebert, M.; Kantapareddy, P.; Meyronet, D.; Rousseau, D.; Guyotat, J.; Montcel, B. Spectral complexity of 5-ALA induced PpIX fluorescence in guided surgery: A clinical study towards the discrimination of healthy tissue and margin boundaries in high and low grade gliomas. Biomed. Opt. Express 2019, 10, 2478–2492. [Google Scholar] [CrossRef]
- Gautheron, A.; Bernstock, J.D.; Picart, T.; Guyotat, J.; Valdés, P.A.; Montcel, B. 5-ALA induced PpIX fluorescence spectroscopy in neurosurgery: A review. Front. Neurosci. 2024, 18, 1310282. [Google Scholar] [CrossRef] [PubMed]
- Black, D.; Kaneko, S.; Walke, A.; Konig, S.; Stummer, W.; Suero Molina, E. Characterization of autofluorescence and quantitative protoporphyrin IX biomarkers for optical spectroscopy-guided glioma surgery. Sci. Rep. 2021, 11, 20009. [Google Scholar] [CrossRef]
- Elliot, M.M.; Segaud, D.S.; MacCormac, M.O.; Patel, M.S.; Reisz, D.Z.; Lavrador, M.J.; Vergani, M.F.; Bhangoo, M.R.; Ashkan, P.K.; Al-Sarraj, P.S.; et al. An Ex-Vivo Spectral and Lifetime-Decay Analysis of 5-ALA Derived PPIX fluorescence: Objective fluorescence Mapping in Glioma Surgery. Neuro-Oncol. 2024, 26, vii8–vii9. [Google Scholar] [CrossRef]
- Suero Molina, E.; Black, D.; Walke, A.; Azemi, G.; D’Alessandro, F.; König, S.; Stummer, W. Unraveling the blue shift in porphyrin fluorescence in glioma: The 620 nm peak and its potential significance in tumor biology. Front. Neurosci. 2023, 17, 1261679. [Google Scholar] [CrossRef]
- Mowforth, O.D.; Brannigan, J.; El Khoury, M.; Sarathi, C.I.P.; Bestwick, H.; Bhatti, F.; Mair, R. Personalised therapeutic approaches to glioblastoma: A systematic review. Front. Med. 2023, 10, 1166104. [Google Scholar] [CrossRef]
- Duffau, H. Surgery for malignant brain gliomas: Fluorescence-guided resection or functional-based resection? Front. Surg. 2019, 6, 21. [Google Scholar] [CrossRef]
- Schebesch, K.M.; Hoehne, J.; Hohenberger, C.; Proescholdt, M.; Riemenschneider, M.J.; Wendl, C.; Brawanski, A. Fluorescein sodium-guided resection of cerebral metastases-experience with the first 30 patients. Acta Neurochir. 2015, 157, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Teich, W.; Frenzel, F.; Hoffmann, K.; Radke, J.; Rösler, J.; Faust, K.; Blank, A.; Brandenburg, S.; Misch, M.; et al. Optical Characterization of Sodium Fluorescein In Vitro and Ex Vivo. Front. Oncol. 2021, 11, 654300. [Google Scholar] [CrossRef] [PubMed]
- Sjöback, R.; Nygren, J.; Kubista, M. Absorption and fluorescence properties of fluorescein. Spectrochim. Acta Part A Mol. Spectrosc. 1995, 51, L7–L21. [Google Scholar] [CrossRef]
- Suero Molina, E.; Schipmann, S.; Stummer, W. Maximizing safe resections: The roles of 5-aminolevulinic acid and intraoperative MR imaging in glioma surgery—Review of the literature. Neurosurg. Rev. 2019, 42, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Zhang, J.; Liu, L. Fluorescence properties of twenty fluorescein derivatives: Lifetime, quantum yield, absorption and emission spectra. J. Fluoresc. 2014, 24, 819–826. [Google Scholar] [CrossRef]
- Belykh, E.; Shaffer, K.V.; Lin, C.; Byvaltsev, V.A.; Preul, M.C.; Chen, L. Blood-Brain Barrier, Blood-Brain Tumor Barrier, and Fluorescence-Guided Neurosurgical Oncology: Delivering Optical Labels to Brain Tumors. Front. Oncol. 2020, 10, 739. [Google Scholar] [CrossRef]
- Smith, E.J.; Gohil, K.; Thompson, C.M.; Naik, A.; Hassaneen, W. Fluorescein-Guided Resection of High Grade Gliomas: A Meta-Analysis. World Neurosurg. 2021, 155, 181–188. [Google Scholar] [CrossRef]
- Cavallo, C.; De Laurentis, C.; Vetrano, I.G.; Falco, J.; Broggi, M.; Schiariti, M.; Ferroli, P.; Acerbi, F. The utilization of fluorescein in brain tumor surgery: A systematic review. J. Neurosurg. Sci. 2018, 62, 690–703. [Google Scholar] [CrossRef]
- Okuda, T.; Yoshioka, H.; Kato, A. Fluorescence-guided surgery for glioblastoma multiforme using high-dose fluorescein sodium with excitation and barrier filters. J. Clin. Neurosci. 2012, 19, 1719–1722. [Google Scholar] [CrossRef]
- Shinoda, J.; Yano, H.; Yoshimura, S.I.; Okumura, A.; Kaku, Y.; Iwama, T.; Sakai, N. Fluorescence-guided resection of glioblastoma multiforme by using high-dose fluorescein: Technical note. sodium. J. Neurosurg. 2003, 99, 597–603. [Google Scholar] [CrossRef]
- Acerbi, F.; Broggi, M.; Schebesch, K.M.; Hohne, J.; Cavallo, C.; De Laurentis, C.; Eoli, M.; Anghileri, E.; Servida, M.; Boffano, C.; et al. Fluorescein-Guided Surgery for Resection of High-Grade Gliomas: A Multicentric Prospective Phase II Study (FLUOGLIO). Clin. Cancer Res. 2018, 24, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Ling, G.; Guo, T.; Guo, F.; Piao, H. Effectiveness and Safety of Ultra-low-dose Fluorescein Sodium-Guided Resection of Malignant Glioma. World Neurosurg. 2024, 185, e774–e785. [Google Scholar] [CrossRef]
- Schebesch, K.M.; Höhne, J.; Rosengarth, K.; Noeva, E.; Schmidt, N.O.; Proescholdt, M. Fluorescein-guided resection of newly diagnosed high-grade glioma: Impact on extent of resection and outcome. Brain Spine 2022, 2, 101690. [Google Scholar] [CrossRef]
- Folaron, M.; Strawbridge, R.; Samkoe, K.S.; Filan, C.; Roberts, D.W.; Davis, S.C. Elucidating the kinetics of sodium fluorescein for fluorescence-guided surgery of glioma. J. Neurosurg. 2019, 131, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Neira, J.A.; Ung, T.H.; Sims, J.S.; Malone, H.R.; Chow, D.S.; Samanamud, J.L.; Zanazzi, G.J.; Guo, X.; Bowden, S.G.; Zhao, B.; et al. Aggressive resection at the infiltrative margins of glioblastoma facilitated by intraoperative fluorescein guidance. J. Neurosurg. 2017, 127, 111–122. [Google Scholar] [CrossRef]
- Kuroiwa, T.; Kajimoto, Y.; Ohta, T. Development of a fluorescein operative microscope for use during malignant glioma surgery a technical note and prelminary report. Surg. Neurol. 1998, 50, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Acerbi, F.; Broggi, M.; Broggi, G.; Ferroli, P. What is the best timing for fluorescein injection during surgical removal of high-grade gliomas? Acta Neurochir. 2015, 157, 1377–1378. [Google Scholar] [CrossRef]
- Xu, K.; Tzankova, V.; Li, C.; Sharma, S. Intravenous fluorescein angiography–associated adverse reactions. Can. J. Ophthalmol. 2016, 51, 321–325. [Google Scholar] [CrossRef]
- Restelli, F.; Bonomo, G.; Monti, E.; Broggi, G.; Acerbi, F.; Broggi, M. Safeness of sodium fluorescein administration in neurosurgery: Case-report of an erroneous very high-dose administration and review of the literature. Brain Spine 2022, 2, 101703. [Google Scholar] [CrossRef]
- Fan, C.; Jiang, Y.; Liu, R.; Wu, G.; Wu, G.; Xu, K.; Miao, Z. Safety and feasibility of low-dose fluorescein-guided resection of glioblastoma. Clin. Neurol. Neurosurg. 2018, 175, 57–60. [Google Scholar] [CrossRef]
- Maugeri, R.; Villa, A.; Pino, M.; Imperato, A.; Giammalva, G.R.; Costantino, G.; Graziano, F.; Gulì, C.; Meli, F.; Francaviglia, N.; et al. With a little help from my friends: The role of intraoperative fluorescent dyes in the surgical management of high-grade gliomas. Brain Sci. 2018, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Acerbi, F.; Broggi, M.; Eoli, M.; Anghileri, E.; Cuppini, L.; Pollo, B.; Schiariti, M.; Visintini, S.; Orsi, C.; Franzini, A.; et al. Fluorescein-guided surgery for grade IV gliomas with a dedicated filter on the surgical microscope: Preliminary results in 12 cases. Acta Neurochir. 2013, 155, 1277–1286. [Google Scholar] [CrossRef]
- Zhang, X.; Habib, A.; Jaman, E.; Mallela, A.N.; Amankulor, N.M.; Zinn, P.O. Headlight and loupe-based fluorescein detection system in brain tumor surgery: A first-in-human experience. J. Neurosurg. Sci. 2023, 67, 374–379. [Google Scholar] [CrossRef]
- Blair, N.P.; Evans, M.A.; Lesar, T.S.; Zeimer, R.C. Fluorescein and fluorescein glucuronide pharmacokinetics after intravenous injection. Investig. Ophthalmol. Vis. Sci. 1986, 27, 1107–1114. [Google Scholar]
- Ichioka, T.; Miyatake, S.I.; Asai, N.; Kajimoto, Y.; Nakagawa, T.; Hayashi, H.; Kuroiwa, T. Enhanced detection of malignant glioma xenograft by fluorescein-human serum albumin conjugate. J. Neuro-Oncol. 2004, 67, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W. Poor man’s fluorescence? Acta Neurochir. 2015, 157, 1379–1381. [Google Scholar] [CrossRef]
- Molina, E.S.; Brokinkel, B. Sodium fluorescein versus 5-aminolevulinic acid to visualize high-grade gliomas. J. Neurosurg. 2020, 133, 1627–1630. [Google Scholar] [CrossRef]
- Sweeney, J.F.; Rosoklija, G.; Sheldon, B.L.; Bondoc, M.; Bandlamuri, S.; Adamo, M.A. Comparison of sodium fluorescein and intraoperative ultrasonography in brain tumor resection. J. Clin. Neurosci. 2022, 106, 141–144. [Google Scholar] [CrossRef]
- Luzzi, S.; Lucifero, A.G.; Martinelli, A.; Maestro, M.D.; Savioli, G.; Simoncelli, A.; Lafe, E.; Preda, L.; Galzio, R. Supratentorial high-grade gliomas: Maximal safe anatomical resection guided by augmented reality high-definition fiber tractography and fluorescein. Neurosurg. Focus 2021, 51, E5. [Google Scholar] [CrossRef]
- Hansen, R.W.; Pedersen, C.B.; Halle, B.; Korshoej, A.R.; Schulz, M.K.; Kristensen, B.W.; Poulsen, F.R. Comparison of 5-aminolevulinic acid and sodium fluorescein for intraoperative tumor visualization in patients with high-grade gliomas: A single-center retrospective study. J. Neurosurg. 2019, 133, 1324–1331. [Google Scholar] [CrossRef]
- Katsevman, G.A.; Turner, R.C.; Urhie, O.; Voelker, J.L.; Bhatia, S. Utility of sodium fluorescein for achieving resection targets in glioblastoma: Increased gross- Or near-total resections and prolonged survival. J. Neurosurg. 2020, 132, 914–920. [Google Scholar] [CrossRef]
- Diaz, R.J.; Dios, R.R.; Hattab, E.M.; Burrell, K.; Rakopoulos, P.; Sabha, N.; Hawkins, C.; Zadeh, G.; Rutka, J.T.; Cohen-Gadol, A.A. Study of the biodistribution of fluorescein in glioma-infiltrated mouse brain and histopathological correlation of intraoperative findings in high-grade gliomas resected under fluorescein fluorescence guidance. J. Neurosurg. 2015, 122, 1360–1369. [Google Scholar] [CrossRef]
- Falco, J.; Cavallo, C.; Vetrano, I.G.; de Laurentis, C.; Siozos, L.; Schiariti, M.; Broggi, M.; Ferroli, P.; Acerbi, F. Fluorescein Application in Cranial and Spinal Tumors Enhancing at Preoperative MRI and Operated with a Dedicated Filter on the Surgical Microscope: Preliminary Results in 279 Patients Enrolled in the FLUOCERTUM Prospective Study. Front. Surg. 2019, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Falco, J.; Rubiu, E.; Broggi, M.; Farinotti, M.; Vetrano, I.G.; Schiariti, M.; Anghileri, E.; Eoli, M.; Pollo, B.; Moscatelli, M.; et al. Towards an Established Intraoperative Oncological Favorable Tool: Results of Fluorescein-Guided Resection from a Monocentric, Prospective Series of 93 Primary Glioblastoma Patients. J. Clin. Med. 2023, 12, 178. [Google Scholar] [CrossRef]
- Chen, B.; Wang, H.; Ge, P.; Zhao, J.; Li, W.; Gu, H.; Wang, G.; Luo, Y.; Chen, D. Gross total resection of glioma with the intraoperative fluorescence-Guidance of fluorescein sodium. Int. J. Med Sci. 2012, 9, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Koc, K.; Anik, I.; Cabuk, B.; Ceylan, S. Fluorescein sodium-guided surgery in glioblastoma multiforme: A prospective evaluation. Br. J. Neurosurg. 2008, 22, 99–103. [Google Scholar] [CrossRef]
- Teng, C.W.; Huang, V.; Arguelles, G.R.; Zhou, C.; Cho, S.S.; Harmsen, S.; Lee, J.Y.K. Applications of indocyanine green in brain tumor surgery: Review of clinical evidence and emerging technologies. Neurosurg. Focus 2021, 50, E4. [Google Scholar] [CrossRef] [PubMed]
- Raabe, A.; Beck, J.; Gerlach, R.; Zimmermann, M.; Seifert, V.; Macdonald, R.L.; Meyer, B.; Selman, W.R. Near-infrared indocyanine green video angiography: A new method for intraoperative assessment of vascular flow. Neurosurgery 2003, 52, 132–139. [Google Scholar] [CrossRef]
- Scerrati, A.; Della Pepa, G.M.; Conforti, G.; Sabatino, G.; Puca, A.; Albanese, A.; Maira, G.; Marchese, E.; Esposito, G. Indocyanine green video-angiography in neurosurgery: A glance beyond vascular applications. Clin. Neurol. Neurosurg. 2014, 124, 106–113. [Google Scholar] [CrossRef]
- Haglund, M.M.; Berger, M.S.; Hochman, D.W. Enhanced optical imaging of human gliomas and tumor margins. Neurosurgery 1996, 38, 308–317. [Google Scholar] [CrossRef]
- Kim, E.H.; Cho, J.M.; Chang, J.H.; Kim, S.H.; Lee, K.S. Application of intraoperative indocyanine green videoangiography to brain tumor surgery. Acta Neurochir. 2011, 153, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Ferroli, P.; Acerbi, F.; Albanese, E.; Tringali, G.; Broggi, M.; Franzini, A.; Broggi, G. Application of intraoperative indocyanine green angiography for CNS tumors: Results on the first 100 cases. In Acta Neurochirurgica Supplementum; Springer Nature: Berlin/Heidelberg, Germany, 2011; Volume 109. [Google Scholar] [CrossRef]
- Acerbi, F.; Vetrano, I.G.; Sattin, T.; Falco, J.; de Laurentis, C.; Zattra, C.M.; Bosio, L.; Rossini, Z.; Broggi, M.; Schiariti, M.; et al. Use of ICG videoangiography and FLOW 800 analysis to identify the patient-specific venous circulation and predict the effect of venous sacrifice: A retrospective study of 172 patients. Neurosurg. Focus 2018, 45, E7. [Google Scholar] [CrossRef] [PubMed]
- Eyupoglu, I.Y.; Hore, N.; Fan, Z.; Buslei, R.; Merkel, A.; Buchfelder, M.; Savaskan, N.E. Intraoperative vascular DIVA surgery reveals angiogenic hotspots in tumor zones of malignant gliomas. Sci. Rep. 2015, 5, 7958. [Google Scholar] [CrossRef]
- Lee, J.Y.; Thawani, J.P.; Pierce, J.; Zeh, R.; Martinez-Lage, M.; Chanin, M.; Venegas, O.; Nims, S.; Learned, K.; Keating, J.; et al. Intraoperative Near-Infrared Optical Imaging Can Localize Gadolinium-Enhancing Gliomas During Surgery. Neurosurgery 2016, 79, 856–871. [Google Scholar] [CrossRef]
- Maeda, H.; Sawa, T.; Konno, T. Mechanism of tumor-targeted delivery of macromolecular drugs, including the EPR effect in solid tumor and clinical overview of the prototype polymeric drug SMANCS. J. Control. Release 2001, 74, 47–61. [Google Scholar] [CrossRef]
- Jiang, J.X.; Keating, J.J.; Jesus, E.M.D.; Judy, R.P.; Madajewski, B.; Venegas, O.; Okusanya, O.T.; Singhal, S. Optimization of the enhanced permeability and retention effect for near-infrared imaging of solid tumors with indocyanine green. Am. J. Nucl. Med. Mol. Imaging 2015, 5, 390–400. [Google Scholar] [PubMed]
- Newton, A.D.; Predina, J.D.; Corbett, C.J.; Frenzel-Sulyok, L.G.; Xia, L.; Petersson, E.J.; Tsourkas, A.; Nie, S.; Delikatny, E.J.; Singhal, S. Optimization of Second Window Indocyanine Green for Intraoperative Near-Infrared Imaging of Thoracic Malignancy. J. Am. Coll. Surg. 2019, 228, 188–197. [Google Scholar] [CrossRef]
- Cho, S.S.; Salinas, R.; De Ravin, E.; Teng, C.W.; Li, C.; Abdullah, K.G.; Buch, L.; Hussain, J.; Ahmed, F.; Dorsey, J.; et al. Near-Infrared Imaging with Second-Window Indocyanine Green in Newly Diagnosed High-Grade Gliomas Predicts Gadolinium Enhancement on Postoperative Magnetic Resonance Imaging. Mol. Imaging Biol. 2020, 22, 1427–1437. [Google Scholar] [CrossRef]
- Shen, B.; Zhang, Z.; Shi, X.; Cao, C.; Zhang, Z.; Hu, Z.; Ji, N.; Tian, J. Real-time intraoperative glioma diagnosis using fluorescence imaging and deep convolutional neural networks. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3482–3492. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, Z.; Zhang, Z.; Cao, C.; Cheng, Z.; Hu, Z.; Tian, J.; Ji, N. Near-Infrared Window II Fluorescence Image-Guided Surgery of High-Grade Gliomas Prolongs the Progression-Free Survival of Patients. IEEE Trans. Biomed. Eng. 2022, 69, 1889–1900. [Google Scholar] [CrossRef]
- Zeh, R.; Sheikh, S.; Xia, L.; Pierce, J.; Newton, A.; Predina, J.; Cho, S.; Nasrallah, M.; Singhal, S.; Dorsey, J.; et al. The second window ICG technique demonstrates a broad plateau period for near infrared fluorescence tumor contrast in glioblastoma. PLoS ONE 2017, 12, 0182034. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.S.; Salinas, R.; Lee, J.Y.K. Indocyanine-Green for Fluorescence-Guided Surgery of Brain Tumors: Evidence, Techniques, and Practical Experience. Front. Surg. 2019, 6, 11. [Google Scholar] [CrossRef]
- Meira, J.; Marques, M.L.; Falcão-Reis, F.; Gomes, E.R.; Carneiro, A. Immediate reactions to fluorescein and indocyanine green in retinal angiography: Review of literature and proposal for patient’s evaluation. Clin. Ophthalmol. 2020, 2020, 171–178. [Google Scholar] [CrossRef]
- Pogue, B.W.; Gibbs-Strauss, S.; Valdes, P.A.; Samkoe, K.; Roberts, D.W.; Paulsen, K.D. Review of Neurosurgical Fluorescence Imaging Methodologies. IEEE J. Sel. Top. Quantum Electron. 2010, 16, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Lavrador, J.P.; Marchi, F.; Elhag, A.; Kalyal, N.; Mthunzi, E.; Awan, M.; Wroe-Wright, O.; Díaz-Baamonde, A.; Mirallave-Pescador, A.; Reisz, Z.; et al. In Situ Light-Source Delivery During 5-Aminulevulinic Acid-Guided High-Grade Glioma Resection: Spatial, Functional and Oncological Informed Surgery. Biomedicines 2024, 12, 2748. [Google Scholar] [CrossRef]
- Roque, D.; Kalyal, N.; Chowdhury, Y.A.; Elhag, A.; Elliot, M.; Ashkan, K.; Vergani, F.; Bhangoo, R.; Lavrador, J.P. Letter to Editor: Fluorescence-guided surgery for high-grade gliomas. Brain Spine 2024, 4, 104147. [Google Scholar] [CrossRef]
- Della Pepa, G.M.; Mattogno, P.; Menna, G.; Agostini, L.; Olivi, A.; Doglietto, F. A Comparative Analysis with Exoscope and Optical Microscope for Intraoperative Visualization and Surgical Workflow in 5-Aminolevulinic Acid–Guided Resection of High-Grade Gliomas. World Neurosurg. 2023, 170, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Suero Molina, E.; Hellwig, S.J.; Walke, A.; Jeibmann, A.; Stepp, H.; Stummer, W. Development and validation of a triple-LED surgical loupe device for fluorescence-guided resections with 5-ALA. J. Neurosurg. 2021, 137, 582–590. [Google Scholar] [CrossRef]
- Giantini-Larsen, A.M.; Parker, W.E.; Cho, S.S.; Goldberg, J.L.; Carnevale, J.A.; Michael, A.P.; Teng, C.W.; De Ravin, E.; Brennan, C.W.; Lee, J.Y.; et al. The Evolution of 5-Aminolevulinic Acid Fluorescence Visualization: Time for a Headlamp/Loupe Combination. World Neurosurg. 2022, 159, 136–143. [Google Scholar] [CrossRef]
- Croce, A.C.; Fiorani, S.; Locatelli, D.; Nano, R.; Ceroni, M.; Tancioni, F.; Giombelli, E.; Benericetti, E.; Bottiroli, G. Diagnostic Potential of Autofluorescence for an Assisted Intraoperative Delineation of Glioblastoma Resection Margins. Photochem. Photobiol. 2003, 77, 309–318. [Google Scholar] [CrossRef]
- Toms, S.A.; Lin, W.C.; Weil, R.J.; Johnson, M.D.; Jansen, E.D.; Mahadevan-Jansen, A. Intraoperative optical spectroscopy identifies infiltrating glioma margins with high sensitivity. Neurosurgery 2005, 57, 382–391. [Google Scholar] [CrossRef]
- Vasefi, F.; MacKinnon, N.; Farkas, D.L.; Kateb, B. Review of the potential of optical technologies for cancer diagnosis in neurosurgery: A step toward intraoperative neurophotonics. Neurophotonics 2016, 4, 011010. [Google Scholar] [CrossRef]
- Valdes, P.A.; Juvekar, P.; Agar, N.Y.; Gioux, S.; Golby, A.J. Quantitative wide-field imaging techniques for fluorescence guided neurosurgery. Front. Surg. 2019, 6, 31. [Google Scholar] [CrossRef]
- Shapey, J.; Xie, Y.; Nabavi, E.; Ebner, M.; Saeed, S.R.; Kitchen, N.; Dorward, N.; Grieve, J.; McEvoy, A.W.; Miserocchi, A.; et al. Optical properties of human brain and tumour tissue: An ex vivo study spanning the visible range to beyond the second near-infrared window. J. Biophotonics 2022, 15, e202100072. [Google Scholar] [CrossRef]
- Kim, A.; Khurana, M.; Moriyama, Y.; Wilson, B.C. Quantification of in vivo fluorescence decoupled from the effects of tissue optical properties using fiber-optic spectroscopy measurements. J. Biomed. Opt. 2010, 15, 67006. [Google Scholar] [CrossRef] [PubMed]
- Valdes, P.A.; Kim, A.; Brantsch, M.; Niu, C.; Moses, Z.B.; Tosteson, T.D.; Wilson, B.C.; Paulsen, K.D.; Roberts, D.W.; Harris, B.T. delta-aminolevulinic acid-induced protoporphyrin IX concentration correlates with histopathologic markers of malignancy in human gliomas: The need for quantitative fluorescence-guided resection to identify regions of increasing malignancy. Neuro Oncol. 2011, 13, 846–856. [Google Scholar] [CrossRef]
- Haj-Hosseini, N.; Richter, J.; Andersson-Engels, S.; Wardell, K. Optical touch pointer for fluorescence guided glioblastoma resection using 5-aminolevulinic acid. Lasers Surg. Med. 2010, 42, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Valdes, P.A.; Leblond, F.; Kim, A.; Wilson, B.C.; Paulsen, K.D.; Roberts, D.W. A spectrally constrained dual-band normalization technique for protoporphyrin IX quantification in fluorescence-guided surgery. Opt. Lett. 2012, 37, 1817–1819. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Rodrigues, F.; Schucht, P.; Preuss, M.; Wiewrodt, D.; Nestler, U.; Stein, M.; Artero, J.M.; Platania, N.; Skjoth-Rasmussen, J.; et al. Predicting the “usefulness” of 5-ALA-derived tumor fluorescence for fluorescence-guided resections in pediatric brain tumors: A European survey. Acta Neurochir. 2014, 156, 2315–2324. [Google Scholar] [CrossRef]
- Valdes, P.A.; Angelo, J.P.; Choi, H.S.; Gioux, S. qF-SSOP: Real-time optical property corrected fluorescence imaging. Biomed. Opt. Express 2017, 8, 3597–3605. [Google Scholar] [CrossRef]
- Xie, Y.; Thom, M.; Ebner, M.; Wykes, V.; Desjardins, A.; Miserocchi, A.; Ourselin, S.; McEvoy, A.W.; Vercauteren, T. Wide-field spectrally resolved quantitative fluorescence imaging system: Toward neurosurgical guidance in glioma resection. J. Biomed. Opt. 2017, 22, 1–14. [Google Scholar] [CrossRef] [PubMed]
- MacCormac, O.; Noonan, P.; Janatka, M.; Horgan, C.C.; Bahl, A.; Qiu, J.; Elliot, M.; Trotouin, T.; Jacobs, J.; Patel, S.; et al. Lightfield hyperspectral imaging in neuro-oncology surgery: An IDEAL 0 and 1 study. Front. Neurosci. 2023, 17, 1239764. [Google Scholar] [CrossRef]
- Lozovaya, G.I.; Masinovsky, Z.; Sivash, A.A. Protoporphyrin ix as a possible ancient photosensitizer: Spectral and photochemical studies. Orig. Life Evol. Biosph. 1990, 20, 321–330. [Google Scholar] [CrossRef]
- Marcu, L.; Hartl, B.A. Fluorescence Lifetime Spectroscopy and Imaging in Neurosurgery. IEEE J. Sel. Top. Quantum Electron. 2012, 18, 1465–1477. [Google Scholar] [CrossRef]
- Erkkila, M.T.; Bauer, B.; Hecker-Denschlag, N.; Madera Medina, M.J.; Leitgeb, R.A.; Unterhuber, A.; Gesperger, J.; Roetzer, T.; Hauger, C.; Drexler, W.; et al. Widefield fluorescence lifetime imaging of protoporphyrin IX for fluorescence-guided neurosurgery: An ex vivo feasibility study. J. Biophotonics 2019, 12, e201800378. [Google Scholar] [CrossRef] [PubMed]
- Erkkila, M.T.; Reichert, D.; Gesperger, J.; Kiesel, B.; Roetzer, T.; Mercea, P.A.; Drexler, W.; Unterhuber, A.; Leitgeb, R.A.; Woehrer, A.; et al. Macroscopic fluorescence-lifetime imaging of NADH and protoporphyrin IX improves the detection and grading of 5-aminolevulinic acid-stained brain tumors. Sci. Rep. 2020, 10, 20492. [Google Scholar] [CrossRef] [PubMed]
- Reichert, D.; Erkkilae, M.T.; Gesperger, J.; Wadiura, L.I.; Lang, A.; Roetzer, T.; Woehrer, A.; Andreana, M.; Unterhuber, A.; Wilzbach, M.; et al. Fluorescence Lifetime Imaging and Spectroscopic Co-Validation for Protoporphyrin IX-Guided Tumor Visualization in Neurosurgery. Front. Oncol. 2021, 11, 741303. [Google Scholar] [CrossRef]
- Russell, J.A.; Diamond, K.R.; Collins, T.J.; Tiedje, H.F.; Hayward, J.E.; Farrell, T.J.; Patterson, M.S.; Fang, Q. Characterization of fluorescence lifetime of photofrin and delta-aminolevulinic acid induced protoporphyrin IX in living cells using single- and two-photon excitation. IEEE J. Sel. Top. Quantum Electron. 2008, 14, 158–166. [Google Scholar] [CrossRef]
- Kantelhardt, S.R.; Diddens, H.; Leppert, J.; Rohde, V.; Hüttmann, G.; Giese, A. Multiphoton excitation fluorescence microscopy of 5-aminolevulinic acid induced fluorescence in experimental gliomas. Lasers Surg. Med. 2008, 40, 273–281. [Google Scholar] [CrossRef]
- Restelli, F.; Mathis, A.M.; Höhne, J.; Mazzapicchi, E.; Acerbi, F.; Pollo, B.; Quint, K. Confocal laser imaging in neurosurgery: A comprehensive review of sodium fluorescein-based CONVIVO preclinical and clinical applications. Front. Oncol. 2022, 12, 998384. [Google Scholar] [CrossRef]
- Wagner, A.; Brielmaier, M.C.; Kampf, C.; Baumgart, L.; Aftahy, A.K.; Meyer, H.S.; Kehl, V.; Höhne, J.; Schebesch, K.M.; Schmidt, N.O.; et al. Fluorescein-stained confocal laser endomicroscopy versus conventional frozen section for intraoperative histopathological assessment of intracranial tumors. Neuro-Oncol. 2024, 26, 922–932. [Google Scholar] [CrossRef]
- Kotwal, A.; Saragadam, V.; Bernstock, J.D.; Sandoval, A.; Veeraraghavan, A.; Valdés, P.A. Hyperspectral imaging in neurosurgery: A review of systems, computational methods, and clinical applications. J. Biomed. Opt. 2024, 30, 023512. [Google Scholar] [CrossRef] [PubMed]
- Valdés, P.A.; Leblond, F.; Anthony, K.; Harris, B.T.; Wilson, B.C.; Fan, X.; Tosteson, T.D.; Hartov, A.; Ji, S.; Erkmen, K.; et al. Quantitative fluorescence in intracranial tumor: Implications for ALA-induced PpIX as an intraoperative biomarker—Clinical article. J. Neurosurg. 2011, 115, 11–17. [Google Scholar] [CrossRef]
- Fabelo, H.; Ortega, S.; Kabwama, S.; Callico, G.M.; Bulters, D.; Szolna, A.; Pineiro, J.F.; Sarmiento, R. HELICoiD project: A new use of hyperspectral imaging for brain cancer detection in real-time during neurosurgical operations. In Proceedings of the Hyperspectral Imaging Sensors: Innovative Applications and Sensor Standards 2016, Baltimore, MD, USA, 17–21 April 2016; Volume 9860. [Google Scholar] [CrossRef]
- Kravchenko, Y.; Sikora, K.; Wireko, A.A.; Lyndin, M. Fluorescence visualization for cancer DETECTION: EXPERIENCE and perspectives. Heliyon 2024, 10, e2439. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, J.; Zhang, Y.; Zhuang, J.; Ge, M.; Shi, B.; Li, J.; Xu, G.; Xu, S.; Fan, C.; et al. Visualizing glioma margins by real-time tracking of γ-glutamyltranspeptidase activity. Biomaterials 2018, 173, 1–10. [Google Scholar] [CrossRef]
- Urano, Y.; Asanuma, D.; Hama, Y.; Koyama, Y.; Barrett, T.; Kamiya, M.; Nagano, T.; Watanabe, T.; Hasegawa, A.; Choyke, P.L.; et al. Selective molecular imaging of viable cancer cells with pH-activatable fluorescence probes. Nat. Med. 2009, 15, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.E.; Tummers, W.S.; Teraphongphom, N.; van den Berg, N.S.; Hasan, A.; Ertsey, R.D.; Nagpal, S.; Recht, L.D.; Plowey, E.D.; Vogel, H.; et al. First-in-human intraoperative near-infrared fluorescence imaging of glioblastoma using cetuximab-IRDye800. J. Neurooncol. 2018, 139, 135–143. [Google Scholar] [CrossRef]
- Sexton, K.; Tichauer, K.; Samkoe, K.S.; Gunn, J.; Hoopes, P.J.; Pogue, B.W. Fluorescent Affibody Peptide Penetration in Glioma Margin Is Superior to Full Antibody. PLoS ONE 2013, 8, 0060390. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; van den Berg, N.S.; Rosenthal, E.L.; Iv, M.; Zhang, M.; Vega Leonel, J.C.M.; Walters, S.; Nishio, N.; Granucci, M.; Raymundo, R.; et al. EGFR-targeted intraoperative fluorescence imaging detects high-grade glioma with panitumumab-IRDye800 in a phase 1 clinical trial. Theranostics 2021, 11, 7130–7143. [Google Scholar] [CrossRef]
- Xie, R.; Wu, Z.; Zeng, F.; Cai, H.; Wang, D.; Gu, L.; Zhu, H.; Lui, S.; Guo, G.; Song, B.; et al. Retro-enantio isomer of angiopep-2 assists nanoprobes across the blood-brain barrier for targeted magnetic resonance/fluorescence imaging of glioblastoma. Signal Transduct. Target. Ther. 2021, 6, 309. [Google Scholar] [CrossRef]
- Taniguchi, M.; Lindsey, J.S. Database of Absorption and Fluorescence Spectra of >300 Common Compounds for use in PhotochemCAD. Photochem. Photobiol. 2018, 94, 290–327. [Google Scholar] [CrossRef] [PubMed]
- Patil, C.G.; Walker, D.G.; Miller, D.M.; Butte, P.; Morrison, B.; Kittle, D.S.; Hansen, S.J.; Nufer, K.L.; Byrnes-Blake, K.A.; Yamada, M.; et al. Phase 1 Safety, Pharmacokinetics, and Fluorescence Imaging Study of Tozuleristide (BLZ-100) in Adults with Newly Diagnosed or Recurrent Gliomas. Clin. Neurosurg. 2019, 85, 439–440. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.M.; Lee, A.; Ojemann, J.; Cole, B.; Poliachik, S.; Ishak, L.; Hansen, S.; Novak, J.; Leary, S. Phase 1 dose escalation and expansion safety study of BLZ-100 in pediatric subjects with primary central nervous system tumors. J. Clin. Oncol. 2016, 34, 15. [Google Scholar] [CrossRef]
- Li, D.; Zhang, J.; Chi, C.; Xiao, X.; Wang, J.; Lang, L.; Ali, I.; Niu, G.; Zhang, L.; Tian, J.; et al. First-in-human study of PET and optical dual-modality image-guided surgery in glioblastoma using 68Ga-IRDye800CW-BBN. Theranostics 2018, 8, 2508–2520. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, J.; Chi, C.; Che, W.; Dong, G.; Wang, J.; Du, Y.; Wang, R.; Zhu, Z.; Tian, J.; et al. Lower-grade gliomas surgery guided by GRPR-targeting PET/NIR dual-modality image probe: A prospective and single-arm clinical trial. Theranostics 2024, 14, 819–829. [Google Scholar] [CrossRef]
- Gil, H.M.; Price, T.W.; Chelani, K.; Bouillard, J.G.; Calaminus, S.D.J.; Stasiuk, G.J. NIR-quantum dots in biomedical imaging and their future. iScience 2021, 24, 102189. [Google Scholar] [CrossRef]
- Singh, A.; Kim, W.; Kim, Y.; Jeong, K.; Kang, C.S.; Kim, Y.S.; Koh, J.; Mahajan, S.D.; Prasad, P.N.; Kim, S. Multifunctional Photonics Nanoparticles for Crossing the Blood–Brain Barrier and Effecting Optically Trackable Brain Theranostics. Adv. Funct. Mater. 2016, 26, 7057–7066. [Google Scholar] [CrossRef]
- Roller, B.T.; Munson, J.M.; Brahma, B.; Santangelo, P.J.; Pai, S.B.; Bellamkonda, R.V. Evans blue nanocarriers visually demarcate margins of invasive gliomas. Drug Deliv. Transl. Res. 2015, 5, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Roque, D.; Cruz, N.; Ferreira, H.A.; Reis, C.P.; Matela, N.; Herculano-Carvalho, M.; Cascão, R.; Faria, C.C. Nanoparticle-Based Treatment in Glioblastoma. J. Pers. Med. 2023, 13, 1328. [Google Scholar] [CrossRef]
- Nunn, A.D. The cost of developing imaging agents for routine clinical use. Investig. Radiol. 2006, 41, 205. [Google Scholar] [CrossRef]
- Nicholson, J.G.; Fine, H.A. Diffuse glioma heterogeneity and its therapeutic implications. Cancer Discov. 2021, 11, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Oliva, R.; Domínguez-García, S.; Carrascal, L.; Abalos-Martínez, J.; Pardillo-Díaz, R.; Verástegui, C.; Castro, C.; Nunez-Abades, P.; Geribaldi-Doldán, N. Evolution of Experimental Models in the Study of Glioblastoma: Toward Finding Efficient Treatments. Front. Oncol. 2021, 10, 614295. [Google Scholar] [CrossRef]
- Purshouse, K.; Bulbeck, H.J.; Rooney, A.G.; Noble, K.E.; Carruthers, R.D.; Thompson, G.; Hamerlik, P.; Yap, C.; Kurian, K.M.; Jefferies, S.J.; et al. Adult brain tumour research in 2024: Status, challenges and recommendations. Neuropathol. Appl. Neurobiol. 2024, 50, e12979. [Google Scholar] [CrossRef]
- Rezai, A.R.; D’Haese, P.F.; Finomore, V.; Carpenter, J.; Ranjan, M.; Wilhelmsen, K.; Mehta, R.I.; Wang, P.; Najib, U.; Vieira Ligo Teixeira, C.; et al. Ultrasound Blood–Brain Barrier Opening and Aducanumab in Alzheimer’s Disease. N. Engl. J. Med. 2024, 390, 55–62. [Google Scholar] [CrossRef]
- Pogue, B.W.; Zhu, T.C.; Ntziachristos, V.; Wilson, B.C.; Paulsen, K.D.; Gioux, S.; Nordstrom, R.; Pfefer, T.J.; Tromberg, B.J.; Wabnitz, H.; et al. AAPM Task Group Report 311: Guidance for performance evaluation of fluorescence-guided surgery systems. Med. Phys. 2024, 51, 740–771. [Google Scholar] [CrossRef] [PubMed]
- Dirven, L.; Armstrong, T.S.; Blakeley, J.O.; Brown, P.D.; Grant, R.; Jalali, R.; Leeper, H.; Mendoza, T.; Nayak, L.; Reijneveld, J.C.; et al. Working plan for the use of patient-reported outcome measures in adults with brain tumours: A Response Assessment in Neuro-Oncology (RANO) initiative. Lancet Oncol. 2018, 19, e173–e180. [Google Scholar] [CrossRef]
- Panciani, P.P.; Fontanella, M.; Schatlo, B.; Garbossa, D.; Agnoletti, A.; Ducati, A.; Lanotte, M. Fluorescence and image guided resection in high grade glioma. Clin. Neurol. Neurosurg. 2012, 114, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Diez Valle, R.; Tejada Solis, S.; Idoate Gastearena, M.A.; Garcia de Eulate, R.; Dominguez Echavarri, P.; Aristu Mendiroz, J. Surgery guided by 5-aminolevulinic fluorescence in glioblastoma: Volumetric analysis of extent of resection in single-center experience. J. Neurooncol. 2011, 102, 105–113. [Google Scholar] [CrossRef]
- Roberts, D.W.; Valdés, P.A.; Harris, B.T.; Fontaine, K.M.; Hartov, A.; Fan, X.; Ji, S.; Lollis, S.S.; Pogue, B.W.; Leblond, F.; et al. Coregistered fluorescence-enhanced tumor resection of malignant glioma: Relationships between δ-aminolevulinic acid-induced protoporphyrin IX fluorescence, magnetic resonance imaging enhancement, and neuropathological parameters: Clinical article. J. Neurosurg. 2011, 114, 595–603. [Google Scholar] [CrossRef]
- Nabavi, A.; Thurm, H.; Zountsas, B.; Pietsch, T.; Lanfermann, H.; Pichlmeier, U.; Mehdorn, M. Five-aminolevulinic acid for fluorescence-guided resection of recurrent malignant gliomas: A phase II study. Neurosurgery 2009, 65, 1070–1077. [Google Scholar] [CrossRef]
- Hefti, M.; von Campe, G.; Moschopulos, M.; Siegner, A.; Looser, H.; Landolt, H. 5-aminolevulinic acid induced protoporphyrin IX fluorescence in high-grade glioma surgery: A one-year experience at a single institutuion. Swiss. Med. Wkly. 2008, 138, 180–185. [Google Scholar] [PubMed]
- Hong, J.; Chen, B.; Yao, X.; Yang, Y. Outcome comparisons of high-grade glioma resection with or without fluorescein sodium-guidance. Curr. Probl. Cancer 2019, 43, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, X.; Zhu, X.; Wu, L.; Ma, S.; Yan, J.; Yan, D. Diffusion Tensor Imaging with Fluorescein Sodium Staining in the Resection of High-Grade Gliomas in Functional Brain Areas. World Neurosurg. 2019, 124, e595–e603. [Google Scholar] [CrossRef] [PubMed]
- Catapano, G.; Sgulò, F.G.; Seneca, V.; Lepore, G.; Columbano, L.; di Nuzzo, G. Fluorescein-Guided Surgery for High-Grade Glioma Resection: An Intraoperative “Contrast-Enhancer”. World Neurosurg. 2017, 104, 239–247. [Google Scholar] [CrossRef]
- Murray, K.J. Improved surgical resection of human brain tumors: Part 1. A preliminary study. Surg. Neurol. 1982, 17, 316–319. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).