The Natural Killer Cell Line NK-92 and Its Genetic Variants: Impact on NK Cell Research and Cancer Immunotherapy
Simple Summary
Abstract
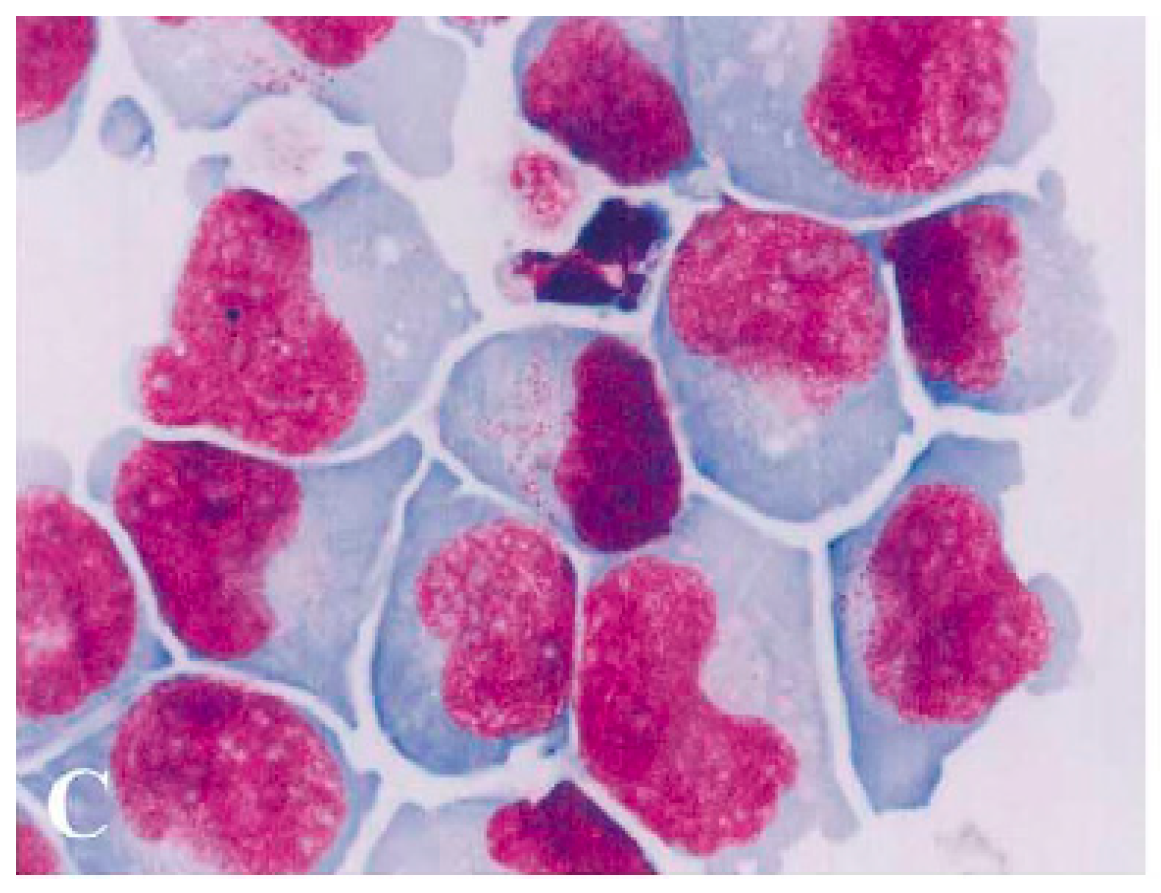
1. Brief History of the Origin and Development of NK-92
2. NK-92 as a Tool in Cancer Research
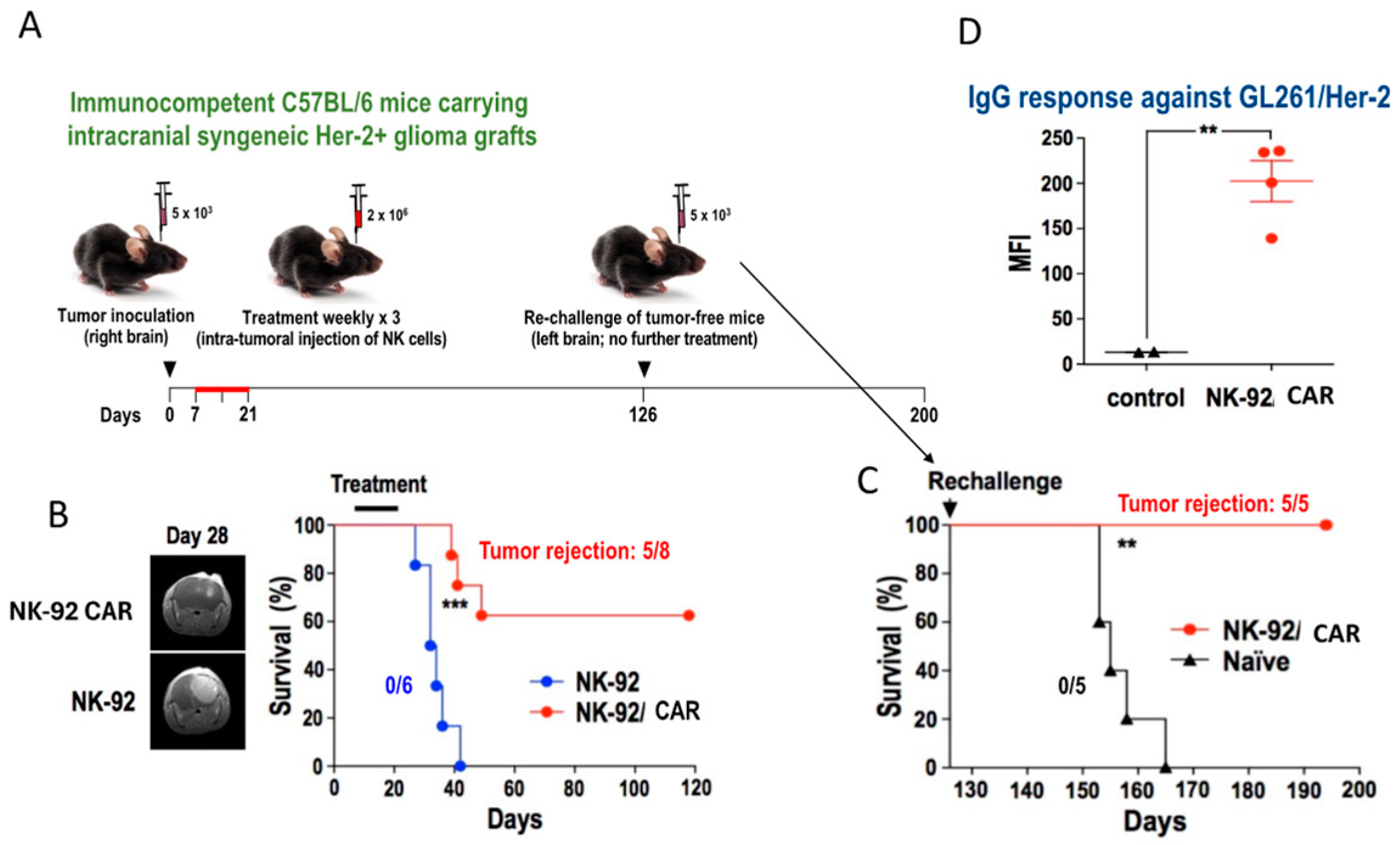
3. NK-92 Cells Can Induce a Vaccine Effect upon Intra-Tumor Injection
4. NK-92 Cells for Infectious Diseases?
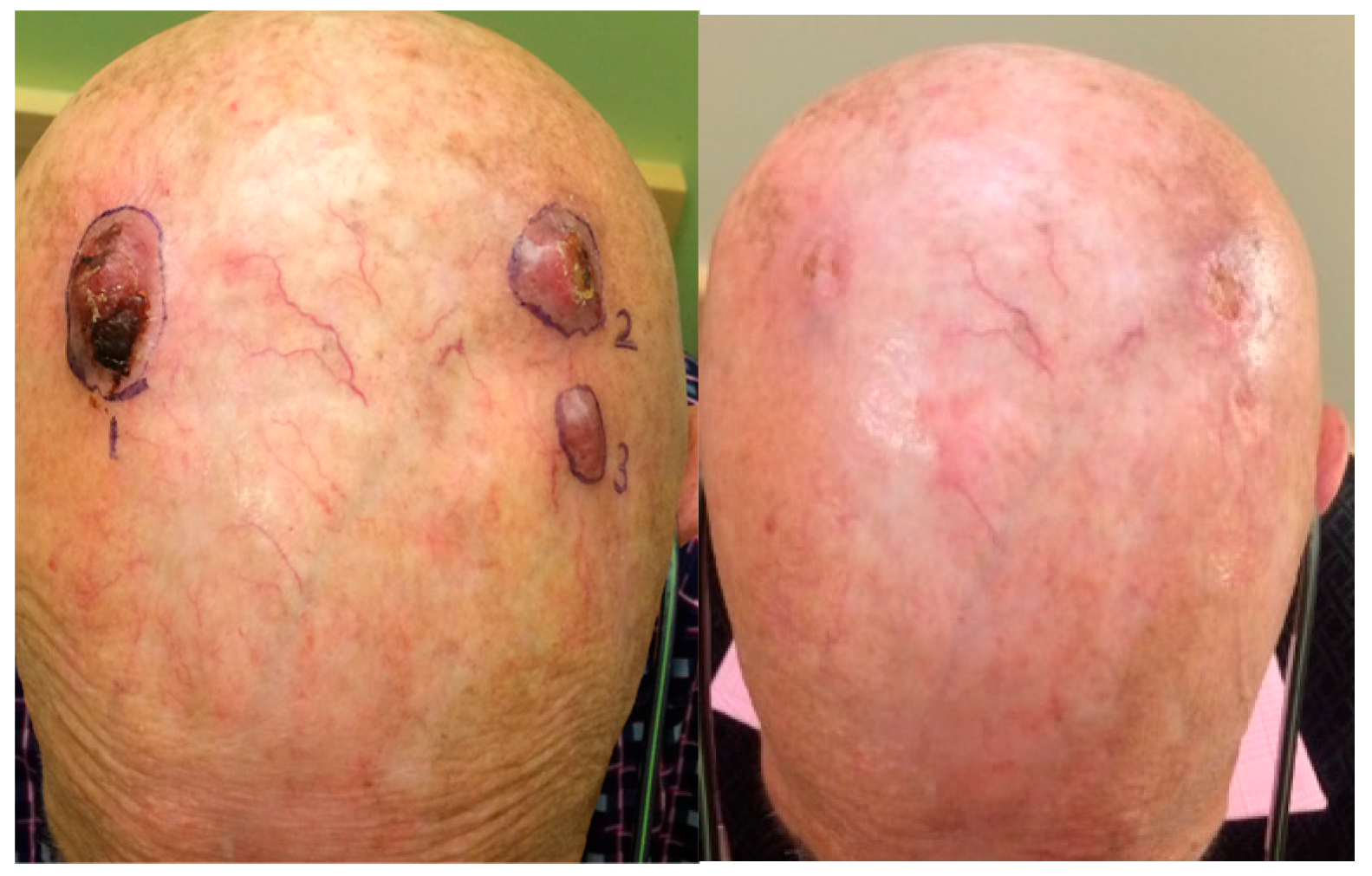
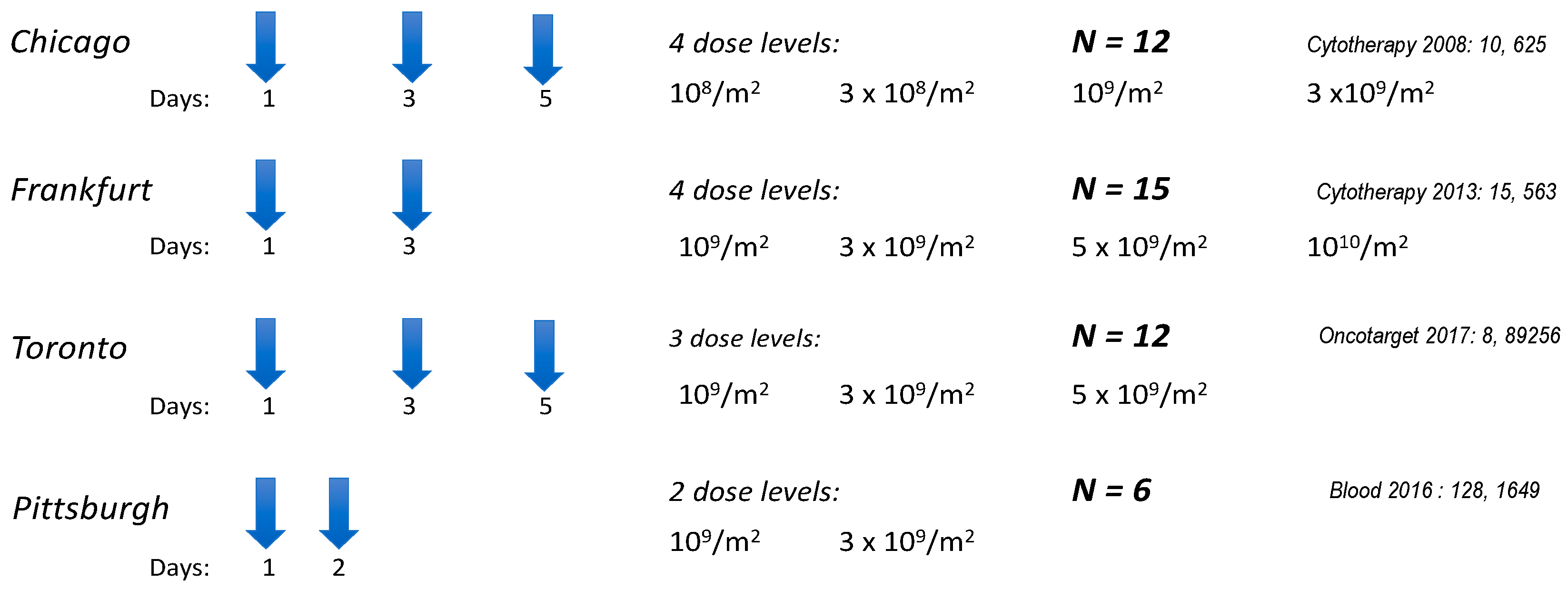
5. Clinical Trials with NK-92 and Its Engineered Variants
6. Engineering NK-92 with a Switch-Off Mechanism
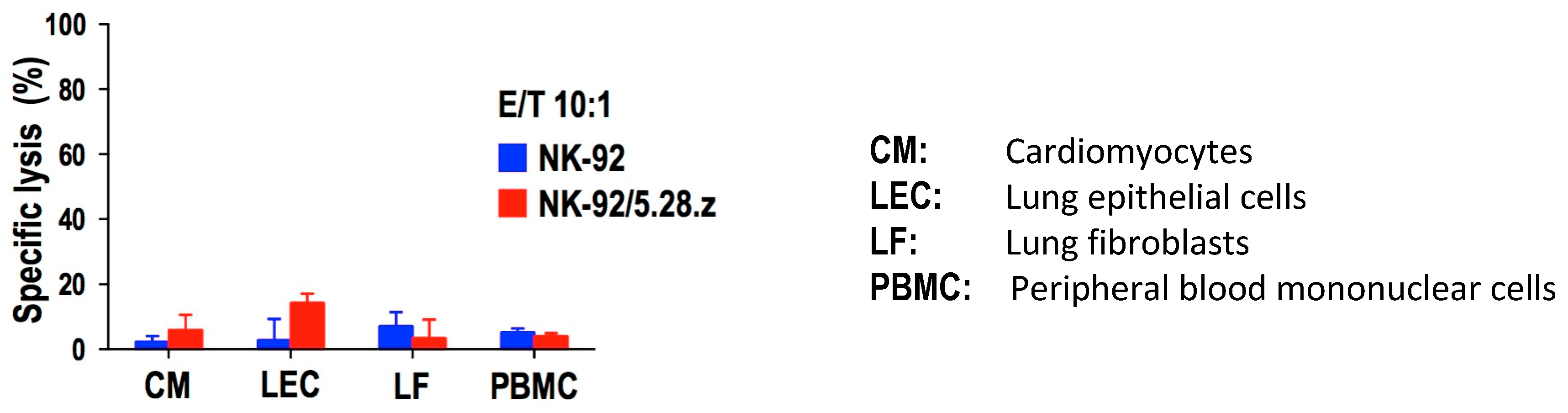
7. Making NK-92 Cells Immune–Neutral
8. A Novel NK-92 Product: Cellular Lysate
9. NK-92 Cells and Their Lysates Have Cytotoxic and Cytostatic Activities Across Species
Funding
Acknowledgments
Conflicts of Interest
References
- Gong, J.H.; Maki, G.; Klingemann, H.G. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia 1994, 8, 652–658. [Google Scholar] [PubMed]
- Klingemann, H. The NK-92 cell line-30 years later: Its impact on natural killer cell research and treatment of cancer. Cytotherapy 2023, 25, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Tam, Y.K.; Maki, G.; Miyagawa, B.; Hennemann, B.; Tonn, T.; Klingemann, H.G. Characterization of genetically altered, interleukin 2-independent natural killer cell lines suitable for adoptive cellular immunotherapy. Hum. Gene Ther. 1999, 10, 1359–1373. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidis, K.V.; Alici, E.; Aints, A.; Christensson, B.; Ljunggren, H.G.; Dilber, M.S. Targeting IL-2 to the endoplasmic reticulum confines autocrine growth stimulation to NK-92 cells. Exp. Hematol. 2005, 33, 159–164. [Google Scholar] [CrossRef]
- Boissel, L.; Klingemann, H.; Campbell, K.; Nichols, K.; Toneguzzo, F.; Marcus, P.; Williams, B.; Keating, A.; Soon-Shiong, P. An ‘off the shelf,’ GMP-grade, IL-2- independent NK cell line expressing the high-affinity Fc-receptor to augment antibody therapeutics. Cancer Res. 2016, 76 (Suppl. 14), 2302. [Google Scholar] [CrossRef]
- Jochems, C.; Hodge, J.W.; Fantini, M.; Fujii, R.; Morillon, I.I.Y.; Greiner, J.W.; Padget, M.R.; Tritsch, S.R.; Yok Tsang, K.; Campbell, K.S.; et al. An NK cell line (haNK) expressing high levels of granzyme and engineered to express the high affinity CD16 allele. Oncotarget 2016, 7, 86359–86373. [Google Scholar] [CrossRef] [PubMed]
- Hullsiek, R.; Li, Y.; Snyder, K.M.; Wang, S.; Di, D.; Borgatti, A.; Lee, C.; Moore, P.F.; Zhu, C.; Fattori, C.; et al. Examination of IgG Fc Receptor CD16A and CD64 expression by canine leukocytes and their ADCC activity in engineered NK Cells. Front. Immunol. 2022, 13, 841859. [Google Scholar] [CrossRef]
- Bhatia, S.; Church, C.; Paulson, K.G.; Pierce, R.H.; Nghiem, P.; Lee, J.H.; Adcoc, B.M.; Soon-Shiong, P.; Chandra, S. Final results from a phase 2 study using off-the shelf activated natural killer (aNK) cells in combination with N-803, an IL-15 superagonist, in patients with metastatic Merkel cell carcinoma (MCC). J. Immunother. Cancer 2019, 7. [Google Scholar]
- Boissel, L.; Betancur, M.; Lu, W.; Krause, D.; Van Etten, R.; Wels, W.; Klingemann, H. Retargeting NK-92 cells by means of CD19-and CD20-specific chimeric antigen receptors compares favorably with antibody-dependent cellular cytotoxicity. Oncoimmunology 2013, 10, e26527. [Google Scholar] [CrossRef]
- Schomer, N.T.; Jiang, Z.K.; Lloyd, M.I.; Klingemann, H.; Boissel, L. CCR7 expression in CD19 chimeric antigen receptor-engineered natural killer cells improves migration toward CD-19-expressing lymphoma cells and increases tumor control in mice with human lymphoma. Cytotherapy 2022, 24, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Moini, K.; Seery, T.; Nangia, C.; MacDiarmid, J.; Brahmbhatt, H.; Spilman, P.; Sender, L.; Soon-Shiong, P. Recurrent pancreatic cancer treated with N-803 and PD-L1 t-haNK followed by an EGFR-targeted nanocell drug conjugate. Oncologist 2025, 30, oyae267. [Google Scholar] [CrossRef]
- Klingemann, H. Are natural killer cells superior CAR drivers? Oncoimmunology 2014, 3, e28147. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, C.; Oberoi, P.; Oelsner, S.; Waldmann, A.; Lindner, A.; Tonn, T.; Wels, W.S. Chimeric antigen receptor-engineered NK-92 Cells: An off-the-shelf cellular therapeutic for targeted elimination of cancer cells and induction of protective antitumor immunity. Front. Immunol. 2017, 8, 533. [Google Scholar] [CrossRef]
- Kim, H.; Han, M.; Kim, M.; Kim, H.; Im, H.J.; Kim, N.; Koh, K.-N. CD19/CD22 bispecific chimeric antigen receptor-NK-92 cells are developed and evaluated. Oncol. Lett. 2023, 25, 236. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Romanski, A.; Uherek, C.; Bug, G.; Seifried, E.; Klingemann, H.; Wels, W.S.; Ottmann, O.G.; Tonn, T. CD19-CAR engineered NK-92 cells are sufficient to overcome NK cell resistance in B-cell malignancies. J. Cell Mol. Med. 2016, 20, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Uherek, C.; Tonn, T.; Uherek, B.; Becker, S.; Schnierle, B.; Klingemann, H.G.; Wels, W. Retargeting of natural killer-cell cytolytic activity to ErbB2-expressing cancer cells results in efficient and selective tumor cell destruction. Blood 2002, 100, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Schönfeld, K.; Sahm, C.; Zhang, C.; Naundorf, S.; Brendel, C.; Odendahl, M.; Nowakowska, P.; Bönig, H.; Köhl, U.; Kloess, S.; et al. Selective inhibition of tumor growth by clonal NK cells expressing an ErbB2/HER2-specific chimeric antigen receptor. Mol. Ther. 2015, 23, 330–338. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, C.; Burger, M.C.; Jennewein, L.; Genßler, S.; Schönfeld, K.; Zeiner, P.; Hattingen, E.; Harter, P.N.; Mittelbronn, M.; Tonn, T.; et al. ErbB2/HER2-Specific NK Cells for Targeted Therapy of Glioblastoma. J. Natl. Cancer Inst. 2015, 108, djv375. [Google Scholar] [CrossRef] [PubMed]
- Burger, M.C.; Forster, M.-T.; Romanski, A.; Straßheimer, F.; Macas, J.; Zeiner, P.S.; Steidl, E.; Herkt, S.; Weber, K.J.; Schupp, J.; et al. Intracranial injection of natural killer cells engineered with a HER2-targeted chimeric antigen receptor in patients with recurrent glioblastoma. Neuro Oncol. 2023, 25, 2058–2071. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shin, M.H.; Kim, J.; Lim, S.A.; Kim, J.; Kim, S.J.; Lee, K.M. NK cell-based immunotherapies in cancer. Immune Netw. 2020, 20, e14. [Google Scholar] [CrossRef] [PubMed]
- Klingemann, H. Development and testing of NK cell lines. In Natural Killer Cells; Lotze, T., Thomson, A.W., Eds.; Academic Press: Cambridge, MA, USA, 2010; pp. 169–175. ISBN 9780123704542. [Google Scholar] [CrossRef]
- Eitler, J.; Wotschel, N.; Miller, N.; Boissel, L.; Klingemann, H.G.; Wels, W.; Tonn, T. Inability of granule polarization by NK cells defines tumor resistance and can be overcome by CAR or ADCC mediated targeting. J. Immunother. Cancer 2021, 9, e001334. [Google Scholar] [CrossRef] [PubMed]
- Boissel, L.; Klingemann, H.; Khan, J.; Soon-Shiong, P. Intra-Tumor injection of CAR-engineered NK cells induces tumor regression and protection against tumor re-challenge. Blood 2016, 128, 466. [Google Scholar] [CrossRef]
- Wu, Z.; Sinzger, C.; Reichel, J.J.; Just, M.; Mertens, T. Natural Killer Cells Can Inhibit the Transmission of Human Cytomegalovirus in Cell Culture by Using Mechanisms from Innate and Adaptive Immune Responses. J. Virol. 2015, 89, 2906–2917. [Google Scholar] [CrossRef]
- Schmidt, S.; Luckowitsch, M.; Hogardt, M.; Lehrnbecher, T. Natural Killer Cell Line NK-92-Mediated Damage of Medically Important Fungi. J. Fungi 2021, 7, 144. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baratin, M.; Roetynck, S.; Pouvelle, B.; Lemmers, C.; Viebig, N.K.; Johansson, S.; Bierling, P.; Scherf, A.; Gysin, J.; Vivier, E.; et al. Dissection of the role of PfEMP1 and ICAM-1 in the sensing of Plasmodium-falciparum-infected erythrocytes by natural killer cells. PLoS ONE 2007, 2, e228. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arai, S.; Meagher, R.; Swearingen, M.; Myint, H.; Rich, E.; Martinson, J.; Klingemann, H. Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: A phase I trial. Cytotherapy 2008, 10, 625–632. [Google Scholar] [CrossRef]
- Tonn, T.; Schwabe, D.; Klingemann, H.G.; Becker, S.; Esser, R.; Koehl, U.; Suttorp, M.; Seifried, E.; Ottmann, O.G.; Bug, G. Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy 2013, 15, 1563–1570. [Google Scholar] [CrossRef]
- Boyiadzis, M.; Agha, M.; Redner, R.L.; Sehgal, A.; Im, A.; Hou, J.-Z.; Farah, R.; Dorritie, K.A.; Raptis, A.; Lim, S.H.; et al. Phase 1 clinical trial of adoptive immunotherapy using “off-the-shelf” activated natural killer cells in patients with refractory and relapsed acute myeloid leukemia. Cytotherapy 2017, 19, 1225–1232. [Google Scholar] [CrossRef]
- Williams, B.A.; Law, A.D.; Routy, B.; Denhollander, N.; Gupta, V.; Wang, X.-H.; Chaboureau, A.; Viswanathan, S.; Keating, A. A phase I trial of NK-92 cells for refractory hematological malignancies relapsing after autologous hematopoietic cell transplantation shows safety and evidence of efficacy. Oncotarget 2017, 51, 89256–89268. [Google Scholar] [CrossRef]
- Yalcin, K.; Ovali, E.; Ozdamarlar, U.; Celen, S.; Karasu, G.; Yesilipek, A.; Hazar, V. NK-92 cellular therapy for pediatric relapsed/refractory Ewing sarcoma. Int. Cancer Conf. J. 2020, 9, 137–140. [Google Scholar] [CrossRef]
- Fung, I.T.; Boissel, L.; Soon-Shiong, P.; Klingemann, H. Generation and characterization of NK-92 cells with hypoimmunogenic modifications. Cancer Res. 2025, 85 (Suppl. 8), 885. [Google Scholar] [CrossRef]
- Chinnapen, H.; Boissel, L.; Bickett, T.; Fleenor, C.; Godbole, V.; Saxena, M.; Soon-Shiong, P.; Klingemann, H. Characterization of lysate from NK-92 cells and its potential use as an immunotherapeutic modality. Cell. Immunol. 2025, 413, 104951. [Google Scholar] [CrossRef] [PubMed]
- Deol, S.; Donahue, P.S.; Mitrut, R.E.; Hammitt-Kess, I.J.; Ahn, J.; Zhang, B.; Leonard, J.N. Comparative evaluation of synthetic cytokines for enhancing production and performance of NK-92 cell-based therapies. GEN Biotechnol. 2023, 3, 228–246. [Google Scholar] [CrossRef]
- Klingemann, H. Human immuno—Therapeutics for cancer treatment of dogs? Front. Vet. Med. 2025; in press. [Google Scholar]
- Helfand, S.C.; Soergel, S.A.; MacWilliams, P.S.; Hank, J.A.; Sondel, P. Clinical and immunological effects of human recombinant interleukin-2 given by repetitive weekly infusion to normal dogs. Cancer Immunol. Immunother. 1994, 39, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Passos Barbosa, M.M.; Kamerer, R.L.; Schmit, J.; Lopez, A.J.; Uyehara, R.; Tighe, R.; Battula, S.; Kaufman, H.L.; Fan, T.M. Preclinical evaluation of an anchored immunotherapy strategy with aluminum hydroxide-tethered interleukin-12 in dogs with advanced malignant melanoma. Mol. Cancer Ther. 2024, 24, 406–418. [Google Scholar] [CrossRef] [PubMed]
- Rebhun, R.B.; York, D.; Cruz, S.M.; Judge, S.J.; Razmara, A.M.; Farley, L.E.; Brady, R.V.; Johnson, E.G.; Burton, J.H.; Willcox, J.; et al. Inhaled recombinant human IL-15 in dogs with naturally occurring pulmonary metastases from osteosarcoma or melanoma: A phase 1 study of clinical activity and correlates of response. J. Immunother. Cancer 2022, 10, e004493. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| NCI Trial Number | Disease | NK-92 Cell | Target Antigen | Phase | Status |
|---|---|---|---|---|---|
| NCT02742727 | CD7+ leukemia and lymphoma | taNK | CD7 | I/II | Unknown |
| NCT02892695 | CD19+ leukemia and lymphoma | taNK | CD19 | I/II | Unknown |
| NCT02944162 | AML | taNK | CD33 | I/II | Unknown |
| NCT03940833 | MM | taNK | BCMA | I/II | Unknown |
| NCT02839954 | Solid tumors | taNK | MUC-1 | I/II | Unknown |
| NCT03383978 | Glioblastoma | taNK | HER2 (ErbB2) | I | Recruiting |
| NCT05528341 | Solid tumors | taNK | NKG2D- L | I | Recruiting |
| NCT03656705 | Non-small cell lung cancer | NK-92 with PD-1 switch receptor | Undefined | I | Enrolling by invitation |
| NCT05618925 | NHL | t-haNK | CD19 | I | Recruiting |
| NCT03228667 | Solid tumors | t-haNK | PD-L1 | IIb | Active, not recruiting |
| NCT04050709 | Solid tumors | t-haNK | PD-L1 | I | Active, not recruiting |
| NCT04390399 | Pancreatic cancer | t-haNK | PD-L1 | II | Recruiting |
| NCT04847466 | Gastric cancer and HNSCC | t-haNK | PD-L1 | II | Recruiting |
| NCT04927884 | TNBC | t-haNK | PD-L1 | Ib/II | Terminated |
| Tumor Type | Response |
|---|---|
| Melanoma | 1 PR |
| Renal Cell Cancer | 1 MR, 5 SD |
| Acute Myeloid Leukemia (relapsed, resistant) | 1 PR |
| Myeloma failing HSCT | 1 CR |
| HD failing HSCT | 1 CR, 3 MR |
| Lung Cancer | 1 MR, 2 PR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klingemann, H. The Natural Killer Cell Line NK-92 and Its Genetic Variants: Impact on NK Cell Research and Cancer Immunotherapy. Cancers 2025, 17, 1968. https://doi.org/10.3390/cancers17121968
Klingemann H. The Natural Killer Cell Line NK-92 and Its Genetic Variants: Impact on NK Cell Research and Cancer Immunotherapy. Cancers. 2025; 17(12):1968. https://doi.org/10.3390/cancers17121968
Chicago/Turabian StyleKlingemann, Hans. 2025. "The Natural Killer Cell Line NK-92 and Its Genetic Variants: Impact on NK Cell Research and Cancer Immunotherapy" Cancers 17, no. 12: 1968. https://doi.org/10.3390/cancers17121968
APA StyleKlingemann, H. (2025). The Natural Killer Cell Line NK-92 and Its Genetic Variants: Impact on NK Cell Research and Cancer Immunotherapy. Cancers, 17(12), 1968. https://doi.org/10.3390/cancers17121968




