Dosimetric Advantage of Scanning Beam Proton Therapy in Gynecologic Patients Receiving Adjuvant Radiotherapy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Proton Treatment Planning
2.2. Photon Treatment Planning
2.3. Plan Comparisons and Robustness Evaluations
2.4. NTCP Evaluations
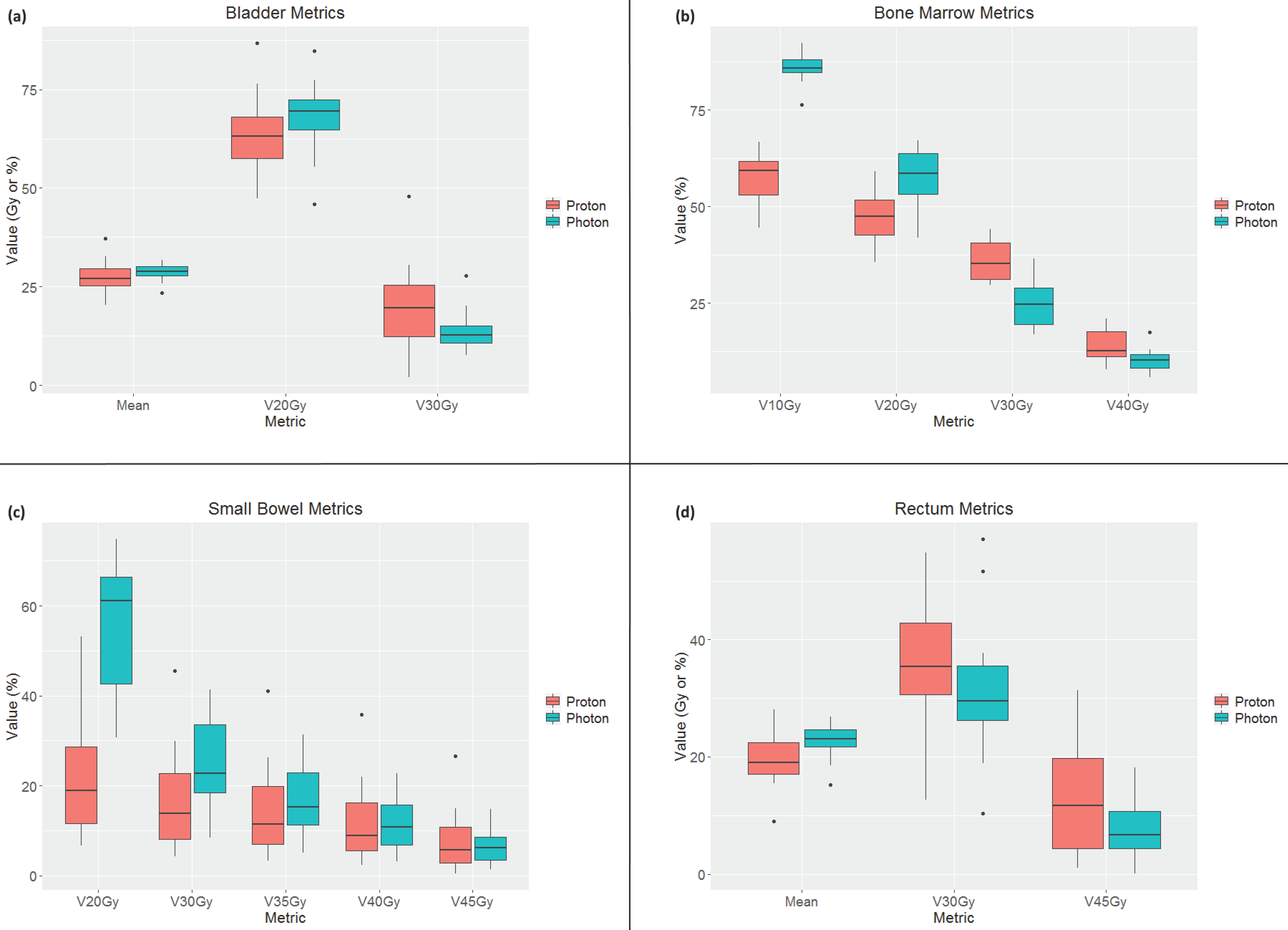
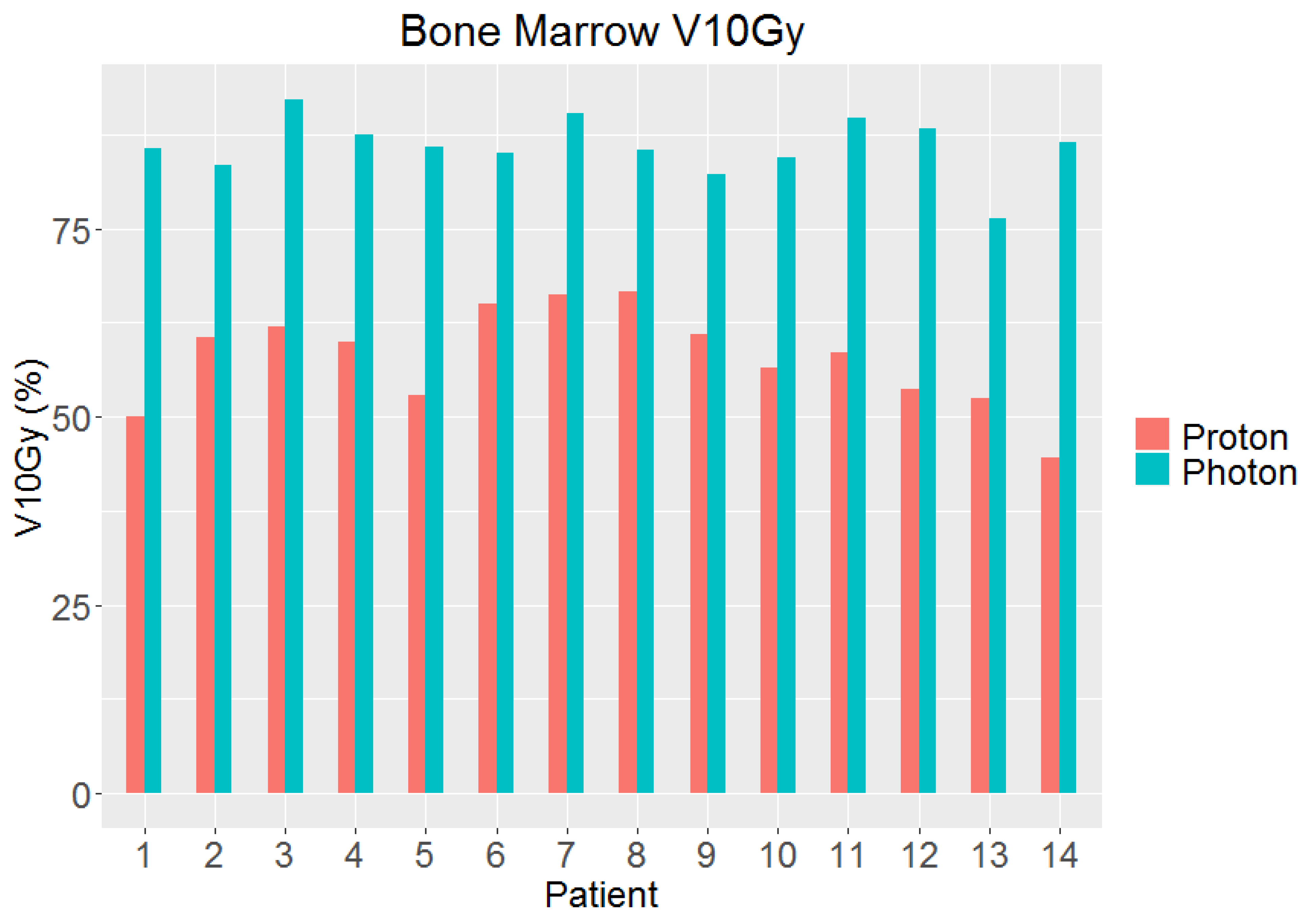
3. Results
| Organ | Metric | Photon Mean (Range) | Proton Mean (Range) | p-Value |
|---|---|---|---|---|
| Bladder | Mean | 28.6 Gy (23.4–37.6 Gy) | 27.6 Gy (20.3–37.2 Gy) | 0.17 |
| V20Gy | 67.9% (45.8–84.7%) | 64.0% (47.3–86.7%) | 0.15 | |
| V45Gy | 14.0% (7.6–27.8%) | 19.9% (2.1–47.9%) | 0.97 | |
| Bone Marrow | V10Gy | 85.9% (76.3–92.2%) | 57.9% (44.5–66.7%) | 4.5 × 10−10 |
| V20Gy | 58.2% (41.9–67.0%) | 47.4% (35.7–59.2%) | 8.3 × 10−6 | |
| V30Gy | 25.2% (16.9–36.6%) | 36.1% (29.8–44.1%) | 1.0 | |
| V40Gy | 10.2% (5.9–17.5%) | 14.0% (7.8–21.1%) | 1.0 | |
| Femoral Head (left) | Mean | 12.9 Gy (8.2–18.2 Gy) | 11.2 Gy (4.7–16.9 Gy) | 0.032 |
| Femoral Head (right) | Mean | 12.7 Gy (8.6–18.8 Gy) | 10.9 Gy (4.7–16.3 Gy) | 0.022 |
| Rectum | Mean | 22.9 Gy (15.2–26.8 Gy) | 19.7 Gy (9.0–28.1 Gy) | 7.4 × 10−3 |
| V30Gy | 31.3% (10.4–57.1%) | 36.1% (12.6–54.9%) | 0.88 | |
| V45Gy | 8.0% (0.1–18.1%) | 12.9% (1.0–31.4%) | 0.98 | |
| Small Bowel | V20Gy | 56.0% (30.6–74.8%) | 21.3% (6.6–53.2%) | 3.73 × 10−8 |
| V30Gy | 24.5% (8.4–41.4%) | 16.9% (4.2–45.5%) | 3.59 × 10−5 | |
| V35Gy | 16.9% (5.0–31.4%) | 14.7% (3.2–41.1%) | 0.037 | |
| V40Gy | 11.8% (3.1−22.7%) | 12.1% (2.3–35.8%) | 0.59 | |
| V45Gy | 6.6% (1.4–14.8%) | 7.5% (0.5–26.6%) | 0.77 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OAR | Organ at risk |
| IFSO | Individual Field Simultaneous Optimization |
| VMAT | Volumetric modulated arc therapy |
| NTCP | Normal tissue complication probability |
| sPBT | Scanning proton radiotherapy |
| CTV | Clinical target volume |
| OTV | Optimization target volume |
| STV | Scanning target volume |
| PTV | Planning target volume |
| DVH | Dose–volume histogram |
| gEUD | Generalized equivalent uniform dose |
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global Cancer Statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Mell, L.K.; Schomas, D.A.; Salama, J.K.; Devisetty, K.; Aydogan, B.; Miller, R.C.; Jani, A.B.; Kindler, H.L.; Mundt, A.J.; Roeske, J.C.; et al. Association Between Bone Marrow Dosimetric Parameters and Acute Hematologic Toxicity in Anal Cancer Patients Treated With Concurrent Chemotherapy and Intensity-Modulated Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 1431–1437. [Google Scholar] [CrossRef]
- Salama, J.K.; Mell, L.K.; Schomas, D.A.; Miller, R.C.; Devisetty, K.; Jani, A.B.; Mundt, A.J.; Roeske, J.C.; Liauw, S.L.; Chmura, S.J. Concurrent Chemotherapy and Intensity-Modulated Radiation Therapy for Anal Canal Cancer Patients: A Multicenter Experience. J. Clin. Oncol. 2007, 25, 4581–4586. [Google Scholar] [CrossRef]
- Milano, M.T.; Jani, A.B.; Farrey, K.J.; Rash, C.; Heimann, R.; Chmura, S.J. Intensity-Modulated Radiation Therapy (IMRT) in the Treatment of Anal Cancer: Toxicity and Clinical Outcome. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 354–361. [Google Scholar] [CrossRef] [PubMed]
- van de Sande, M.A.E.; Creutzberg, C.L.; van de Water, S.; Sharfo, A.W.; Hoogeman, M.S. Which Cervical and Endometrial Cancer Patients Will Benefit Most from Intensity-Modulated Proton Therapy? Radiother. Oncol. 2016, 120, 397–403. [Google Scholar] [CrossRef]
- De Boer, P.; Van de Schoot, A.J.A.J.; Westerveld, H.; Smit, M.; Buist, M.R.; Bel, A.; Rasch, C.R.N.; Stalpers, L.J.A. PO-0833: Reducing Small Bowel Dose for Cervical Cancer Using IMPT and Target Tailoring in Treatment Planning. Radiother. Oncol. 2017, 123, S448–S449. [Google Scholar] [CrossRef]
- Anand, A.; Bues, M.; Rule, W.G.; Keole, S.R.; Beltran, C.J.; Yin, J.; Haddock, M.G.; Hallemeier, C.L.; Miller, R.C.; Ashman, J.B. Scanning Proton Beam Therapy Reduces Normal Tissue Exposure in Pelvic Radiotherapy for Anal Cancer. Radiother. Oncol. 2015, 117, 505–508. [Google Scholar] [CrossRef]
- Arians, N.; Lindel, K.; Krisam, J.; Oelmann-Avendano, J.T.; Meixner, E.; König, L.; Hoerner-Rieber, J.; Wark, A.; Forster, T.; Weykamp, F.; et al. Treatment Tolerability and Toxicity of Postoperative Proton Beam Therapy for Gynecologic Malignancies: Results of the Prospective Phase 2 APROVE Trial. Int. J. Radiat. Oncol. Biol. Phys. 2023, 116, 825–836. [Google Scholar] [CrossRef]
- Berlin, E.; Yegya-Raman, N.; Garver, E.; Li, T.; Lin, L.L.; Taunk, N.K. Acute and Long-Term Toxicity of Whole Pelvis Proton Radiation Therapy for Definitive or Adjuvant Management of Gynecologic Cancers. Gynecol. Oncol. 2023, 172, 92–97. [Google Scholar] [CrossRef]
- Taunk, N. The Role of Proton Therapy in Gynecological Radiation Oncology. Int. J. Gynecol. Cancer 2022, 32, 414–420. [Google Scholar] [CrossRef]
- Anderson, J.D.; Voss, M.M.; Laughlin, B.S.; Garda, A.E.; Aziz, K.; Mullikin, T.C.; Haddock, M.G.; Petersen, I.A.; DeWees, T.A.; Vora, S.A. Outcomes of Proton Beam Therapy Compared With Intensity-Modulated Radiation Therapy for Uterine Cancer. Int. J. Part. Ther. 2023, 9, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Song, W.Y.; Huh, S.N.; Liang, Y.; White, G.; Nichols, R.C.; Watkins, W.T.; Mundt, A.J.; Mell, L.K. Dosimetric Comparison Study between Intensity Modulated Radiation Therapy and Three-Dimensional Conformal Proton Therapy for Pelvic Bone Marrow Sparing in the Treatment of Cervical Cancer. J. Appl. Clin. Med. Phys. 2010, 11, 83–92. [Google Scholar] [CrossRef]
- Dinges, E.; Felderman, N.; McGuire, S.; Gross, B.; Bhatia, S.; Mott, S.; Buatti, J.; Wang, D. Bone Marrow Sparing in Intensity Modulated Proton Therapy for Cervical Cancer: Efficacy and Robustness under Range and Setup Uncertainties. Radiother. Oncol. 2015, 115, 373–378. [Google Scholar] [CrossRef]
- Wark, A.; Gupta, A.; Meixner, E.; König, L.; Hörner-Rieber, J.; Forster, T.; Lang, K.; Ellerbrock, M.; Herfarth, K.; Debus, J.; et al. Bone Marrow Sparing by Intensity Modulated Proton Beam Therapy in Postoperative Irradiation of Gynecologic Malignancies. Technol. Cancer Res. Treat. 2024, 23, 1–11. [Google Scholar] [CrossRef]
- Lin, L.L.; Kirk, M.; Scholey, J.; Taku, N.; Kiely, J.B.; White, B.; Both, S. Initial Report of Pencil Beam Scanning Proton Therapy for Posthysterectomy Patients with Gynecologic Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Clivio, A.; Kluge, A.; Cozzi, L.; Köhler, C.; Neumann, O.; Vanetti, E.; Wlodarczyk, W.; Marnitz, S. Intensity Modulated Proton Beam Radiation for Brachytherapy in Patients with Cervical Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Marnitz, S.; Wlodarczyk, W.; Neumann, O.; Koehler, C.; Weihrauch, M.; Budach, V.; Cozzi, L. Which Technique for Radiation Is Most Beneficial for Patients with Locally Advanced Cervical Cancer? Intensity Modulated Proton Therapy versus Intensity Modulated Photon Treatment, Helical Tomotherapy and Volumetric Arc Therapy for Primary Radiation—An Intraindividual Comparison. Radiat. Oncol. 2015, 10, 91. [Google Scholar] [CrossRef]
- Milby, A.B.; Both, S.; Ingram, M.; Lin, L.L. Dosimetric Comparison of Combined Intensity-Modulated Radiotherapy (IMRT) and Proton Therapy versus IMRT Alone for Pelvic and Para-Aortic Radiotherapy in Gynecologic Malignancies. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, e477–e484. [Google Scholar] [CrossRef]
- Georg, D.; Georg, P.; Hillbrand, M.; Pötter, R.; Mock, U. Assessment of Improved Organ at Risk Sparing for Advanced Cervix Carcinoma Utilizing Precision Radiotherapy Techniques. Strahlenther. Onkologie 2008, 184, 586–591. [Google Scholar] [CrossRef]
- Verma, V.; Simone, C.B.; Wahl, A.O.; Beriwal, S.; Mehta, M.P. Proton Radiotherapy for Gynecologic Neoplasms. Acta Oncol. 2016, 55, 1257–1265. [Google Scholar] [CrossRef]
- Hashimoto, S.; Shibamoto, Y.; Iwata, H.; Ogino, H.; Shibata, H.; Toshito, T.; Sugie, C.; Mizoe, J.E. Whole-Pelvic Radiotherapy with Spot-Scanning Proton Beams for Uterine Cervical Cancer: A Planning Study. J. Radiat. Res. 2016, 57, 524–532. [Google Scholar] [CrossRef]
- Anand, A.; Bues, M.; Gamez, M.E.; Stefan, C.; Patel, S.H. Individual Field Simultaneous Optimization (IFSO) in Spot Scanning Proton Therapy of Head and Neck Cancers. Med. Dosim. 2019, 44, 375–378. [Google Scholar] [CrossRef]
- Taku, N.; Dise, J.; Kenton, O.; Yin, L.; Teo, B.K.K.; Lin, L.L. Quantification of Vaginal Motion Associated with Daily Endorectal Balloon Placement during Whole Pelvis Radiotherapy for Gynecologic Cancers. Radiother. Oncol. 2016, 120, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Michalski, J.M.; Gay, H.; Jackson, A.; Tucker, S.L.; Deasy, J.O. Radiation Dose-Volume Effects in Radiation-Induced Rectal Injury. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S123–S129. [Google Scholar] [CrossRef] [PubMed]
- Klopp, A.H.; Moughan, J.; Portelance, L.; Miller, B.E.; Salehpour, M.R.; Hildebrandt, E.; Nuanjing, J.; D’Souza, D.; Souhami, L.; Small, W.; et al. Hematologic Toxicity in RTOG 0418: A Phase 2 Study of Postoperative IMRT for Gynecologic Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 83–90. [Google Scholar] [CrossRef]
- Kavanagh, B.D.; Pan, C.C.; Dawson, L.A.; Das, S.K.; Li, X.A.; Ten Haken, R.K.; Miften, M. Radiation Dose-Volume Effects in the Stomach and Small Bowel. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S101–S107. [Google Scholar] [CrossRef]
- Klopp, A.H.; Yeung, A.R.; Deshmukh, S.; Gil, K.M.; Wenzel, L.; Westin, S.N.; Gifford, K.; Gaffney, D.K.; Small, W.; Thompson, S.; et al. Patient-Reported Toxicity during Pelvic Intensity-Modulated Radiation Therapy: NRG Oncology-RTOG 1203. J. Clin. Oncol. 2018, 36, 2538–2544. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Tao, C.; Zhu, J.; Chen, J.; Yu, G.; Qin, S.; Yin, Y.; Li, D. An Integrated Strategy of Biological and Physical Constraints in Biological Optimization for Cervical Carcinoma. Radiat. Oncol. 2017, 12, 64. [Google Scholar] [CrossRef]
- Gulliford, S.L.; Partridge, M.; Sydes, M.R.; Webb, S.; Evans, P.M.; Dearnaley, D.P. Parameters for the Lyman Kutcher Burman (LKB) Model of Normal Tissue Complication Probability (NTCP) for Specific Rectal Complications Observed in Clinical Practise. Radiother. Oncol. 2012, 102, 347–351. [Google Scholar] [CrossRef]
- Yoshimura, T.; Kinoshita, R.; Onodera, S.; Toramatsu, C.; Suzuki, R.; Ito, Y.M.; Takao, S.; Matsuura, T.; Matsuzaki, Y.; Umegaki, K.; et al. NTCP Modeling Analysis of Acute Hematologic Toxicity in Whole Pelvic Radiation Therapy for Gynecologic Malignancies—A Dosimetric Comparison of IMRT and Spot-Scanning Proton Therapy (SSPT). Phys. Medica 2016, 32, 1095–1102. [Google Scholar] [CrossRef]
- Alevronta, E.; Skokic, V.; Wilderäng, U.; Dunberger, G.; Sjöberg, F.; Bull, C.; Bergmark, K.; Jörnsten, R.; Steineck, G. Dose-Response Relationships of the Sigmoid for Urgency Syndrome after Gynecological Radiotherapy. Acta Oncol. 2018, 57, 1352–1358. [Google Scholar] [CrossRef] [PubMed]


| Patient | Age (years) | FIGO † | T | N | M | Volume of CTV (cm3) |
|---|---|---|---|---|---|---|
| 1 | 61 | IIIC1 | T2 | N1 | M0 | 339.2 |
| 2 | 65 | II | T2 | N0 | M0 | 327.1 |
| 3 | 78 | IIIB | T3b | N0 | M1 | 463.5 |
| 4 | 66 | IIIC2 | T1 | N2 | Mx | 415.0 |
| 5 * | 49 | T4 | N1 | M0 | 599.3 | |
| 6 | 69 | IIIC1 | T1c | N1 | M0 | 315.3 |
| 7 | 83 | IIIC1 | T1b | N1b | M0 | 478.9 |
| 8 | 81 | IIIC1 | T1b | N1 | M0 | 464.7 |
| 9 | 65 | IIIC2 | T1b | N2a | M0 | 487.3 |
| 10 | 83 | IA | T1a | N0 | M0 | 244.5 |
| 11 | 72 | IA | T1a | Nx | M0 | 325.2 |
| 12 | 67 | IA | T1a | N0 | M0 | 316.2 |
| 13 | 76 | IA | T1a | N0 | M0 | 348.4 |
| 14 | 76 | IIIA | T3a | N0 | M0 | 376.8 |
| Organ | Metric | Photon 5 mm Robustness Mean (Range) | Proton 5 mm Robustness Mean (Range) | Proton 5 mm/3% Robustness Mean (Range) |
|---|---|---|---|---|
| Bladder | Mean | 32.1 Gy (27.5–35.4 Gy) | 31.8 Gy (25.3–40.5 Gy) | 32.9 Gy (26.6–41.0 Gy) |
| V20Gy | 75.2% (57.1–88.3%) | 73.5% (58.4–92.9%) | 76.1% (63.9–96.8%) | |
| V45Gy | 27.0% (17.5–42.7%) | 29.8% (11.3–60.1%) | 33.5% (13.2–63.0%) | |
| Bone Marrow | V10Gy | 87.0% (77.2–93.0%) | 58.9% (46.1–67.9%) | 59.1% (46.3–68.0%) |
| V20Gy | 59.3% (43.7–68.0%) | 47.9% (36.0–59.4%) | 48.3% (36.5–59.6%) | |
| V30Gy | 28.1% (19.5–39.2%) | 36.9% (30.1–45.1%) | 37.8% (30.2–45.6%) | |
| V40Gy | 13.2% (8.1–21.1%) | 15.5% (9.0–22.5%) | 16.8% (9.8–23.0%) | |
| Femoral Head (left) | Mean | 14.1 Gy (8.9–19.5 Gy) | 12.3 Gy (5.8–18.8 Gy) | 12.6 Gy (5.9–19.0 Gy) |
| Femoral Head (right) | Mean | 14.0 Gy (9.4–20.1 Gy) | 12.2 Gy (5.8–18.6 Gy) | 12.4 Gy (5.9–18.8 Gy) |
| Rectum | Mean | 25.4 Gy (16.8–30.4 Gy) | 22.4 Gy (11.0–28.9 Gy) | 24.1 Gy (11.7–33.7 Gy) |
| V30Gy | 39.8% (16.1–63.9%) | 42.6% (17.3–59.4%) | 46.7% (19.2–69.1%) | |
| V45Gy | 15.7% (2.8–25.7%) | 16.0% (5.1–31.1%) | 21.0% (4.7–38.6%) | |
| Small Bowel | V20Gy | 59.6% (32.7–79.1%) | 22.9% (6.7–56.4%) | 26.9% (10.7–62.2%) |
| V30Gy | 28.7% (12.0–47.0%) | 18.4% (4.3–48.8%) | 22.1% (7.5–54.6%) | |
| V35Gy | 20.8% (7.9–36.9%) | 16.1% (3.4–43.7%) | 19.6% (6.1–49.2%) | |
| V40Gy | 15.3% (5.4–28.3%) | 13.4% (2.6–37.6%) | 16.6% (4.6–43.2%) | |
| V45Gy | 9.7% (3.2–19.3%) | 8.7% (0.9–28.0%) | 10.5% (1.6–31.4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ger, R.B.; Lentz, J.M.; Niedzielski, J.S.; Vora, S.A.; Bues, M.; Hernandez Morales, D.; Anderson, J.D.; Kutyreff, C.J.; Schulz, C.A.; Lara, P.R.; et al. Dosimetric Advantage of Scanning Beam Proton Therapy in Gynecologic Patients Receiving Adjuvant Radiotherapy. Cancers 2025, 17, 2010. https://doi.org/10.3390/cancers17122010
Ger RB, Lentz JM, Niedzielski JS, Vora SA, Bues M, Hernandez Morales D, Anderson JD, Kutyreff CJ, Schulz CA, Lara PR, et al. Dosimetric Advantage of Scanning Beam Proton Therapy in Gynecologic Patients Receiving Adjuvant Radiotherapy. Cancers. 2025; 17(12):2010. https://doi.org/10.3390/cancers17122010
Chicago/Turabian StyleGer, Rachel B., Jarrod M. Lentz, Joshua S. Niedzielski, Sujay A. Vora, Martin Bues, Danairis Hernandez Morales, Justin D. Anderson, Christopher J. Kutyreff, Christie A. Schulz, Pedro R. Lara, and et al. 2025. "Dosimetric Advantage of Scanning Beam Proton Therapy in Gynecologic Patients Receiving Adjuvant Radiotherapy" Cancers 17, no. 12: 2010. https://doi.org/10.3390/cancers17122010
APA StyleGer, R. B., Lentz, J. M., Niedzielski, J. S., Vora, S. A., Bues, M., Hernandez Morales, D., Anderson, J. D., Kutyreff, C. J., Schulz, C. A., Lara, P. R., Ridgway, A. K., Lemish, P. R., Gagneur, J. D., & Anand, A. (2025). Dosimetric Advantage of Scanning Beam Proton Therapy in Gynecologic Patients Receiving Adjuvant Radiotherapy. Cancers, 17(12), 2010. https://doi.org/10.3390/cancers17122010






