Accelerated Radiotherapy for Complicated Bone Metastases: SHARON Bone Randomized Phase III Trial Shows Non-Inferiority Compared to Standard Palliative Fractionation (NCT03503682)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
- (1)
- Patients with painful, complicated BMs, defined as those with neuraxis involvement, pathologic fractures, and/or a soft tissue component at the symptomatic site [5].
- (2)
- Pain at the treatment site distinguishable from pain related to other lesions.
- (3)
- A minimum pain intensity of ≥2 when assessed using a numeric rating scale (NRS).
- (4)
- Patients eligible only for palliative treatment and not suitable for definitive treatment.
- (5)
- Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) score less than 4.
- (6)
- Age equal to or greater than 18 years.
- (7)
- No changes made to the analgesic medication during the preceding week.
The Exclusion Criteria Were as Follows:
- (1)
- Previous RT performed at the same site.
- (2)
- Pregnancy or lactation.
- (3)
- Patient unavailability for follow-up visits.
- (4)
- The presence of comorbid conditions that, at the discretion of the physicians, made RT inadvisable.
2.3. Sample Size Calculation
2.4. Randomization
2.5. Interventions
2.6. Evaluations
2.7. Primary Endpoint
2.8. Secondary Endpoints
- (1)
- Differences in toxicity between arms.
- (2)
- Treatment interruption and re-treatment at the same site in both the study arms.
- (3)
- Overall survival rates in the two study arms.
2.9. Statistical Analysis
3. Results
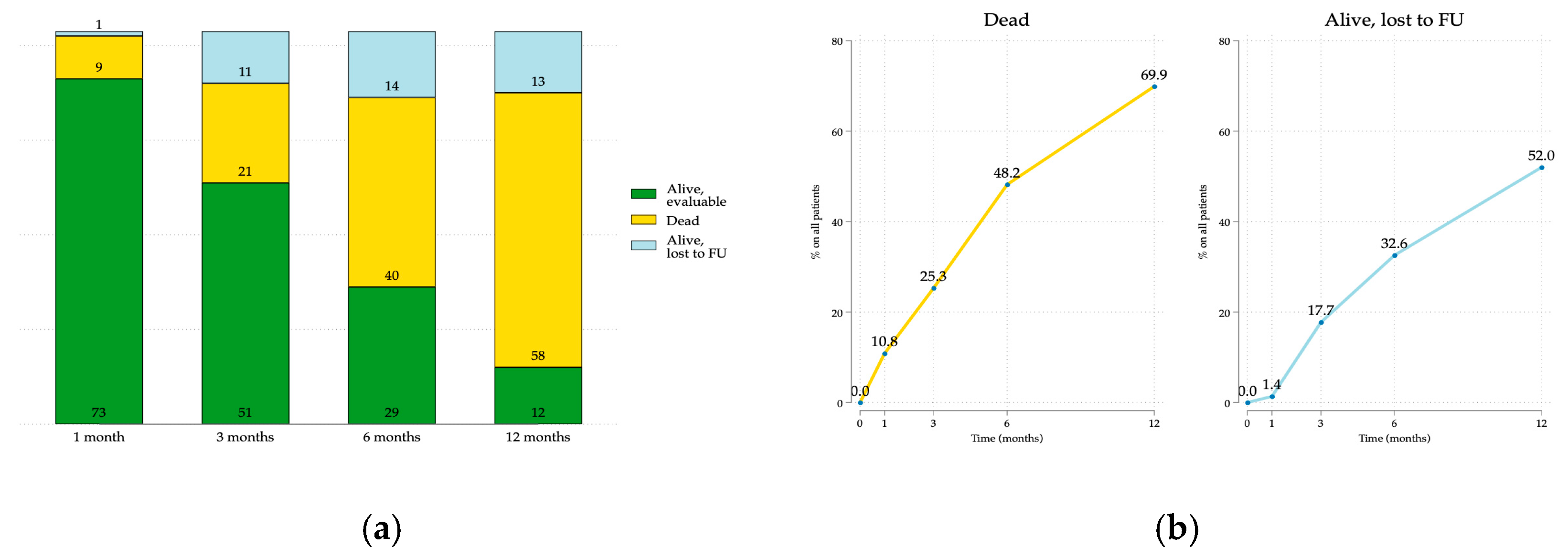
3.1. Patients’ Characteristics and Retention in the Study
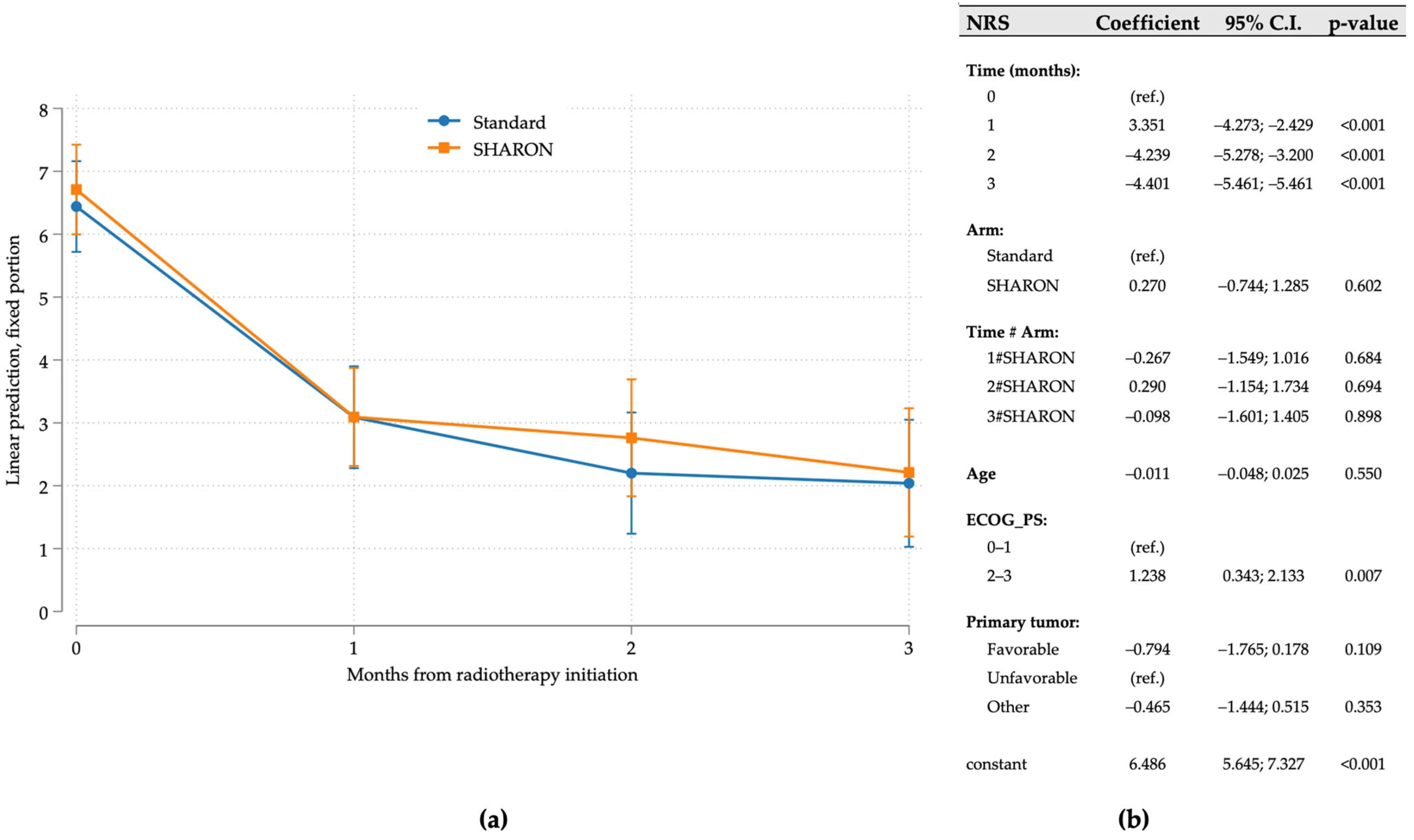
3.2. Pain Response
3.3. Toxicity
3.4. Treatment Interruption
3.5. Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riihimäki, M.; Thomsen, H.; Sundquist, K.; Sundquist, J.; Hemminki, K. Clinical Landscape of Cancer Metastases. Cancer Med. 2018, 7, 5534. [Google Scholar] [CrossRef] [PubMed]
- Mundy, G.R. Metastasis to Bone: Causes, Consequences and Therapeutic Opportunities. Nat. Rev. Cancer 2002, 2, 584–593. [Google Scholar] [CrossRef] [PubMed]
- van der Velden, J.; Willmann, J.; Spałek, M.; Oldenburger, E.; Brown, S.; Kazmierska, J.; Andratschke, N.; Menten, J.; van der Linden, Y.; Hoskin, P. ESTRO ACROP Guidelines for External Beam Radiotherapy of Patients with Uncomplicated Bone Metastases. Radiother. Oncol. 2022, 173, 197–206. [Google Scholar] [CrossRef]
- Oldenburger, E.; Brown, S.; Willmann, J.; van der Velden, J.M.; Spałek, M.; van der Linden, Y.M.; Kazmierska, J.; Menten, J.; Andratschke, N.; Hoskin, P. ESTRO ACROP Guidelines for External Beam Radiotherapy of Patients with Complicated Bone Metastases. Radiother. Oncol. 2022, 173, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Alcorn, S.R.; Elledge, C.R.; Wright, J.L.; Smith, T.J.; McNutt, T.R.; Fiksel, J.; Zeger, S.L.; DeWeese, T.L. Frequency of Complicated Symptomatic Bone Metastasis Over a Breadth of Operational Definitions HHS Public Access. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 800–810. [Google Scholar] [CrossRef]
- Farina, E.; Macchia, G.; Buwenge, M.; Siepe, G.; Zamagni, A.; Cammelli, S.; Cilla, S.; Wondemagegnhu, T.; Woldemariam, A.A.; Uddin, A.F.M.K.; et al. Radiotherapy in Palliation of Thoracic Tumors: A Phase I–II Study (SHARON Project). Clin. Exp. Metastasis 2018, 35, 739–746. [Google Scholar] [CrossRef]
- Farina, E.; Macchia, G.; Siepe, G.; Zamagni, A.; Buwenge, M.; Scirocco, E.; Cellini, F.; Deressa, B.T.; Tigeneh, W.; Uddin, K.A.F.M.; et al. Palliative Short-Course Radiotherapy in Advanced Pelvic Cancer: A Phase II Study (SHARON Project). Anticancer. Res. 2019, 39, 4237–4242. [Google Scholar] [CrossRef]
- Capuccini, J.; Macchia, G.; Farina, E.; Buwenge, M.; Genovesi, D.; Caravatta, L.; Nguyen, N.P.; Cammelli, S.; Cilla, S.; Wondemagegnhu, T.; et al. Short-Course Regimen of Palliative Radiotherapy in Complicated Bone Metastases: A Phase i-Ii Study (SHARON Project). Clin. Exp. Metastasis 2018, 35, 605–611. [Google Scholar] [CrossRef]
- Chow, E.; Harris, K.; Fan, G.; Tsao, M.; Sze, W.M. Palliative Radiotherapy Trials for Bone Metastases: A Systematic Review. J. Clin. Oncol. 2007, 25, 1423–1436. [Google Scholar] [CrossRef]
- Sze, W.M.; Shelley, M.; Held, I.; Mason, M. Cochrane Database of Systematic Reviews Palliation of Metastatic Bone Pain: Single Fraction versus Multifraction Radiotherapy. Cochrane Database Syst. Rev. 2011, 2002, CD004721. [Google Scholar] [CrossRef]
- Wu, J.S.Y.; Wong, R.; Johnston, M.; Bezjak, A.; Whelan, T. Meta-Analysis of Dose-Fractionation Radiotherapy Trials for the Palliation of Painful Bone Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2003, 55, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Blackwelder, W.C. “Proving the Null Hypothesis” in Clinical Trials. Control Clin. Trials 1982, 3, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Jones, D. ICRU Report 50—Prescribing, Recording and Reporting Photon Beam Therapy. Med. Phys. 1994, 21, 833–834. [Google Scholar] [CrossRef]
- Salazar, O.M.; Sandhu, T.; Da Motta, N.W.; Escutia, M.Á.P.; Lanzós-Gonzales, E.; Mouelle-Sone, A.; Moscol, A.; Zaharia, M.; Zaman, S. Fractionated Half-Body Irradiation (HBI) for the Rapid Palliation of Widespread, Symptomatic, Metastatic Bone Disease: A Randomized Phase III Trial of the International Atomic Energy Agency (IAEA). Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 765–775. [Google Scholar] [CrossRef]
- Cox, J.D.; Stetz, J.; Pajak, T.F. Toxicity Criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1341–1346. [Google Scholar] [CrossRef]
- Alcorn, S.; Cortés, Á.A.; Bradfield, L.; Brennan, M.; Dennis, K.; Diaz, D.A.; Doung, Y.C.; Elmore, S.; Hertan, L.; Johnstone, C.; et al. External Beam Radiation Therapy for Palliation of Symptomatic Bone Metastases: An ASTRO Clinical Practice Guideline. Pr. Radiat. Oncol. 2024, 14, 377–397. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, D.M.; Harvey, R.D.; Hurley, P.; Levit, L.A.; Kim, E.S.; Klepin, H.D.; Mileham, K.F.; Nowakowski, G.; Schenkel, C.; Davis, C.; et al. Early Impact of COVID-19 on the Conduct of Oncology Clinical Trials and Long-Term Opportunities for Transformation: Findings From an American Society of Clinical Oncology Survey. JCO Oncol. Pr. 2020, 16, 417–421. [Google Scholar] [CrossRef]
- Unger, J.M.; Xiao, H.; LeBlanc, M.; Hershman, D.L.; Blanke, C.D. Cancer Clinical Trial Participation at the 1-Year Anniversary of the Outbreak of the COVID-19 Pandemic. JAMA Netw. Open 2021, 4, e2118433. [Google Scholar] [CrossRef]
- Chow, E.; Hoskin, P.; Mitera, G.; Zeng, L.; Lutz, S.; Roos, D.; Hahn, C.; Van Der Linden, Y.; Hartsell, W.; Kumar, E. Update of the International Consensus on Palliative Radiotherapy Endpoints for Future Clinical Trials in Bone Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 1730–1737. [Google Scholar] [CrossRef]
- Maranzano, E.; Trippa, F.; Casale, M.; Costantini, S.; Lupattelli, M.; Bellavita, R.; Marafioti, L.; Pergolizzi, S.; Santacaterina, A.; Mignogna, M.; et al. 8Gy Single-Dose Radiotherapy Is Effective in Metastatic Spinal Cord Compression: Results of a Phase III Randomized Multicentre Italian Trial. Radiother. Oncol. 2009, 93, 174–179. [Google Scholar] [CrossRef]
- Rades, D.; Stalpers, L.J.A.; Hulshof, M.C.; Borgmann, K.; Karstens, J.H.; Koning, C.C.E.; Alberti, W. Comparison of 1 × 8 Gy and 10 × 3 Gy for Functional Outcome in Patients with Metastatic Spinal Cord Compression. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Rades, D.; Huttenlocher, S.; Šegedin, B.; Perpar, A.; Conde, A.J.; Garcia, R.; Veninga, T.; Stalpers, L.J.A.; Cacicedo, J.; Rudat, V.; et al. Single-Fraction Versus 5-Fraction Radiation Therapy for Metastatic Epidural Spinal Cord Compression in Patients With Limited Survival Prognoses: Results of a Matched-Pair Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Rades, D.; Conde-Moreno, A.J.; Cacicedo, J.; Veninga, T.; Segedin, B.; Stanic, K.; Rudat, V.; Schild, S.E. 1 × 8 Gy versus 5 × 4 Gy for Metastatic Epidural Spinal Cord Compression: A Matched-Pair Study of Three Prognostic Patient Subgroups. Radiat. Oncol. 2018, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, P.J.; Hopkins, K.; Misra, V.; Holt, T.; McMenemin, R.; Dubois, D.; McKinna, F.; Foran, B.; Madhavan, K.; MacGregor, C.; et al. Effect of Single-Fraction vs. Multifraction Radiotherapy on Ambulatory Status Among Patients With Spinal Canal Compression From Metastatic Cancer. JAMA 2019, 322, 2084. [Google Scholar] [CrossRef]

| Treatment Arm | ||||
|---|---|---|---|---|
| All | Standard | SHARON | p-Value | |
| 83 (100.0%) | 41 (49.4%) | 42 (50.6%) | ||
| Age at randomization | 64.0 ± 11.3 | 62.7 ± 10.7 | 65.2 ± 11.8 | 0.323 * |
| Age class | ||||
| <65 y | 44 (53.0%) | 25 (61.0%) | 19 (45.2%) | 0.151 § |
| ≥65 | 39 (47.0%) | 16 (39.0%) | 23 (54.8%) | |
| Sex | ||||
| Male | 52 (62.7%) | 24 (58.5%) | 28 (66.7%) | 0.444 § |
| Female | 31 (37.3%) | 17 (41.5%) | 14 (33.3%) | |
| ECOG_PS | ||||
| 0–1 | 57 (68.7%) | 30 (73.2%) | 27 (64.3%) | 0.383 § |
| 2–3 | 26 (31.3%) | 11 (26.8%) | 15 (35.7%) | |
| Primary tumor | ||||
| Favorable | 21 (25.3%) | 10 (24.4%) | 11 (26.2%) | 0.559 § |
| Unfavorable | 40 (48.2%) | 22 (53.7%) | 18 (42.9%) | |
| Other | 22 (26.5%) | 9 (22.0%) | 13 (31.0%) | |
| Metastases site | ||||
| Spine | 38 (45.8%) | 20 (48.8%) | 18 (42.9%) | 0.624 ° |
| Pelvis | 23 (27.7%) | 9 (22.0%) | 14 (33.3%) | |
| Thorax | 17 (20.5%) | 10 (24.4%) | 7 (16.7%) | |
| Extremities | 5 (6.0%) | 2 (4.9%) | 3 (7.1%) | |
| Type of complication | ||||
| Spinal compression | 8 (9.9%) | 4 (9.8%) | 4 (10.0%) | 0.970 ° |
| Nerve compression | 18 (22.2%) | 10 (24.4%) | 8 (20.0%) | |
| Pathological fracture | 11 (13.6%) | 5 (12.2%) | 6 (15.0%) | |
| Extraosseous extention | 44 (54.3%) | 22 (53.7%) | 22 (55.0%) | |
| Intention-to-Treat Analysis | All 83 (100.0%) | Standard 41 (49.4%) | SHARON 42 (50.6%) | p-Value |
|---|---|---|---|---|
| NRS response (4 groups) | ||||
| Complete response | 19 (22.9%) | 8 (19.5%) | 11 (26.2%) | 0.501 |
| Partial response | 37 (44.6%) | 18 (43.9%) | 19 (45.2%) | |
| Stable disease | 10 (12.0%) | 4 (9.8%) | 6 (14.3%) | |
| Progression | 17 (20.5%) | 11 (26.8%) | 6 (14.3%) | |
| NRS response (2 groups) | ||||
| Overall response (complete + partial) | 56 (67.5%) | 26 (63.4%) | 30 (71.4%) | 0.436 |
| No response (stable + progression) | 27 (32.5%) | 15 (36.6%) | 12 (28.6%) | |
| NRS + DS response (4 groups) | ||||
| Complete response | 17 (20.5%) | 6 (14.6%) | 11 (26.2%) | 0.465 |
| Partial response | 28 (33.7%) | 13 (31.7%) | 15 (35.7%) | |
| Stable disease | 17 (20.5%) | 10 (24.4%) | 7 (16.7%) | |
| Progression | 21 (25.3%) | 12 (29.3%) | 9 (21.4%) | |
| NRS + DS response (2 groups) | ||||
| Overall response (complete + partial) | 45 (54.2%) | 19 (46.3%) | 26 (61.9%) | 0.155 |
| No response (stable + progression) | 38 (45.8%) | 22 (53.7%) | 16 (38.1%) | |
| PER-PROTOCOL ANALYSIS | All 73 (100.0%) | Standard 35 (47.9%) | SHARON 38 (52.1%) | p-value |
| NRS response (4 groups) | ||||
| Complete response | 19 (26.0%) | 8 (22.9%) | 11 (28.9%) | 0.571 * |
| Partial response | 37 (50.7%) | 18 (51.4%) | 19 (50.0%) | |
| Stable disease | 10 (13.7%) | 4 (11.4%) | 6 (15.8%) | |
| Progression | 7 (9.6%) | 5 (14.3%) | 2 (5.3%) | |
| NRS response (2 groups) | ||||
| Overall response (complete + partial) | 56 (76.7%) | 26 (74.3%) | 30 (78.9%) | 0.638 |
| No response (stable + progression) | 17 (23.3%) | 9 (25.7%) | 8 (21.1%) | |
| NRS + DS response (4 groups) | ||||
| Complete response | 17 (23.3%) | 6 (17.1%) | 11 (28.9%) | 0.549 |
| Partial response | 28 (38.4%) | 13 (37.1%) | 15 (39.5%) | |
| Stable disease | 17 (23.3%) | 10 (28.6%) | 7 (18.4%) | |
| Progression | 11 (15.1%) | 6 (17.1%) | 5 (13.2%) | |
| NRS + DS response (2 groups) | ||||
| Overall response (complete + partial) | 45 (61.6%) | 19 (54.3%) | 26 (68.4%) | 0.215 |
| No response (stable + progression) | 28 (38.4%) | 16 (45.7%) | 12 (31.6%) |
| Total Population, n: 83 | Standard Arm, n: 41 | Sharon Arm, n: 42 | p-Value ° | |
|---|---|---|---|---|
| Acute toxicity | 0.190 | |||
| NO | 51 (61.4%) | 22 (53.7%) | 29 (69.0%) | |
| YES | 31 (37.3%) | 18 (43.9%) | 13 (31.0%) | |
| N.A. | 1 (1.2%) | 1 (2.4%) | 0 (0.0%) | |
| Treatment completed | 0.026 | |||
| NO | 5 (6.0%) | 5 (12.2%) | 0 (0.0%) | |
| YES | 78 (94.0%) | 36 (87.8%) | 42 (100.0%) | |
| Re-treatment (same site) | 0.313 | |||
| NO | 74 (89.2%) | 35 (85.4%) | 39 (92.9%) | |
| YES | 9 (10.8%) | 6 (14.6%) | 3 (7.1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamagni, A.; Siepe, G.; Gibertoni, D.; Donati, C.M.; Cellini, F.; Fiorica, F.; Pezzulla, D.; Deodato, F.; Candoli, F.; Bisello, S.; et al. Accelerated Radiotherapy for Complicated Bone Metastases: SHARON Bone Randomized Phase III Trial Shows Non-Inferiority Compared to Standard Palliative Fractionation (NCT03503682). Cancers 2025, 17, 2000. https://doi.org/10.3390/cancers17122000
Zamagni A, Siepe G, Gibertoni D, Donati CM, Cellini F, Fiorica F, Pezzulla D, Deodato F, Candoli F, Bisello S, et al. Accelerated Radiotherapy for Complicated Bone Metastases: SHARON Bone Randomized Phase III Trial Shows Non-Inferiority Compared to Standard Palliative Fractionation (NCT03503682). Cancers. 2025; 17(12):2000. https://doi.org/10.3390/cancers17122000
Chicago/Turabian StyleZamagni, Alice, Giambattista Siepe, Dino Gibertoni, Costanza M. Donati, Francesco Cellini, Francesco Fiorica, Donato Pezzulla, Francesco Deodato, Filippo Candoli, Silvia Bisello, and et al. 2025. "Accelerated Radiotherapy for Complicated Bone Metastases: SHARON Bone Randomized Phase III Trial Shows Non-Inferiority Compared to Standard Palliative Fractionation (NCT03503682)" Cancers 17, no. 12: 2000. https://doi.org/10.3390/cancers17122000
APA StyleZamagni, A., Siepe, G., Gibertoni, D., Donati, C. M., Cellini, F., Fiorica, F., Pezzulla, D., Deodato, F., Candoli, F., Bisello, S., Scirocco, E., Manfrida, S., Gabbani, M., Cilla, S., Macchia, G., & Morganti, A. G. (2025). Accelerated Radiotherapy for Complicated Bone Metastases: SHARON Bone Randomized Phase III Trial Shows Non-Inferiority Compared to Standard Palliative Fractionation (NCT03503682). Cancers, 17(12), 2000. https://doi.org/10.3390/cancers17122000











