Functional and Oncologic Outcomes in Single-Kidney Patients Treated with Robot-Assisted Partial Nephrectomy for Renal Tumors: Results from a Prospectively Maintained Dataset of a Single Tertiary Referral Center
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
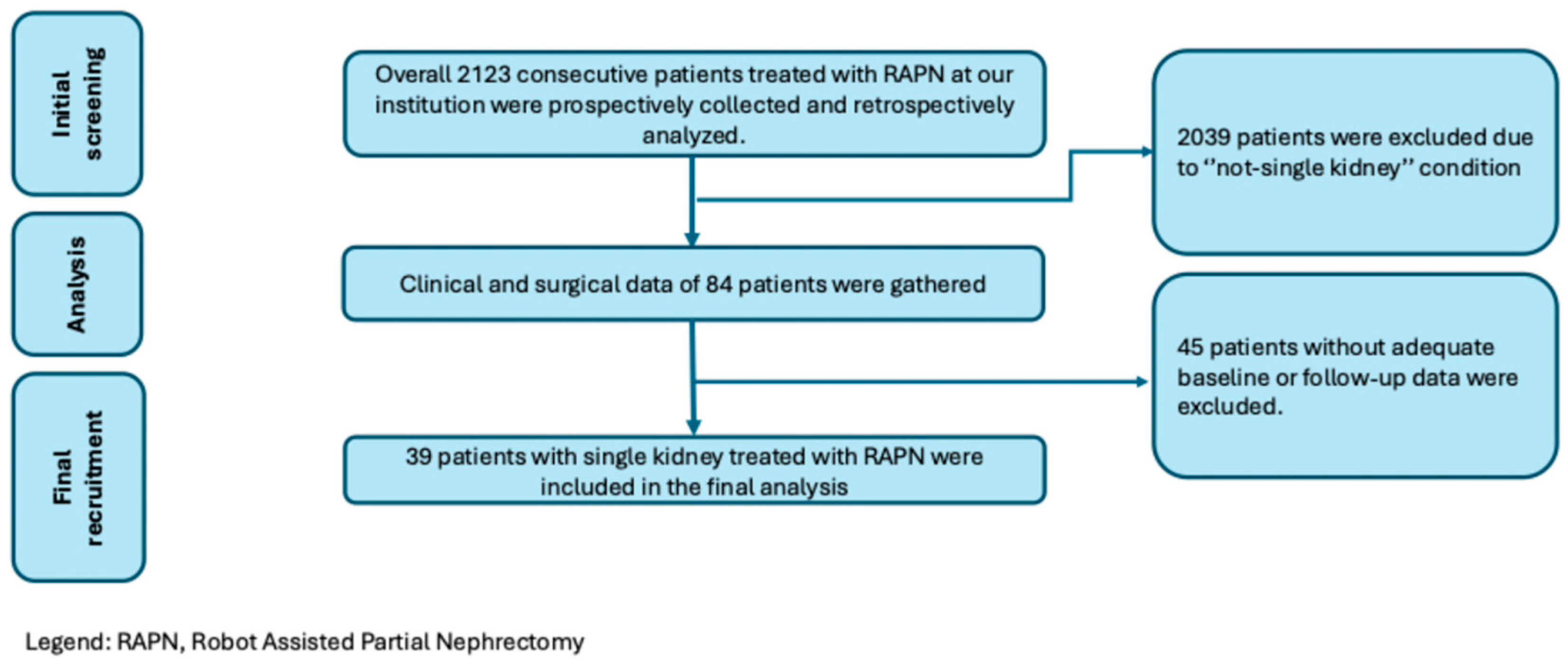
2.1. Patients, Dataset and Selection
2.2. Follow-Up
2.3. Outcomes
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RAPN | Robot-Assisted Partial Nephrectomy |
| NSS | Nephron-Sparing Surgery |
| RCC | Renal Cell Carcinoma |
| BMI | Body Mass Index |
| CCI | Charlson Comorbidity Index |
| ASA | American Society of Anesthesiologists |
| CT | Computed Tomography |
| MRI | Magnetic Resonance Imaging |
| PADUA | Preoperative Aspects and Dimensions Used for Anatomy |
| RENAL | Radius–Exophycity–Nearness–Anterior–Location |
| CD | Clavien–Dindo |
| EAU | European Association of Urology |
| LR | Local Recurrence |
| SR | Systemic Recurrence |
| EGFR | Estimated Glomerular Filtration Rate |
| IQR | Interquartile Range |
| ISUP | International Society of Urological Pathology |
| PSMs | Positive Surgical Margins |
| MM | Millimeters |
| EBL | Estimated Blood Loss |
| OD | Odds Ratio |
| HR | Hazard Ratio |
| CI | Confidence Interval |
| POD | Postoperative Day |
| MAP | Mayo Adhesive Probability |
References
- Rod, X.; Peyronnet, B.; Seisen, T.; Pradere, B.; Gomez, F.D.; Verhoest, G.; Vaessen, C.; De La Taille, A.; Bensalah, K.; Roupret, M. Impact of ischaemia time on renal function after partial nephrectomy: A systematic review. BJU Int. 2016, 118, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Carbonara, U.; Simone, G.; Minervini, A.; Sundaram, C.P.; Larcher, A.; Lee, J.; Checcucci, E.; Fiori, C.; Patel, D.; Meagher, M.; et al. Outcomes of robot-assisted partial nephrectomy for completely endophytic renal tumors: A multicenter analysis. Eur. J. Surg. Oncol. 2021, 47, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Minervini, A.; Campi, R.; Di Maida, F.; Mari, A.; Montagnani, I.; Tellini, R.; Tuccio, A.; Siena, G.; Vittori, G.; Lapini, A.; et al. Tumor-parenchyma interface and long-term oncologic outcomes after robotic tumor enucleation for sporadic renal cell carcinoma. Urol. Oncol. 2018, 36, 527.e1–527.e11. [Google Scholar] [CrossRef]
- Schiavina, R.; Serni, S.; Mari, A.; Antonelli, A.; Bertolo, R.; Bianchi, G.; Brunocilla, E.; Borghesi, M.; Carini, M.; Longo, N.; et al. A prospective, multicenter evaluation of predictive factors for positive surgical margins after nephron-sparing surgery for renal cell carcinoma: The RECORd1 Italian Project. Clin. Genitourin. Cancer. 2015, 13, 165–170. [Google Scholar] [CrossRef]
- Soputro, N.A.; Mikesell, C.D.; Younis, S.K.; Rai, S.; Wang, L.; Ionson, A.C.; Pedraza, A.M.; Weight, C.J.; Kaouk, J. Functional outcomes of robot-assisted partial nephrectomy in patients with a solitary kidney. BJU Int. 2025. [Google Scholar] [CrossRef]
- Ito, H.; Muraoka, K.; Uemura, K.; Jikuya, R.; Kondo, T.; Tatenuma, T.; Kawahara, T.; Komeya, M.; Ito, Y.; Hasumi, H.; et al. Impact of chronic kidney disease stages on surgical and functional outcomes in robot-assisted partial nephrectomy for localized renal tumors. J. Robot. Surg. 2024, 18, 109. [Google Scholar] [CrossRef]
- Pandolfo, S.D.; Cerrato, C.; Wu, Z.; Franco, A.; Del Giudice, F.; Sciarra, A.; Verze, P.; Lucarelli, G.; Imbimbo, C.; Perdonà, S.; et al. A systematic review of robot-assisted partial nephrectomy outcomes for advanced indications: Large tumors (cT2-T3), solitary kidney, completely endophytic, hilar, recurrent, and multiple renal tumors. Asian J. Urol. 2023, 10, 390–406. [Google Scholar] [CrossRef] [PubMed]
- Benichou, Y.; Audenet, F.; Bensalah, K.; Roupret, M.; Paparel, P.; Lebacle, C.; Bruyère, F.; Beauval, J.-B.; Villers, A.; Lang, H.; et al. Partial nephrectomy in solitary kidneys: Comparison between open surgery and robotic-assisted laparoscopy on perioperative and functional outcomes (UroCCR-54 study). World J. Urol. 2023, 41, 315–324. [Google Scholar] [CrossRef]
- Badani, K.K.; Kothari, P.D.; Okhawere, K.E.; Eun, D.; Hemal, A.; Abaza, R.; Porter, J.; Lovallo, G.; Ahmed, M.; Munver, R.; et al. Selective clamping during robot-assisted partial nephrectomy in patients with a solitary kidney: Is it safe and does it help? BJU Int. 2020, 125, 893–897. [Google Scholar] [CrossRef]
- Arora, S.; Abaza, R.; Adshead, J.M.; Ahlawat, R.K.; Challacombe, B.J.; Dasgupta, P.; Gandaglia, G.; Moon, D.A.; Yuvaraja, T.B.; Capitanio, U.; et al. ‘Trifecta’ outcomes of robot-assisted partial nephrectomy in solitary kidney: A Vattikuti Collective Quality Initiative (VCQI) database analysis. BJU Int. 2018, 121, 119–123. [Google Scholar] [CrossRef]
- Ficarra, V.; Novara, G.; Secco, S.; Macchi, V.; Porzionato, A.; De Caro, R.; Artibani, W. Preoperative aspects and dimensions used for an anatomical (PADUA) classification of renal tumours in patients who are candidates for nephron-sparing surgery. Eur. Urol. 2009, 56, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Kutikov, A.; Uzzo, R.G. The R.E.N.A.L. nephrometry score: A comprehensive standardized system for quantitating renal tumor size, location and depth. J. Urol. 2009, 182, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Novara, G.; Ficarra, V.; Antonelli, A.; Artibani, W.; Bertini, R.; Carini, M.; Cunico, S.C.; Imbimbo, C.; Longo, N.; Martignoni, G.; et al. Validation of the 2009 TNM version in a large multi-institutional cohort of patients treated for renal cell carcinoma: Are further improvements needed? Eur. Urol. 2010, 58, 588. [Google Scholar] [CrossRef] [PubMed]
- Rioux-Leclercq, N.; Ferran, A.; Mahul, A.; Argani, P.; Billis, A.; Bonsib, S.; Cheng, L.; Cheville, J.; Eble, J.; Egevad, L.; et al. Les tumeurs rénales: Recommandations de la conférence de consensus de l’International Society of Urologic Pathology (ISUP) 2012 [Renal tumors: The International Society of Urologic Pathology (ISUP) 2012 consensus conference recommendations]. Ann. Pathol. 2014, 34, 448–461. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Yasuda, Y.; Zhang, J.; Attawettayanon, W.; Rathi, N.; Wilkins, L.; Roversi, G.; Zhang, A.; Accioly, J.P.E.; Shah, S.; Munoz-Lopez, C.; et al. Comprehensive Management of Renal Masses in Solitary Kidneys. Eur. Urol. Oncol. 2023, 6, 84–94. [Google Scholar] [CrossRef]
- Cacciamani, G.E.; Gill, T.; Medina, L.; Ashrafi, A.; Winter, M.; Sotelo, R.; Artibani, W.; Gill, I.S. Impact of Host Factors on Robotic Partial Nephrectomy Outcomes: Comprehensive Systematic Review and Meta-Analysis. J. Urol. 2018, 200, 716–730. [Google Scholar] [CrossRef]
- Lambertini, L.; Mari, A.; Sandulli, A.; Amparore, D.; Antonelli, A.; Barale, M.; Bove, P.; Brunocilla, E.; Capitanio, U.; DA Pozzo, L.F.; et al. Minimally invasive transperitoneal partial versus radical nephrectomy in obese patients: Perioperative and long-term functional outcomes from a large perspective contemporary series (RECORd2 project). Minerva Urol. Nephrol. 2024, 76, 185–194. [Google Scholar] [CrossRef]
- Flammia, R.S.; Anceschi, U.; Tuderti, G.; Di Maida, F.; Grosso, A.A.; Lambertini, L.; Mari, A.; Mastroianni, R.; Bove, A.; Capitanio, U.; et al. Development and internal validation of a nomogram predicting 3-year chronic kidney disease upstaging following robot-assisted partial nephrectomy. Int. Urol. Nephrol. 2024, 56, 913–921. [Google Scholar] [CrossRef]
- Dawidek, M.T.; Calderon, L.P.; Villada, J.S.A.; Ucpinar, B.A.; Eismann, L.; Reese, S.W.; Ganz, M.; Akin, O.; Reznik, E.; Coleman, J.A.; et al. Tumors in Solitary Kidneys Are Not All Equal: Outcomes of Partial Nephrectomy in High-Risk Cases. Urol Pract. 2025. [Google Scholar] [CrossRef]
- Antonelli, A.; Mari, A.; Longo, N.; Novara, G.; Porpiglia, F.; Schiavina, R.; Ficarra, V.; Carini, M.; Minervini, A.; Collaborators. Role of Clinical and Surgical Factors for the Prediction of Immediate, Early and Late Functional Results, and its Relationship with Cardiovascular Outcome after Partial Nephrectomy: Results from the Prospective Multicenter RECORd 1 Project. J. Urol. 2018, 199, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Simone, G.; Gill, I.S.; Mottrie, A.; Kutikov, A.; Patard, J.-J.; Alcaraz, A.; Rogers, C.G. Indications, techniques, outcomes, and limitations for minimally ischemic and off-clamp partial nephrectomy: A systematic review of the literature. Eur. Urol. 2015, 68, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Stout, T.E.; Gellhaus, P.T.; Tracy, C.R.; Steinberg, R.L. Robotic Partial vs Radical Nephrectomy for Clinical T3a Tumors: A Narrative Review. J. Endourol. 2023, 37, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Pandolfo, S.D.; Wu, Z.; Campi, R.; Bertolo, R.; Amparore, D.; Mari, A.; Verze, P.; Manfredi, C.; Franco, A.; Ditonno, F.; et al. Outcomes and Techniques of Robotic-Assisted Partial Nephrectomy (RAPN) for Renal Hilar Masses: A Comprehensive Systematic Review. Cancers 2024, 16, 693. [Google Scholar] [CrossRef]
- Buffi, N.; Uleri, A.; Paciotti, M.; Lughezzani, G.; Casale, P.; Diana, P.; DE Groote, R.; Sarchi, L.; Mottaran, A.; Bravi, C.; et al. Techniques and outcomes of robot-assisted partial nephrectomy for the treatment of multiple ipsilateral renal masses. Minerva Urol. Nephrol. 2023, 75, 223–230. [Google Scholar] [CrossRef]
- Grosso, A.A.; Di Maida, F.; Lambertini, L.; Cadenar, A.; Coco, S.; Ciaralli, E.; Salamone, V.; Vittori, G.; Tuccio, A.; Mari, A.; et al. Three-dimensional virtual model for robot-assisted partial nephrectomy: A propensity-score matching analysis with a contemporary control group. World J. Urol. 2024, 42, 338. [Google Scholar] [CrossRef]
- Amparore, D.; Piana, A.; Piramide, F.; De Cillis, S.; Checcucci, E.; Fiori, C.; Porpiglia, F. 3D anatomical digital twins: New generation virtual models to navigate robotic partial nephrectomy. BJUI Compass. 2025, 6, e453. [Google Scholar] [CrossRef]
- Volpi, G.; Gatti, C.; Quarà, A.; Piramide, F.; Amparore, D.; Alessio, P.; De Cillis, S.; Colombo, M.; Busacca, G.; Sica, M.; et al. Metaverse surgical planning for robotic surgery: Preliminary experience and users’ perception. Ther. Adv. Urol. 2024, 16, 17562872241297524. [Google Scholar] [CrossRef]
- Amparore, D.; Piramide, F.; Verri, P.; Checcucci, E.; Piana, A.; Basile, G.; Larcher, A.; Gallioli, A.; Territo, A.; Gaya, J.M.; et al. Nephrometry Scores Based on Three-dimensional Virtual Models Improve the Accuracy of Predicting Postoperative Complications After Robotic Partial Nephrectomy: Results from a Collaborative ERUS Validation Study. Eur. Urol. Open Sci. 2025, 74, 11–20. [Google Scholar] [CrossRef]
- Beksac, A.T.; Okhawere, K.E.; Zeinab, M.A.; Harrison, B.; Stifelman, M.D.; Eun, D.D.; Abaza, R.; Badani, K.K.; Kaouk, J.H. Robotic partial nephrectomy for management of renal mass in patients with a solitary kidney: Can we expand the indication to T2 and T3 disease? Minerva Urol. Nephrol. 2022, 74, 203–208. [Google Scholar] [CrossRef]
- Meng, M. Frozen section during partial nephrectomy: Does it predict positive margins? Gordetsky J, Gorin MA, Canner J, Ball MW, Pierorazio PM, Allaf ME, Epstein JI. BJU Int. 2015 Dec; 116(6):868-72. [Epub 2015 Mar 23]. doi: 10.1111/bju.13011. Urol. Oncol. 2017, 35, 312–313. [Google Scholar] [CrossRef]
- Carbonara, U.; Amparore, D.; Gentile, C.; Bertolo, R.; Erdem, S.; Ingels, A.; Marchioni, M.; Muselaers, C.H.; Kara, O.; Marandino, L.; et al. Current strategies to diagnose and manage positive surgical margins and local recurrence after partial nephrectomy. Asian J. Urol. 2022, 9, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Tomczak, P.; Park, S.H.; Venugopal, B.; Ferguson, T.; Symeonides, S.N.; Hajek, J.; Chang, Y.-H.; Lee, J.-L.; Sarwar, N.; et al. Overall Survival with Adjuvant Pembrolizumab in Renal-Cell Carcinoma. N. Engl. J. Med. 2024, 390, 1359–1371. [Google Scholar] [CrossRef] [PubMed]
- Grosso, A.A.; Lambertini, L.; Di Maida, F.; Gallo, M.L.; Mari, A.; Minervini, A. Three-dimensional reconstruction and intraoperative ultrasonography: Crucial tools to safely approach highly complex renal masses. Int. Braz. J. Urol. 2022, 48, 996–997. [Google Scholar] [CrossRef] [PubMed]
- Minervini, A.; Grosso, A.A.; Di Maida, F.; Mari, A.; Vittori, G.; Muto, G.; Carini, M. How far is too far? Exploring the indications for robotic partial nephrectomy in a highly complex kidney tumor. Int. Braz. J. Urol. 2020, 46, 871–872. [Google Scholar] [CrossRef]
- Andras, I.; Territo, A.; Telecan, T.; Medan, P.; Perciuleac, I.; Berindean, A.; Stanca, D.V.; Buzoianu, M.; Coman, I.; Crisan, N. Role of the Laparoscopic Approach for Complex Urologic Surgery in the Era of Robotics. J. Clin. Med. 2021, 10, 1812. [Google Scholar] [CrossRef]

| Overall Number (n = 39) | %/IQR | ||
|---|---|---|---|
| Age, years—median (IQR) | 65 | 56–71 | |
| Sex—n. (%) | Male | 28 | 71.8 |
| Female | 11 | 28.2 | |
| Side—n. (%) | Right | 19 | 48.7 |
| Left | 20 | 51.3 | |
| BMI, kg/m2—median (IQR) | 27.2 | 25.8–30.9 | |
| ASA score—n. (%) | 1 | 4 | 10.2 |
| 2 | 20 | 51.2 | |
| 3 | 15 | 38.6 | |
| CCI—median (IQR) | 2 | 4–5 | |
| Baseline creatinine (mg/dL)—median (IQR) | 1.5 | 1.0—2.0 | |
| Baseline eGFR (mL/min/1.73 m²)—median (IQR) | 45 | 35–80 | |
| Baseline chronic kidney disease stage—n. (%) | 1 | 0 | 0 |
| 2 | 11 | 28.2 | |
| 3a | 9 | 23.1 | |
| 3b | 14 | 35.9 | |
| 4 | 5 | 12.8 | |
| Single kidney reason—n. (%) | Surgical | 21 | 53.8 |
| Congenital | 10 | 25.6 | |
| Functional | 8 | 20.6 | |
| PADUA score, median (IQR) | 7 | 8–9 | |
| RENAL score, median (IQR) | 5 | 6–7 | |
| Tumor diameter, mm—median (IQR) | 32 | 21–49 | |
| MAP-score, n. (%) | 0 | 10 | 25.6 |
| 1 | 7 | 18.0 | |
| 2 | 11 | 28.2 | |
| 3 | 6 | 15.3 | |
| 4 | 3 | 7.6 | |
| 5 | 2 | 5.3 | |
| Clinical T-stage, n. (%) | 1a | 24 | 61.5 |
| 1b | 9 | 23.0 | |
| 2 | 5 | 13.8 | |
| 3a | 0 | 0 | |
| 3b | 1 | 2.7 | |
| Overall Number (n = 39) | %/IQR | ||
|---|---|---|---|
| Surgical access, n. (%) | Transperitoneal | 31 | 79.4 |
| Retroperitoneal | 8 | 20.6 | |
| Operative time, minutes—median (IQR) | 87 | 115–160 | |
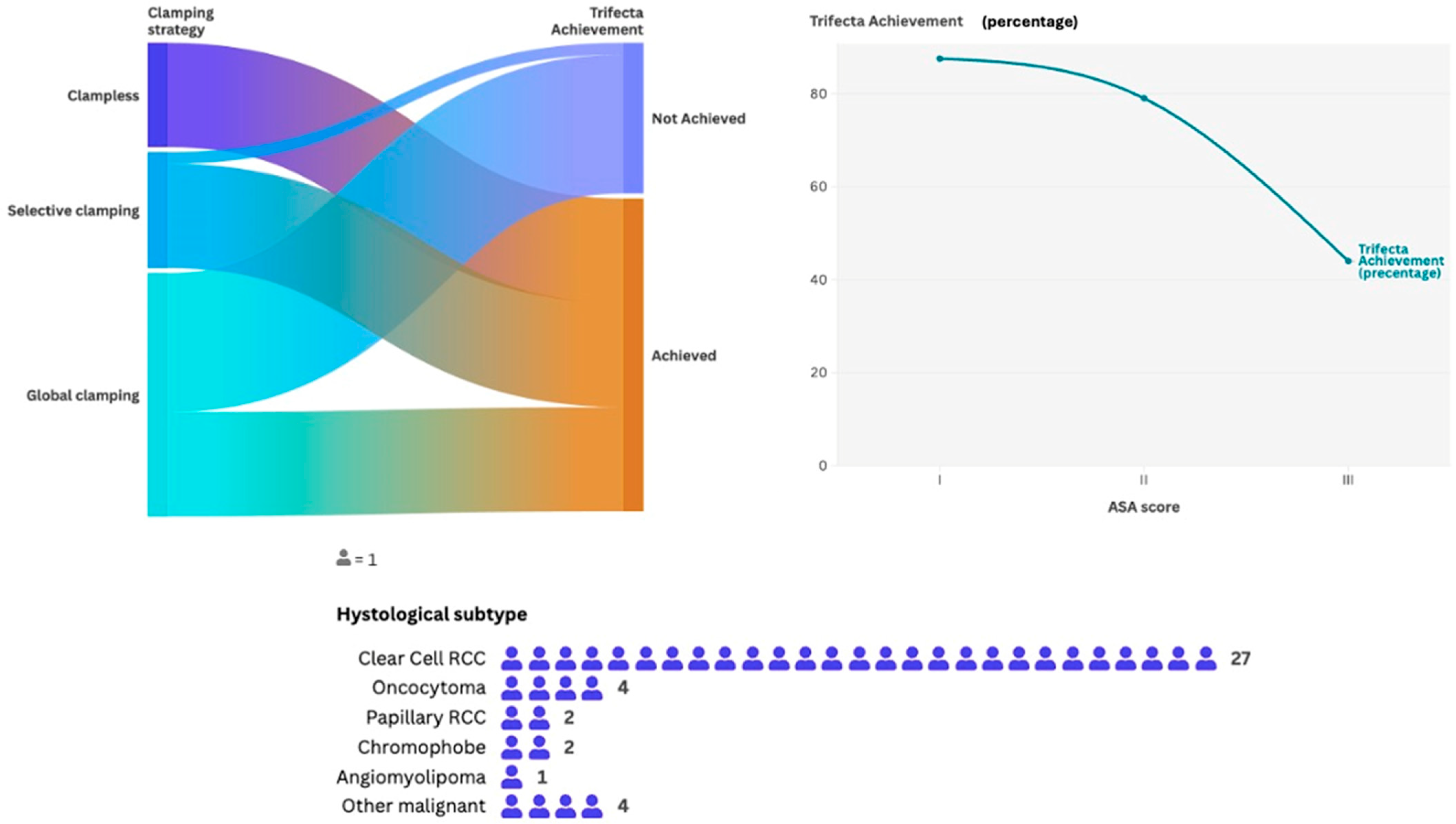
| Clamping—n. (%) | Global | 21 | 53.8 |
| Selective | 10 | 25.6 | |
| No clamp | 8 | 20.6 | |
| Ischemia time, minutes—median (IQR) | 12 | 10–18 | |
| Intraoperative complication, n. (%) | 0 | 0 | |
| EBL, mL—median (IQR) | 300 | 250–450 | |
| Postoperative complication—n. (%) | 25 | 64.0 | |
| CD classification—n. (%) | I | 9 | 23.1 |
| II | 12 | 30.7 | |
| III | 3 | 7.6 | |
| IV | 1 | 2.6 | |
| Hospital stay, days—median (IQR) | 4 | 3–5 | |
| Drainage time, days—median (IQR) | 2 | 2–3 | |
| Δ Hemoglobin blood level, g/dL—median (IQR) | −2.9 | −2.2–−3.5 | |
| Δ Creatinine serum level at 3rd postoperative day, mg/dL—median (IQR) | 1.5 | 1.1–1.9 | |
| Δ eGFR serum level at 3rd postoperative day, mg/dL—median (IQR) | 20 | 5–45 | |
| Positive surgical margin, n. (%) | 6 | 15.3 | |
| Trifecta rate, n. (%) | 26 | 66.6 | |
| Overall Number (n = 39) | %/IQR | ||
|---|---|---|---|
| Malignant histotype—n. (%) | Clear cell RCC | 27 | 69.2 |
| Papillary RCC | 2 | 5.1 | |
| Chromophobe RCC | 2 | 5.1 | |
| Other malignant histotype | 3 | 7.9 | |
| Benign histotype—n. (%) | Oncocytoma | 4 | 10.2 |
| Angiomyolipoma | 1 | 2.5 | |
| Other benign histotype | 0 | 0 | |
| Pathological tumor diameter, mm—median (IQR) | 30 | 20–49 | |
| Nucleolar grade—median (IQR) | I | 4 | 11.7 |
| II | 16 | 47.1 | |
| III | 9 | 26.4 | |
| IV | 5 | 14.8 | |
| Pathological T-stage—n. (%) | 1a | 16 | 47.2 |
| 1b | 10 | 29.3 | |
| 2 | 2 | 5.9 | |
| 3a | 5 | 14.7 | |
| 3b | 1 | 2.9 | |
| Presence of tumor necrosis, n. (%) | 3 | 8.8 | |
| Sarcomatoid differentiation, n (%) | 3 | 8.8 | |
| Patients Reaching Trifecta (n = 26) | Patients Not Reaching Trifecta (n = 13) | p-Value | ||
|---|---|---|---|---|
| Age, years—median (IQR) | 65 (56–71) | 67 (58–71) | 0.18 | |
| ASA score, n. (%) | 1 | 8 (30.7) | 1 (7.6) | <0.001 |
| 2 | 14 (53.8) | 3 (23.0) | ||
| 3 | 4 (15.5) | 9 (69.4) | ||
| Clinical tumor diameter (mm), median (IQR) | 30 (21–47) | 32 (22–49) | 0.07 | |
| PADUA score, median (IQR) | 7 (8–9) | 7 (8–10) | 0.18 | |
| Clinical T/stage, n. (%) | 1a | 18 (69.2) | 6 (46.1) | 0.09 |
| 1b | 5 (19.2) | 4 (30.7) | ||
| 2 | 2 (7.6) | 3 (23.2) | ||
| 3a | 0 (0.0) | 0 (0.0) | ||
| 3b | 1 (4.0) | 0 (0.0) | ||
| Operative time, minutes—median (IQR) | 85 (111–159) | 89 (118–162) | 0.31 | |
| MAP-score, n. (%) | 0 | 5 | 5 | 0.32 |
| 1 | 3 | 4 | ||
| 2 | 6 | 5 | ||
| 3 | 3 | 3 | ||
| 4 | 2 | 1 | ||
| 5 | 0 | 2 | ||
| Clamping, n. (%) | Global | 9 (34.6) | 12 (92.3) | <0.001 |
| Selective | 9 (34.6) | 1 (7.7) | ||
| No clamp | 8 (30.8) | 0 (0.0) | ||
| EBL, mL—median (IQR) | 350 (300–450) | 250 (300–350) | 0.06 | |
| (% eGFR Loss > 30% at 3rd POD | Trifecta Failure | (% eGFR Loss > 30% at Last Follow-Up | Disease Recurrence | |
|---|---|---|---|---|
| OR, (95% CI), p | OR, (95% CI), p | HR, (95% CI), p | HR, (95% CI), p | |
| ASA score (>II vs. ≤II) | 1.19, (1.13–4.21), 0.002 | 1.24, (1.10–3.87), 0.001 | 2.21, (1.47–6.81), <0.0001 | - |
| Global clamping (vs. selective/clampless) | 1.82, (1.43–2.31), <0.0001 | 1.64, (1.18–3.27), 0.003 | 1.63, (0.87–5.71), 0.11 | - |
| Nucleolar grade > 2 | - | - | - | 1.14, (1.05–1.21), 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grosso, A.A.; Lambertini, L.; Di Maida, F.; Carli, G.; Ramos, P.; Sandulli, A.; Salamone, V.; Conte, F.; Lipparini, F.; Ciaralli, E.; et al. Functional and Oncologic Outcomes in Single-Kidney Patients Treated with Robot-Assisted Partial Nephrectomy for Renal Tumors: Results from a Prospectively Maintained Dataset of a Single Tertiary Referral Center. Cancers 2025, 17, 1978. https://doi.org/10.3390/cancers17121978
Grosso AA, Lambertini L, Di Maida F, Carli G, Ramos P, Sandulli A, Salamone V, Conte F, Lipparini F, Ciaralli E, et al. Functional and Oncologic Outcomes in Single-Kidney Patients Treated with Robot-Assisted Partial Nephrectomy for Renal Tumors: Results from a Prospectively Maintained Dataset of a Single Tertiary Referral Center. Cancers. 2025; 17(12):1978. https://doi.org/10.3390/cancers17121978
Chicago/Turabian StyleGrosso, Antonio Andrea, Luca Lambertini, Fabrizio Di Maida, Giulia Carli, Pedro Ramos, Alessandro Sandulli, Vincenzo Salamone, Francesca Conte, Filippo Lipparini, Elena Ciaralli, and et al. 2025. "Functional and Oncologic Outcomes in Single-Kidney Patients Treated with Robot-Assisted Partial Nephrectomy for Renal Tumors: Results from a Prospectively Maintained Dataset of a Single Tertiary Referral Center" Cancers 17, no. 12: 1978. https://doi.org/10.3390/cancers17121978
APA StyleGrosso, A. A., Lambertini, L., Di Maida, F., Carli, G., Ramos, P., Sandulli, A., Salamone, V., Conte, F., Lipparini, F., Ciaralli, E., Paganelli, D., Giudici, S., Oriti, R., Fantechi, R., Salvi, M., Vittori, G., Raspollini, M. R., Nesi, G., Minervini, A., & Mari, A. (2025). Functional and Oncologic Outcomes in Single-Kidney Patients Treated with Robot-Assisted Partial Nephrectomy for Renal Tumors: Results from a Prospectively Maintained Dataset of a Single Tertiary Referral Center. Cancers, 17(12), 1978. https://doi.org/10.3390/cancers17121978








