Risk Factors for Wound Dehiscence After Spinal Metastasis Surgery and a New Approach to Prevention—Curved Skin Incision
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Surgical Methods
2.2. Study Variables and Outcomes
2.3. Statistical Analysis
3. Results
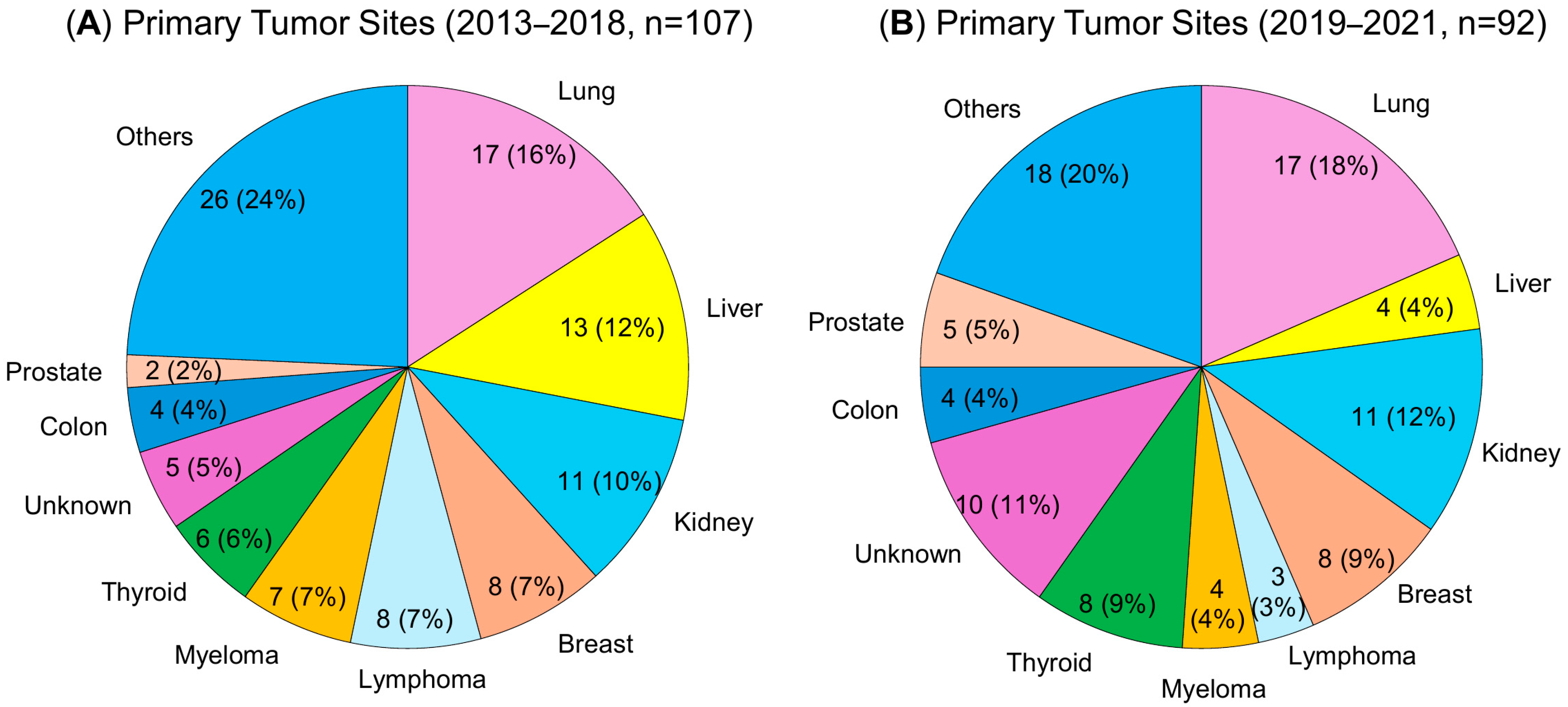
3.1. Patient Characteristics
3.2. Postoperative Wound Dehiscence in Patients with MI (Enrolled in 2013–2017)
3.3. Postoperative Wound Dehiscence in Patients with MI or CSI (Enrolled in 2019–2021)
3.4. Efficacy of CSI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CSI | curved skin incision |
| MI | midline incision |
| SSM | symptomatic spinal metastases |
| PS | performance status |
| ADL | activities of daily living |
| QOL | quality of life |
| BMI | body mass index |
| MTD | molecular target drug |
| VEGF | vascular endothelial growth factor |
References
- Coleman, R.E. Metastatic bone disease: Clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 2001, 27, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Kim, H.I.; Park, J.K.; Choi, Y.S.; Han, J.H.; Lim, S.D. Contemporary trends in the incidence and timing of spinal metastases. J. Neurosurg. Spine 2024, 30, 45–52. [Google Scholar] [CrossRef]
- Long, N.; Woodlock, D.; D’Agostino, R.; Nguyen, G.; Gangai, N.; Sevilimedu, V.; Do, R.K.G. Incidence and prevalence of bone metastases in different solid tumors determined by natural language processing of CT reports. Cancers 2025, 17, 218. [Google Scholar] [CrossRef] [PubMed]
- Kakutani, K.; Kanda, Y.; Yurube, T.; Maeno, K.; Takada, T.; Kurakawa, T.; Takeoka, Y.; Miyazaki, S.; Hoshino, Y.; Nishida, K.; et al. The identification of risk factors for symptomatic spinal metastasis onset: A prospective cohort study of 128 asymptomatic spinal metastasis patients. Cancers 2023, 15, 1251. [Google Scholar] [CrossRef]
- Lutz, S.; Berk, L.; Chang, E.; Chow, E.; Hahn, C.; Hoskin, P.; Howell, D.; Konski, A.; Kachnic, L.; Lo, S.; et al. Palliative radiotherapy for bone metastases: An ASTRO evidence-based guideline. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 965–976. [Google Scholar] [CrossRef]
- Patchell, R.A.; Tibbs, P.A.; Regine, W.F.; Payne, R.; Saris, S.; Kryscio, R.J.; Mohiuddin, M.; Young, B. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: A randomised trial. Lancet 2005, 366, 643–648. [Google Scholar] [CrossRef]
- Choi, D.; Crockard, A.; Bunger, C.; Harms, J.; Kawahara, N.; Mazel, C.; Melcher, R.; Tomita, K.; Global Spine Tumor Study Group. Review of metastatic spine tumour classification and indications for surgery: The consensus statement of the Global Spine Tumour Study Group. Eur. Spine J. 2010, 19, 215–222. [Google Scholar] [CrossRef]
- Kanda, Y.; Kakutani, K.; Sakai, Y.; Miyazaki, K.; Matsuo, T.; Yurube, T.; Takeoka, Y.; Ohnishi, H.; Ryu, M.; Kumagai, N.; et al. Clinical characteristics and surgical outcomes of metastatic spine tumors in the very elderly: A prospective cohort study in a super-aged society. J. Clin. Med. 2023, 12, 4747. [Google Scholar] [CrossRef]
- Fomchenko, E.I.; Bayley, J.C.; Alvarez-Breckenridge, C.; Rhines, L.D.; Tatsui, C.E. Spinal metastases and the evolving role of molecular targeted therapy, chemotherapy, and immunotherapy. Neurospine 2022, 19, 978–993. [Google Scholar] [CrossRef]
- Finkelstein, J.A.; Zaveri, G.; Wai, E.; Vidmar, M.; Kreder, H.; Chow, E. A population-based study of surgery for spinal metastases: Survival rates and complications. J. Bone Jt. Surg. Br. 2003, 85, 1045–1050. [Google Scholar] [CrossRef]
- Matsuo, T.; Kanda, Y.; Sakai, Y.; Yurube, T.; Takeoka, Y.; Miyazaki, K.; Ohnishi, H.; Ryu, M.; Kumagai, N.; Kuroshima, K.; et al. Modified frailty index as a novel predictor for the incidence and severity of postoperative complications after spinal metastases surgery: A prospective cohort study. Bone Jt. J. 2024, 106-B, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, J.; Peng, S.; Wang, J.; Zhang, L. Radiation-induced skin injury: Pathogenesis, treatment, and management. Aging 2020, 12, 11627–11647. [Google Scholar] [CrossRef]
- Lau, D.; Chou, D. Posterior thoracic corpectomy with cage reconstruction for metastatic spinal tumors: Comparing the mini-open approach to the open approach. J. Neurosurg. Spine 2015, 23, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.J.; Thayaparan, G.K.; Fairhall, J.M.; Mobbs, R.J. Minimally invasive percutaneous fixation techniques for metastatic spinal disease. Orthop. Surg. 2014, 6, 187–195. [Google Scholar] [CrossRef]
- Borrelli, M.R.; Shen, A.H.; Lee, G.K.; Momeni, A.; Longaker, M.T.; Wan, D.C. Radiation-induced skin fibrosis: Pathogenesis, current treatment options, and emerging therapeutics. Ann. Plast Surg. 2019, 83 (Suppl. S1), S59–S64. [Google Scholar] [CrossRef]
- Katagiri, H.; Okada, R.; Takagi, T.; Takahashi, M.; Murata, H.; Harada, H.; Nishimura, T.; Asakura, H.; Ogawa, H. New prognostic factors and scoring system for patients with skeletal metastasis. Cancer Med. 2014, 3, 1359–1367. [Google Scholar] [CrossRef]
- Carl, H.M.; Ahmed, A.K.; Abu-Bonsrah, N.; De la Garza Ramos, R.; Sankey, E.W.; Pennington, Z.; Bydon, A.; Witham, T.F.; Wolinsky, J.P.; Gokaslan, Z.L.; et al. Risk factors for wound-related reoperations in patients with metastatic spine tumor. J. Neurosurg. Spine 2018, 28, 663–668. [Google Scholar] [CrossRef]
- Sugita, S.; Hozumi, T.; Yamakawa, K.; Goto, T.; Kondo, T. Risk factors for surgical site infection after posterior fixation surgery and intraoperative radiotherapy for spinal metastases. Eur. Spine J. 2016, 25, 1034–1038. [Google Scholar] [CrossRef]
- Ghogawala, Z.; Mansfield, F.L.; Borges, L.F. Spinal radiation before surgical stabilization and instrumentation: A study of wound complications. J. Neurosurg. 2001, 95, 247–250. [Google Scholar] [CrossRef]
- Bose, D.; Meric-Bernstam, F.; Hofstetter, W.; Reardon, D.A.; Flaherty, K.T.; Ellis, L.M. Vascular endothelial growth factor targeted therapy in the perioperative setting: Implications for patient care. Lancet Oncol. 2010, 11, 373–382. [Google Scholar] [CrossRef]
- Scappaticci, F.A.; Fehrenbacher, L.; Cartwright, T.; Hainsworth, J.D.; Heim, W.; Berlin, J.; Kabbinavar, F.F.; Hurwitz, H.I.; Novotny, W.F.; Malik, I.; et al. Surgical wound healing complications in metastatic colorectal cancer patients treated with bevacizumab. J. Surg. Oncol. 2005, 91, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Mahfud, M.; Breitenstein, S.; El-Badry, A.M.; Puhan, M.A.; Slankamenac, K.; Graf, R.; Clavien, P.A. Impact of preoperative bevacizumab on complications after resection of colorectal liver metastases: Case-matched control study. World J. Surg. 2010, 34, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Ladha, H.; Pawar, T.; Gilbert, M.R.; Sloan, A.E.; Weil, R.J. Impact of bevacizumab chemotherapy on craniotomy wound healing in patients with brain tumors. J. Neurosurg. 2011, 114, 1609–1616. [Google Scholar] [CrossRef]
- Chieng, L.O.; Hubbard, Z.; Salgado, C.J.; Levi, A.D.; Chim, H. Reconstruction of open wounds as a complication of spinal surgery with flaps: A systematic review. Neurosurg. Focus 2015, 39, E17. [Google Scholar] [CrossRef]
- Itshayek, E.; Yamada, J.; Bilsky, M.; Schmidt, M.; Shaffrey, C.; Gerszten, P.; Polly, D.; Gokaslan, Z.; Varga, P.P.; Fisher, C.G. Timing of surgery and radiotherapy in the management of metastatic spine disease: A systematic review. Int. J. Oncol. 2010, 36, 533–544. [Google Scholar] [CrossRef]
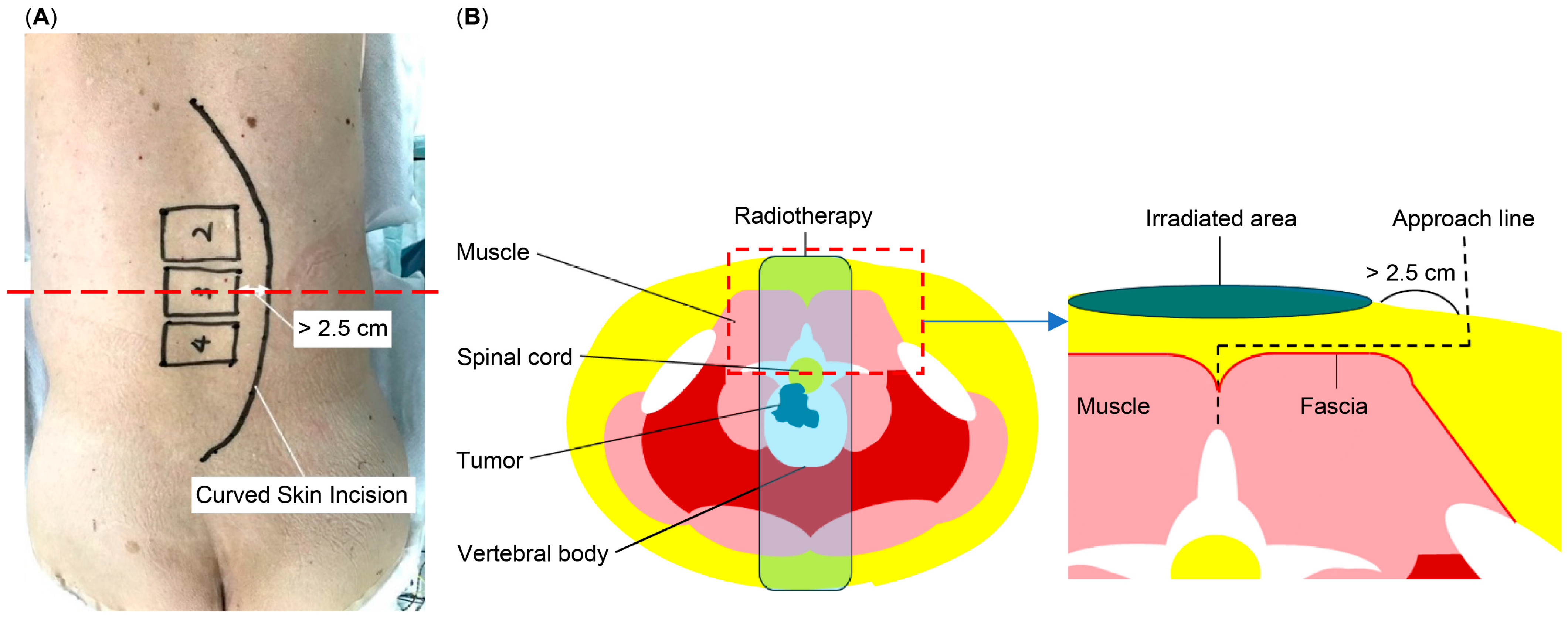

| MI, Enrolled in 2013–2018 | MI/CSI, Enrolled in 2019–2021 | |||||
|---|---|---|---|---|---|---|
| Characteristics | Total | Dehiscence | No Dehiscence | Total | Dehiscence | No Dehiscence |
| (n = 107) | (n = 9) | (n = 98) | (n = 92) | (n = 8) | (n = 84) | |
| Age, years (range) | 69 (38–90) | 69 (59–83) | 69 (38–90) | 71 (42–88) | 70 (64–74) | 71 (42–88) |
| Sex (male/female) | 65:42 | 7:2 | 58:40 | 57:35 | 4:4 | 53:31 |
| BMI, kg/m2 (range) | 20.4 (13.8–38.3) | 19.7 (15.6–23.8) | 20.4 (13.8–38.3) | 20.4 (14.7–33.9) | 22.1 (14.9–26.0) | 20.1 (14.7–33.9) |
| Revised Katagiri score (range) | 6 (0–9) | 6 (3–7) | 6 (0–9) | 5 (0–9) | 5 (3–8) | 5 (0–9) |
| Smoking history, n (%) | 45 (42.1%) | 3 (33.3%) | 42 (42.9%) | 31 (33.7%) | 6 (75.0%) | 25 (29.8%) |
| Preoperative radiotherapy, n (%) | 3 (29.0%) | 8 (88.9%) | 23 (23.5%) | 22 (23.9%) | 4 (50.0%) | 18 (21.4%) |
| Preoperative chemotherapy, n (%) | 50 (45.9%) | 7 (77.8%) | 44 (44.9%) | 42 (45.7%) | 3 (37.5%) | 39 (46.4%) |
| Preoperative MTD therapy, n (%) | 17 (15.9%) | 6 (66.7%) | 11 (11.2%) | 18 (19.6%) | 2 (25.0%) | 16 (19.0%) |
| Surgical information | ||||||
| Blood loss, mL (range) | 280 (0–2500) | 460 (0–600) | 258 (0–2500) | 200 (0–1268) | 312 (90–560) | 190 (0–1268) |
| Operation time, min (range) | 197 (73–450) | 168 (73–450) | 201 (99–373) | 208 (108–424) | 196 (167–254) | 212 (108–424) |
| Complication | N (%) |
|---|---|
| Wound dehiscence | 9 (8.4%) |
| Fracture | 4 (3.7%) |
| Pneumonia | 4 (3.7%) |
| Rod failure | 3 (2.8%) |
| DVT | 3 (2.8%) |
| Paralysis | 3 (2.8%) |
| Depression | 2 (1.9%) |
| Cerebrospinal fluid leaking | 2 (1.9%) |
| Others | 5 (4.7%) |
| Risk Factor | Odds Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Age (>65 years) | 1.063 | 0.133–8.492 | 0.954 |
| Sex (male) | 2.684 | 0.253–28.482 | 0.413 |
| BMI | 1.058 | 0.767–1.459 | 0.731 |
| Revised Katagiri score | 1.052 | 0.562–1.966 | 0.875 |
| Smoking history | 0.546 | 0.054–5.489 | 0.607 |
| Preoperative radiotherapy | 32.599 | 2.968–358.060 | 0.004 * |
| Preoperative chemotherapy | 0.314 | 0.024–4.092 | 0.376 |
| Preoperative MTD therapy | 25.242 | 1.802–353.617 | 0.017 * |
| Blood loss | 1.001 | 0.998–1.004 | 0.615 |
| Operation time | 0.997 | 0.986–1.009 | 0.668 |
| Before Propensity Score Matching | After Propensity Score Matching | |||||
| MI (n = 59) | CSI (n = 33) | p-Value | MI (n = 29) | CSI (n = 29) | p-Value | |
| Age ≥ 65 years | 42 (71.2%) | 24 (72.7%) | >0.999 | 21 (72.4%) | 21 (72.4%) | >0.999 |
| Sex (male) | 34 (57.6%) | 23 (69.7%) | 0.273 | 20 (69.0%) | 20 (69.0%) | >0.999 |
| BMI, kg/m2 | 21.3 ± 3.8 | 21.1 ± 3.5 | 0.880 | 21.4 ± 3.8 | 21.2 ± 3.8 | 0.820 |
| Revised Katagiri score | 4.6 ± 2.2 | 5.2 ± 2.2 | 0.294 | 5.2 ± 2.2 | 5.1 ± 2.3 | 0.589 |
| Smoking | 18 (30.5%) | 13 (39.4%) | 0.491 | 11 (37.9%) | 10 (34.5%) | 0.787 |
| Preoperative radiotherapy | 11 (18.3%) | 11 (33.3%) | 0.132 | 10 (34.5%) | 9 (31.0%) | >0.999 |
| Preoperative chemotherapy | 27 (45.8%) | 15 (45.5%) | >0.999 | 14 (48.3%) | 15 (58.6%) | 0.599 |
| Preoperative MTD therapy | 10 (16.9%) | 6 (18.2%) | >0.999 | 8 (27.6%) | 6 (27.6%) | >0.999 |
| Blood loss, mL | 240 ± 221 | 354 ± 330 | 0.051 | 295 ± 255 | 300 ± 291 | 0.204 |
| Operation time, min | 206 ± 54 | 214 ± 64 | 0.518 | 205 ± 54 | 206 ± 54 | 0.961 |
| Dehiscence | 7 (11.9%) | 1 (3.0%) | 6 (20.7%) | 0 (0.0%) | 0.024 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyazaki, K.; Kanda, Y.; Yurube, T.; Takeoka, Y.; Tsujimoto, T.; Matsuo, T.; Ryu, M.; Kumagai, N.; Kuroshima, K.; Hiranaka, Y.; et al. Risk Factors for Wound Dehiscence After Spinal Metastasis Surgery and a New Approach to Prevention—Curved Skin Incision. Cancers 2025, 17, 1973. https://doi.org/10.3390/cancers17121973
Miyazaki K, Kanda Y, Yurube T, Takeoka Y, Tsujimoto T, Matsuo T, Ryu M, Kumagai N, Kuroshima K, Hiranaka Y, et al. Risk Factors for Wound Dehiscence After Spinal Metastasis Surgery and a New Approach to Prevention—Curved Skin Incision. Cancers. 2025; 17(12):1973. https://doi.org/10.3390/cancers17121973
Chicago/Turabian StyleMiyazaki, Kunihiko, Yutaro Kanda, Takashi Yurube, Yoshiki Takeoka, Takeru Tsujimoto, Tomoya Matsuo, Masao Ryu, Naotoshi Kumagai, Kohei Kuroshima, Yoshiaki Hiranaka, and et al. 2025. "Risk Factors for Wound Dehiscence After Spinal Metastasis Surgery and a New Approach to Prevention—Curved Skin Incision" Cancers 17, no. 12: 1973. https://doi.org/10.3390/cancers17121973
APA StyleMiyazaki, K., Kanda, Y., Yurube, T., Takeoka, Y., Tsujimoto, T., Matsuo, T., Ryu, M., Kumagai, N., Kuroshima, K., Hiranaka, Y., Kuroda, R., & Kakutani, K. (2025). Risk Factors for Wound Dehiscence After Spinal Metastasis Surgery and a New Approach to Prevention—Curved Skin Incision. Cancers, 17(12), 1973. https://doi.org/10.3390/cancers17121973







