Benefits from 18F-FDG PET-CT-Based Radiotherapy Planning in Stage III Non-Small-Cell Lung Cancer: A Prospective Single-Center Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Details
2.2. Immobilization Equipment
2.3. Staff Training
2.4. Patient Simulation
2.5. PET-CT Interpretation
2.6. Radiotherapy Schedule Details
2.7. Chemotherapy Details
2.8. Radiotherapy Treatment Planning
2.9. Target Volume Delineation
2.10. Statistical Analysis
3. Results
3.1. Comparison Between RT Plans
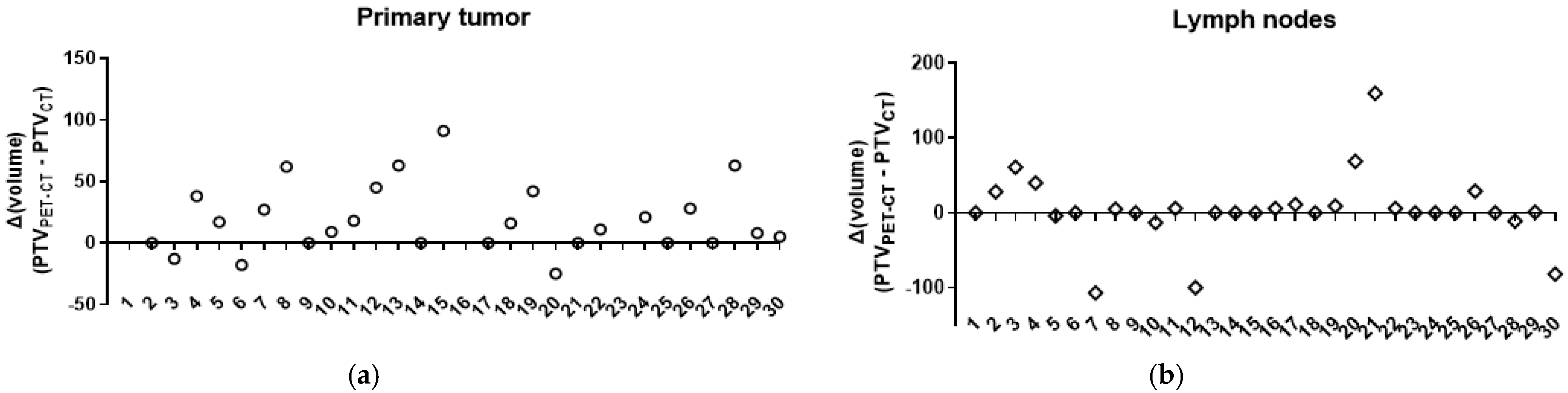
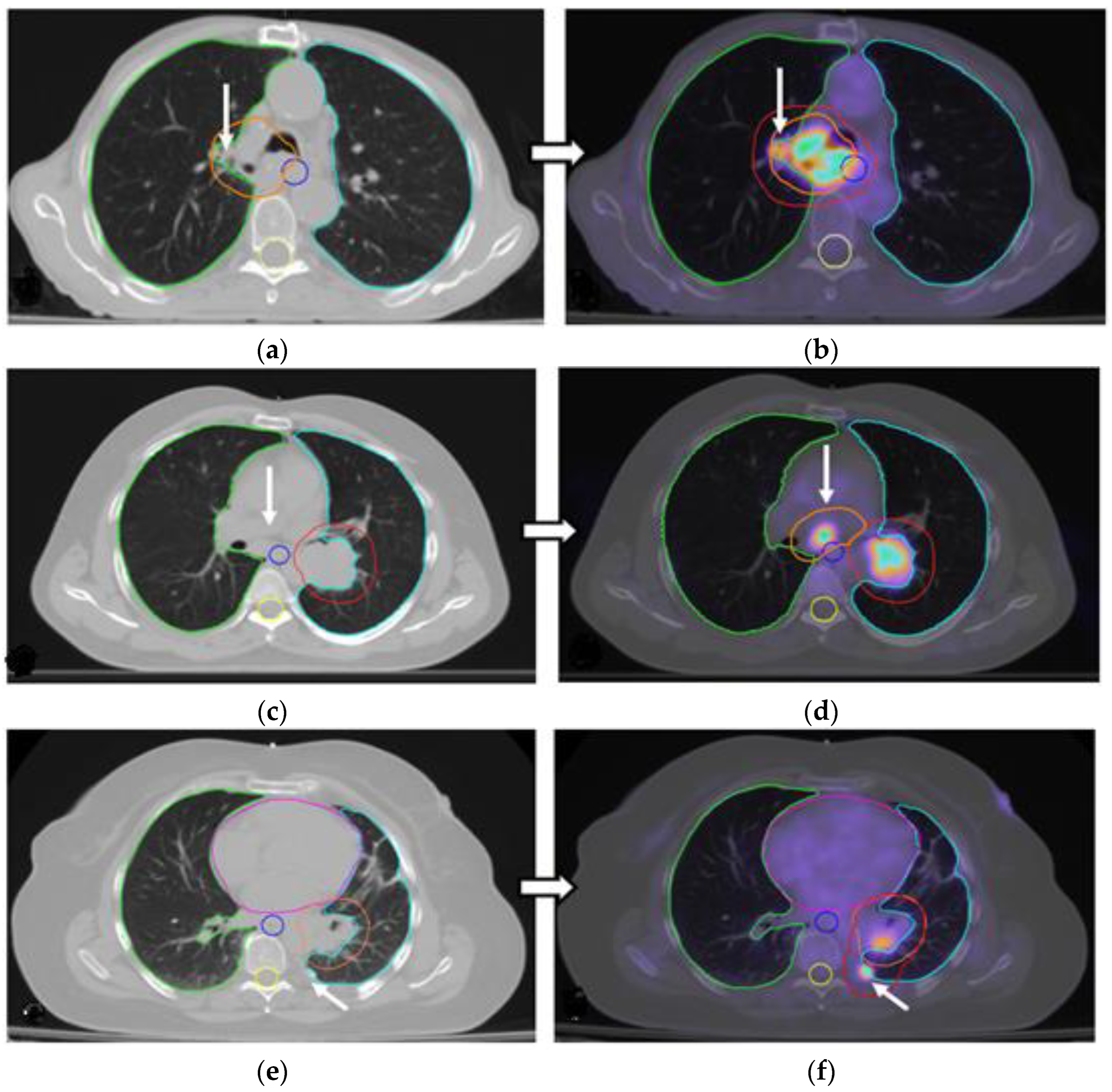
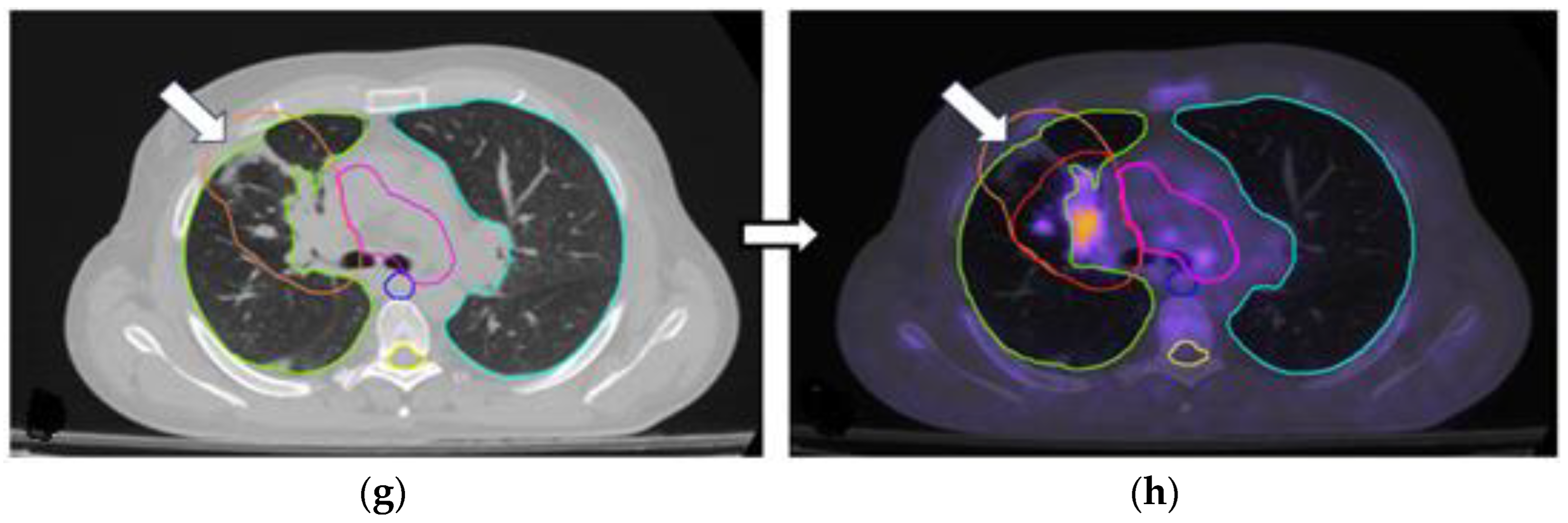
3.2. Changes in the Primary Tumor and Nodal Coverage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IARC | International Agency for Research on Cancer |
| NSCLC | Non-Small-Cell Lung Carcinoma |
| SCLC | Small-Cell Lung Carcinoma |
| NCCN | National Comprehensive Cancer Network |
| IASLC | International Association for the Study of Lung Cancer |
| LRC | Locoregional Control |
| RT | Radiotherapy |
| VMAT | Volumetric Modulated Arc Therapy |
| IGRT | Image-Guided Radiation Therapy |
| LINAC | Linear Accelerator |
| CT | Computed Tomography |
| 18F-FDG PET-CT or PET-CT | 18Fluorine-Labeled Fluorodeoxyglucose Positron Emission Tomography |
| PTV | Planning Target Volume |
| PS | Performance Status |
| AJCC TNM | American Joint Committee on Cancer—Tumor, Nodes, Metastasis |
| 4D | Four-Dimensional |
| FOV | Field of View |
| OSEM | Ordered Subset Expectation Maximization |
| TOF | Time-of-Flight |
| PSF | Point Spread Function |
| SUVmax | Maximal Standardized Uptake Value |
| ROI | Region of Interest |
| SUV | Standardized Uptake Values |
| 18F-FDG | Fluorodeoxyglucose Labeled with Fluorine-18 |
| ICRU | International Commission on Radiation Units and Measurements |
| CBCT | Cone-Beam Computed Tomography |
| OARs | Organs at Risk |
| TPS | Treatment Planning System |
| GTV | Gross Tumor Volume |
| CTV | Clinical Target Volume |
| ESTRO | European Society for Radiotherapy and Oncology |
| ACROP | Advisory Committee on Radiation Oncology Practice |
Appendix A. System Calibration
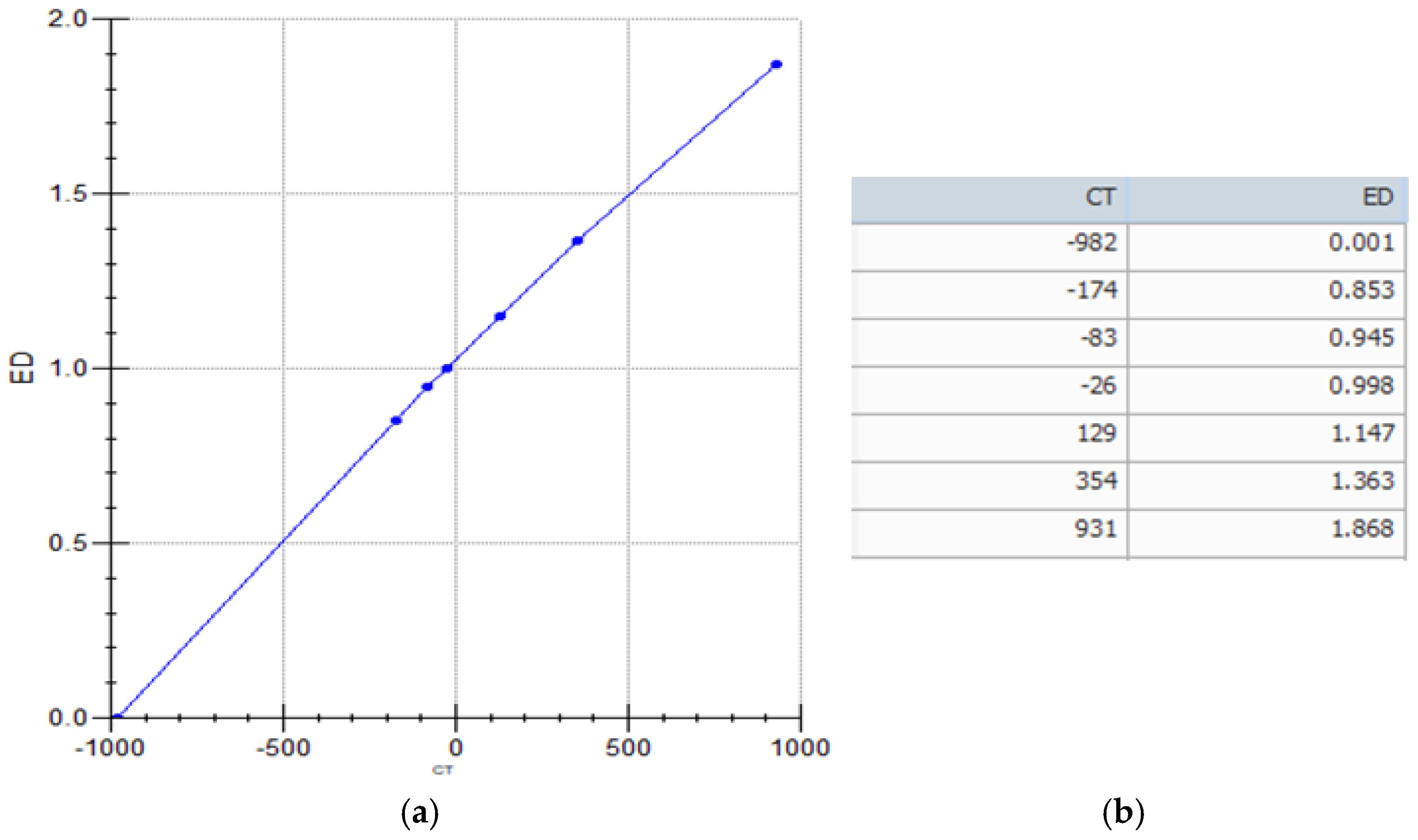
References
- Lung Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/lung-cancer (accessed on 21 January 2025).
- Cancer (IARC) TIA for R on. Global Cancer Observatory. Available online: https://gco.iarc.fr/ (accessed on 21 January 2025).
- Guidelines Detail. NCCN. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1450 (accessed on 21 January 2025).
- IASLC|International Association for the Study of Lung Cancer. 2018. Available online: https://www.iaslc.org/ (accessed on 21 January 2025).
- Spigel, D.R.; Faivre-Finn, C.; Gray, J.E.; Vicente, D.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Garassino, M.C.; Hui, R.; Quantin, X.; et al. Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Sanuki, N.; Takeda, A.; Eriguchi, T.; Tsurugai, Y.; Tateishi, Y.; Kibe, Y.; Akiba, T.; Fukuzawa, T.; Horita, N. Local control correlates with overall survival in radiotherapy for early-stage non-small cell lung cancer: A systematic review. Radiother. Oncol. 2023, 183, 109664. [Google Scholar] [CrossRef]
- Kilburn, J.M.; Soike, M.H.; Lucas, J.T.; Ayala-Peacock, D.; Blackstock, W.; Isom, S.; Kearns, W.T.; Hinson, W.H.; Miller, A.A.; Petty, W.J.; et al. Image guided radiotherapy may result in improved local control in locally advanced lung cancer patients. Pract. Radiat. Oncol. 2016, 6, e73–e80. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Hallac, R.R.; Yuan, Q.; Ding, Y.; Zhang, Z.; Xie, X.-J.; Francis, F.; Roehrborn, C.G.; Sims, R.D.; Costa, D.N.; et al. Incorporating Oxygen-Enhanced MRI into Multi-Parametric Assessment of Human Prostate Cancer. Diagnostics 2017, 7, 48. [Google Scholar] [CrossRef]
- Appel, S.; Weizman, N.; Davidson, T.; Urban, D.; Lawrence, Y.R.; Symon, Z.; Goldstein, J. Reexpansion of atelectasis caused by use of continuous positive airway pressure (CPAP) before radiation therapy (RT). Adv. Radiat. Oncol. 2016, 1, 136–140. [Google Scholar] [CrossRef][Green Version]
- Tennyson, N.; Weiss, E.; Sleeman, W.; Rosu, M.; Jan, N.; Hugo, G.D. Effect of variations in atelectasis on tumor displacement during radiation therapy for locally advanced lung cancer. Adv. Radiat. Oncol. 2017, 2, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Eaton, D.J.; Byrne, J.P.; Cosgrove, V.P.; Thomas, S.J. Unintended doses in radiotherapy—Over, under and outside? Br. J. Radiol. 2018, 91, 20170863. [Google Scholar] [CrossRef]
- Bradley, J.; Thorstad, W.L.; Mutic, S.; Miller, T.R.; Dehdashti, F.; Siegel, B.A.; Bosch, W.; Bertrand, R.J. Impact of FDG-PET on radiation therapy volume delineation in non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 78–86. [Google Scholar] [CrossRef]
- Zilli, T.; Achard, V.; Dal Pra, A.; Schmidt-Hegemann, N.; Jereczek-Fossa, B.A.; Lancia, A.; Ingrosso, G.; Alongi, F.; Aluwini, S.; Arcangeli, S.; et al. Recommendations for radiation therapy in oligometastatic prostate cancer: An ESTRO-ACROP Delphi consensus. Radiother. Oncol. 2022, 176, 199–207. [Google Scholar] [CrossRef]
- Evaluating Inclusion and Exclusion Criteria in Clinical Trials; Workshop Report; Availability. Federal Register. Available online: https://www.federalregister.gov/documents/2018/08/23/2018-18232/evaluating-inclusion-and-exclusion-criteria-in-clinical-trials-workshop-report-availability (accessed on 9 June 2025).
- Hwang, J.K.; Page, B.J.; Flynn, D.; Passmore, L.; McCaul, E.; Brady, J.; Yang, I.A.; Marshall, H.; Windsor, M.; Bowman, R.V.; et al. Validation of the Eighth Edition TNM Lung Cancer Staging System. J. Thorac. Oncol. 2020, 15, 649–654. [Google Scholar] [CrossRef]
- Tsoutsou, P.G.; Froudarakis, M.E.; Bouros, D.; Koukourakis, M.I. Hypofractionated/accelerated radiotherapy with cytoprotection (HypoARC) combined with vinorelbine and liposomal doxorubicin for locally advanced non-small cell lung cancer (NSCLC). Anticancer Res. 2008, 28, 1349–1354. [Google Scholar] [PubMed]
- Radiotherapy Dose Fractionation, Fourth Edition. Available online: https://www.rcr.ac.uk/our-services/all-our-publications/clinical-oncology-publications/radiotherapy-dose-fractionation-fourth-edition/ (accessed on 6 May 2025).
- Nestle, U.; De Ruysscher, D.; Ricardi, U.; Geets, X.; Belderbos, J.; Pöttgen, C.; Dziadiuszko, R.; Peeters, S.; Lievens, Y.; Hurkmans, C.; et al. ESTRO ACROP guidelines for target volume definition in the treatment of locally advanced non-small cell lung cancer. Radiother. Oncol. 2018, 127, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Hunte, S.O.; Clark, C.H.; Zyuzikov, N.; Nisbet, A. Volumetric modulated arc therapy (VMAT): A review of clinical outcomes—What is the clinical evidence for the most effective implementation? Br. J. Radiol. 2022, 95, 20201289. [Google Scholar] [CrossRef]
- Diwanji, T.P.; Mohindra, P.; Vyfhuis, M.; Iii, J.W.S.; Kalavagunta, C.; Mossahebi, S.; Yu, J.; Feigenberg, S.; Badiyan, S.N. Advances in radiotherapy techniques and delivery for non-small cell lung cancer: Benefits of intensity-modulated radiation therapy, proton therapy, and stereotactic body radiation therapy. Transl. Lung Cancer Res. 2017, 6, 131. [Google Scholar] [CrossRef]
- Keidar, Z.; Haim, N.; Guralnik, L.; Wollner, M.; Bar-Shalom, R.; Ben-Nun, A.; Israel, O. PET/CT Using 18F-FDG in Suspected Lung Cancer Recurrence: Diagnostic Value and Impact on Patient Management. J. Nucl. Med. 2004, 45, 1640–1646. [Google Scholar] [PubMed]
- Ergonul, A.G.; Akcam, T.I.; Özdil, A.; Turhan, K.; Cakan, A.; Cagirici, U. Diagnostic value of 18F-FDG-PET/CT in benign lung diseases. Kardiochir. Torakochirurgia Pol. 2018, 15, 1–4. [Google Scholar]
- MacManus, M.; Nestle, U.; Rosenzweig, K.E.; Carrio, I.; Messa, C.; Belohlavek, O.; Danna, M.; Inoue, T.; Deniaud-Alexandre, E.; Schipani, S.; et al. Use of PET and PET/CT for Radiation Therapy Planning: IAEA expert report 2006–2007. Radiother. Oncol. 2009, 91, 85–94. [Google Scholar] [CrossRef]
- Flanderijn, M.; van Leer, B.; Slart, R.H.J.A.; Pillay, J. 18F-fluorodeoxyglucose positron emission tomography/computed tomography differentiates between pneumonia and atelectasis in a mechanically ventilated patient. Intensive Care Med. 2024, 50, 1361–1362. [Google Scholar] [CrossRef]
- Erasmus, L.T.; Strange, T.A.; Agrawal, R.; Strange, C.D.; Ahuja, J.; Shroff, G.S.; Truong, M.T. Lung Cancer Staging: Imaging and Potential Pitfalls. Diagnostics 2023, 13, 3359. [Google Scholar] [CrossRef]
- Prathipati, A.; Manthri, R.G.; Subramanian, B.V.; Das, P.; Jilla, S.; Mani, S.; Sarala, S.; Kottu, R.; Kalawat, T.C.; Naidu, K.V.J.R. A Prospective Study Comparing Functional Imaging (18F-FDG PET) Versus Anatomical Imaging (Contrast Enhanced CT) in Dosimetric Planning for Non-small Cell Lung Cancer. Asia Ocean. J. Nucl. Med. Biol. 2017, 5, 75–84. [Google Scholar]
- Mandal, B.; Basu, A.; Manna, A.; Mondal, J.; Ghosh, D.; Chakraborty, I.; Biswas, J.; Chakraborty, A. A Prospective Study Comparing Dosimetry Between Computed Tomography (CT) Based Radiation Planning and Positron Emission Computed Tomography (PET-CT) Based Radiation Planning in Treatment of Non-Metastatic Non Small Cell Lung Carcinoma. Asian Pac. J. Cancer Prev. 2023, 24, 2543–2550. [Google Scholar] [CrossRef] [PubMed]
- Chi, A.; Nguyen, N.P. The Utility of Positron Emission Tomography in the Treatment Planning of Image-Guided Radiotherapy for Non-Small Cell Lung Cancer. Front. Oncol. 2014, 4, 273. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shao, Y.; Wang, H.; Chen, H.; Gu, H.; Duan, Y.; Feng, A.; Li, X.; Xu, Z. Dosimetric comparison and biological evaluation of PET- and CT-based target delineation for LA-NSCLC using auto-planning. Phys. Med. 2019, 67, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Vojtíšek, R.; Mužík, J.; Šlampa, P.; Budíková, M.; Hejsek, J.; Smolák, P.; Ferda, J.; Fínek, J. The impact of PET/CT scanning on the size of target volumes, radiation exposure of organs at risk, TCP and NTCP, in the radiotherapy planning of non-small cell lung cancer. Rep. Pract. Oncol. Radiother. 2013, 19, 182–190. [Google Scholar] [CrossRef]
- Schrevens, L.; Lorent, N.; Dooms, C.; Vansteenkiste, J. The role of PET scan in diagnosis, staging, and management of non-small cell lung cancer. Oncologist 2004, 9, 633–643. [Google Scholar] [CrossRef]
- Guerra, A.; Wang, H.; Orton, M.R.; Konidari, M.; Papanikolaou, N.K.; Koh, D.M.; Donato, H.; Alves, F.C. Prediction of extracapsular extension of prostate cancer by MRI radiomic signature: A systematic review. Insights Imaging 2024, 15, 217. [Google Scholar] [CrossRef]
- de Margerie-Mellon, C.; Chassagnon, G. Artificial intelligence: A critical review of applications for lung nodule and lung cancer. Diagn. Interv. Imaging 2023, 104, 11–17. [Google Scholar] [CrossRef]
- Cellina, M.; Cè, M.; Irmici, G.; Ascenti, V.; Khenkina, N.; Toto-Brocchi, M.; Martinenghi, C.; Papa, S.; Carrafiello, G. Artificial Intelligence in Lung Cancer Imaging: Unfolding the Future. Diagnostics 2022, 12, 2644. [Google Scholar] [CrossRef]
- Chen, M.; Copley, S.J.; Viola, P.; Lu, H.; Aboagye, E.O. Radiomics and artificial intelligence for precision medicine in lung cancer treatment. Semin. Cancer Biol. 2023, 93, 97–113. [Google Scholar] [CrossRef]
| Feature | |
|---|---|
| Patients (number) | 34 |
| Patient characteristics | |
| Male | 31 |
| Female | 3 |
| Age (median ± SD) | 69.75 ± 9 years |
| Height (cm) (median ± SD) | 172 ± 6.8 cm |
| Weight (kg) (median ± SD) | 73.5 ± 16.5 kg |
| Blood glucose | 116.03 ± 17.64 mg/dL |
| Tumor location | |
| Left upper lobe | 15 |
| Left lower lobe | 2 |
| Right upper lobe | 9 |
| Right middle lobe | 5 |
| Right lower lobe | 3 |
| Central location | 22 |
| Peripheral location | 12 |
| Histology | |
| Adenocarcinoma | 11 |
| Squamous cell carcinoma | 21 |
| Large-cell carcinoma | 2 |
| CT-based TNM staging | |
| Τ0 Ν2 * | 2 |
| Τ2 Ν0 * | 2 |
| Τ2Ν3 | 1 |
| Τ3 Ν0 | 4 |
| Τ3 Ν1 | 3 |
| Τ3 Ν2 | 7 |
| Τ3Ν3 | 3 |
| Τ4 Ν0 | 2 |
| Τ4 Ν2 | 3 |
| Τ4 Ν3 | 7 |
| Parameter | Number of Patients Requiring Therapeutic Plan Modification | Number of Patients Requiring RT Plan Modification |
|---|---|---|
| Identification of extensive distant metastasis | 4/34 (11.8%) | (*) |
| Detection of oligometastatic disease included in the RT planning | - | 3/34 (8.8%) |
| Inadequate coverage of the primary tumor area | - | 17/34 (50%) |
| Detection of lymph node involvement in cases with negative CT findings | - | 3/34 (8.8%) |
| Exclusion of lymph node involvement in cases with suspected CT findings | - | 3/34 (8.8%) |
| Identification of additional involved nodes demanding larger RT fields and/or higher RT doses. | - | 12/34 (35.3%) |
| Accurate identification of the primary tumor location in patients with pleural effusion and/or lung atelectasis | - | 3/34 (8.8%) |
| Primary Tumor PTV | Number of pts (%) | Median Change/Range—p-Value |
|---|---|---|
| Decrease | 3/30 (10%) | −18 (−25 to −13) |
| Stable | 7/30 (23.3%) | 0 (0) |
| Increase | 20/30 (66.7%) | +33 (5 to 581) |
| p < 0.0001 | ||
| Lymph node PTV * | No of pts (%) | Median change/range—p-value |
| Decrease | 6/30 (20%) | −47.5 (−107 to −4) |
| Stable | 11/30 (36.7%) | 0 (0) |
| Increase | 13/30 (43.3%) | 11 (1 to 160) |
| p < 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulita, A.; Valsamaki, P.; Bekou, E.; Anevlavis, S.; Nanos, C.; Zisimopoulos, A.; Giatromanolaki, A.; Koukourakis, M.I. Benefits from 18F-FDG PET-CT-Based Radiotherapy Planning in Stage III Non-Small-Cell Lung Cancer: A Prospective Single-Center Study. Cancers 2025, 17, 1969. https://doi.org/10.3390/cancers17121969
Mulita A, Valsamaki P, Bekou E, Anevlavis S, Nanos C, Zisimopoulos A, Giatromanolaki A, Koukourakis MI. Benefits from 18F-FDG PET-CT-Based Radiotherapy Planning in Stage III Non-Small-Cell Lung Cancer: A Prospective Single-Center Study. Cancers. 2025; 17(12):1969. https://doi.org/10.3390/cancers17121969
Chicago/Turabian StyleMulita, Admir, Pipitsa Valsamaki, Eleni Bekou, Stavros Anevlavis, Christos Nanos, Athanasios Zisimopoulos, Alexandra Giatromanolaki, and Michael I. Koukourakis. 2025. "Benefits from 18F-FDG PET-CT-Based Radiotherapy Planning in Stage III Non-Small-Cell Lung Cancer: A Prospective Single-Center Study" Cancers 17, no. 12: 1969. https://doi.org/10.3390/cancers17121969
APA StyleMulita, A., Valsamaki, P., Bekou, E., Anevlavis, S., Nanos, C., Zisimopoulos, A., Giatromanolaki, A., & Koukourakis, M. I. (2025). Benefits from 18F-FDG PET-CT-Based Radiotherapy Planning in Stage III Non-Small-Cell Lung Cancer: A Prospective Single-Center Study. Cancers, 17(12), 1969. https://doi.org/10.3390/cancers17121969







