Therapeutic Consequences of 68Ga-PSMA-11-PET/CT in Prostate Cancer in Correlation to the Gleason Score, PSA Value, and D’Amico-Defined Risk Groups
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Investigation Protocol
2.3. Analysis
3. Results
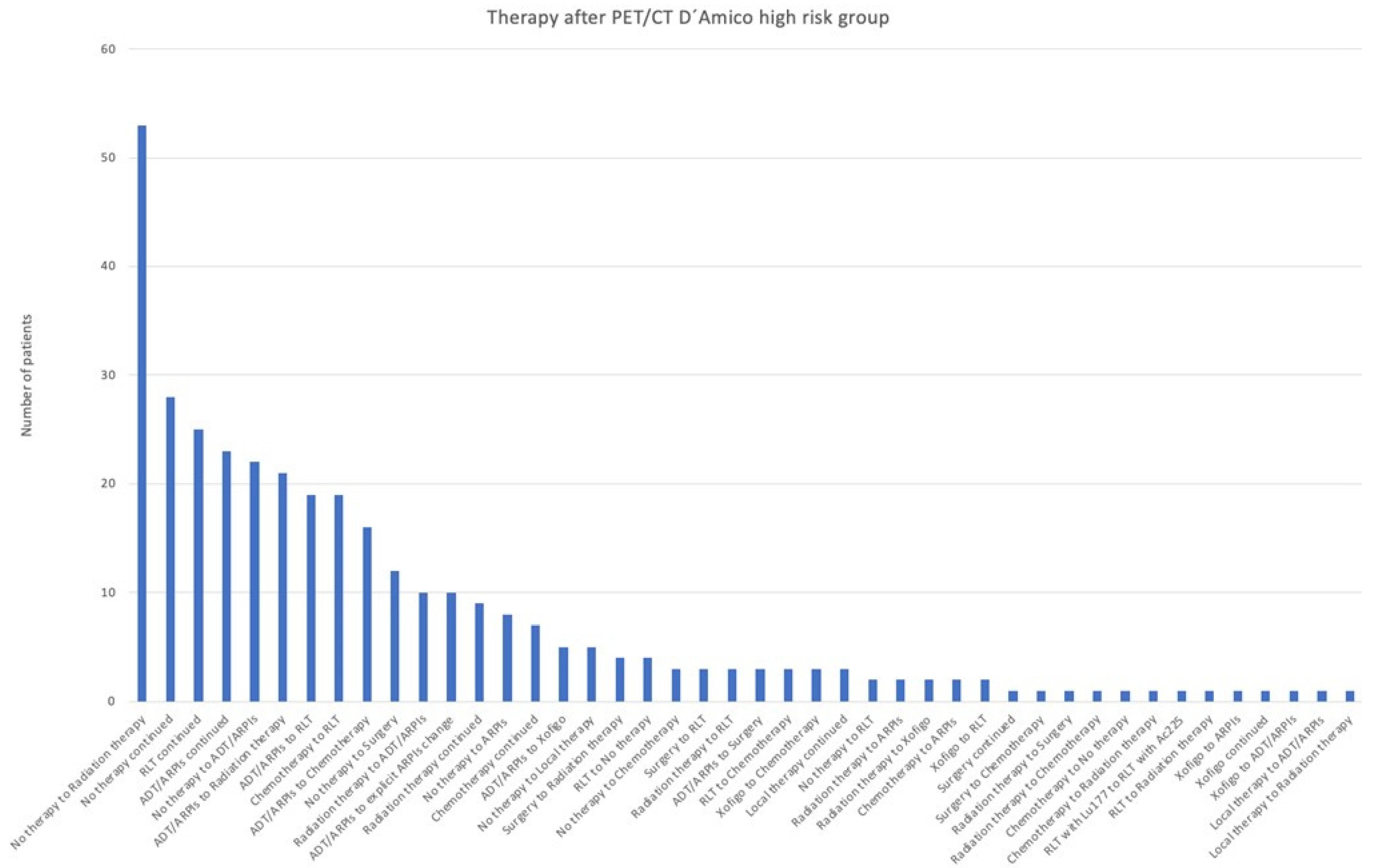
3.1. Adjustments in Treatment After the 68Ga-PSMA-11-PET/CT
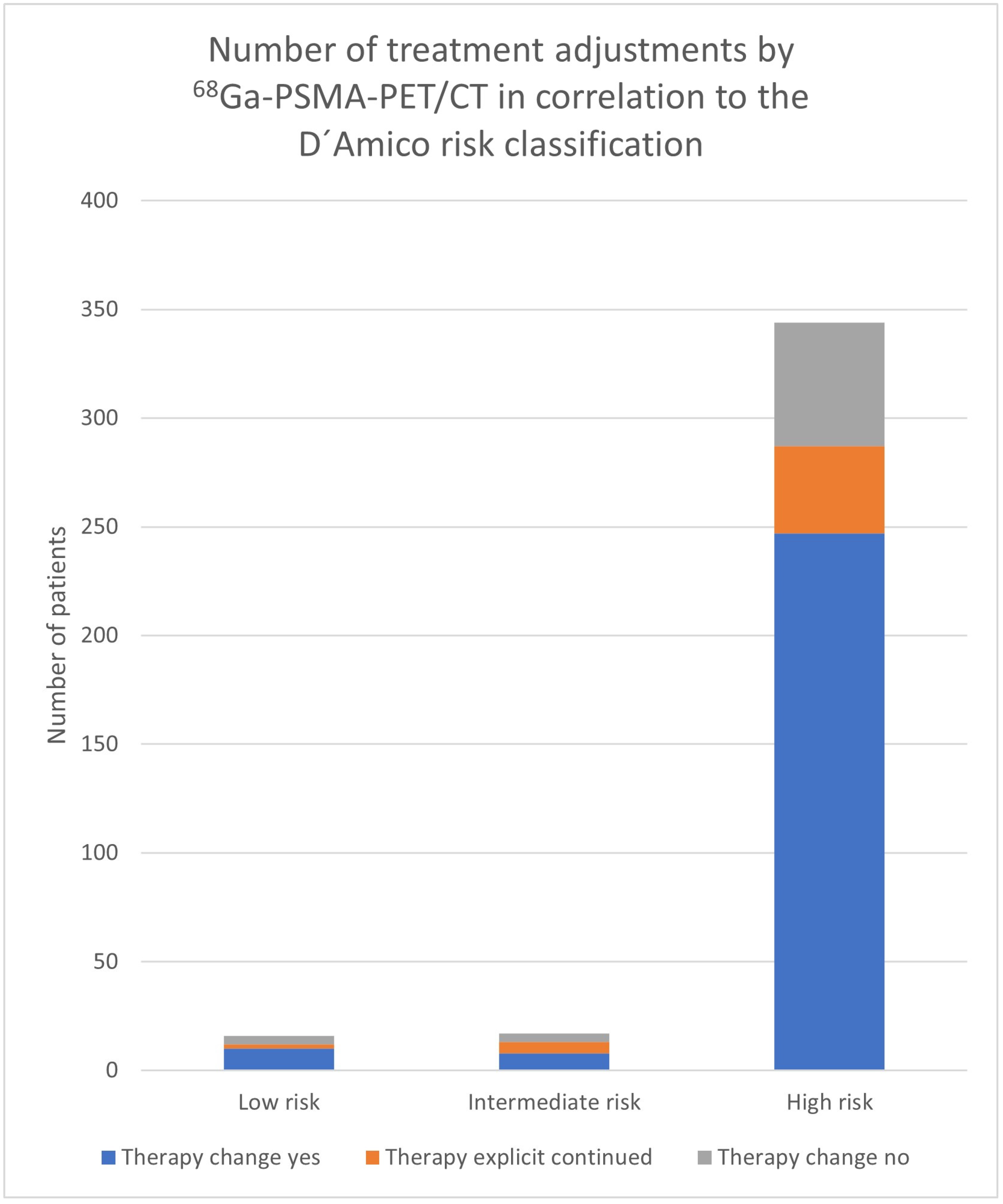
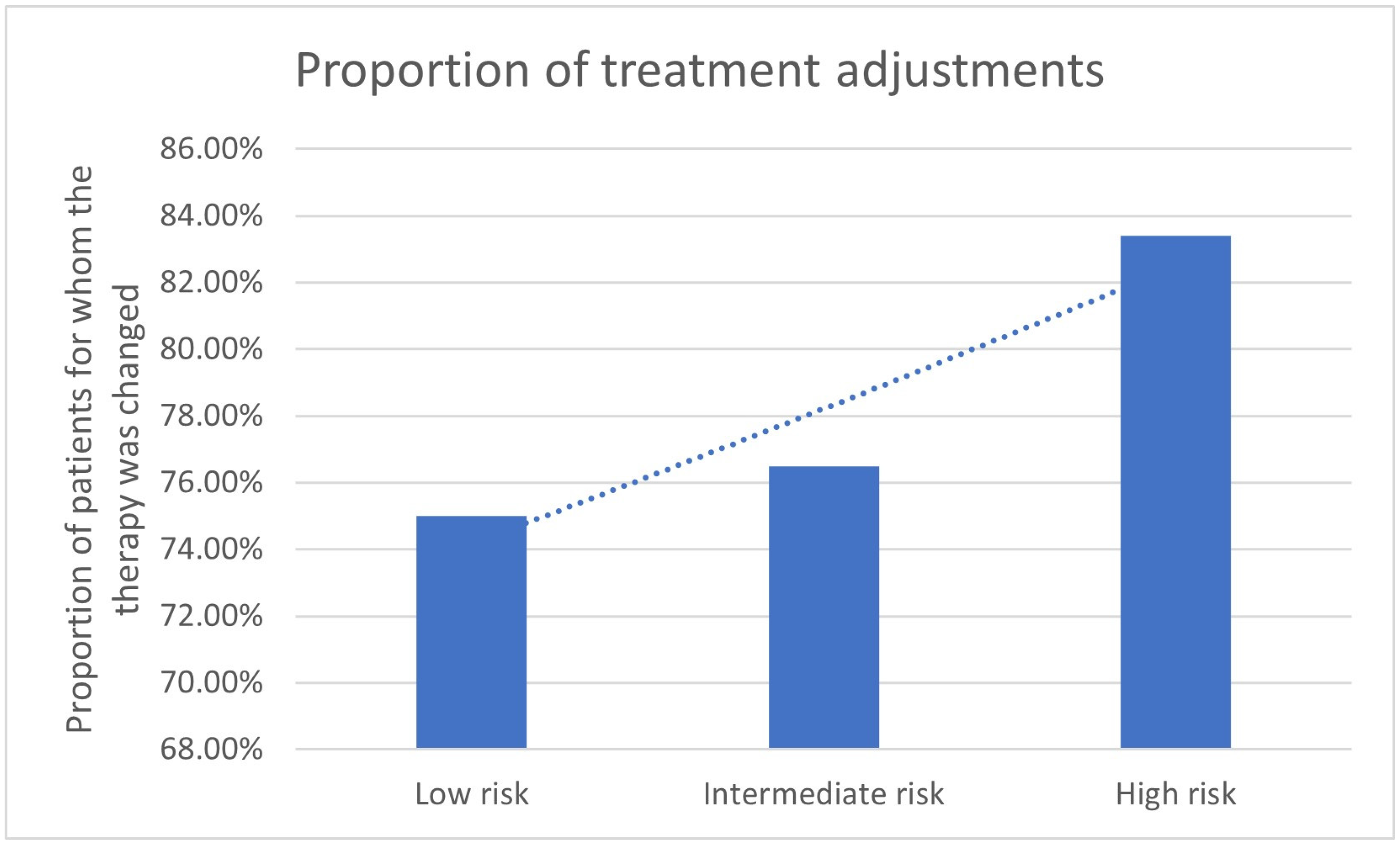
3.2. Impact of D’Amico Risk
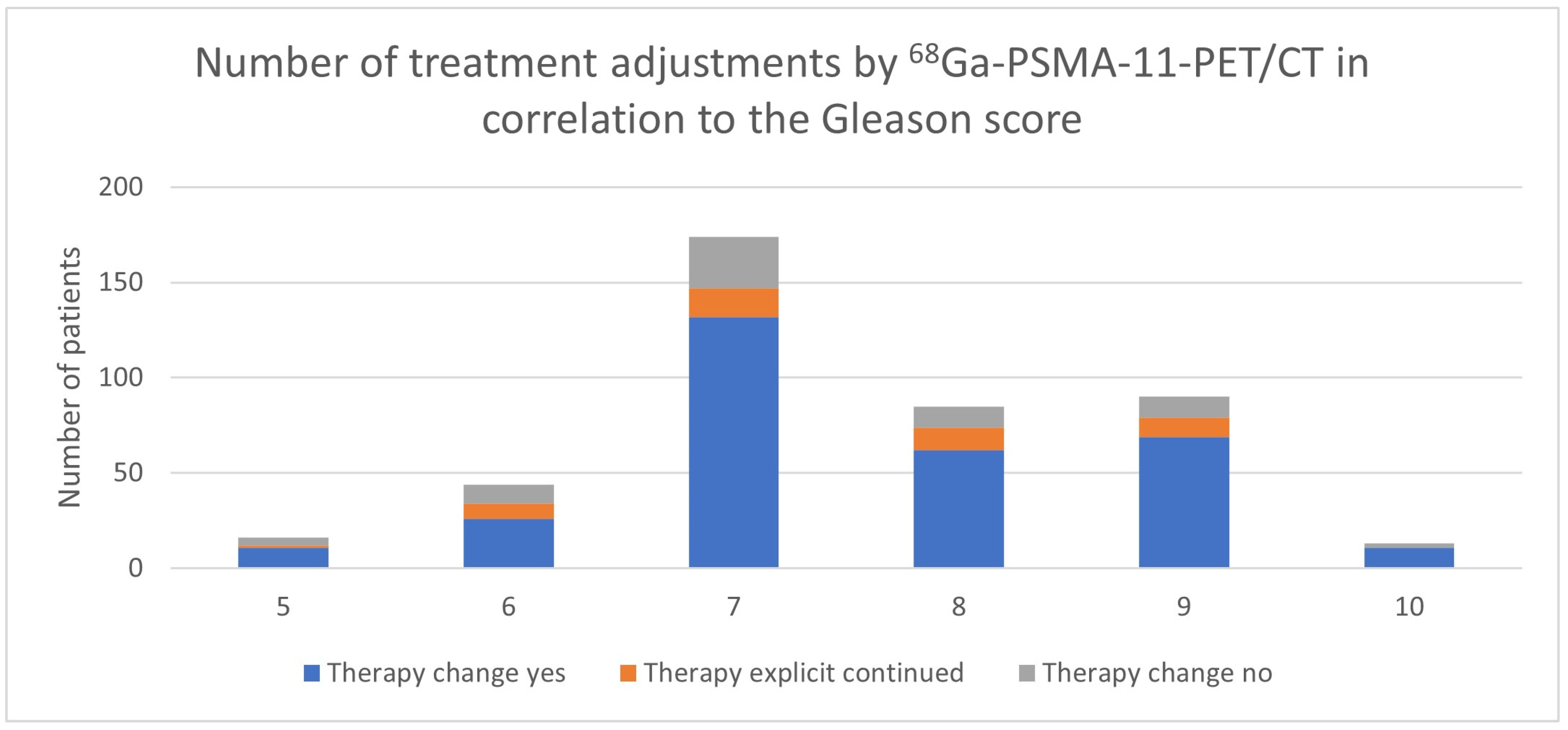
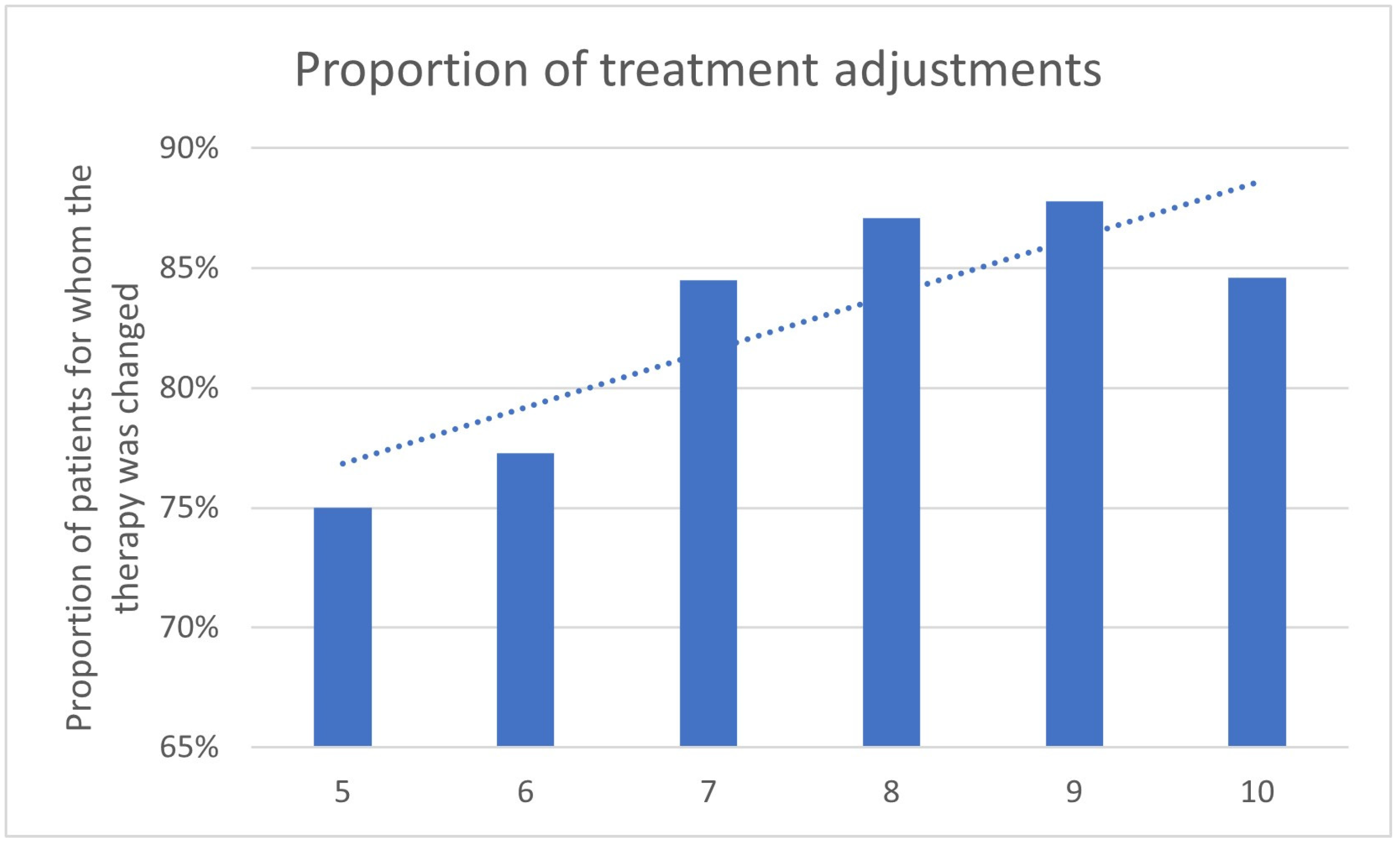
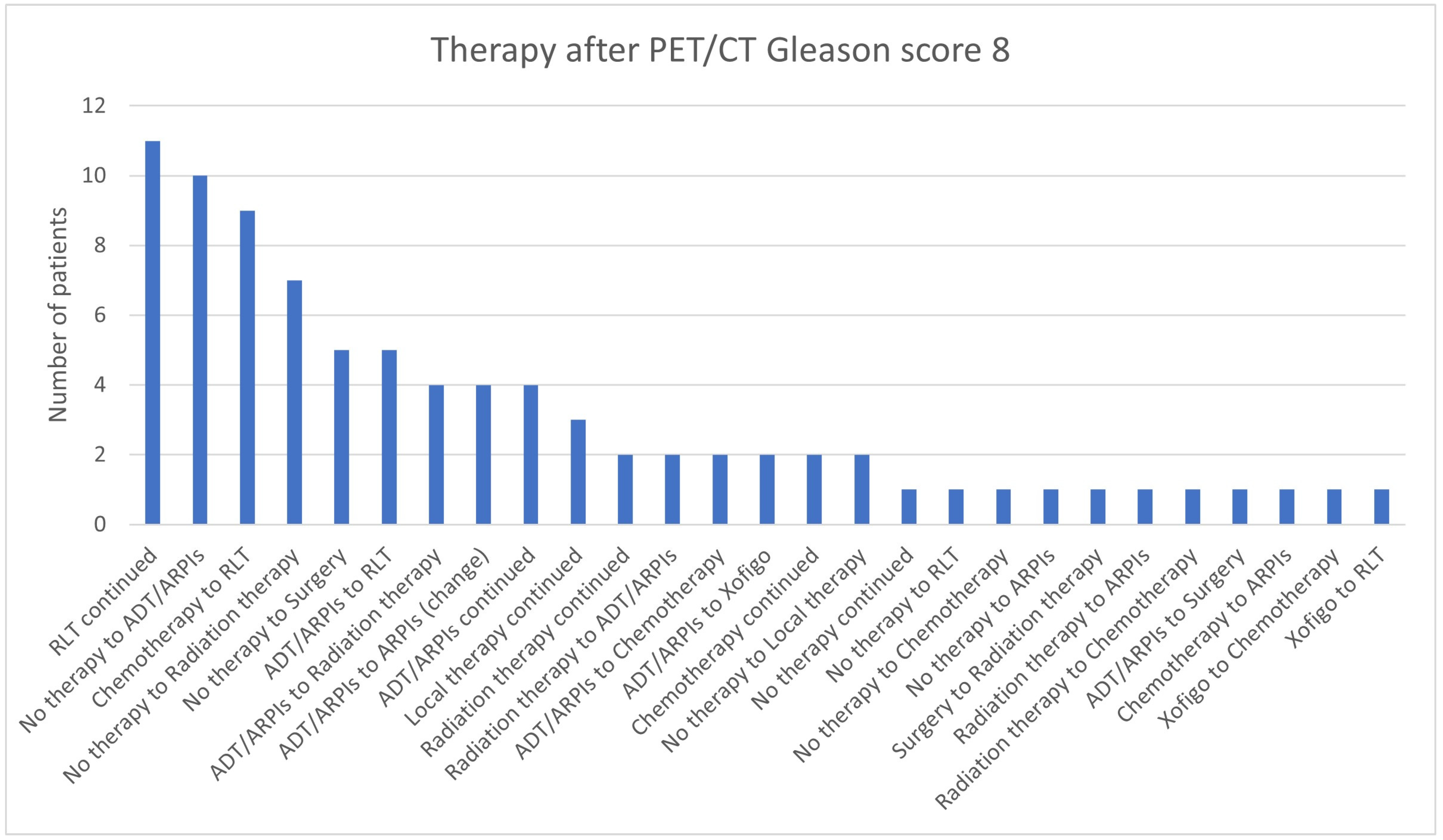
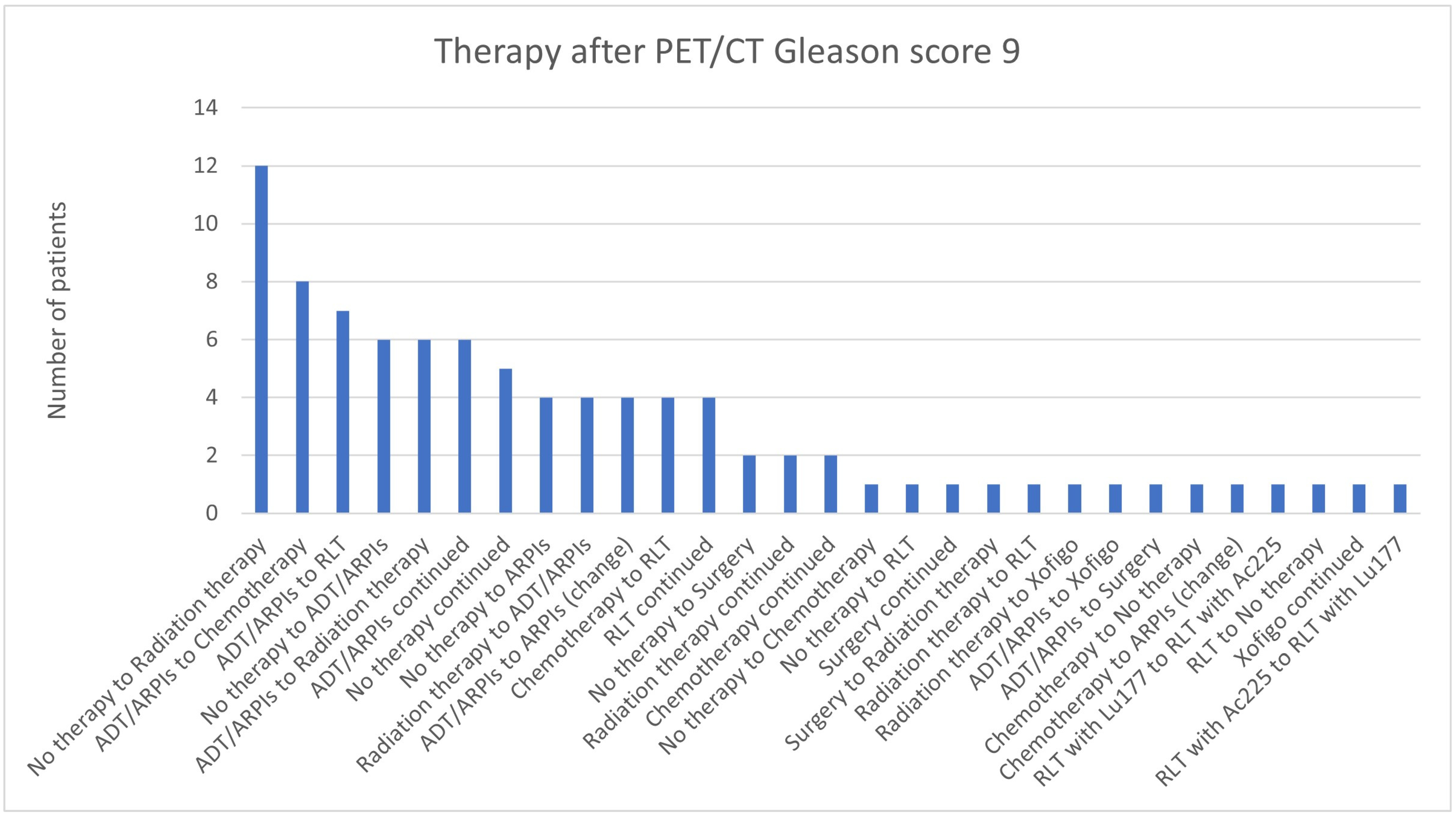
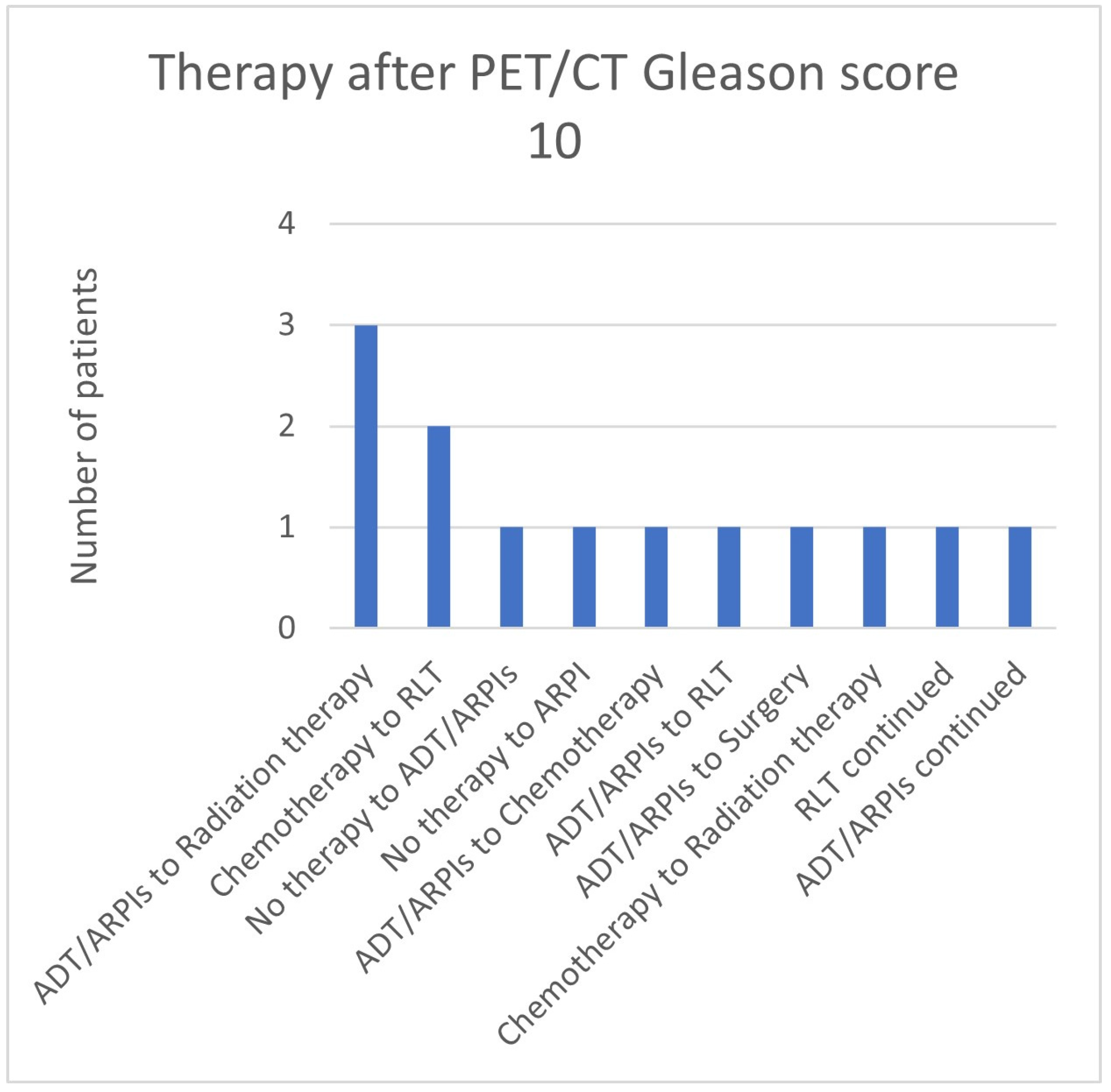
3.3. Impact of the Gleason Score
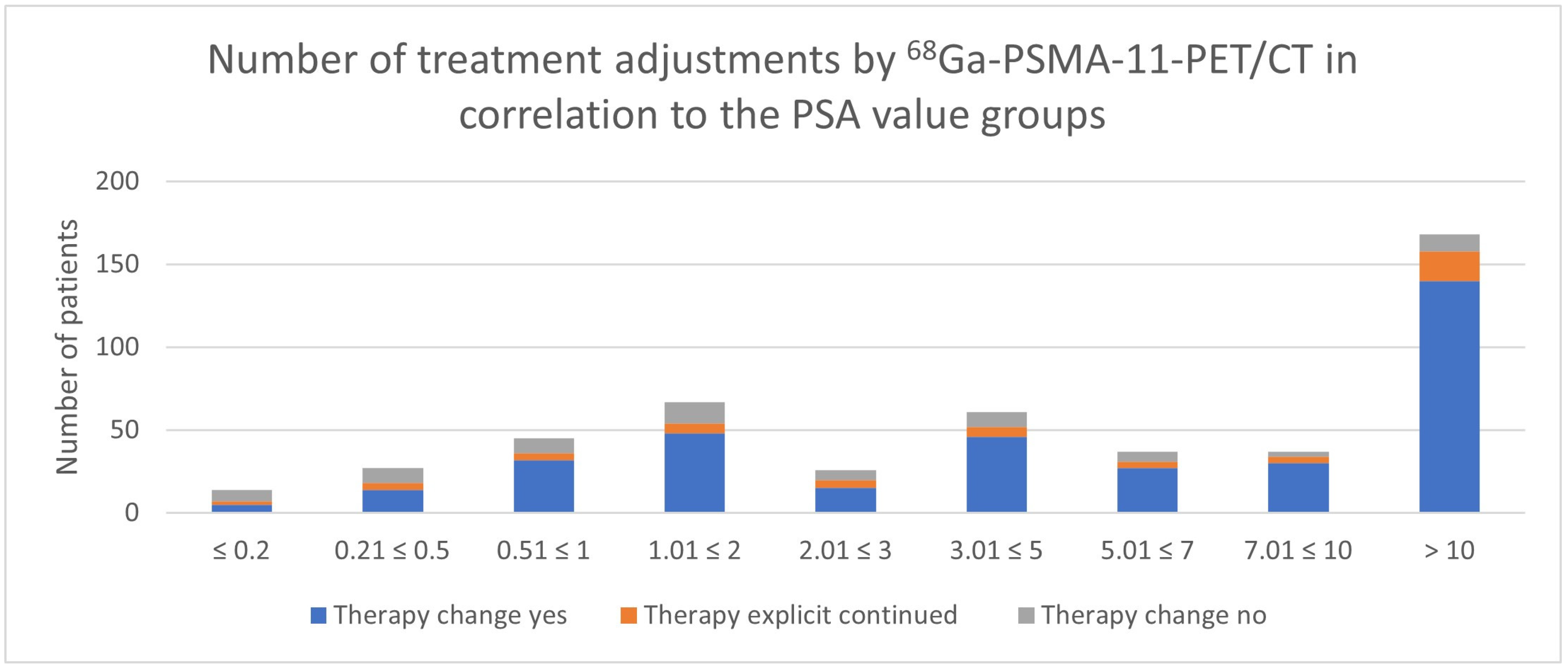
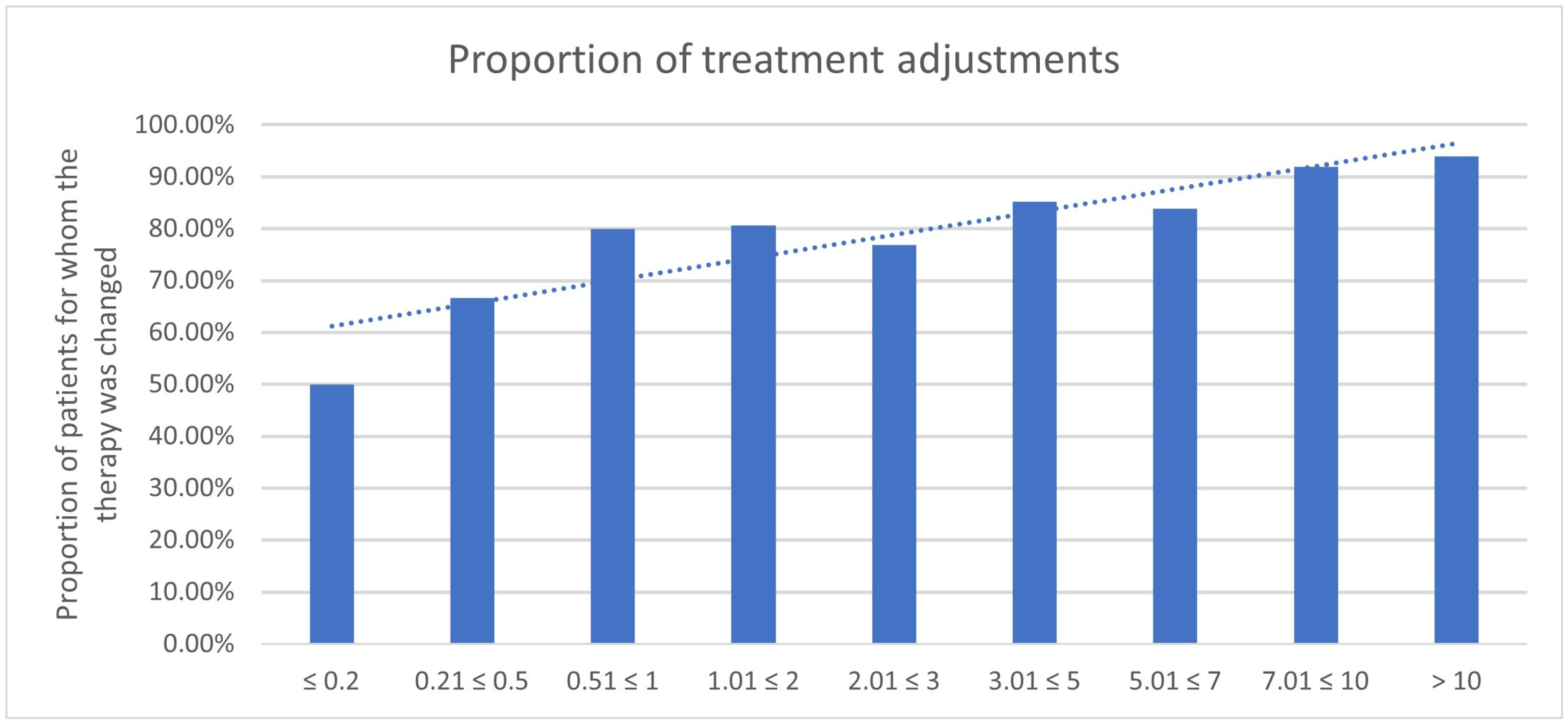
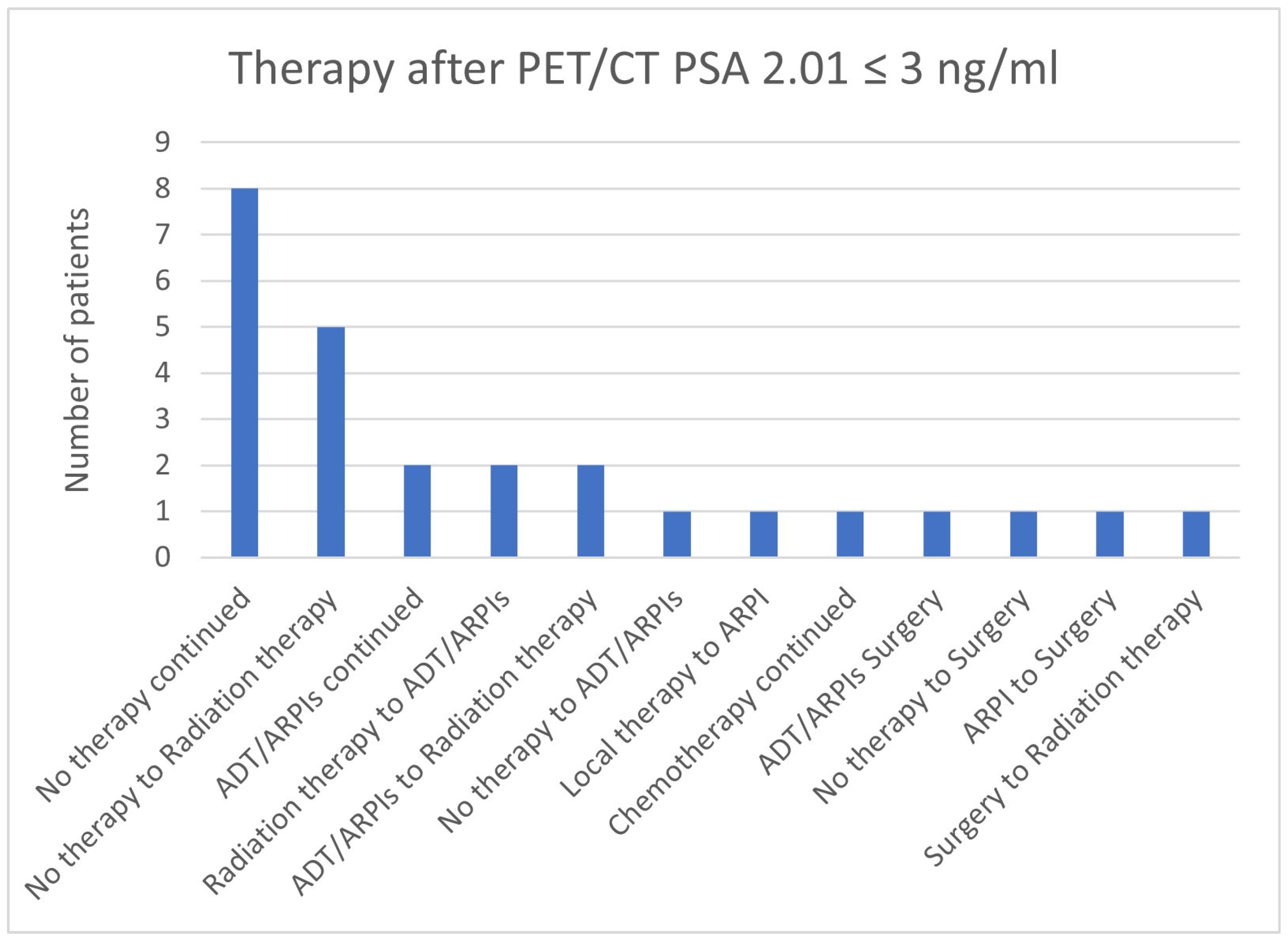
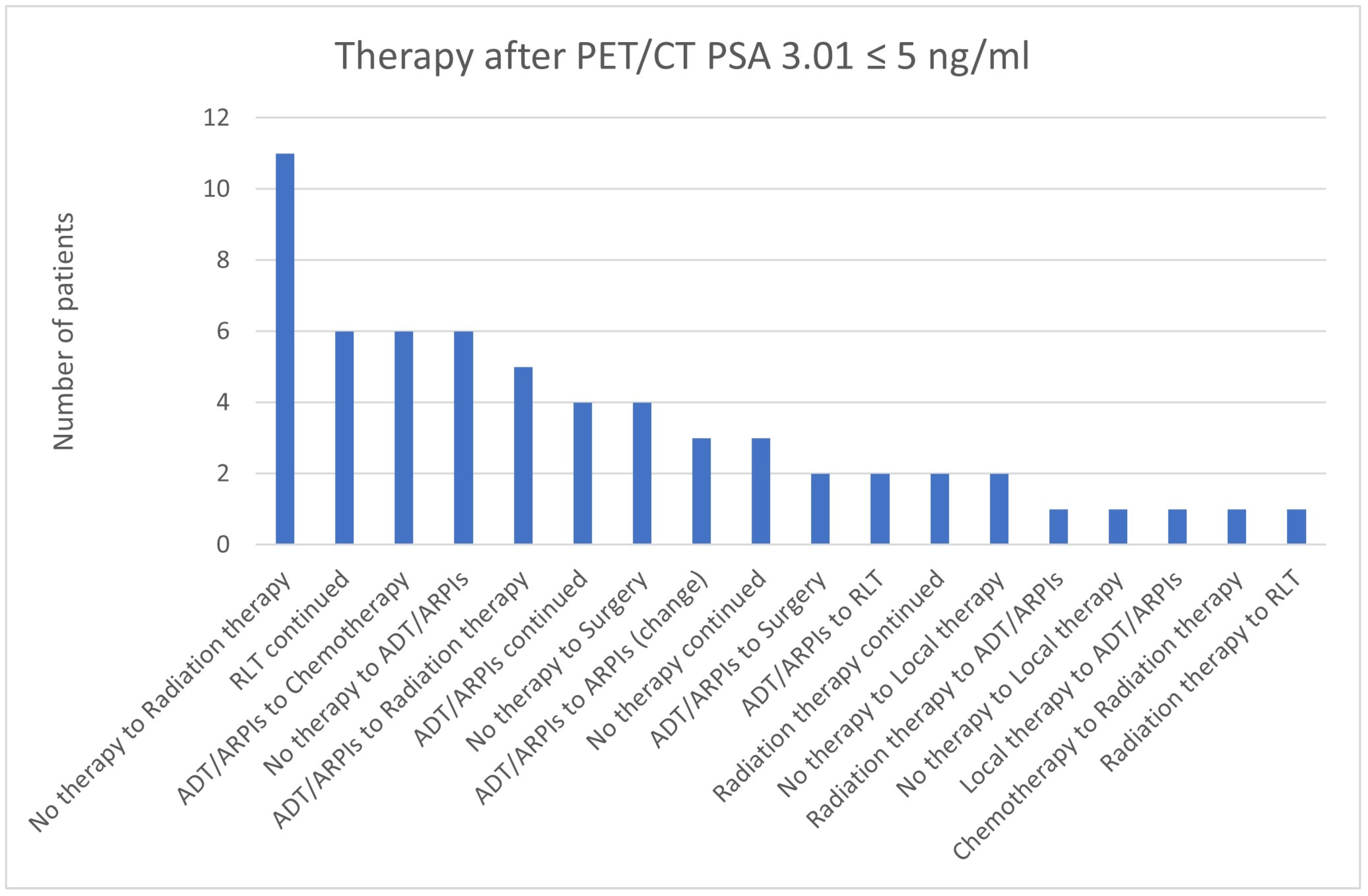
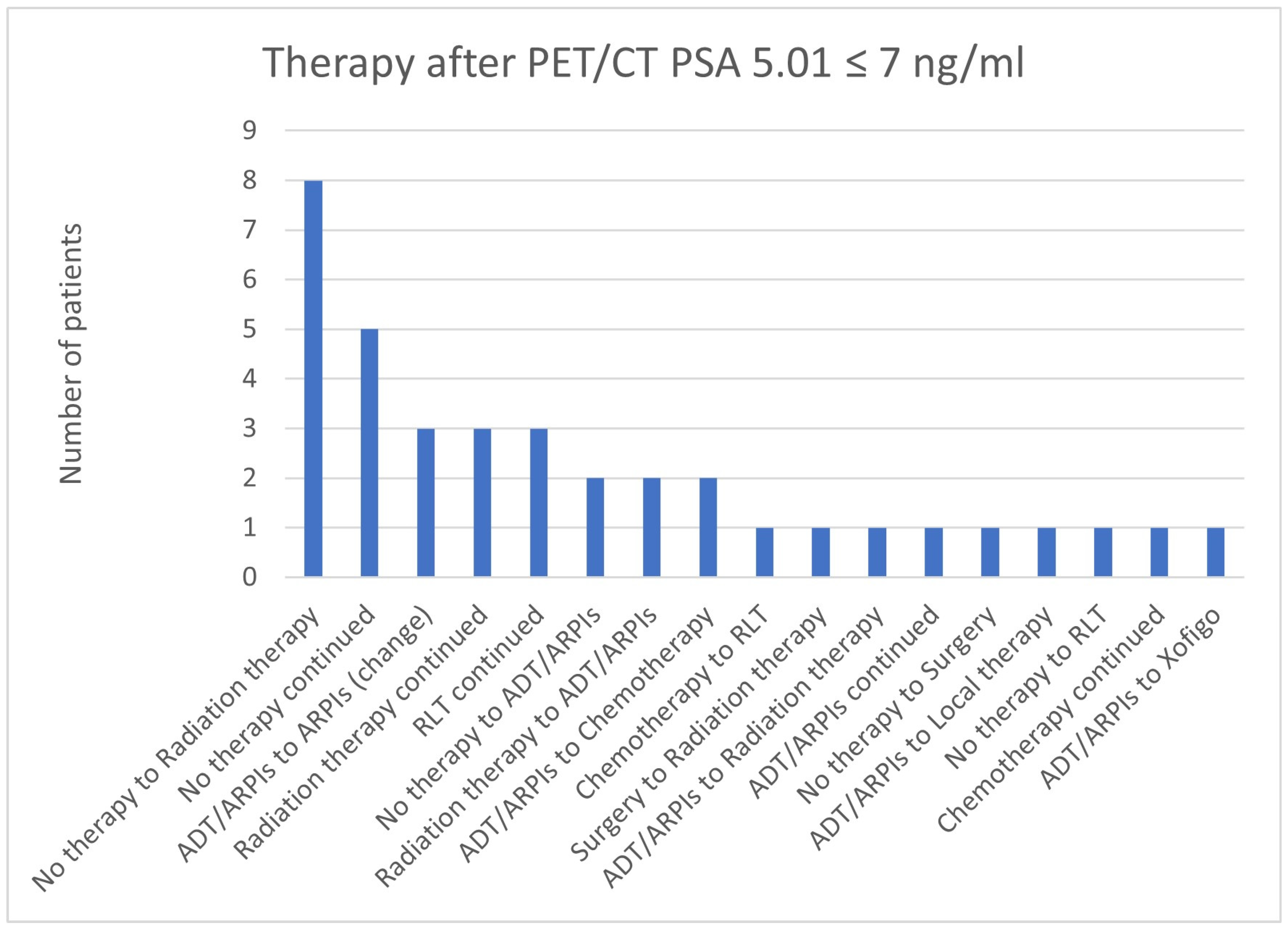
3.4. Impact of PSA Value
3.5. Continuation of Therapy Based on the 68Ga-PSMA-PET/CT Findings
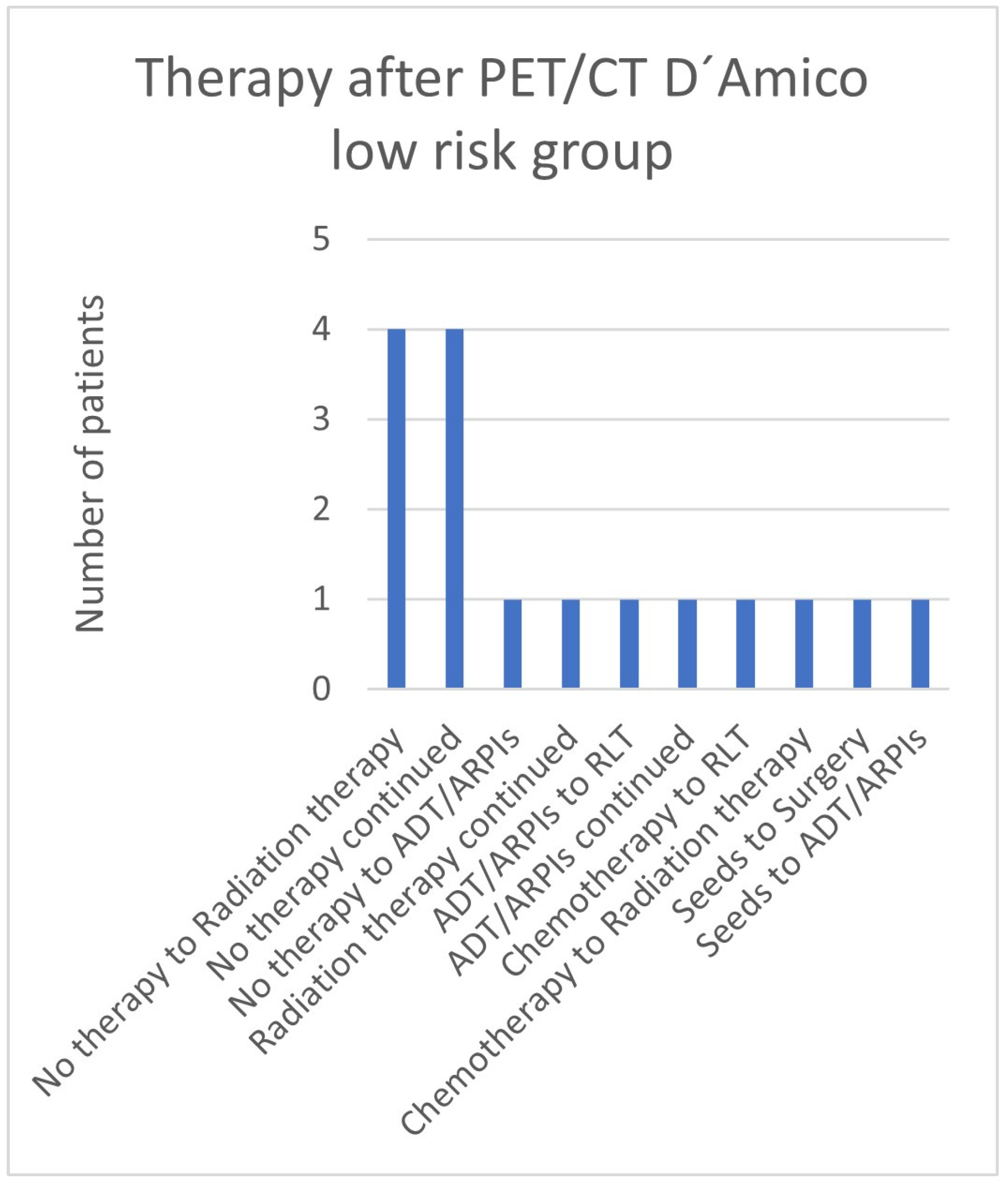
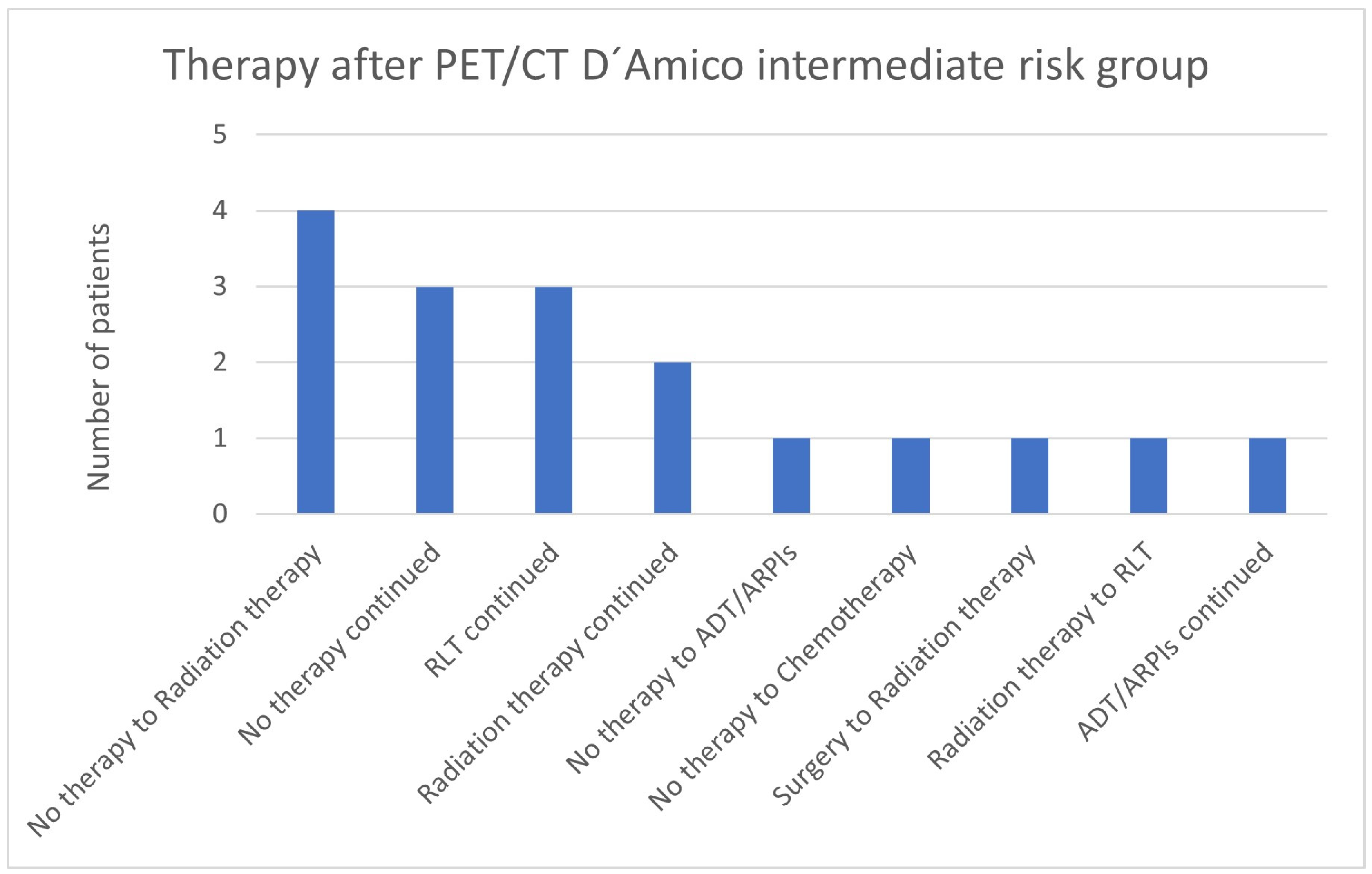
3.6. Therapies Based on the 68Ga-PSMA-PET/CT Findings in Low- and Intermediate-Risk Patients
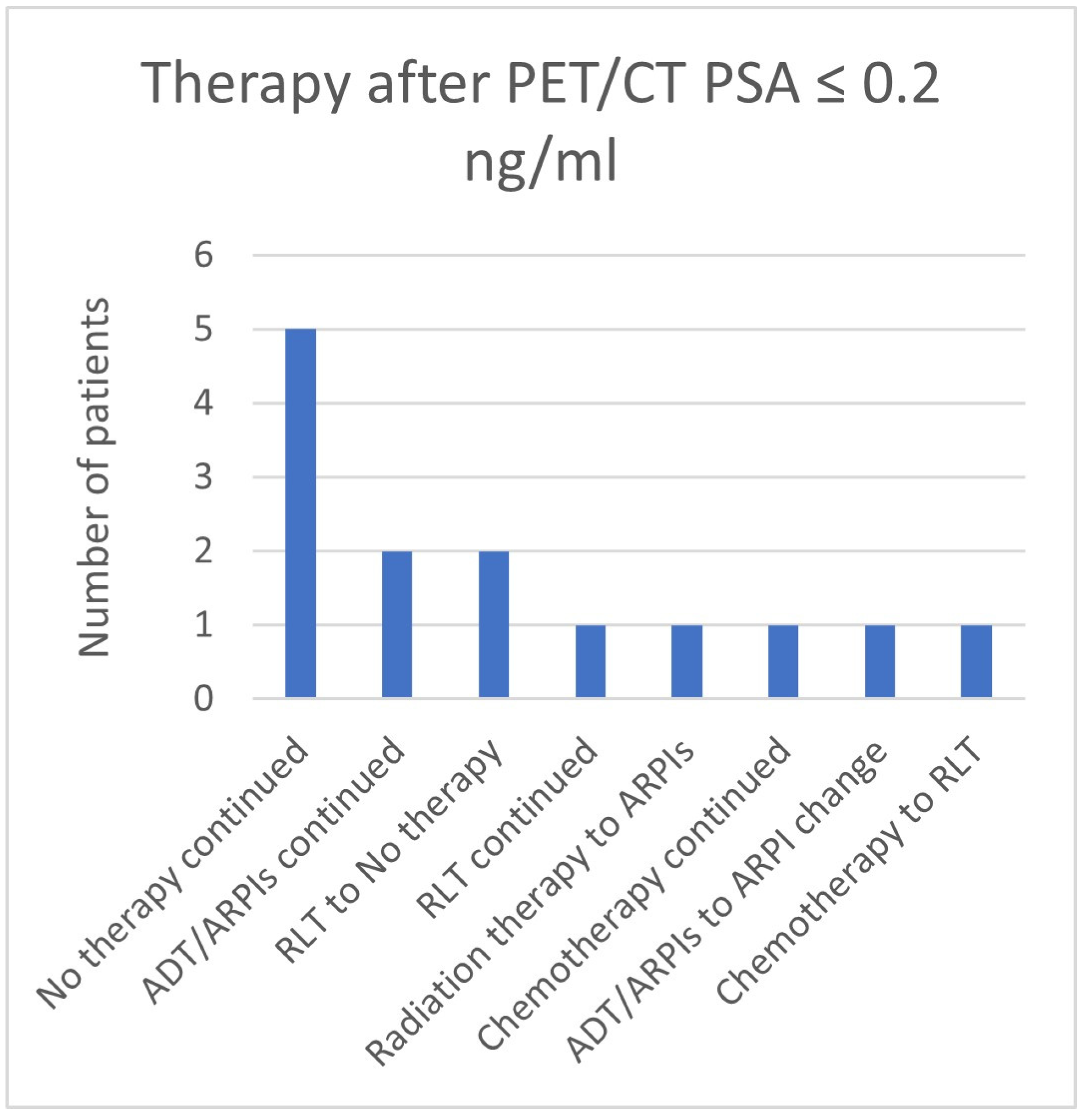
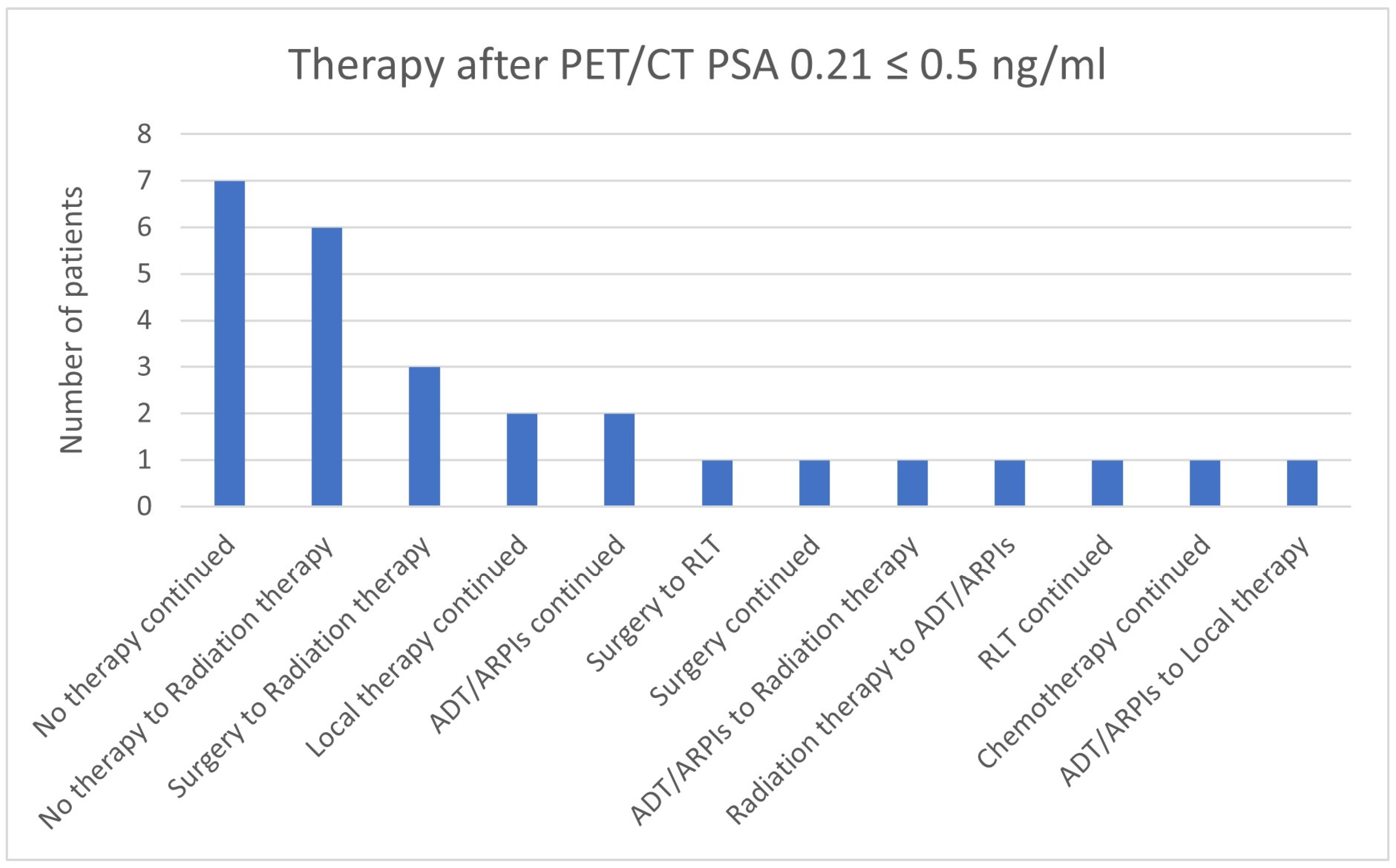
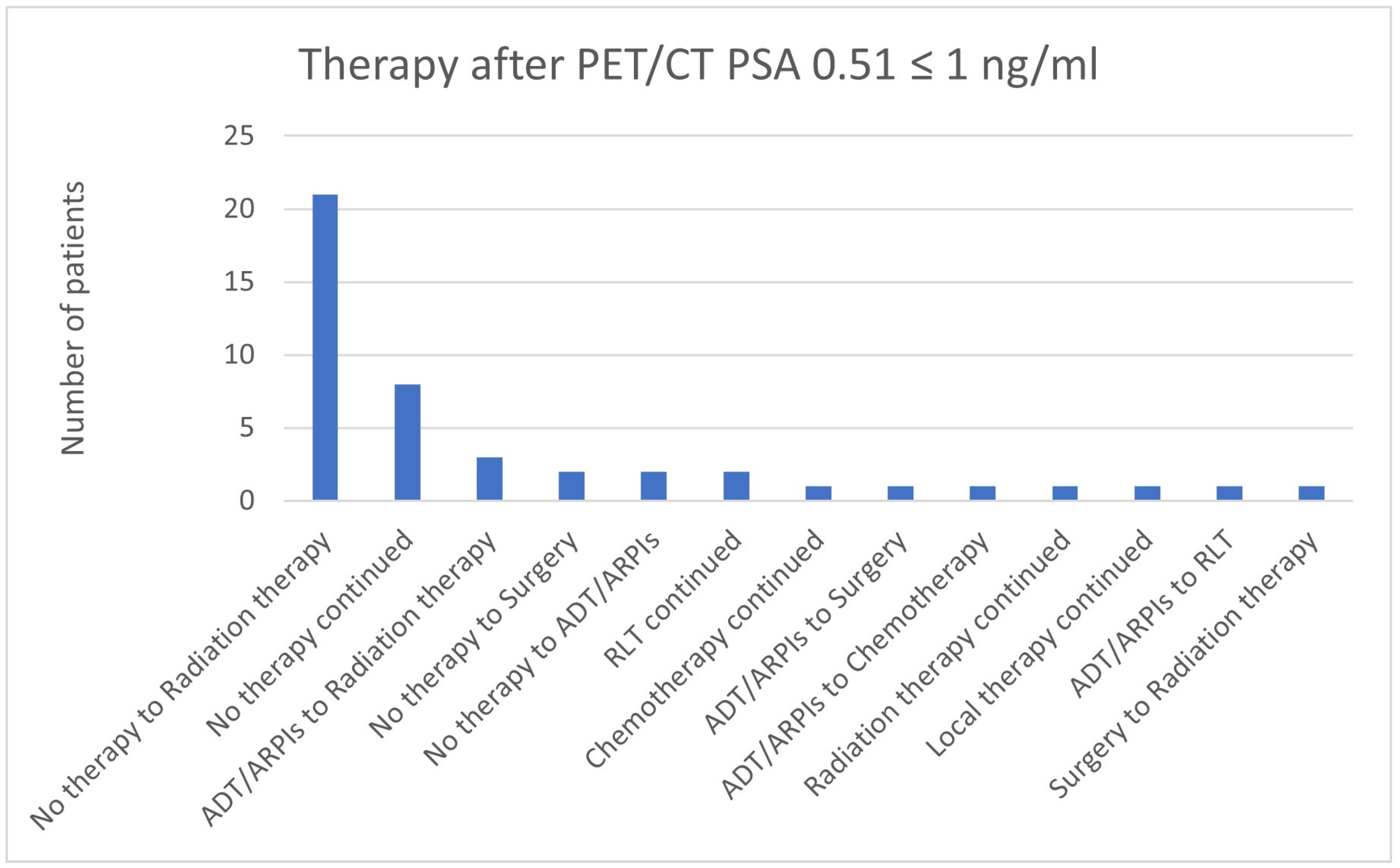
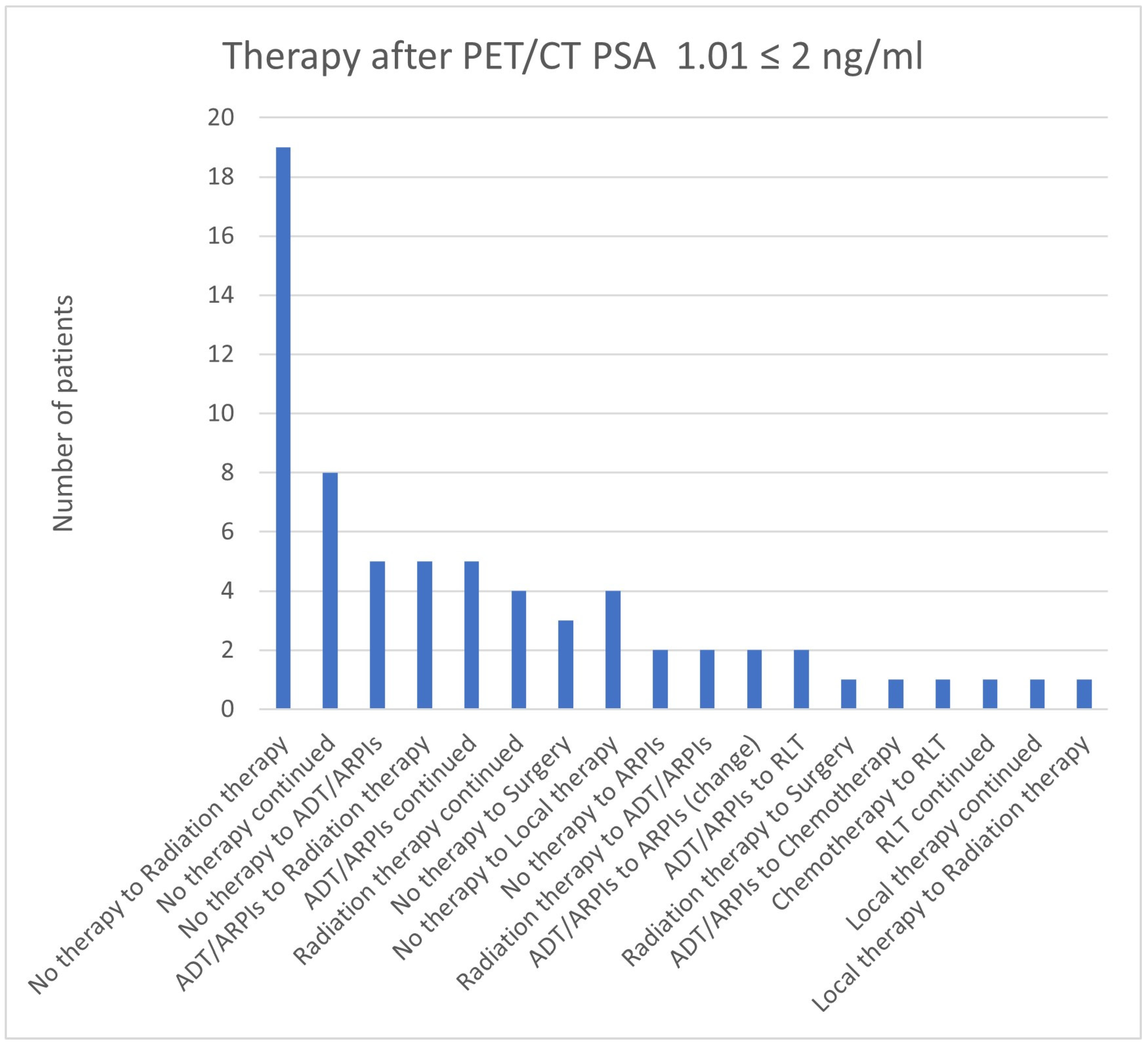
3.7. Therapies Based on the 68Ga-PSMA-PET/CT Findings in Patients with a PSA ≤ 2 ng/mL
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pozdnyakov, A.; Kulanthaivelu, R.; Bauman, G.; Ortega, C.; Veit-Haibach, P.; Metser, U. The impact of PSMA PET on the treatment and outcomes of men with biochemical recurrence of prostate cancer: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2023, 26, 240–248. [Google Scholar] [CrossRef] [PubMed]
- S3 Leitlinie Prostatakarzinom (S3 Guideline Prostate Cancer) Version 7.0 May 2024, AWMF-Reg.Nr.: 043-022OL. Available online: https://www.leitlinienprogramm-onkologie.de/fileadmin/user_upload/Downloads/Leitlinien/Prostatatkarzinom/Version_7/LL_Prostatakarzinom_Langversion_7.0.pdf (accessed on 10 January 2025).
- Han, S.; Woo, S.; Kim, Y.J.; Suh, C.H. Impact of 68Ga-PSMA PET on the Management of Patients with Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. 2018, 74, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Fendler, W.P.; Eiber, M.; Beheshti, M.; Bomanji, J.; Ceci, F.; Cho, S.; Giesel, F.; Haberkorn, U.; Hope, T.A.; Kopka, K.; et al. 68Ga-PSMA PET/CT: Joint EANM and SNMMI procedure guideline for prostate cancer imaging: Version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Oromieh, A.; Eiber, M.; Fendler, W.; Schmidt, M.; Rahbar, K.; Ahmadzadehfar, H.; Umutlu, L.; Hadaschik, B.; Hakenberg, O.W.; Fornara, P.; et al. DGN-Handlungsempfehlung (S1-Leitlinie)—PSMA-Liganden-PET/CT in der Diagnostik des Prostatakarzinoms—Stand: 01/2022—AWMF-Registernummer: 031-055 [Procedure Guideline for Prostate Cancer Imaging with PSMA-ligand PET/CT]. Nuklearmedizin 2023, 62, 5–19. (In German) [Google Scholar] [CrossRef] [PubMed]
- Afshar-Oromieh, A.; da Cunha, M.L.; Wagner, J.; Haberkorn, U.; Debus, N.; Weber, W.; Eiber, M.; Holland-Letz, T.; Rauscher, I. Performance of [68Ga]Ga-PSMA-11 PET/CT in patients with recurrent prostate cancer after prostatectomy multi-centre evaluation of 2533 patients. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2925–2934. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- D’Amico, A.V.; Whittington, R.; Malkowicz, S.B.; Schultz, D.; Blank, K.; Broderick, G.A.; Tomaszewski, J.E.; Renshaw, A.A.; Kaplan, I.; Beard, C.J.; et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998, 280, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Ulas Babacan, O.; Hasbek, Z.; Seker, K. The Relationship between D’Amico and ISUP Risk Classifications and 68Ga-PSMA PET/CT SUVmax Values in Newly Diagnosed Prostate Cancers. Curr. Oncol. 2024, 31, 5307–5317. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Leeuwen, P.J.; Stricker, P.; Hruby, G.; Kneebone, A.; Ting, F.; Thompson, B.; Nguyen, Q.; Ho, B.; Emmett, L. 68Ga-PSMA has a high detection rate of prostate cancer recurrence outside the prostatic fossa in patients being considered for salvage radiation treatment. BJU Int. 2016, 117, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Morigi, J.J.; Stricker, P.D.; van Leeuwen, P.J.; Tang, R.; Ho, B.; Nguyen, Q.; Hruby, G.; Fogarty, G.; Jagavkar, R.; Kneebone, A.; et al. Prospective Comparison of 18F-Fluoromethylcholine Versus 68Ga-PSMA PET/CT in Prostate Cancer Patients Who Have Rising PSA After Curative Treatment and Are Being Considered for Targeted Therapy. J. Nucl. Med. 2015, 56, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Shakespeare, T.P. Effect of prostate-specific membrane antigen positron emission tomography on the decision-making of radiation oncologists. Radiat. Oncol. 2015, 10, 233. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bock, F.; Frerker, B.; Schubert, L.; Rennau, H.; Kurth, J.; Krause, B.J.; Hildebrandt, G.; Schwarzenböck, S.M. Impact of 68Ga-PSMA PET/CT on radiation treatment planning of prostate cancer patients. Nuklearmedizin 2024, 63, 199–206. (In English) [Google Scholar] [CrossRef] [PubMed]
- Roach, P.J.; Francis, R.; Emmett, L.; Hsiao, E.; Kneebone, A.; Hruby, G.; Eade, T.; Nguyen, Q.A.; Thompson, B.D.; Cusick, T.; et al. The Impact of 68Ga-PSMA PET/CT on Management Intent in Prostate Cancer: Results of an Australian Prospective Multicenter Study. J. Nucl. Med. 2018, 59, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Hope, T.A.; Aggarwal, R.; Chee, B.; Tao, D.; Greene, K.L.; Cooperberg, M.R.; Feng, F.; Chang, A.; Ryan, C.J.; Small, E.J.; et al. Impact of 68Ga-PSMA-11 PET on Management in Patients with Biochemically Recurrent Prostate Cancer. J. Nucl. Med. 2017, 58, 1956–1961. [Google Scholar] [CrossRef] [PubMed]
- Calais, J.; Fendler, W.P.; Eiber, M.; Gartmann, J.; Chu, F.-I.; Nickols, N.G.; Reiter, R.E.; Rettig, M.B.; Marks, L.S.; Ahlering, T.E.; et al. Impact of 68Ga-PSMA-11 PET/CT on the Management of Prostate Cancer Patients with Biochemical Recurrence. J. Nucl. Med. 2018, 59, 434–441. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fendler, W.P.; Ferdinandus, J.; Czernin, J.; Eiber, M.; Flavell, R.R.; Behr, S.C.; Wu, I.-W.K.; Lawhn-Heath, C.; Pampaloni, M.H.; Reiter, R.E.; et al. Impact of 68Ga-PSMA-11 PET on the Management of Recurrent Prostate Cancer in a Prospective Single-Arm Clinical Trial. J. Nucl. Med. 2020, 61, 1793–1799. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sterzing, F.; Kratochwil, C.; Fiedler, H.; Katayama, S.; Habl, G.; Kopka, K.; Afshar-Oromieh, A.; Debus, J.; Haberkorn, U.; Giesel, F.L. 68Ga-PSMA-11 PET/CT: A new technique with high potential for the radiotherapeutic management of prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 34–41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dewes, S.; Schiller, K.; Sauter, K.; Eiber, M.; Maurer, T.; Schwaiger, M.; Gschwend, J.E.; Combs, S.E.; Habl, G. Integration of 68Ga-PSMA-PET imaging in planning of primary definitive radiotherapy in prostate cancer: A retrospective study. Radiat. Oncol. 2016, 11, 73. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Calais, J.; Kishan, A.U.; Cao, M.; Fendler, W.P.; Eiber, M.; Herrmann, K.; Ceci, F.; Reiter, R.E.; Rettig, M.B.; Hegde, J.V.; et al. Potential Impact of 68Ga-PSMA-11 PET/CT on the Planning of Definitive Radiation Therapy for Prostate Cancer. J. Nucl. Med. 2018, 59, 1714–1721. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schmidt-Hegemann, N.S.; Eze, C.; Li, M.; Rogowski, P.; Schaefer, C.; Stief, C.; Buchner, A.; Zamboglou, C.; Fendler, W.; Ganswindt, U.; et al. Einfluss der Ga-68 PSMA-PET/CT auf das strahlentherapeutische Vorgehen bei Patienten mit Prostatakarzinomen. In 57. Jahrestagung der Deutschen Gesellschaft für Nuklearmedizin; Georg Thieme Verlag KG: Stuttgart, Germany, 2019. [Google Scholar]
- Schmidt-Hegemann, N.S.; Fendler, W.P.; Buchner, A.; Stief, C.; Rogowski, P.; Niyazi, M.; Eze, C.; Li, M.; Bartenstein, P.; Belka, C.; et al. Detection level and pattern of positive lesions using PSMA PET/CT for staging prior to radiation therapy. Radiat. Oncol. 2017, 12, 176. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schmidt-Hegemann, N.S.; Eze, C.; Li, M.; Rogowski, P.; Schaefer, C.; Stief, C.; Buchner, A.; Zamboglou, C.; Fendler, W.P.; Ganswindt, U.; et al. Impact of 68Ga-PSMA PET/CT on the Radiotherapeutic Approach to Prostate Cancer in Comparison to CT: A Retrospective Analysis. J. Nucl. Med. 2018, 60, 963–970. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marzec, J.; Becker, J.; Paulsen, F.; Wegener, D.; Olthof, S.-C.; Pfannenberg, C.; Schwenck, J.; Bedke, J.; Stenzl, A.; Nikolaou, K.; et al. 68Ga-PSMA-PET/CT-directed IGRT/SBRT for oligometastases of recurrent prostate cancer after initial surgery. Acta Oncol. 2020, 59, 149–156. [Google Scholar] [CrossRef] [PubMed]
- King, C.R. The timing of salvage radiotherapy after radical prostatectomy: A systematic review. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Sonni, I.; Eiber, M.; Fendler, W.P.; Alano, R.M.; Vangala, S.S.; Kishan, A.U.; Nickols, N.; Rettig, M.B.; Reiter, R.E.; Czernin, J.; et al. Impact of 68Ga-PSMA-11 PET/CT on Staging and Management of Prostate Cancer Patients in Various Clinical Settings: A Prospective Single-Center Study. J. Nucl. Med. 2020, 61, 1153–1160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morigi, J.J.; Anderson, J.; DENunzio, C.; Fanti, S. Prostate specific membrane antigen positron emission tomography/computed tomography and staging high risk prostate cancer: A non-systematic review of high clinical impact literature. Minerva Urol. Nephrol. 2021, 73, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Weiner, A.B.; Agrawal, R.; Valle, L.F.; Sonni, I.; Kishan, A.U.; Rettig, M.B.; Raman, S.S.; Calais, J.; Boutros, P.C.; Reiter, R.E. Impact of PSMA PET on Prostate Cancer Management. Curr. Treat. Options Oncol. 2024, 25, 191–205. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ploussard, G.; Roubaud, G.; Barret, E.; Beauval, J.B.; Brureau, L.; Créhange, G.; Dariane, C.; Fiard, G.; Fromont, G.; Gauthé, M.; et al. French AFU Cancer Committee Guidelines—Update 2022–2024: Prostate cancer—Management of metastatic disease and castration resistance. Prog. Urol. 2022, 32, 1373–1419. [Google Scholar] [CrossRef] [PubMed]
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Brunckhorst, O.; Darraugh, J.; Eberli, D.; De Meerleer, G.; De Santis, M.; Farolfi, A.; et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer-2024 Update. Part I: Screening, Diagno-sis, and Local Treatment with Curative Intent. Eur. Urol. 2024, 86, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, J.; Tombal, B.; Collette, L.; Van Nieuwenhove, S.; Pasoglou, V.; Gérard, T.; Jamar, F.; Lhommel, R.; Lecouvet, F.E. Comparison of 68Ga-Prostate Specific Membrane Antigen (PSMA) Positron Emission Tomography Computed Tomography (PET-CT) and Whole-Body Magnetic Resonance Imaging (WB-MRI) with Diffusion Sequences (DWI) in the Staging of Advanced Prostate Cancer. Cancers 2021, 13, 5286. [Google Scholar] [CrossRef]
- von Eyben, F.E.; Picchio, M.; von Eyben, R.; Rhee, H.; Bauman, G. 68Ga-Labeled Prostate-specific Membrane Antigen Ligand Positron Emission Tomography/Computed Tomography for Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. Focus 2018, 4, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.; Amiel, G.E. The Impact of PSMA PET/CT on Modern Prostate Cancer Management and Decision Making-The Urological Perspective. Cancers 2023, 15, 3402. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Uslu, H.; Şahin, D.; İbişoğlu, E.; Tatoğlu, M.T. PRIMARY scoring in 68Ga-PSMA PET/CT: Correlation with prostate cancer risk groups and its potential impact on active surveillance. Ann. Nucl. Med. 2025, 39, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Shagera, Q.A.; Gil, T.; Barraco, E.; Boegner, P.; Kristanto, P.; El Ali, Z.; Sideris, S.; Martinez Chanza, N.; Roumeguère, T.; Flamen, P.; et al. Evaluating response to radium-223 using 68Ga-PSMA PET/CT imaging in patients with metastatic castration-resistant prostate cancer. Ann. Nucl. Med. 2025, 39, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Karpinski, M.J.; Rahbar, K.; Bögemann, M.; Nikoukar, L.R.; Schäfers, M.; Hoberück, S.; Miederer, M.; Hölscher, T.; Rasul, S.; Miszczyk, M.; et al. Updated Prostate Cancer Risk Groups by Prostate-specific Membrane Antigen Positron Emission Tomography Prostate Cancer Molecular Imaging Standardized Evaluation (PPP2): Results from an International Multicentre Registry Study. Eur. Urol. 2025; Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
| N = 562 | ||
|---|---|---|
| Year of birth of patients median (min; max) | 1947 (1929–1967) | |
| Activity median (min; max) in MBq | 188 (106–343) | |
| Acquisition time after application in minutes | 60 (30–145) | |
| PSA value in ng/mL | 5.1 (0.01–45.839) | |
| T at initial diagnosis | 1 | 17 (3.0%) |
| 2 | 100 (17.8%) | |
| 2a | 11 (2.0%) | |
| 2b | 12 (2.1%) | |
| 2c | 65 (11.6%) | |
| 2x | 12 (2.1%) | |
| 3 | 183 (32.6%) | |
| 3a | 43 (7.7%) | |
| 3b | 91 (16.2%) | |
| 3c | 2 (2.1%) | |
| 3x | 47 (8.4%) | |
| 4 | 17 (3.0%) | |
| x | 245 (43.6%) | |
| N at initial diagnosis | 0 | 193 (34.3%) |
| 1 | 83 (14.8%) | |
| x | 286 (50.9%) | |
| M at initial diagnosis | 0 | 63 (11.2%) |
| 1 | 21 (3.7%) | |
| x | 478 (85.1%) | |
| D’ Amico risk group | N = 377 | |
| Low risk | 16 (4.2%) | |
| Intermediate risk | 17 (4.5%) | |
| High risk | 344 (91.2%) | |
| Gleason score groups | N = 422 | |
| Gleason score 5 | 16 (3.8%) | |
| Gleason score 6 | 44 (10.4%) | |
| Gleason score 7 | 174 (41.2%) | |
| Gleason score 8 | 85 (20.1%) | |
| Gleason score 9 | 90 (21.3%) | |
| Gleason score 10 | 13 (3.1%) | |
| PSA value groups | N = 482 | |
| ≤0.2 | 14 (2.9%) | |
| 0.21 ≤ 0.5 | 27 (5.6%) | |
| 0.51 ≤ 1 | 45 (9.3%) | |
| 1.01 ≤ 2 | 67 (13.9%) | |
| 2.01 ≤ 3 | 26 (5.4%) | |
| 3.01 ≤ 5 | 61 (12.7%) | |
| 5.01 ≤ 7 | 37 (7.7%) | |
| 7.01 ≤ 10 | 37 (7.7%) | |
| >10 | 168 (34.9%) | |
| Results N = 562 | ||
|---|---|---|
| Therapy change | Yes | 415 (73.8%) |
| No | 147 (26.2%) | |
| In 68, no therapy change explicit because of PET/CT result. | ||
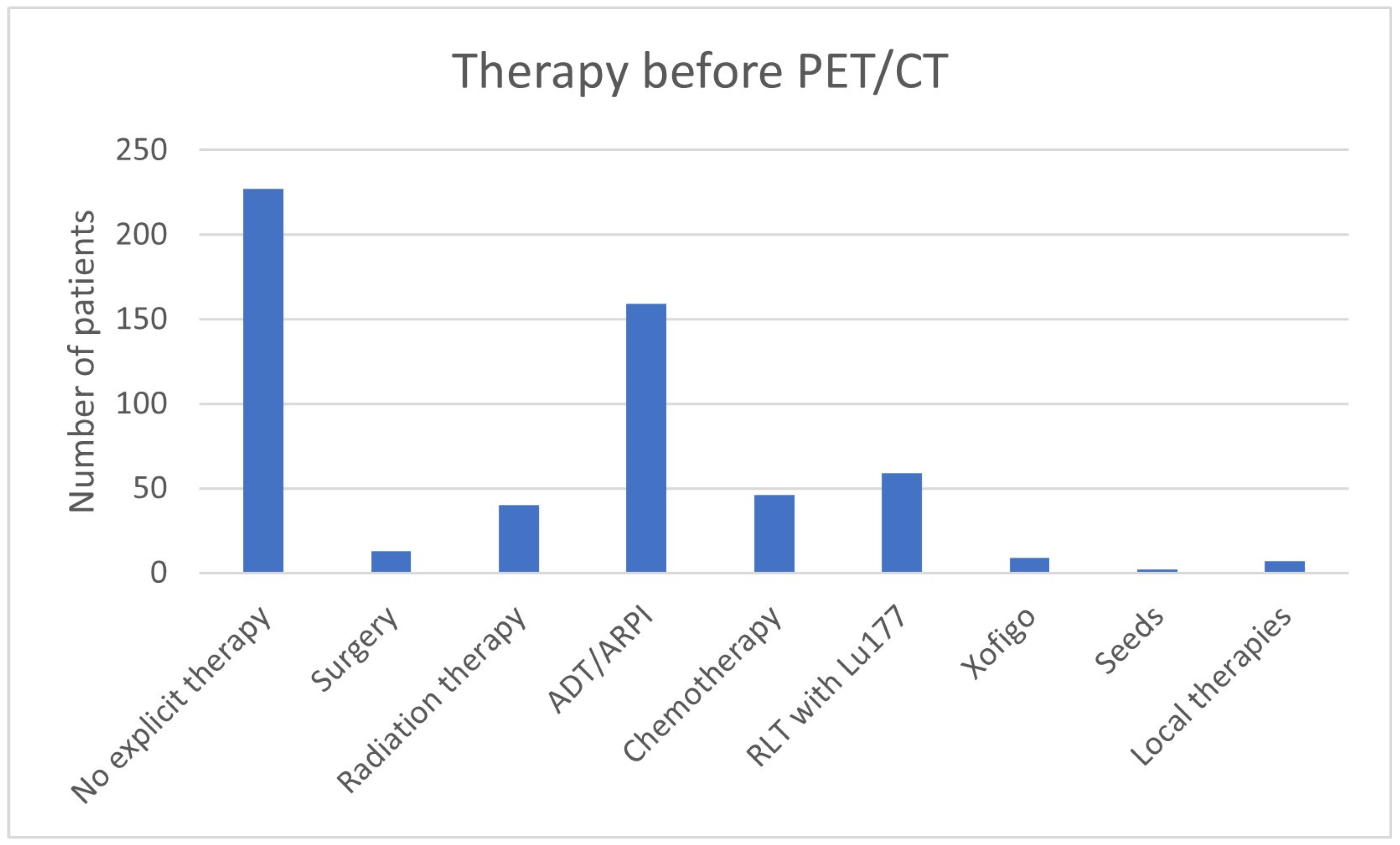
| Therapy before PET/CT | No explicit therapy | 227 (40.4%) |
| Surgery | 13 (2.3%) | |
| Radiation therapy | 40 (7.1%) | |
| ADT/ARPI | 159 (28.2%) | |
| Chemotherapy | 46 (8.2%) | |
| RLT with 177Lu | 59 (10.5%) | |
| Xofigo | 9 (1.6%) | |
| Seeds | 2 (0.4%) | |
| Local therapies | 7 (1.2%) | |
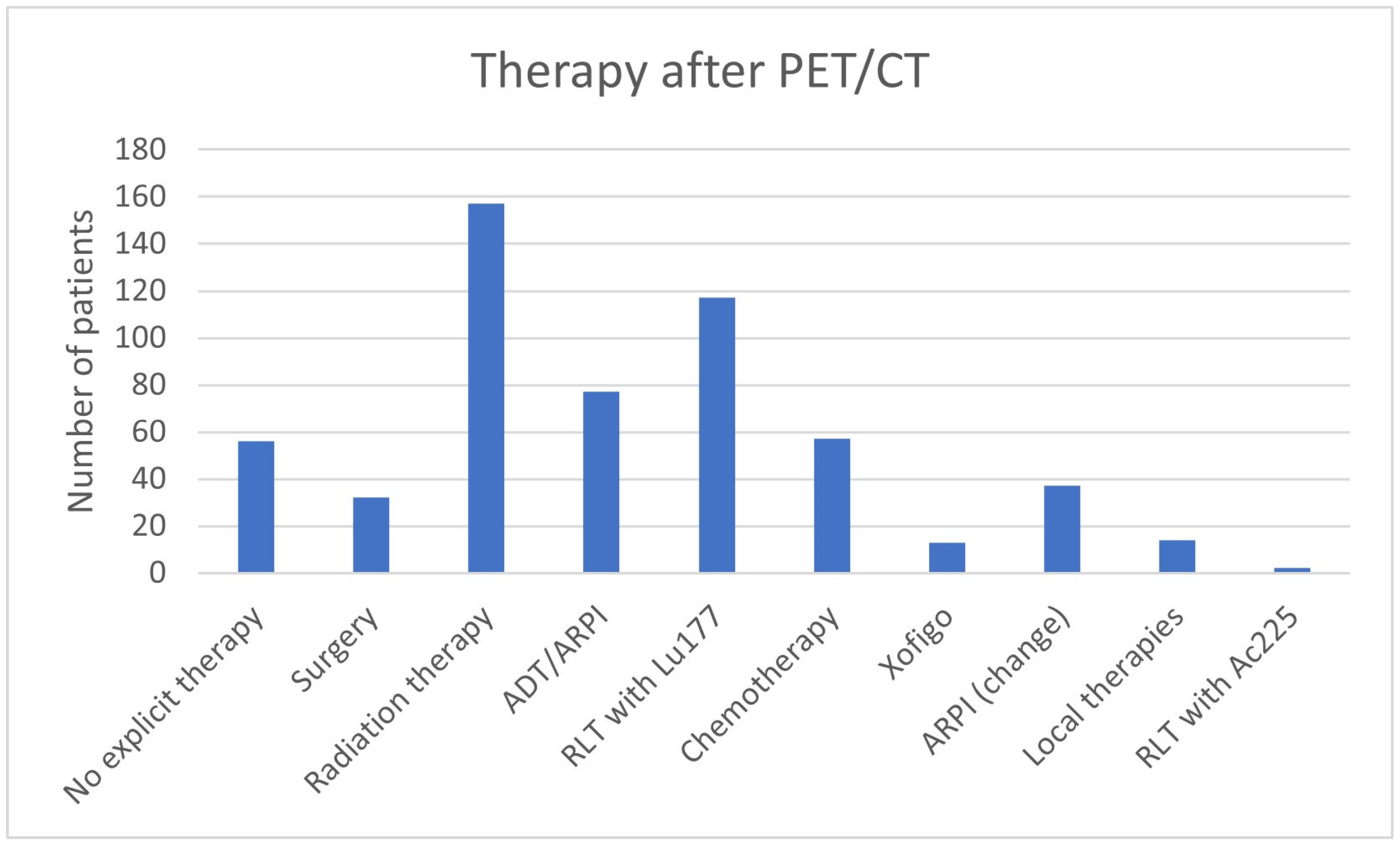
| Therapy after PET/CT | No explicit therapy | 56 (10.0%) |
| Surgery | 32 (5.7%) | |
| Radiation therapy | 157 (27.9%) | |
| ADT/ARPI | 77 (13.7%) | |
| RLT with 177Lu | 117 (20.8%) | |
| Chemotherapy | 57 (10.1%) | |
| Xofigo | 13 (2.3%) | |
| ARPI (change) | 37 (6.6%) | |
| Local therapies | 14 (2.5%) | |
| RLT with 225Ac | 2 (0.4%) | |
| Risk Group | Therapy Change Yes | Therapy Change No | N | Therapy Continued Because of PET/CT | Decision because of PET/CT |
|---|---|---|---|---|---|
| Low risk | 10 | 6 | 16 | 2 | 12/16 (75.0%) |
| Intermediate risk | 8 | 9 | 17 | 5 | 13/17 (76.5%) |
| High risk | 247 | 97 | 344 | 40 | 287/344 (83.4%) |
| 272 | 105 | 377 | |||
| Gleason Score | Therapy Change Yes | Therapy Change No | N | Therapy Continued Because of PET/CT | Decision Because of PET/CT |
|---|---|---|---|---|---|
| 5 | 11 | 5 | 16 | 1 | 12/16 (75%) |
| 6 | 26 | 18 | 44 | 8 | 34/44 (77.3%) |
| 7 | 132 | 42 | 174 | 15 | 147/174 (84.5%) |
| 8 | 62 | 23 | 85 | 12 | 74/85 (87.1%) |
| 9 | 69 | 21 | 90 | 10 | 79/90 (87.8%) |
| 10 | 11 | 2 | 13 | 0 | 11/13 (84.6%) |
| 422 | |||||
| PSA in ng/mL | Therapy Change Yes | Therapy Change No | N | Therapy Continued Because of PET/CT | Decision Because of PET/CT |
|---|---|---|---|---|---|
| ≤0.2 | 5 | 9 | 14 | 2 | 7/14 (50.0%) |
| 0.21 ≤ 0.5 | 14 | 13 | 27 | 4 | 18/27 (66.7%) |
| 0.51 ≤ 1 | 32 | 13 | 45 | 4 | 36/45 (80.0%) |
| 1.01 ≤ 2 | 48 | 19 | 67 | 6 | 54/67 (80.6%) |
| 2.01 ≤ 3 | 15 | 11 | 26 | 5 | 20/26 (76.9%) |
| 3.01 ≤ 5 | 46 | 15 | 61 | 6 | 52/61 (85.2%) |
| 5.01 ≤ 7 | 27 | 10 | 37 | 4 | 31/37 (83.8%) |
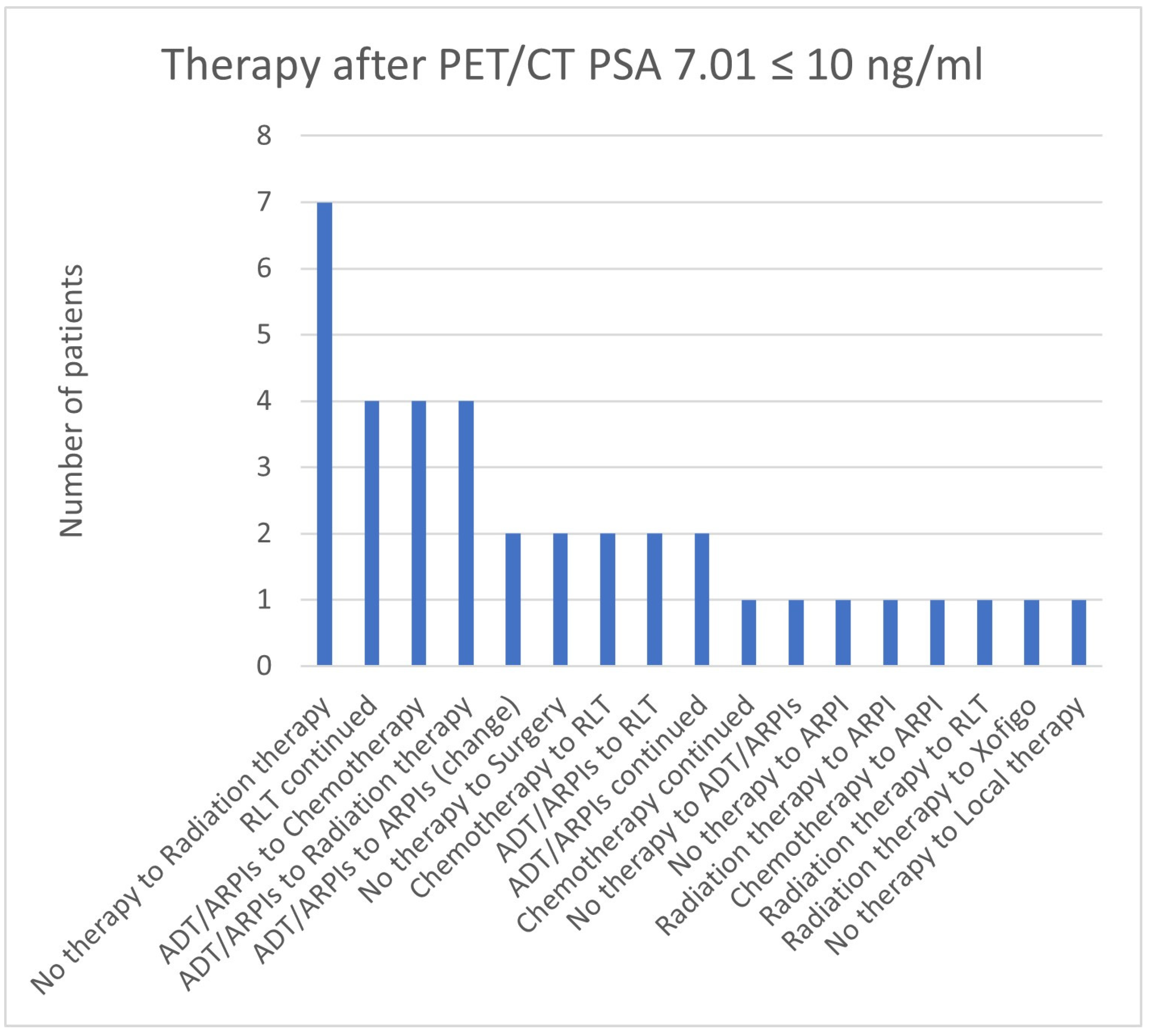
| 7.01 ≤ 10 | 30 | 7 | 37 | 4 | 34/37 (91.9%) |
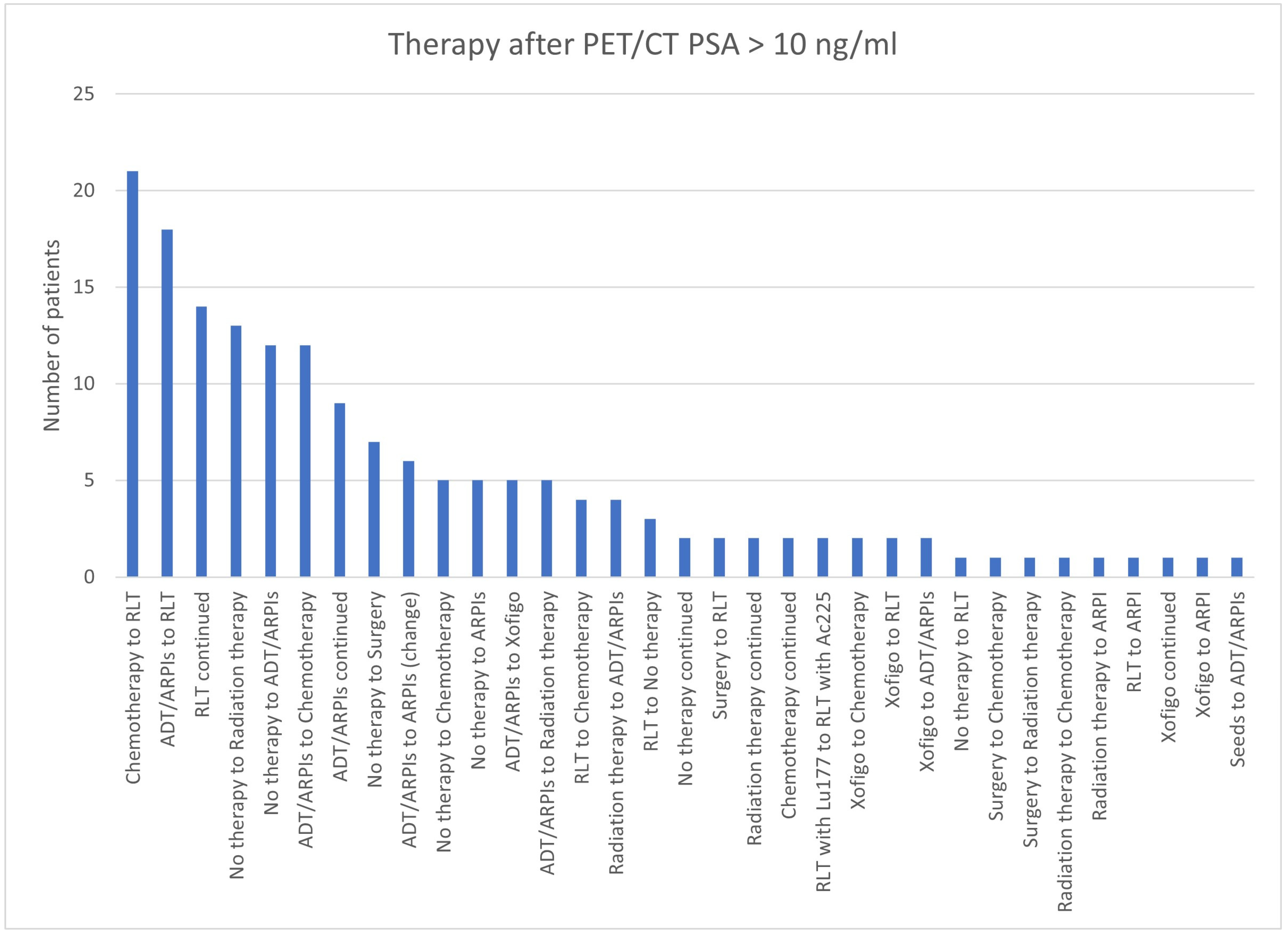
| >10 | 140 | 28 | 168 | 18 | 158/168 (94.0%) |
| 482 | |||||
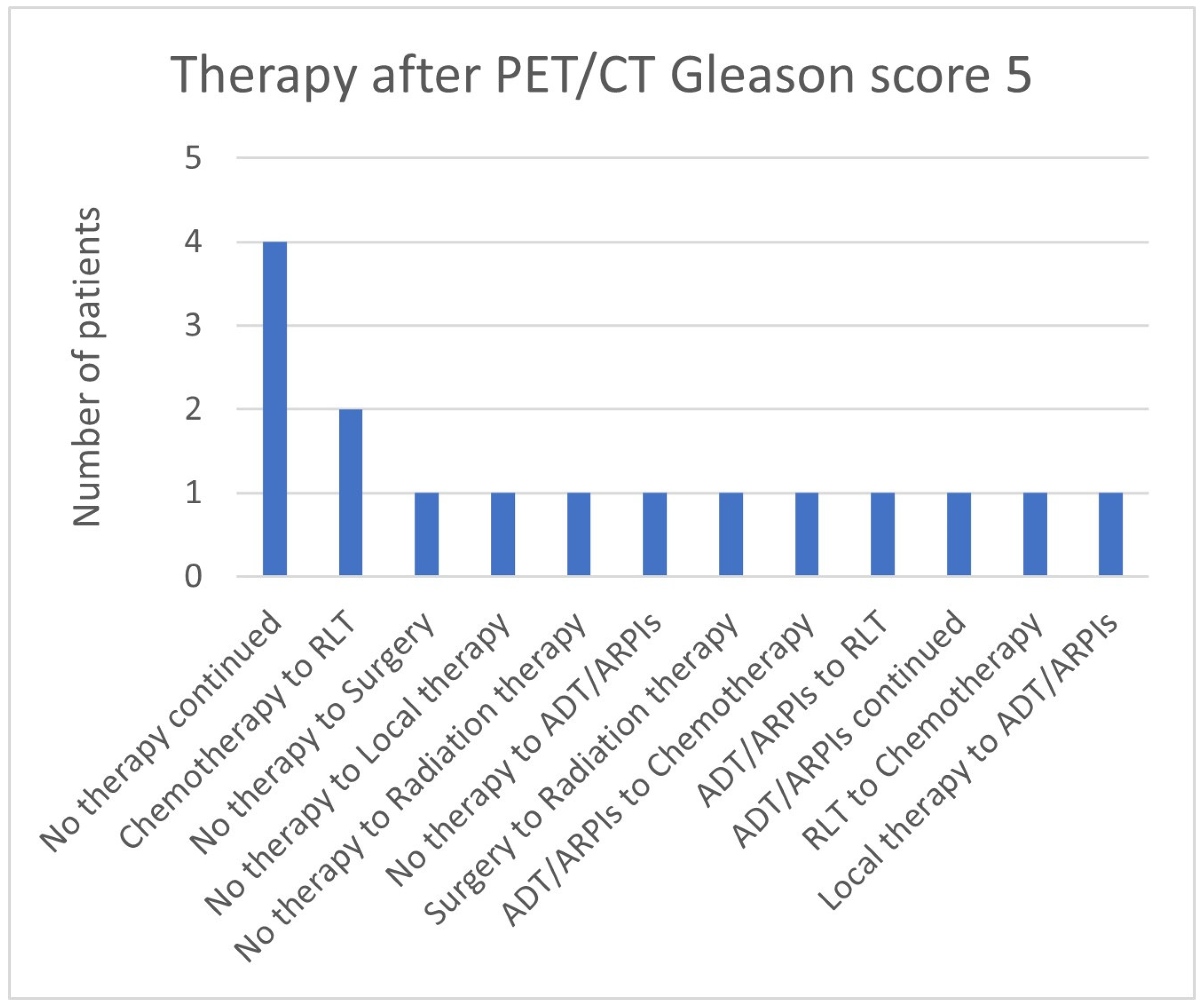
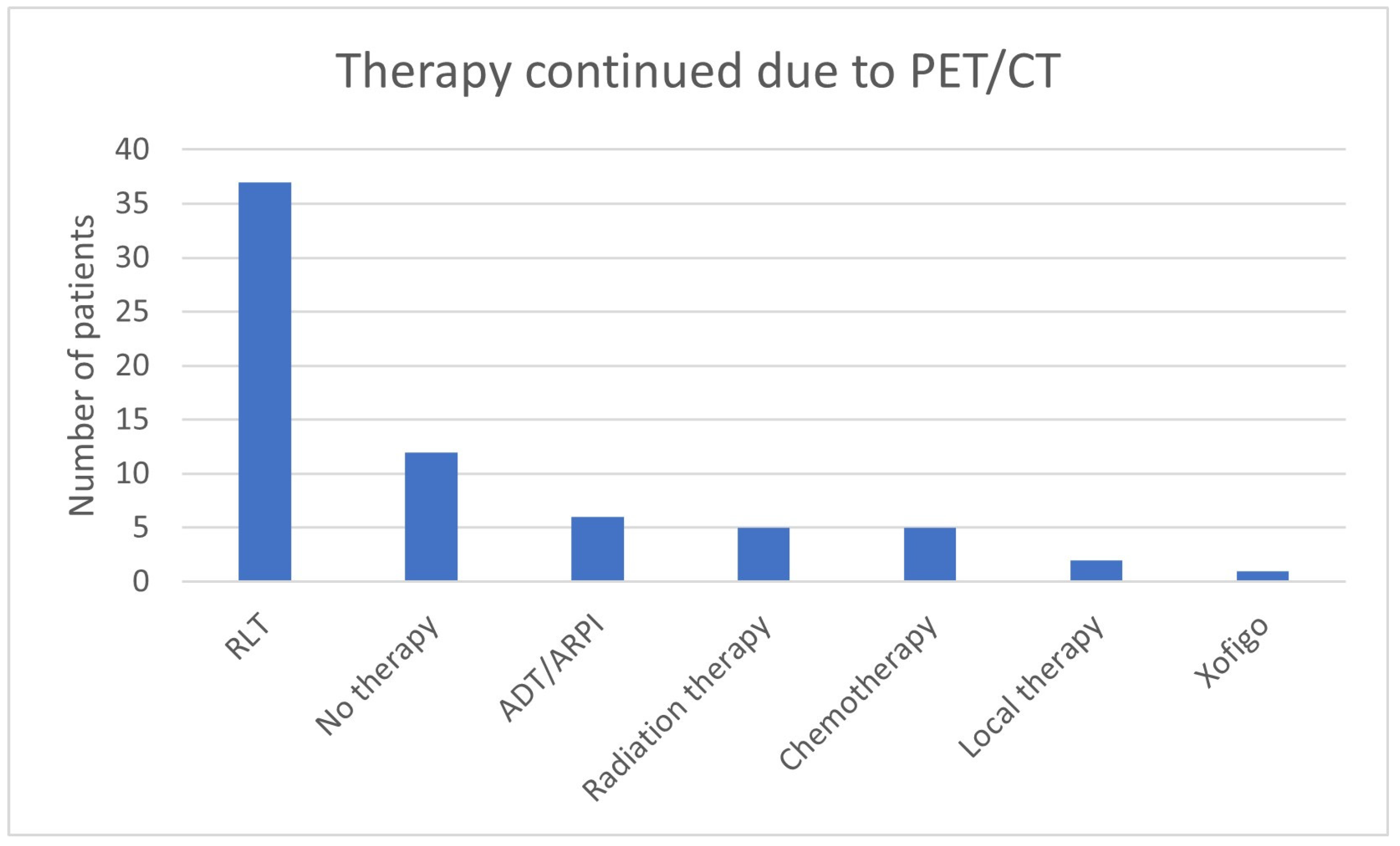
| Therapy Continued Explicitly Due to 68Ga-PSMA-11-PET/CT Findings N = 68 | ||
|---|---|---|
| RLT continued N = 37 | PSA value in ng/mL | |
| Median (Min, Max) | 8.7 (0.2–334) | |
| Gleason Score | ||
| Median (Min, Max) | 8 (6–9) | |
| Unknown | 7 (18.9%) | |
| 6 | 4 (10.1%) | |
| 7 | 5 (13.5%) | |
| 8 | 7 (18.9%) | |
| 9 | 4 (10.1%) | |
| D’Amico | ||
| Unknown | 15 (40.5%) | |
| Intermediate risk | 3 (8.1%) | |
| High risk | 19 (51.4%) | |
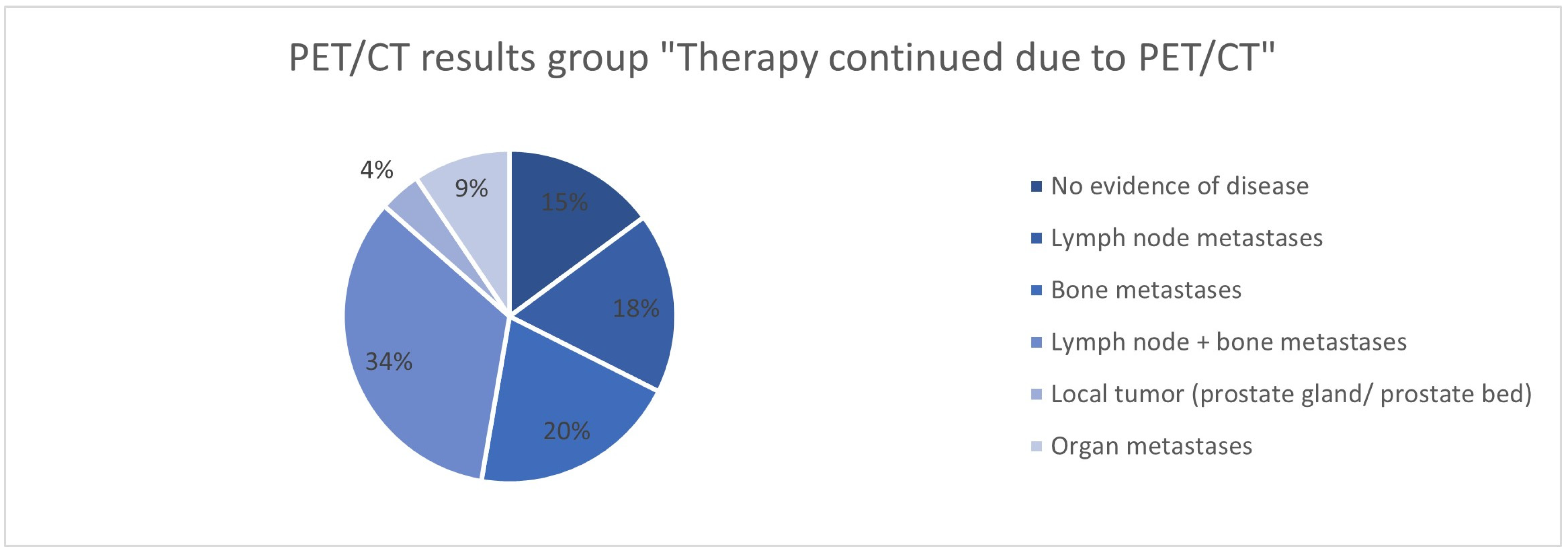
| PET/CT result | ||
| LN Metastases | 5 (13.5%) | |
| Bone Metastases | 10 (27.0%) | |
| LN + Bone Metastases | 21 (56.8%) | |
| Organ Metastases | 5 (13.5%) | |
| No therapy continued N = 12 | PSA value in ng/mL | |
| Median (Min, Max) | 1.7 (0.24–13.5) | |
| Gleason Score | ||
| Median (Min, Max) | 7 (5–9) | |
| Unknown | 2 (16.7%) | |
| 5 | 1 (8.3%) | |
| 6 | 2 (16.7%) | |
| 7 | 6 (50.0%) | |
| 9 | 1 (8.3%) | |
| D’Amico | ||
| Unknown | 2 (16.7%) | |
| Low risk | 2 (16.7%) | |
| Intermediate risk | 1 (8.3%) | |
| High risk | 7 (58.3%) | |
| PET/CT result | ||
| No evidence of disease | 9 (75.0%) | |
| LN Metastases | 2 (16.7%) | |
| Local tumor | 1 (8.3%) | |
| ADT/ARPI continued N = 6 | PSA value in ng/mL | |
| Median (Min, Max) | 2.55 (0.3–546) | |
| Gleason Score | ||
| Median (Min, Max) | 8 (7–9) | |
| Unknown | 1 (16.7%) | |
| 7 | 2 (33.3%) | |
| 8 | 1 (16.7%) | |
| 9 | 2 (33.3%) | |
| D’Amico | ||
| Unknown | 1 (16.7%) | |
| High risk | 5 (83.3%) | |
| PET/CT result | ||
| No evidence of disease | 1 (16.7%) | |
| LN Metastases | 4 (66.7%) | |
| LN + Bone Metastases | 1 (16.7%) | |
| Local tumor | 1 (16.7%) | |
| Radiation therapy continued N = 5 | PSA value in ng/mL | |
| Median (Min, Max) | 8 (0.78–26.3) | |
| Gleason Score | ||
| Median (Min, Max) | 7 (6–9) | |
| Unknown | 2 (40%) | |
| 6 | 1 (20%) | |
| 7 | 1 (20%) | |
| 9 | 1 (20%) | |
| D’Amico | ||
| Unknown | 1 (20%) | |
| Intermediate risk | 1 (20%) | |
| High risk | 3 (60%) | |
| PET/CT result | ||
| No evidence of disease | 1 (20%) | |
| LN Metastases | 2 (40%) | |
| LN + Bone Metastases | 1 (20%) | |
| Local tumor | 1 (20%) | |
| Chemotherapy continued N = 5 | PSA value in ng/mL | |
| Median (Min, Max) | 13.2 (0.04–88) | |
| Gleason Score | ||
| Median (Min, Max) | 8 (6–9) | |
| 6 | 1 (20%) | |
| 7 | 1 (20%) | |
| 8 | 2 | |
| 9 | 1 (20%) | |
| D’Amico | ||
| Unknown | 1 (20%) | |
| High risk | 4 (80%) | |
| PET/CT result | ||
| LN Metastases | 1 (20%) | |
| Bone Metastases | 2 (40%) | |
| LN + Bone Metastases | 1 (20%) | |
| Organ Metastases | 2 4(0%) | |
| Local therapy continued N = 2 | PSA value in ng/mL | |
| Median (Min, Max) | 0.8 (0.5–1.1) | |
| Gleason Score | ||
| Median (Min, Max) | 8 (8.8) | |
| 8 | 2 (100%) | |
| D’Amico | ||
| High risk | 2 (100%) | |
| Bone Metastases | 2 (100%) | |
| Xofigo continued N = 1 | PSA value in ng/mL | 25 |
| Gleason Score | ||
| 9 | 1 (100%) | |
| D’Amico | ||
| High risk | 1 (100%) | |
| Bone Metastases | 1 (100%) | |
| Therapy Decision N = 562 | ||
|---|---|---|
| Therapy continued due to PET/CT N = 68 | PSA value in ng/mL | |
| Median (Min, Max) | 5 (0.04–3349) | |
| Gleason Score | ||
| Median (Min, Max) | 7 (5–9) | |
| Unknown | 22 (32.4%) | |
| 5 | 1 (1.5%) | |
| 6 | 8 (11.8%) | |
| 7 | 15 (10.3%) | |
| 8 | 12 (17.6%) | |
| 9 | 10 (14.7%) | |
| 10 | 0 (0%) | |
| D’Amico | ||
| Unknown | 20 (29.4%) | |
| Low risk | 2 (2.9%) | |
| Intermediate risk | 5 (7.4%) | |
| High risk | 41 (60.3%) | |
| No evidence of disease | 11 (16.2%) | |
| Lymph node metastases | 13 (19.1%) | |
| Bone metastases | 15 (22.1%) | |
| Lymph node + bone metastases | 25 (36.8%) | |
| Local tumor (prostate gland/prostate bed) | 3 (4.4%) | |
| Organ metastases | 7 (10.3%) | |
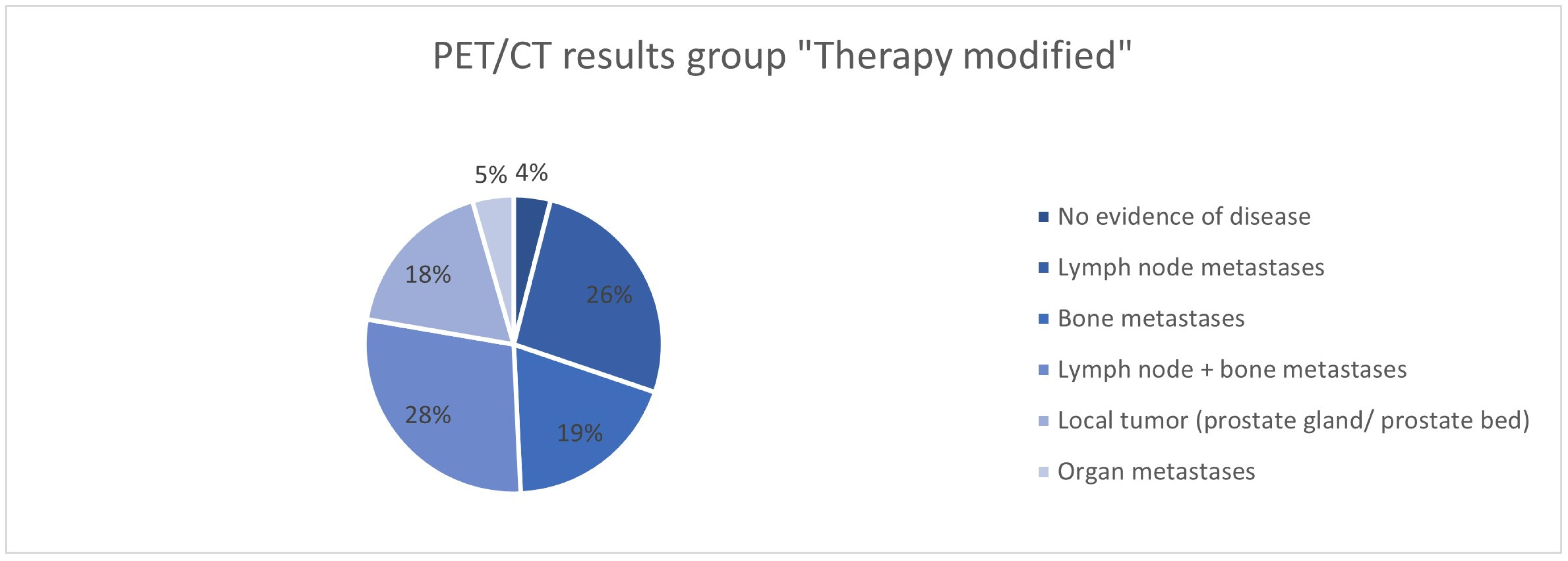
| Therapy modified N = 415 | PSA value in ng/mL | |
| Median (Min, Max) | 6 (0.01–14000) | |
| Gleason Score | ||
| Median (Min, Max) | 7 (5–10) | |
| Unknown | 96 (23.1%) | |
| 5 | 11 (2.7%) | |
| 6 | 28 (6.7%) | |
| 7 | 135 (32.5%) | |
| 8 | 66 (15.9%) | |
| 9 | 69 (16.6%) | |
| 10 | 10 (2.4%) | |
| D’Amico | ||
| Unknown | 142 (34.1%) | |
| Low risk | 11 (2.7%) | |
| Intermediate risk | 9 (2.2%) | |
| High risk | 253 (61.0%) | |
| No evidence of disease | 18 (4.3%) | |
| Lymph node metastases | 117 (28.2%) | |
| Bone metastases | 85 (20.5%) | |
| Lymph node + bone metastases | 127 (30.6%) | |
| Local tumor (prostate gland/prostate bed) | 80 (19.2%) | |
| Organ metastases | 20 (4.8%) | |
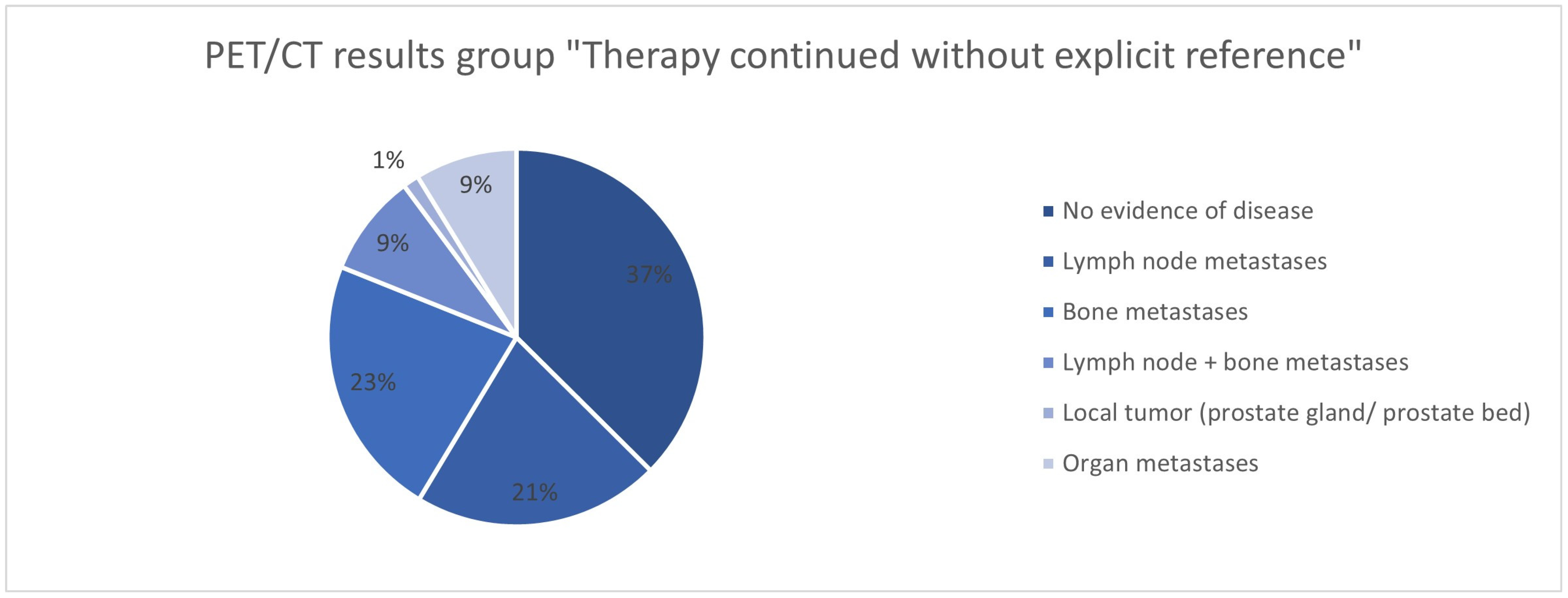
| Therapy continued without explicit reference N = 79 | PSA value in ng/mL | |
| Median (Min, Max) | 2 (0.01–452) | |
| Gleason Score | ||
| Median (Min, Max) | 7 (5–10) | |
| Unknown | 22 (29.3%) | |
| 5 | 4 (5.1%) | |
| 6 | 8 (10.1%) | |
| 7 | 24 (30.4%) | |
| 8 | 7 (8.9%) | |
| 9 | 11 (13.9%) | |
| 10 | 3 (3.8%) | |
| D’Amico | ||
| Unknown | 23 (29.1%) | |
| Low risk | 3 (3.8%) | |
| Intermediate risk | 3 (3.8%) | |
| High risk | 50 (63.3%) | |
| No evidence of disease | 30 (38.0%) | |
| Lymph node metastases | 17 (21.5%) | |
| Bone metastases | 18 (22.8%) | |
| Lymph node + bone metastases | 7 (8.9%) | |
| Local tumor (prostate gland/prostate bed) | 6 (1.4%) | |
| Organ metastases | 7 (8.9%) | |
| PET/CT Result | Therapy |
|---|---|
| Bone and organ metastases | 1 ADT/ARPIs continued |
| Bone metastases | 1 radiation therapy continued |
| Local tumor detection | 4 no therapy to radiation therapy 1 no therapy continued 1 seeds to surgery |
| Lymph node and bone metastases | 1 chemotherapy to RLT 1 seeds to ADT/ARPIs |
| Lymph node metastases | 3 no therapy continued 1 no therapy to ADT/ARPIs 1 chemotherapy to radiation therapy |
| Lymph node, bone, and organ metastases | 1 ADT/ARPIs to chemotherapy |
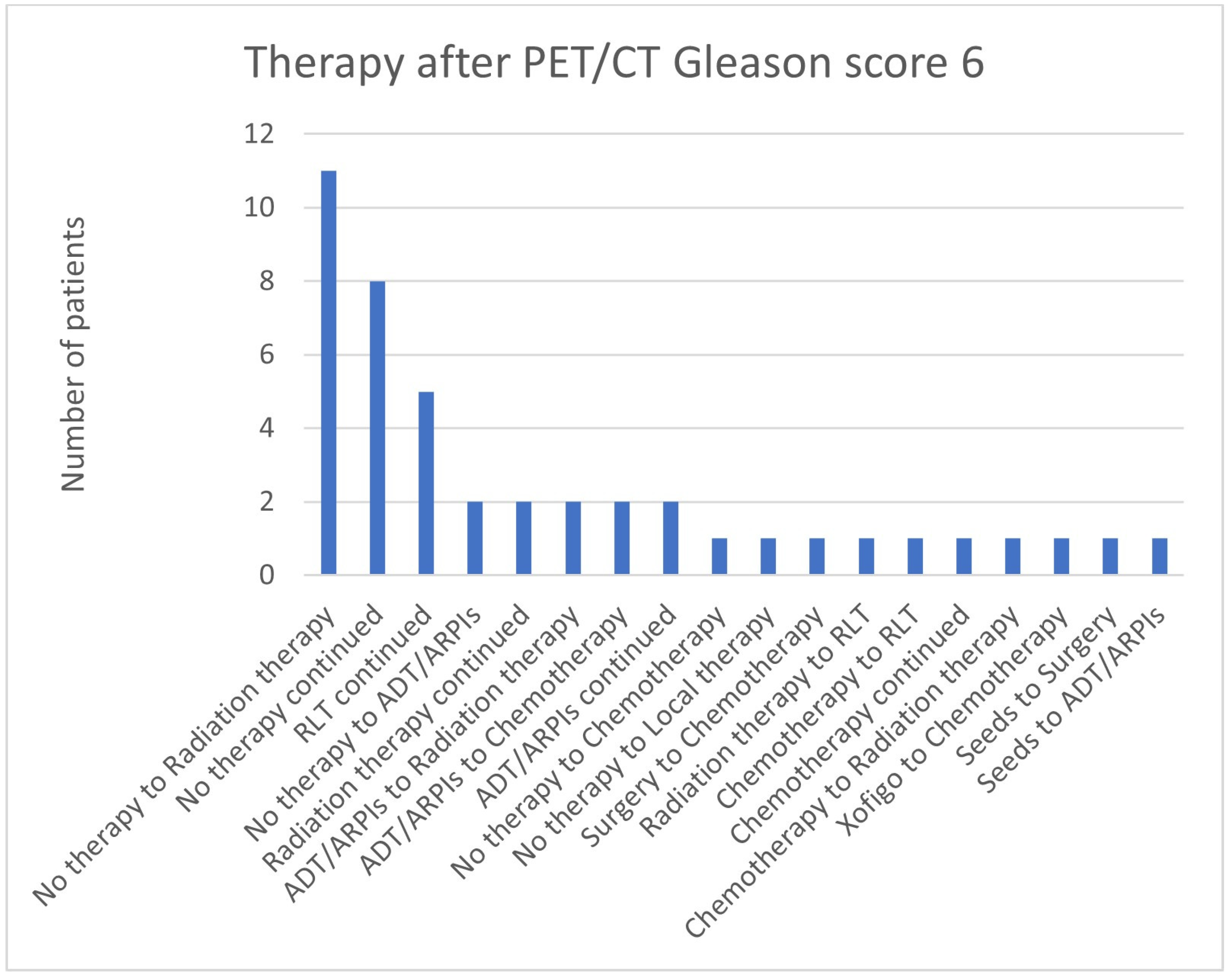
| PET/CT Result | Therapy |
|---|---|
| Bone metastases | 1 no therapy to chemotherapy 3 RLT continued 1 no therapy to radiation therapy |
| Local tumor detection | 2 no therapy to radiation therapy |
| Lymph node and bone metastases | 1 no therapy to ADT/ARPIs 1 radiation therapy to PRRT |
| Lymph node metastases | 1 surgery to radiation therapy 1 radiation therapy continued 1 ADT/ARPIs continued |
| Lymph node metastases and local tumor detection | 1 no therapy to radiation therapy |
| No tumor lesion is imageable | 3 no therapy continued 1 radiation therapy continued |
| PET/CT Result | Therapy |
|---|---|
| Bone metastases | 1 Radiation Therapy to ARPI 1 ADT/ARPIs continued 1 no therapy continued |
| Local tumor detection | 1 ADT/ARPIs continued |
| Lymph node metastases | 1 no therapy continued 1 RLT continued 1 chemotherapy continued 1 chemotherapy to RLT 1 RLT to no therapy |
| No tumor lesion imageable | 1 RLT to no therapy 3 no therapy continued 1 ADT/ARPIs continued |
| PET/CT Result | Therapy |
|---|---|
| Bone metastases | 1 local therapy continued 1 no therapy to radiation therapy |
| Local tumor detection | 1 surgery continued 1 ADT/ARPIs to local therapy 1 no therapy to radiation therapy 1 surgery to radiation therapy |
| Lymph node and bone metastases | 1 RLT continued 1 chemotherapy continued 1 ADT/ARPIs continued |
| Lymph node metastases | 3 no therapy to radiation therapy 1 surgery to RLT |
| No tumor lesion imageable | 7 no therapy continued 2 surgery to radiation therapy 1 ADT/ARPIs continued 1 ADT/ARPIs to radiation therapy 1 no therapy to radiation therapy |
| Organ metastases | 1 local therapy continued 1 radiation therapy to ADT/ARPIs |
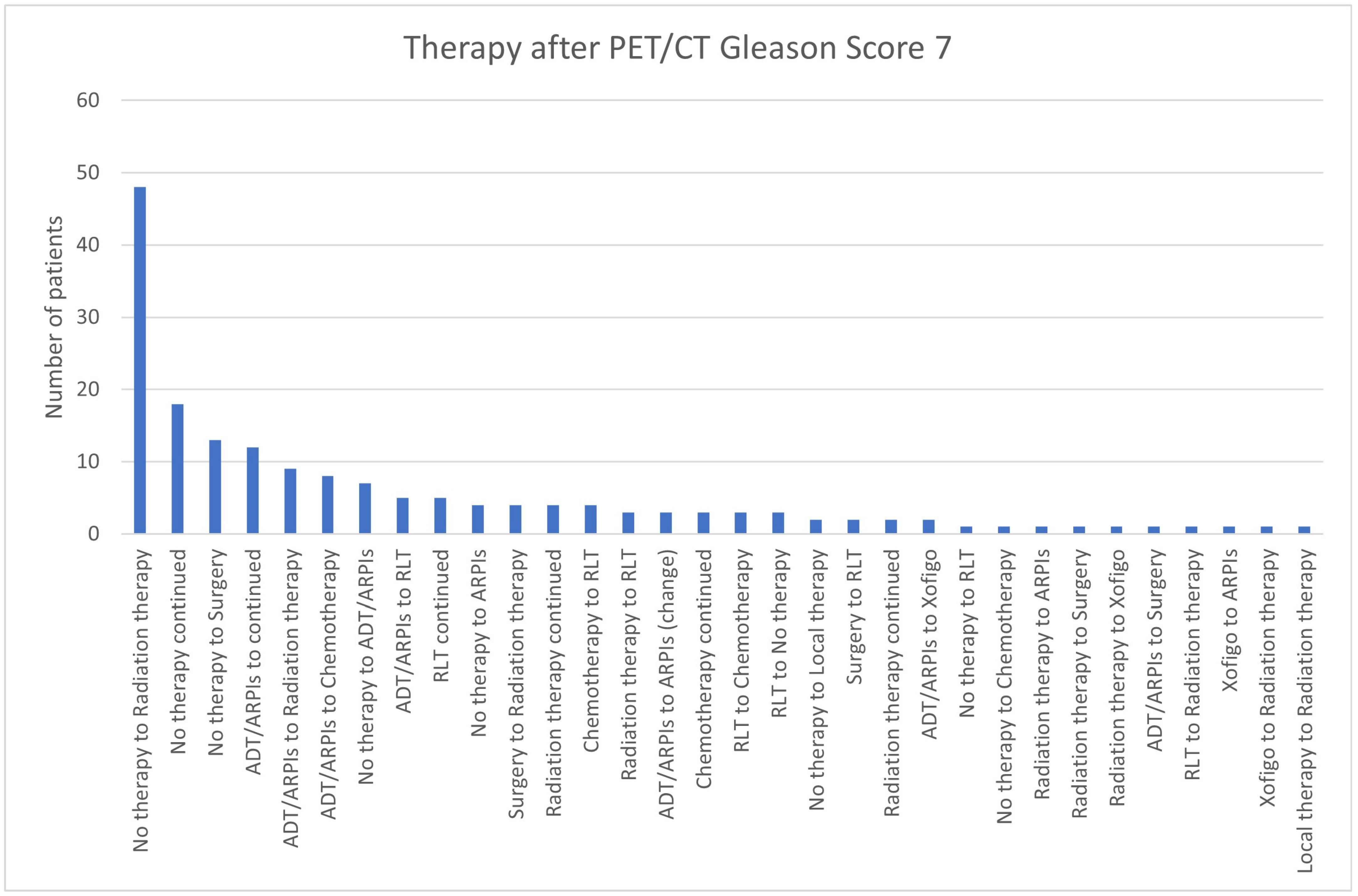
| PET/CT Result | Therapy |
|---|---|
| Bone metastases | 1 no therapy to ADT/ARPIs 2 RLT continued 1 chemotherapy continued 1 ADT/ARPIs to chemotherapy 1 local therapy continued 1 surgery to radiation therapy 1 no therapy to radiation therapy |
| Local tumor detection | 2 ADT/ARPIs to radiation therapy 4 no therapy to radiation therapy |
| Lymph node and bone metastases | 1 radiation therapy continued 1 ADT/ARPIs to radiation therapy 1 no therapy to ADT/ARPIs |
| Lymph node metastases | 13 no therapy to radiation therapy 2 no therapy to surgery 1 ADT/ARPIs to RLT 2 no therapy continued |
| No tumor lesion imageable | 6 no therapy continued 1 ADT/ARPIs to surgery 2 no therapy to radiation therapy |
| Organ metastases | 1 no therapy to radiation therapy |
| PET/CT Result | Therapy |
|---|---|
| Bone metastases | 3 no therapy to radiation therapy 5 no therapy to ARPIs 3 radiation therapy to ADT/ARPIs 2 RLT continued 1 local therapy continued 1 local therapy to radiation therapy 2 no therapy continued |
| Local tumor detection | 1 no therapy to surgery 2 no therapy to local therapy 1 radiation therapy to surgery 2 no therapy to radiation therapy 1 ADT/ARPI to radiation therapy |
| Local tumor detection and organ metastases | 1 no therapy to radiation therapy |
| Lymph node and bone metastases | 1 local therapy continued 1 no therapy to radiation therapy 1 ADT/ARPIs to radiation therapy 1 no therapy to ADT/ARPIs |
| Lymph node and bone metastases and local tumor detection | 1 radiation therapy to ADT/ARPIs |
| Lymph node metastases | 1 no therapy to ADT/ARPIs 7 ADT/ARPIs to radiation therapy 3 ADT/ARPIs continued 1 ADT/ARPIs to ARPIs (change) 2 ADT/ARPIs to RLT 3 ADT/ARPIs to chemotherapy 2 chemotherapy to RLT 3 no therapy to local therapy 1 radiation therapy continued 1 no therapy continued 1 no therapy to surgery 1 no therapy to radiation therapy |
| Lymph node metastases and local tumor detection | 2 no therapy to radiation therapy 1 no therapy to local therapy 1 ADT/ARPIs continued |
| Lymph node, bone, and organ metastases | 1 ADT/ARPIs to RLT |
| No tumor lesion imageable | 1 no therapy continued 1 radiation therapy continued 1 ADT/ARPIs continued 1 no therapy to ADT/ARPIs 2 no therapy to radiation therapy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eilsberger, F.; Ebrahimifard, A.; Spiegel, F.T.; Yousefi, B.H.; Bagheri, S.; Bowl, W.; Wang, Q.; Pfestroff, A.; Müller, L.; Luster, M.; et al. Therapeutic Consequences of 68Ga-PSMA-11-PET/CT in Prostate Cancer in Correlation to the Gleason Score, PSA Value, and D’Amico-Defined Risk Groups. Cancers 2025, 17, 1944. https://doi.org/10.3390/cancers17121944
Eilsberger F, Ebrahimifard A, Spiegel FT, Yousefi BH, Bagheri S, Bowl W, Wang Q, Pfestroff A, Müller L, Luster M, et al. Therapeutic Consequences of 68Ga-PSMA-11-PET/CT in Prostate Cancer in Correlation to the Gleason Score, PSA Value, and D’Amico-Defined Risk Groups. Cancers. 2025; 17(12):1944. https://doi.org/10.3390/cancers17121944
Chicago/Turabian StyleEilsberger, Friederike, Ali Ebrahimifard, Florian Torsten Spiegel, Behrooz Hooshyar Yousefi, Shamim Bagheri, Wadim Bowl, Qi Wang, Andreas Pfestroff, Laura Müller, Markus Luster, and et al. 2025. "Therapeutic Consequences of 68Ga-PSMA-11-PET/CT in Prostate Cancer in Correlation to the Gleason Score, PSA Value, and D’Amico-Defined Risk Groups" Cancers 17, no. 12: 1944. https://doi.org/10.3390/cancers17121944
APA StyleEilsberger, F., Ebrahimifard, A., Spiegel, F. T., Yousefi, B. H., Bagheri, S., Bowl, W., Wang, Q., Pfestroff, A., Müller, L., Luster, M., Di Fazio, P., & Librizzi, D. (2025). Therapeutic Consequences of 68Ga-PSMA-11-PET/CT in Prostate Cancer in Correlation to the Gleason Score, PSA Value, and D’Amico-Defined Risk Groups. Cancers, 17(12), 1944. https://doi.org/10.3390/cancers17121944







