Neutrophil to Lymphocyte Ratio as a Biomarker for the Prediction of Cancer Outcomes and Immune-Related Adverse Events in a CTLA-4-Treated Population
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Acronyms
| CBC | Complete blood count |
| CI | Confidence interval |
| CR | Complete remission |
| CTLA-4 | Cytotoxic T-lymphocyte associated protein 4 |
| DERM | Dermatologic |
| ECOG | Eastern Cooperative Oncology Group performance status |
| ENDO | Endocrine |
| GI/HEP | Gastrointestinal and/or hepatic |
| HR | Hazard ratio |
| ICI | Immune checkpoint inhibitor |
| IPI | Ipilimumab |
| irAE | Immune-related adverse event |
| IRB | Institutional Review Board |
| LAG3 | Lymphocyte activation gene 3 |
| MR | Minimal residual disease |
| NED | No evidence of disease |
| NEURO | Neurological |
| NIVO | Nivolumab |
| NLR | Neutrophil to lymphocyte ratio |
| NSCLC | Non-small cell lung cancer |
| OPHTL | Ophthalmologic |
| OR | Odds ratio |
| PD | Progression of disease |
| PD-1 | Programmed cell death protein 1 |
| PD-L1 | Programmed cell death ligand 1 |
| PULM | Pulmonary |
| RENAL | Renal |
| RHEUM/MSK | Rheumatologic and/or musculoskeletal |
| ROC | Receiver operating characteristic |
| SD | Stable disease |
| ±SD | Standard deviation |
| SE | Standard error |
| UNC | University of North Carolina at Chapel Hill |
References
- Ramos-Casals, M.; Brahmer, J.R.; Callahan, M.K.; Flores-Chavez, A.; Keegan, N.; Khamashta, M.A.; Lambotte, O.; Mariette, X.; Prat, A.; Suarez-Almazor, M.E. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Primers 2020, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Adam, K.; Butler, S.C.; Workman, C.J.; Vignali, D.A.A. Advances in LAG3 cancer immunotherapeutics. Trends Cancer 2025, 11, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Borgeaud, M.; Sandoval, J.; Obeid, M.; Banna, G.; Michielin, O.; Addeo, A.; Friedlaender, A. Novel targets for immune-checkpoint inhibition in cancer. Cancer Treat. Rev. 2023, 120, 102614. [Google Scholar] [CrossRef]
- Kraehenbuehl, L.; Weng, C.H.; Eghbali, S.; Wolchok, J.D.; Merghoub, T. Enhancing immunotherapy in cancer by targeting emerging immunomodulatory pathways. Nat. Rev. Clin. Oncol. 2022, 19, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Zou, W.; Chen, L. Inhibitory B7-family molecules in the tumour microenvironment. Nat. Rev. Immunol. 2008, 8, 467–477. [Google Scholar] [CrossRef]
- Darnell, E.P.; Mooradian, M.J.; Baruch, E.N.; Yilmaz, M.; Reynolds, K.L. Immune-Related Adverse Events (irAEs): Diagnosis, Management, and Clinical Pearls. Curr. Oncol. Rep. 2020, 22, 39. [Google Scholar] [CrossRef]
- Boutros, C.; Tarhini, A.; Routier, E.; Lambotte, O.; Ladurie, F.L.; Carbonnel, F.; Izzeddine, H.; Marabelle, A.; Champiat, S.; Berdelou, A.; et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat. Rev. Clin. Oncol. 2016, 13, 473–486. [Google Scholar] [CrossRef]
- Yin, Q.; Wu, L.; Han, L.; Zheng, X.; Tong, R.; Li, L.; Bai, L.; Bian, Y. Immune-related adverse events of immune checkpoint inhibitors: A review. Front. Immunol. 2023, 14, 1167975. [Google Scholar] [CrossRef]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef]
- Nakamura, Y. Biomarkers for Immune Checkpoint Inhibitor-Mediated Tumor Response and Adverse Events. Front. Med. 2019, 6, 119. [Google Scholar] [CrossRef] [PubMed]
- Chennamadhavuni, A.; Abushahin, L.; Jin, N.; Presley, C.J.; Manne, A. Risk Factors and Biomarkers for Immune-Related Adverse Events: A Practical Guide to Identifying High-Risk Patients and Rechallenging Immune Checkpoint Inhibitors. Front. Immunol. 2022, 13, 779691. [Google Scholar] [CrossRef] [PubMed]
- Les, I.; Martínez, M.; Pérez-Francisco, I.; Cabero, M.; Teijeira, L.; Arrazubi, V.; Torrego, N.; Campillo-Calatayud, A.; Elejalde, I.; Kochan, G.; et al. Predictive Biomarkers for Checkpoint Inhibitor Immune-Related Adverse Events. Cancers 2023, 15, 1629. [Google Scholar] [CrossRef] [PubMed]
- Templeton, A.J.; McNamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocana, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef]
- Cupp, M.A.; Cariolou, M.; Tzoulaki, I.; Aune, D.; Evangelou, E.; Berlanga-Taylor, A.J. Neutrophil to lymphocyte ratio and cancer prognosis: An umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. 2020, 18, 360. [Google Scholar] [CrossRef]
- Owen, D.H.; Wei, L.; Bertino, E.M.; Edd, T.; Villalona-Calero, M.A.; He, K.; Shields, P.G.; Carbone, D.P.; Otterson, G.A. Incidence, Risk Factors, and Effect on Survival of Immune-related Adverse Events in Patients with Non-Small-cell Lung Cancer. Clin. Lung Cancer 2018, 19, e893–e900. [Google Scholar] [CrossRef]
- Pavan, A.; Calvetti, L.; Dal Maso, A.; Attili, I.; Del Bianco, P.; Pasello, G.; Guarneri, V.; Aprile, G.; Conte, P.; Bonanno, L. Peripheral Blood Markers Identify Risk of Immune-Related Toxicity in Advanced Non-Small Cell Lung Cancer Treated with Immune-Checkpoint Inhibitors. Oncologist 2019, 24, 1128–1136. [Google Scholar] [CrossRef]
- Peng, L.; Wang, Y.; Liu, F.; Qui, X.; Zhang, X.; Fang, C.; Qian, X.; Li, Y. Peripheral blood markers predictive of outcome and immune-related adverse events in advanced non-small cell lung cancer treated with PD-1 inhibitors. Cancer Immunol. Immunother. 2020, 69, 1813–1822. [Google Scholar] [CrossRef]
- Eun, Y.; Kim, I.Y.; Sun, J.M.; Lee, J.; Cha, H.S.; Koh, E.M.; Kim, H. Risk factors for immune-related adverse events associated with anti-PD-1 pembrolizumab. Sci. Rep. 2019, 9, 14039. [Google Scholar] [CrossRef]
- Matsukane, R.; Watanabe, H.; Minami, H.; Hata, K.; Suetsugu, K.; Tsuji, T.; Masuda, S.; Okamoto, I.; Nakagawa, T.; Ito, T.; et al. Continuous monitoring of neutrophils to lymphocytes ratio for estimating the onset, severity, and subsequent prognosis of immune related adverse events. Sci. Rep. 2021, 11, 1324. [Google Scholar] [CrossRef]
- Zhang, W.; Tan, Y.; Li, Y.; Liu, J. Neutrophil to Lymphocyte ratio as a predictor for immune-related adverse events in cancer patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Front. Immunol. 2023, 14, 1234142. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xiang, D.; Wan, J.; Yang, L.; Zheng, C. Focus on the Dynamics of Neutrophil-to-Lymphocyte Ratio in Cancer Patients Treated with Immune Checkpoint Inhibitors: A Meta-Analysis and Systematic Review. Cancers 2022, 14, 5297. [Google Scholar] [CrossRef] [PubMed]
- Valero, C.; Lee, M.; Hoen, D.; Weiss, K.; Kelly, D.W.; Adusumilli, P.S.; Paik, P.K.; Plitas, G.; Ladanyi, M.; Postow, M.A.; et al. Pretreatment neutrophil-to-lymphocyte ratio and mutational burden as biomarkers of tumor response to immune checkpoint inhibitors. Nat. Commun. 2021, 12, 729. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Tanaka, R.; Maruyama, H.; Ishitsuka, Y.; Okiyama, N.; Watanabe, R.; Fujimoto, M.; Fujisawa, Y. Correlation between blood cell count and outcome of melanoma patients treated with anti-PD-1 antibodies. Jpn. J. Clin. Oncol. 2019, 49, 431–437. [Google Scholar] [CrossRef]
- Cassidy, M.R.; Wolchok, R.E.; Zheng, J.; Panageas, K.S.; Wolchok, J.D.; Coit, D.; Postow, M.A.; Ariyan, C. Neutrophil to Lymphocyte Ratio is Associated with Outcome During Ipilimumab Treatment. eBioMedicine 2017, 18, 56–61. [Google Scholar] [CrossRef]
- Ferrucci, P.F.; Gandini, S.; Battaglia, A.; Alfieri, S.; Di Giacomo, A.M.; Giannareilli, D.; Cappellini, G.C.; De Galitiis, F.; Marchetti, P.; Amato, G.; et al. Baseline neutrophil-to-lymphocyte ratio is associated with outcome of ipilimumab-treated metastatic melanoma patients. Br. J. Cancer 2015, 112, 1904–1910. [Google Scholar] [CrossRef]
- Matsumura, Y.; Kawarada, Y.; Matsuo, M.; Yokota, K.; Mizoguchi, H.; Akiyama, M.; Yamada, K. Retrospective Analysis of Neutrophil-to-Lymphocyte Ratio in Patients with Melanoma Who Received Ipilimumab Monotherapy or Ipilimumab in Combination with Nivolumab in Japan. Biol. Pharm. Bull. 2023, 46, 427–431. [Google Scholar] [CrossRef]
- Zaragoza, J.; Caille, A.; Beneton, N.; Bens, G.; Christiann, F.; Maillard, H.; Machet, L. High neutrophil to lymphocyte ratio measured before starting ipilimumab treatment is associated with reduced overall survival in patients with melanoma. Br. J. Dermatol. 2016, 174, 146–151. [Google Scholar] [CrossRef]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am. J. Clin. Oncol. 2016, 39, 98–106. [Google Scholar] [CrossRef]
- Chera, A.; Stancu, A.L.; Bucur, O. Thyroid-related adverse events induced by immune checkpoint inhibitors. Front. Endocrinol. 2022, 13, 1010279. [Google Scholar] [CrossRef]
- Jing, Y.; Zhang, Y.; Wang, J.; Li, K.; Chen, X.; Heng, J.; Gao, Q.; Ye, Y.; Zhang, Z.; Liu, Y.; et al. Association Between Sex and Immune-Related Adverse Events During Immune Checkpoint Inhibitor Therapy. J. Natl. Cancer Inst. 2021, 113, 1396–1404. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, X.; Qu, J.; Zuo, W.; Tang, Y.; Zhu, H.; Chen, X. Immune-Related Adverse Events Associated with Outcomes in Patients with NSCLC Treated with Anti-PD-1 Inhibitors: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 708195. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Lee, H.J.; Farmer, J.R.; Reynolds, K.L. Mechanisms Driving Immune-Related Adverse Events in Cancer Patients Treated with Immune Checkpoint Inhibitors. Curr. Cardiol. Rep. 2021, 23, 98. [Google Scholar] [CrossRef] [PubMed]

| Patients with Non-Varying ICI Therapy (N = 111) | Patients Treated for Melanoma with Cancer Response (N = 100) | ||||
|---|---|---|---|---|---|
| Baseline Characteristics | n * | % * | n * | % * | |
| Age, mean (±SD) years (range = 17 to 81) | 57.1 | (±13.0) | 56.7 | (±13.2) | |
| Female | 49 | 44.1 | 42 | 42.0 | |
| NLR, mean (±SD) (range = 0.20 to 45.3) | 4.62 | (±4.95) | 4.25 | (±3.20) | |
| NLR < 4 (lit) | 59 | 53.2 | 55 | 55.0 | |
| NLR < 5 (lit) | 78 | 70.3 | 71 | 71.0 | |
| ECOG status at the start of the ICI | (Missing, N/A) | 6 | 5.4 | 6 | 6.0 |
| 0 | 61 | 55 | 58 | 58.0 | |
| 1 | 36 | 32.4 | 28 | 28.0 | |
| 2 | 7 | 6.3 | 7 | 7.0 | |
| 3 | 1 | 0.9 | 1 | 1.0 | |
| Cancer stage at the start of the ICI | (Missing, limited/no formal staging) | 2 | 1.8 | - | - |
| <3 | 1 | 0.9 | 1 | 1.0 | |
| 3 | 49 | 44.1 | 45 | 45.0 | |
| 4 | 59 | 53.2 | 54 | 54.0 | |
| Patients with Non-Varying ICI Therapy (N = 111) | Patients Treated for Melanoma with Cancer Response (N = 100) | ||||
|---|---|---|---|---|---|
| Characteristics | n * | % * | n * | % * | |
| IPI only | 40 | 36.0 | 38 | 38.0 | |
| IPI with NIVO | 57 | 51.4 | 48 | 48.0 | |
| IPI with PEMBRO | 14 | 12.6 | 14 | 14.0 | |
| irAE by organ type | GI/HEP | 49 | 44.1 | 46 | 46.0 |
| DERM | 43 | 38.7 | 42 | 42.0 | |
| ENDO | 32 | 28.8 | 29 | 29.0 | |
| RHEUM/MSK | 10 | 9.0 | 8 | 8.0 | |
| PULM | 4 | 3.6 | 3 | 3.0 | |
| OPTHL | 3 | 2.7 | 3 | 3.0 | |
| NEURO | 4 | 3.6 | 4 | 4.0 | |
| RENAL | 3 | 2.7 | 2 | 2.0 | |
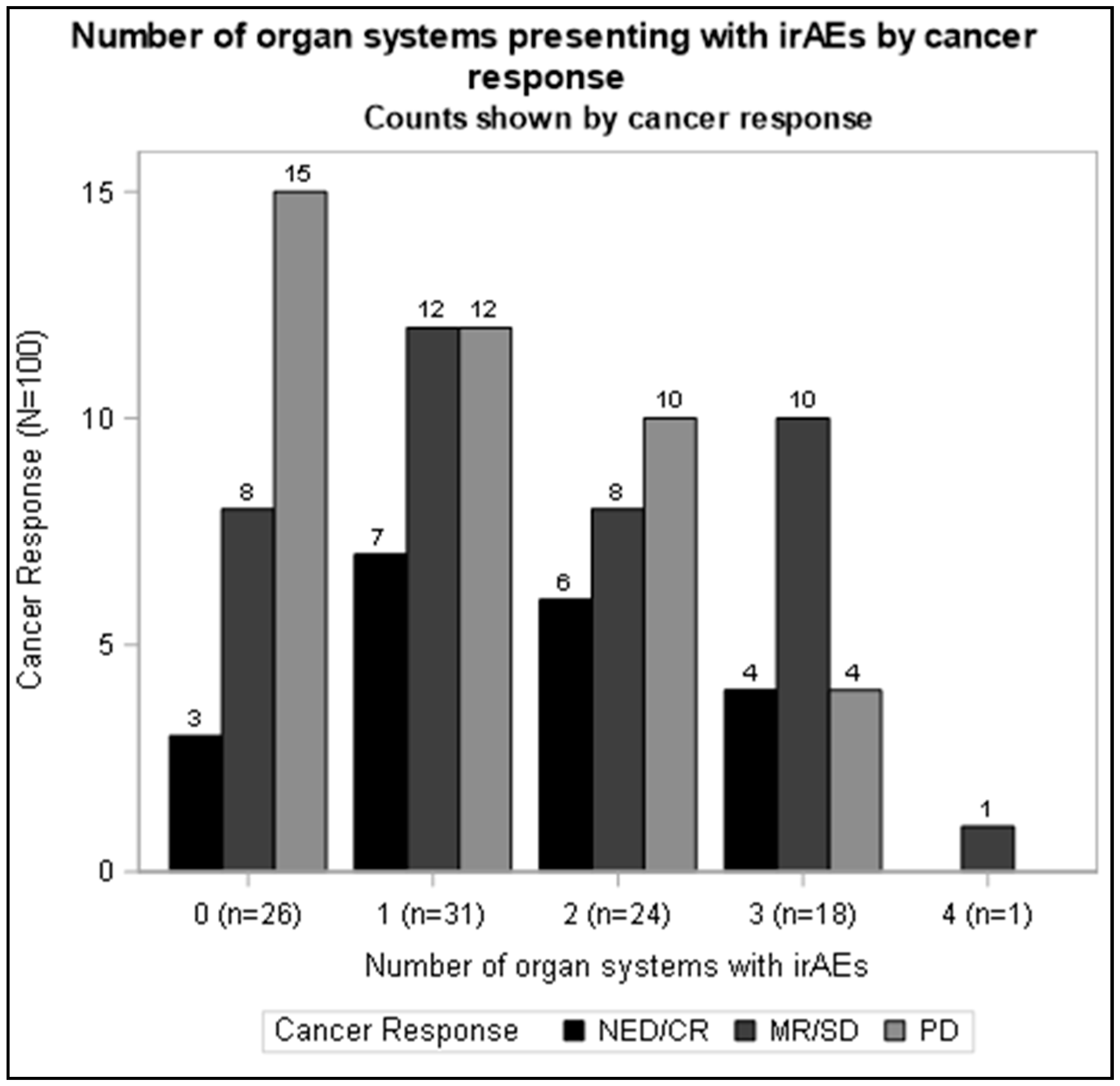
| Count of organ systems with irAEs | 0 | 31 | 27.9 | 26 | 26.0 |
| 1 | 34 | 30.6 | 31 | 31.0 | |
| 2 | 25 | 22.5 | 24 | 24.0 | |
| 3 | 20 | 18.0 | 18 | 18.0 | |
| 4 | 1 | 0.9 | 1 | 1.0 | |
| At least 1 type of irAE | 80 | 72.1 | 74 | 74.0 | |
| At least 2 types of irAEs | 46 | 41.4 | 43 | 43.0 | |
| At least 3 types of irAEs | 21 | 18.9 | 19 | 19.0 | |
| Cancer Response | (Missing, N/A) | 1 | 0.9 | ||
| NED/CR | 20 | 18.0 | 20 | 20.0 | |
| MR/SD | 40 | 36.0 | 39 | 39.0 | |
| PD | 50 | 45.0 | 41 | 41.0 | |
| PD, mean (SE), days | 740 | (79) | 802 | (84) | |
| Mortality | 53 | 47.7 | 43 | 43.0 | |
| Mortality, mean (SE), days | 1066 | (63) | 1142 | (64) | |
| OR (95% CI) 1 | OR (95% CI) 2 | |
|---|---|---|
| Model by irAE count definition | ||
| 1 irAE increase (linear, one additional irAE) | 1.48 (1.02, 2.15) | 1.71 (1.13, 2.57) |
| irAE (categorical) | ||
| 1 (vs. none) | 1.76 (0.60, 5.13) | 1.90 (0.55, 6.50) |
| 2 (vs. none) | 2.86 (0.90, 9.07) | 3.83 (1.11, 13.3) |
| 3 or 4 (vs. none) | 3.10 (0.94, 10.2) | 4.62 (1.24, 17.2) |
| At least 1 (vs. none) | 2.38 (0.96, 5.93) | 3.10 (1.11, 8.63) |
| At least 2 (vs. 1 or none) | 2.22 (0.98, 5.05) | 3.03 (1.24, 7.43) |
| 3 or 4 (vs. 2 or fewer) | 1.83 (0.69, 4.87) | 2.32 (0.82, 6.59) |
| Model by irAE organ type definition | ||
| GI/HEP | 1.39 (0.65, 2.99) | 1.55 (0.62, 3.88) |
| DERM | 1.19 (0.55, 2.57) | 1.16 (0.50, 2.70) |
| ENDO | 2.82 (1.19, 6.67) | 2.51 (0.95, 6.65) |
| RHEUM/MSK | 2.56 (0.63, 10.3) | 4.46 (0.90, 22.2) |
| Mortality (n = 111) | Disease Progression (n = 109) | Mortality (n = 103) | Disease Progression (n = 101) | |
|---|---|---|---|---|
| HR (95% CI) 1 | HR (95% CI) 1 | HR (95% CI) 2 | HR (95% CI) 2 | |
| irAE definition | ||||
| Any/first | 0.58 (0.32, 1.03) | 0.55 (0.31, 0.97) | 0.49 (0.26, 0.93) | 0.40 (0.20, 0.82) |
| irAE (categorical) | ||||
| 1 (vs. none) | 0.68 (0.33, 1.37) | 0.81 (0.40, 1.64) | 0.61 (0.28, 1.32) | 0.62 (0.27, 1.43) |
| 2–4 (vs. none) | 0.52 (0.27, 0.99) | 0.42 (0.22, 0.83) | 0.44 (0.22, 0.88) | 0.31 (0.14, 0.68) |
| Site-specific irAEs | ||||
| GI/HEP | 0.87 (0.49, 1.57) | 0.84 (0.46, 1.55) | 0.77 (0.41, 1.45) | 0.70 (0.35, 1.41) |
| DERM | 0.65 (0.36, 1.17) | 0.67 (0.36, 1.23) | 0.72 (0.39, 1.30) | 0.66 (0.35, 1.26) |
| ENDO | 0.74 (0.37, 1.46) | 0.52 (0.25, 1.11) | 0.71 (0.35, 1.44) | 0.44 (0.19, 1.01) |
| RHEUM/MSK | 0.91 (0.32, 2.59) | 0.60 (0.21, 1.73) | 0.74 (0.22, 2.48) | 0.52 (0.15, 1.74) |
| NLR < 4 | NLR < 5 | NLR < 4 | NLR < 5 | ||
|---|---|---|---|---|---|
| irAE Outcome | irAE Count | OR (95% CI) 1 | OR (95% CI) 1 | OR (95% CI) 2 | OR (95% CI) 2 |
| Any | 80 | 2.35 (0.99, 5.58) | 4.34 (1.73, 10.9) | 2.54 (0.99, 6.54) | 4.11 (1.51, 11.2) |
| GI/HEP | 49 | 1.03 (0.48, 2.22) | 2.03 (0.85, 4.83) | 0.89 (0.39, 2.04) | 1.61 (0.64, 4.05) |
| DERM | 43 | 1.70 (0.77, 3.72) | 2.22 (0.90, 5.48) | 1.57 (0.68, 3.59) | 2.24 (0.87, 5.79) |
| ENDO | 32 | 1.28 (0.55, 3.00) | 0.80 (0.32, 1.97) | 1.46 (0.59, 3.63) | 0.82 (0.31, 2.15) |
| RHEUM/MSK | 10 | 1.35 (0.35, 5.16) | 4.40 (0.53, 36.9) | 1.56 (0.34, 7.25) | 3.52 (0.40, 31.3) |
| 2 or more organ systems | 46 | 1.21 (0.54, 2.68) | 1.66 (0.69, 4.01) | 1.08 (0.47, 2.51) | 1.43 (0.57, 3.61) |
| 3 or 4 organ systems | 21 | 1.12 (0.42, 3.02) | 1.86 (0.59, 5.87) | 1.00 (0.34, 2.97) | 1.37 (0.41, 4.64) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cunningham, M.M.; Romero, R.; Alvarez, C.; Saxena Beem, S.; Schwartz, T.A.; Ishizawar, R.C. Neutrophil to Lymphocyte Ratio as a Biomarker for the Prediction of Cancer Outcomes and Immune-Related Adverse Events in a CTLA-4-Treated Population. Cancers 2025, 17, 2011. https://doi.org/10.3390/cancers17122011
Cunningham MM, Romero R, Alvarez C, Saxena Beem S, Schwartz TA, Ishizawar RC. Neutrophil to Lymphocyte Ratio as a Biomarker for the Prediction of Cancer Outcomes and Immune-Related Adverse Events in a CTLA-4-Treated Population. Cancers. 2025; 17(12):2011. https://doi.org/10.3390/cancers17122011
Chicago/Turabian StyleCunningham, Michael M., Rachel Romero, Carolina Alvarez, Shruti Saxena Beem, Todd A. Schwartz, and Rumey C. Ishizawar. 2025. "Neutrophil to Lymphocyte Ratio as a Biomarker for the Prediction of Cancer Outcomes and Immune-Related Adverse Events in a CTLA-4-Treated Population" Cancers 17, no. 12: 2011. https://doi.org/10.3390/cancers17122011
APA StyleCunningham, M. M., Romero, R., Alvarez, C., Saxena Beem, S., Schwartz, T. A., & Ishizawar, R. C. (2025). Neutrophil to Lymphocyte Ratio as a Biomarker for the Prediction of Cancer Outcomes and Immune-Related Adverse Events in a CTLA-4-Treated Population. Cancers, 17(12), 2011. https://doi.org/10.3390/cancers17122011





