A Closer Look at Radiation Exposure During Percutaneous Cryoablation for T1 Renal Tumors
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Data Collection
2.2. PCA Procedure
2.3. Follow-Up
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
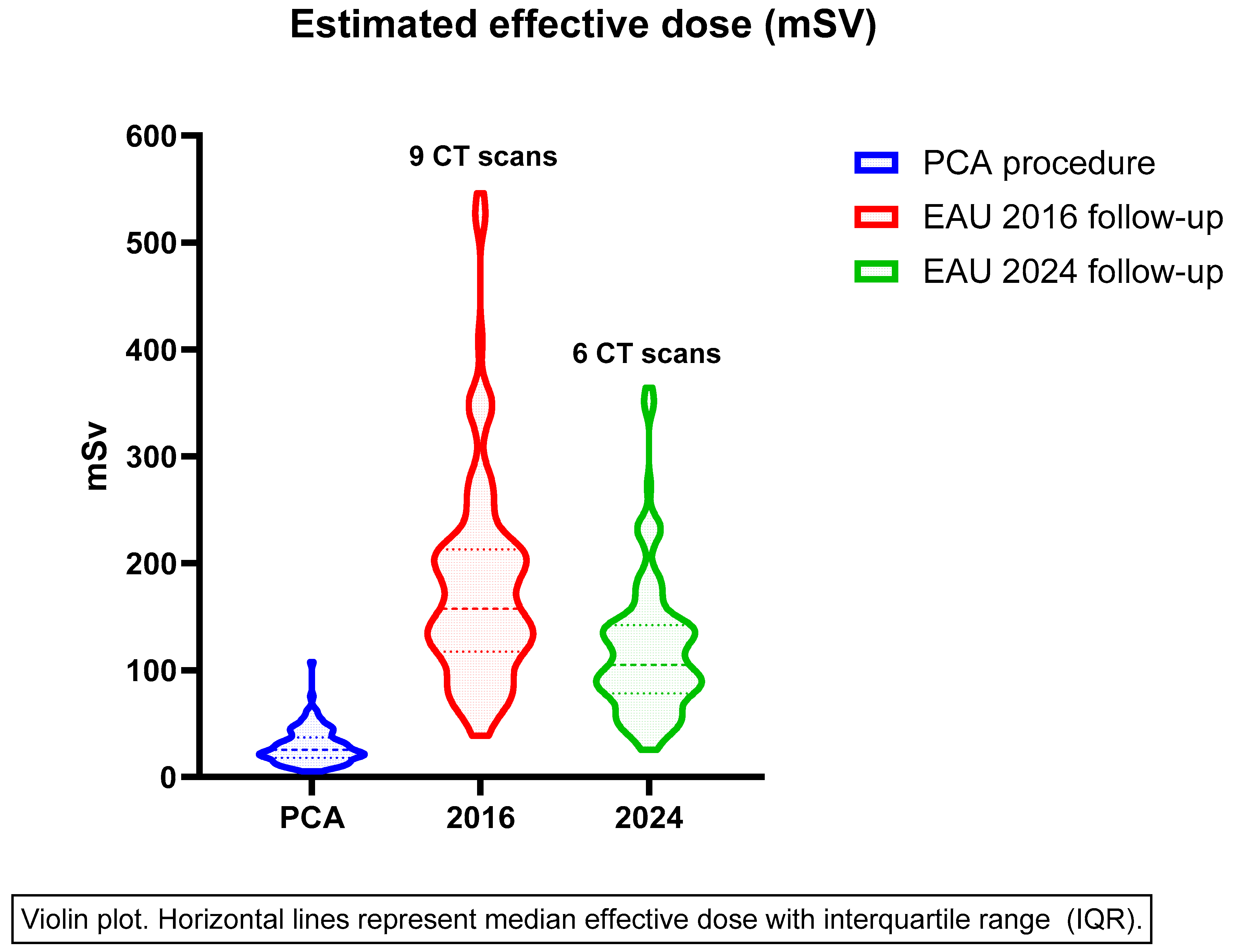
3.2. Effective Dose
3.3. Factors Influencing Radiation Exposure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Capitanio, U.; Bensalah, K.; Bex, A.; Boorjian, S.A.; Bray, F.; Coleman, J.; Gore, J.L.; Sun, M.; Wood, C.; Russo, P. Epidemiology of Renal Cell Carcinoma. Eur. Urol. 2019, 75, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Ljungberg, B.; Albiges, L.; Abu-Ghanem, Y.; Bensalah, K.; Dabestani, S.; Fernández-Pello, S.; Giles, R.H.; Hofmann, F.; Hora, M.; Kuczyk, M.A.; et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2022 Update. Eur. Urol. 2022, 82, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Zondervan, P.J.; Buijs, M.; De Bruin, D.M.; van Delden, O.M.; Van Lienden, K.P. Available ablation energies to treat cT1 renal cell cancer: Emerging technologies. World J. Urol. 2019, 37, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.K.; Lagerveld, B.W.; Keeley, F.; Lughezzani, G.; Sriprasad, S.; Barber, N.J.; Hansen, L.U.; Buffi, N.M.; Guazzoni, G.; van der Zee, J.A.; et al. Oncological outcomes and complication rates after laparoscopic-assisted cryoablation: A European Registry for Renal Cryoablation (EuRECA) multi-institutional study. BJU Int. 2017, 119, 390–395. [Google Scholar] [CrossRef]
- Pickersgill, N.A.; Vetter, J.M.; Kim, E.H.; Cope, S.J.; Du, K.; Venkatesh, R.; Giardina, J.D.; Saad, N.E.; Bhayani, S.B.; Figenshau, R.S. Ten-Year Experience with Percutaneous Cryoablation of Renal Tumors: Tumor Size Predicts Disease Progression. J. Endourol. 2020, 34, 1211–1217. [Google Scholar] [CrossRef]
- Pessoa, R.R.; Autorino, R.; Laguna, M.P.; Molina, W.R.; Gustafson, D.; Nogueira, L.; da Silva, R.D.; Werahera, P.N.; Kim, F.J. Laparoscopic Versus Percutaneous Cryoablation of Small Renal Mass: Systematic Review and Cumulative Analysis of Comparative Studies. Clin. Genitourin. Cancer 2017, 15, 513–519.e5. [Google Scholar] [CrossRef]
- Goyal, J.; Verma, P.; Sidana, A.; Georgiades, C.S.; Rodriguez, R. Single-center comparative oncologic outcomes of surgical and percutaneous cryoablation for treatment of renal tumors. J. Endourol. 2012, 26, 1413–1419. [Google Scholar] [CrossRef]
- Hebbadj, S.; Cazzato, R.L.; Garnon, J.; Shaygi, B.; Buy, X.; Tsoumakidou, G.; Lang, H.; Gangi, A. Safety Considerations and Local Tumor Control Following Percutaneous Image-Guided Cryoablation of T1b Renal Tumors. Cardiovasc. Intervent. Radiol. 2018, 41, 449–458. [Google Scholar] [CrossRef]
- Grange, R.; Tradi, F.; Izaaryene, J.; Daidj, N.; Brunelle, S.; Walz, J.; Gravis, G.; Piana, G. Computed tomography-guided percutaneous cryoablation of T1b renal tumors: Safety, functional and oncological outcomes. Int. J. Hyperth. 2019, 36, 1065–1071. [Google Scholar] [CrossRef]
- Pecoraro, A.; Palumbo, C.; Knipper, S.; Mistretta, F.A.; Tian, Z.; Shariat, S.F.; Saad, F.; Briganti, A.; Fiori, C.; Porpiglia, F.; et al. Cryoablation Predisposes to Higher Cancer Specific Mortality Relative to Partial Nephrectomy in Patients with Nonmetastatic pT1b Kidney Cancer. J. Urol. 2019, 202, 1120–1126. [Google Scholar] [CrossRef]
- Zondervan, P.J.; Buijs, M.; de la Rosette, J.J.; van Delden, O.; van Lienden, K.; Laguna, M.P. Cryoablation of small kidney tumors. Int. J. Surg. 2016, 36, 533–540. [Google Scholar] [CrossRef]
- Garnon, J.; Van Strijen, M.J.; Nielsen, T.K.; King, A.J.; Montauban Van Swijndregt, A.D.; Cazzato, R.L.; Auloge, P.; Rousseau, C.; Dalili, D.; Keeley, F.X., Jr.; et al. Safety of percutaneous renal cryoablation: An international multicentre experience from the EuRECA retrospective percutaneous database. Eur. Radiol. 2019, 29, 6293–6299. [Google Scholar] [CrossRef] [PubMed]
- Linet, M.S.; Slovis, T.L.; Miller, D.L.; Kleinerman, R.; Lee, C.; Rajaraman, P.; Berrington de Gonzalez, A. Cancer risks associated with external radiation from diagnostic imaging procedures. CA Cancer J. Clin. 2012, 62, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Koenig, T.R.; Wolff, D.; Mettler, F.A.; Wagner, L.K. Skin injuries from fluoroscopically guided procedures: Part 1, characteristics of radiation injury. AJR Am. J. Roentgenol. 2001, 177, 3–11. [Google Scholar] [CrossRef]
- Brenner David, J.; Hall Eric, J. Computed Tomography—An Increasing Source of Radiation Exposure. New Engl. J. Med. 2007, 357, 2277–2284. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.F.; Ma, K.L.; Shan, H.; Liu, T.F.; Zhao, S.Q.; Wan, Y.; Wang, H.Q. CT Scans and Cancer Risks: A Systematic Review and Dose-response Meta-analysis. BMC Cancer 2022, 22, 1238. [Google Scholar] [CrossRef]
- Henderickx, M.M.; Brits, T.; Zabegalina, N.S.; Baard, J.; Ballout, M.; Beerlage, H.P.; De Wachter, S.; Kamphuis, G.M. Can operator-controlled imaging reduce fluoroscopy time during flexible ureterorenoscopy? Cent. Eur. J. Urol. 2022, 75, 90–95. [Google Scholar]
- Tracy, C.R.; Kogan, P.; Gupta, A.; Gahan, J.C.; Theckumparampil, N.P.; Elsamra, S.E.; Okunov, Z.; Sun, S.; Lall, C.; Lobko, I.; et al. Radiation Exposure During Percutaneous Ablation of Small Renal Masses: A Multi-Institutional Multimodality Analysis. J. Endourol. 2015, 29, 1314–1320. [Google Scholar] [CrossRef]
- Fukushima, Y.; Nakamura, J.; Seki, Y.; Ando, M.; Miyazaki, M.; Tsushima, Y. Patients’ radiation dose in computed tomography-fluoroscopy-guided percutaneous cryoablation for small renal tumors. Eur. J. Radiol. 2021, 144, 109972. [Google Scholar] [CrossRef]
- Levesque, V.M.; Shyn, P.B.; Tuncali, K.; Tatli, S.; Nawfel, R.D.; Olubiyi, O.; Silverman, S.G. Radiation dose during CT-guided percutaneous cryoablation of renal tumors: Effect of a dose reduction protocol. Eur. J. Radiol. 2015, 84, 2218–2221. [Google Scholar] [CrossRef]
- Tsalafoutas, I.A.; Koukourakis, G.V. Patient dose considerations in computed tomography examinations. World J. Radiol. 2010, 2, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Widdershoven, C.V.; Aarts, B.M.; Zondervan, P.J.; Henderickx, M.M.; Klompenhouwer, E.G.; van Delden, O.M.; Prevoo, W.; Montauban van Swijndregt, A.D.; van Moorselaar, R.J.; Bex, A.; et al. Renal biopsies performed before versus during ablation of T1 renal tumors: Implications for prevention of overtreatment and follow-up. Abdom. Radiol. 2021, 46, 373–379. [Google Scholar] [CrossRef]
- Truesdale, C.M.; Soulen, M.C.; Clark, T.W.; Mondschein, J.I.; Wehrenberg-Klee, E.; Malkowicz, S.B.; Wein, A.J.; Guzzo, T.J.; Stavropoulos, S.W. Percutaneous Computed Tomography–guided Renal Mass Radiofrequency Ablation versus Cryoablation: Doses of Sedation Medication Used. J. Vasc. Interv. Radiol. 2013, 24, 347–350. [Google Scholar] [CrossRef]
- Zondervan, P.J.; Wagstaff, P.G.K.; Desai, M.M.; de Bruin, D.M.; Fraga, A.F.; Hadaschik, B.A.; Köllermann, J.; Liehr, U.B.; Pahernik, S.A.; Schlemmer, H.P.; et al. Follow-up after focal therapy in renal masses: An international multidisciplinary Delphi consensus project. World J. Urol. 2016, 34, 1657–1665. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arnold, D.C.; Schroeder, G.; Smith, J.C.; Wahjudi, I.N.; Heldt, J.P.; Richards, G.D.; Agarwal, G.; Brisbane, W.G.; Farley, D.V.; Baldwin, D.D. Comparing Radiation Exposure Between Ablative Therapies for Small Renal Masses. J. Endourol. 2013, 27, 1435–1439. [Google Scholar] [CrossRef]
- Borgbjerg, J.; Bylling, T.; Andersen, G.; Thygesen, J.; Mikkelsen, A.; Nielsen, T.K. CT-guided cryoablation of renal cancer: Radiation burden and the associated risk of secondary cancer from procedural- and follow-up imaging. Abdom. Radiol. 2020, 45, 3581–3588. [Google Scholar] [CrossRef] [PubMed]
- Park, B.K.; Morrison, P.R.; Tatli, S.; Govindarajulu, U.; Tuncali, K.; Judy, P.; Shyn, P.B.; Silverman, S.G. Estimated effective dose of CT-guided percutaneous cryoablation of liver tumors. Eur. J. Radiol. 2012, 81, 1702–1706. [Google Scholar] [CrossRef][Green Version]
- Aljweber, H.A.; Mamoun, E.; Khouqeer, G.A.; Elgarayhi, A.; Sallah, M. Reducing effective radiation dose with improved image quality of abdominal computed tomography scans for overweight patients. J. Radiat. Res. Appl. Sci. 2024, 17, 100868. [Google Scholar] [CrossRef]
- Leng, S.; Atwell, T.D.; Yu, L.; Mandrekar, J.; Lewis, B.D.; Woodrum, D.A.; McCollough, C.H. Radiation Dose Reduction for CT-Guided Renal Tumor Cryoablation. Am. J. Roentgenol. 2011, 196, W586–W591. [Google Scholar] [CrossRef]
- Zhong, J.; Gallagher, M.; Hounslow, C.; Iball, G.; Wah, T. Radiation dose reduction in CT-guided cryoablation of renal tumors. Diagn. Interv. Radiol. 2021, 27, 244–248. [Google Scholar] [CrossRef]
- X-Ray Risk. Available online: https://www.xrayrisk.com/calculator/calculator-normal-studies.php (accessed on 15 June 2025).
- Sailer, A.M.; Schurink, G.W.H.; Wildberger, J.E.; de Graaf, R.; van Zwam, W.H.; de Haan, M.W.; Kemerink, G.J.; Jeukens, C.R. Radiation exposure of abdominal cone beam computed tomography. Cardiovasc. Intervent. Radiol. 2015, 38, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Won, J.Y.; Park, S.Y. Percutaneous cryoablation for renal cell carcinoma using ultrasound-guided targeting and computed tomography-guided ice-ball monitoring: Radiation dose and short-term outcomes. Acta Radiol. 2019, 60, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Duijn, M.; Ruiter, A.E.C.; van Swijndregt, A.D.M.; van der Hulst, V.P.M.; Lagerveld, B.W. Preliminary Assessment of Cone Beam CT Guided Percutaneous Cryoablation for CT1A Renal Cell Carcinoma: A Relatively Novel and Underutilized Technique. Clin. Genitourin. Cancer 2025, 23, 102329. [Google Scholar] [CrossRef] [PubMed]
- Duijn, M.; Ruiter, A.E.C.; Montauban van Swijndregt, A.D.; Lagerveld, B.W. P174—The efficacy and safety of cone beam computed tomography for percutaneous renal cell carcinoma cryoablation: A single-center long-term follow-up study. Eur. Urol. Open Sci. 2023, 57, S247. [Google Scholar] [CrossRef]
- Breen, D.J.; King, A.J.; Patel, N.; Lockyer, R.; Hayes, M. Image-guided Cryoablation for Sporadic Renal Cell Carcinoma: Three- and 5-year Outcomes in 220 Patients with Biopsy-proven Renal Cell Carcinoma. Radiology 2018, 289, 554–561. [Google Scholar] [CrossRef]
- McEachen, J.C.; Leng, S.; Atwell, T.D.; Tollefson, M.K.; Friese, J.L.; Wang, Z.; Murad, M.H.; Schmit, G.D. Percutaneous Renal Tumor Ablation: Radiation Exposure During Cryoablation and Radiofrequency Ablation. Cardiovasc. Intervent. Radiol. 2016, 39, 233–238. [Google Scholar] [CrossRef]
| Months Post-Ablative Therapy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 6 | 12 | 18 | 24 | 30 | 36 | 48 | 60 | >60 | |
| EAU 2024 | CT | CT | CT | >3 yrs CT once every 2 yrs | ||||||
| EAU 2016 | CT | CT | CT | CT | CT | CT | CT once every 2 yrs up to 10 yrs | |||
| AUA 2021 | CT | CT | CT | CT | CT | CT | Every 2 yrs up to 10 yrs | |||
| NCCN 2024 | CT/MRI/CEUS | CT/MRI/CEUS | CT/MRI/CEUS | CT/MRI/CEUS | CT/MRI/CEUS | CT/MRI/CEUS | CT/MRI/CEUS | |||
| Variable | N = 133 |
|---|---|
| Age (mean, stdev) | 65 (11) |
| Gender, male (%) | 93 (70) |
| Side, left (%) | 63 (47) |
| BMI (mean, stdev) | 29 (5.2) |
| Serum creatinine (med IQR) | 95 (77–116) |
| eGFR (median, IQR) | 70 (50–83) |
| Tumor diameter, mm (mean, sdtev) | 28 (9.6) |
| RENAL group (%) | |
| Low (4–6) | 56 (42) |
| Intermediate (7–9) | 70 (53) |
| High (10–12) | 7 (5.3) |
| No. of needles (mean, stdev) | 3.6 (1.0) |
| Additional procedures | 45 (34) |
| Complications Clavien Dindo ≥3 | 3 (2.3) |
| Variable | OR | 95% CI | p-Value |
|---|---|---|---|
| BMI | 1.723 | 1.315, 2.132 | <0.001 |
| Number of needles | 4.060 | 2.019, 6.102 | <0.001 |
| Additional procedures | 8.056 | 2.019, 6.102 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van den Brink, L.; Henderickx, M.M.E.L.; van Delden, O.M.; Beerlage, H.P.; de Bruin, D.M.; Zondervan, P.J. A Closer Look at Radiation Exposure During Percutaneous Cryoablation for T1 Renal Tumors. Cancers 2025, 17, 2016. https://doi.org/10.3390/cancers17122016
van den Brink L, Henderickx MMEL, van Delden OM, Beerlage HP, de Bruin DM, Zondervan PJ. A Closer Look at Radiation Exposure During Percutaneous Cryoablation for T1 Renal Tumors. Cancers. 2025; 17(12):2016. https://doi.org/10.3390/cancers17122016
Chicago/Turabian Stylevan den Brink, Luna, Michaël M. E. L. Henderickx, Otto M. van Delden, Harrie P. Beerlage, Daniel Martijn de Bruin, and Patricia J. Zondervan. 2025. "A Closer Look at Radiation Exposure During Percutaneous Cryoablation for T1 Renal Tumors" Cancers 17, no. 12: 2016. https://doi.org/10.3390/cancers17122016
APA Stylevan den Brink, L., Henderickx, M. M. E. L., van Delden, O. M., Beerlage, H. P., de Bruin, D. M., & Zondervan, P. J. (2025). A Closer Look at Radiation Exposure During Percutaneous Cryoablation for T1 Renal Tumors. Cancers, 17(12), 2016. https://doi.org/10.3390/cancers17122016






