Upfront Anti-CD38 Monoclonal Antibody-Based Quadruplet Therapy for Multiple Myeloma: A Systematic Review and Meta-Analysis of Clinical Trials
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- ((“Multiple Myeloma”[Mesh] OR “multiple myeloma”[tiab]) AND
- (“Daratumumab”[Mesh] OR “Daratumumab”[tiab] OR “DARA”[tiab]) AND
- ((“Bortezomib”[Mesh] OR “Bortezomib”[tiab] OR “VRD”[tiab] OR “VTD”[tiab]) OR
- (“Carfilzomib”[Mesh] OR “Carfilzomib”[tiab] OR “KRD”[tiab])) AND
- (“Lenalidomide”[Mesh] OR “Lenalidomide”[tiab] OR “Thalidomide”[Mesh] OR “Thalidomide”[tiab]) AND (“Dexamethasone”[Mesh] OR “Dexamethasone”[tiab]))
2.1. Data Abstraction and Effect Estimates
2.2. Statistical Analyses
2.3. Assessment of Study Quality and Risk of Bias
3. Results
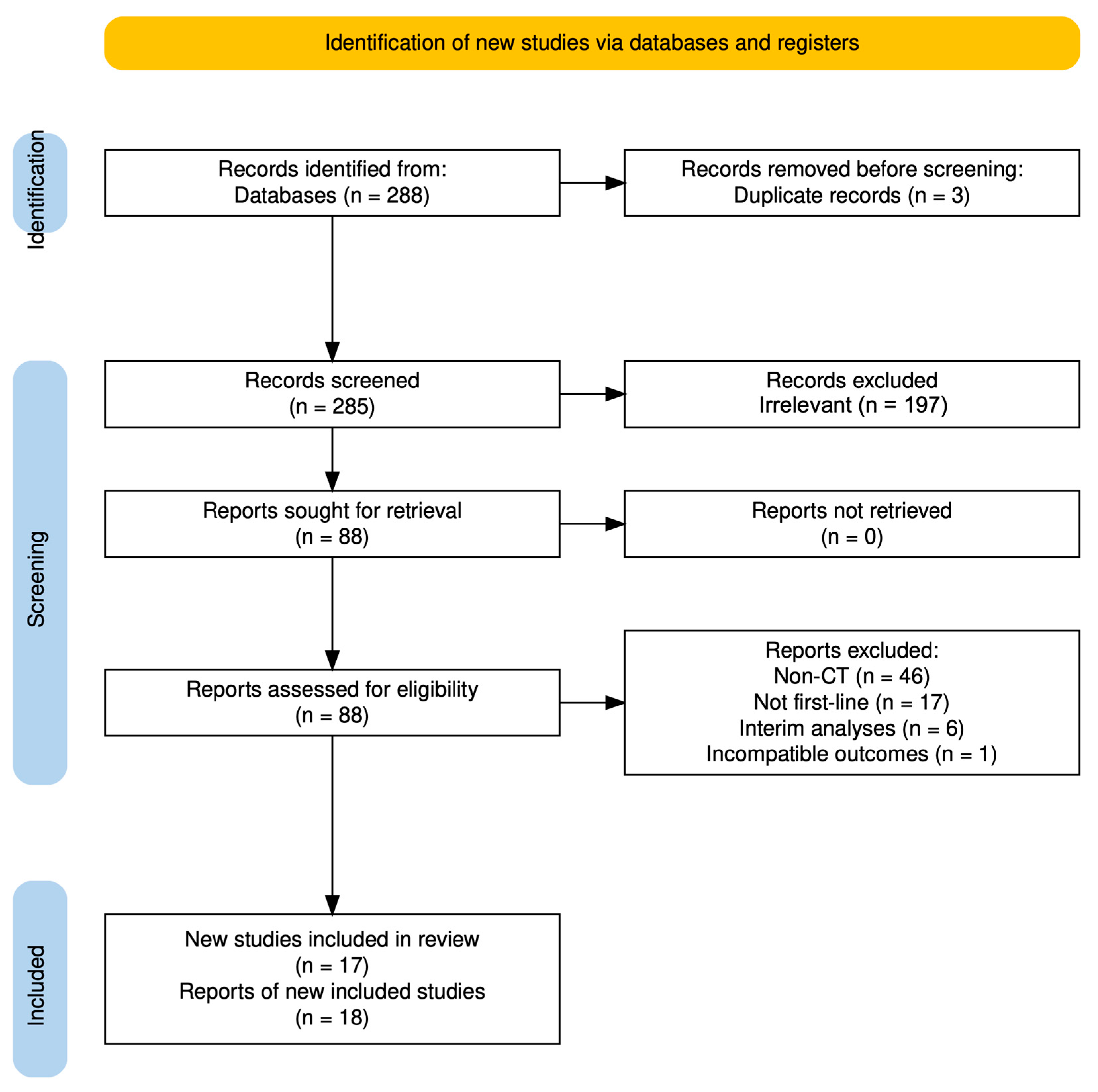
3.1. Selection of Studies
3.2. Progression-Free Survival
3.3. High-Risk Cytogenetic Exploratory PFS Sub-Analysis
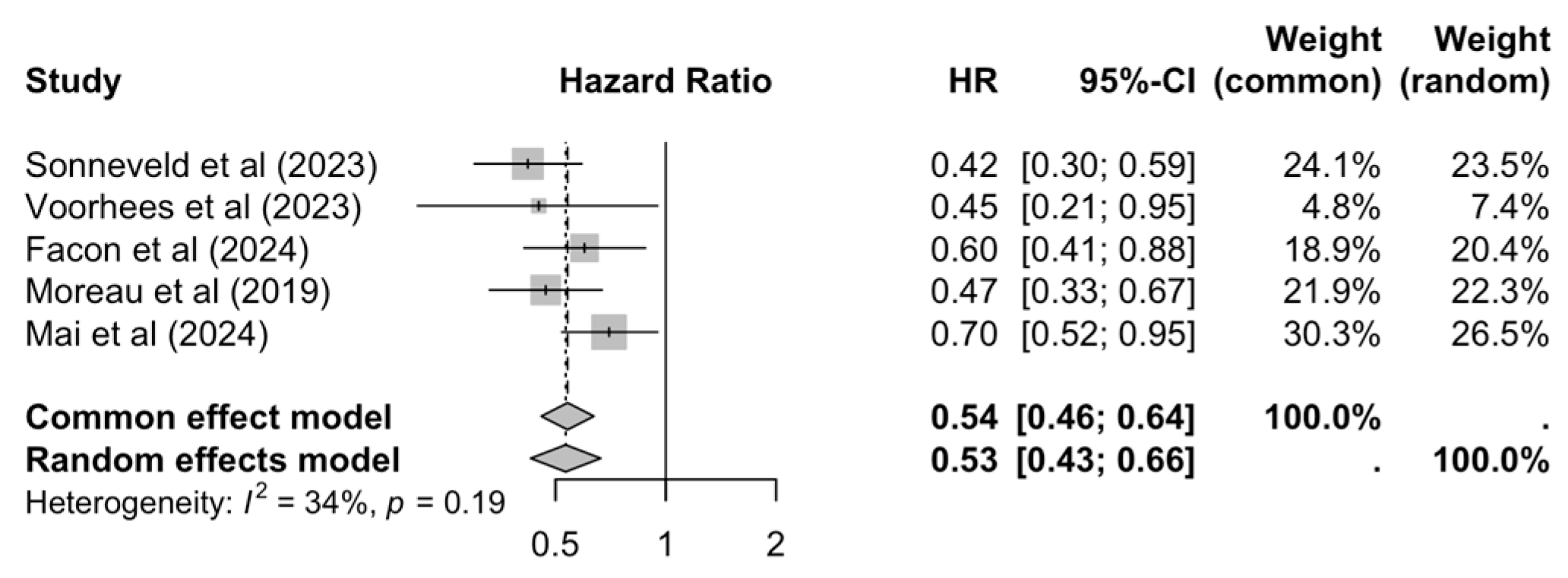
3.4. Overall Survival
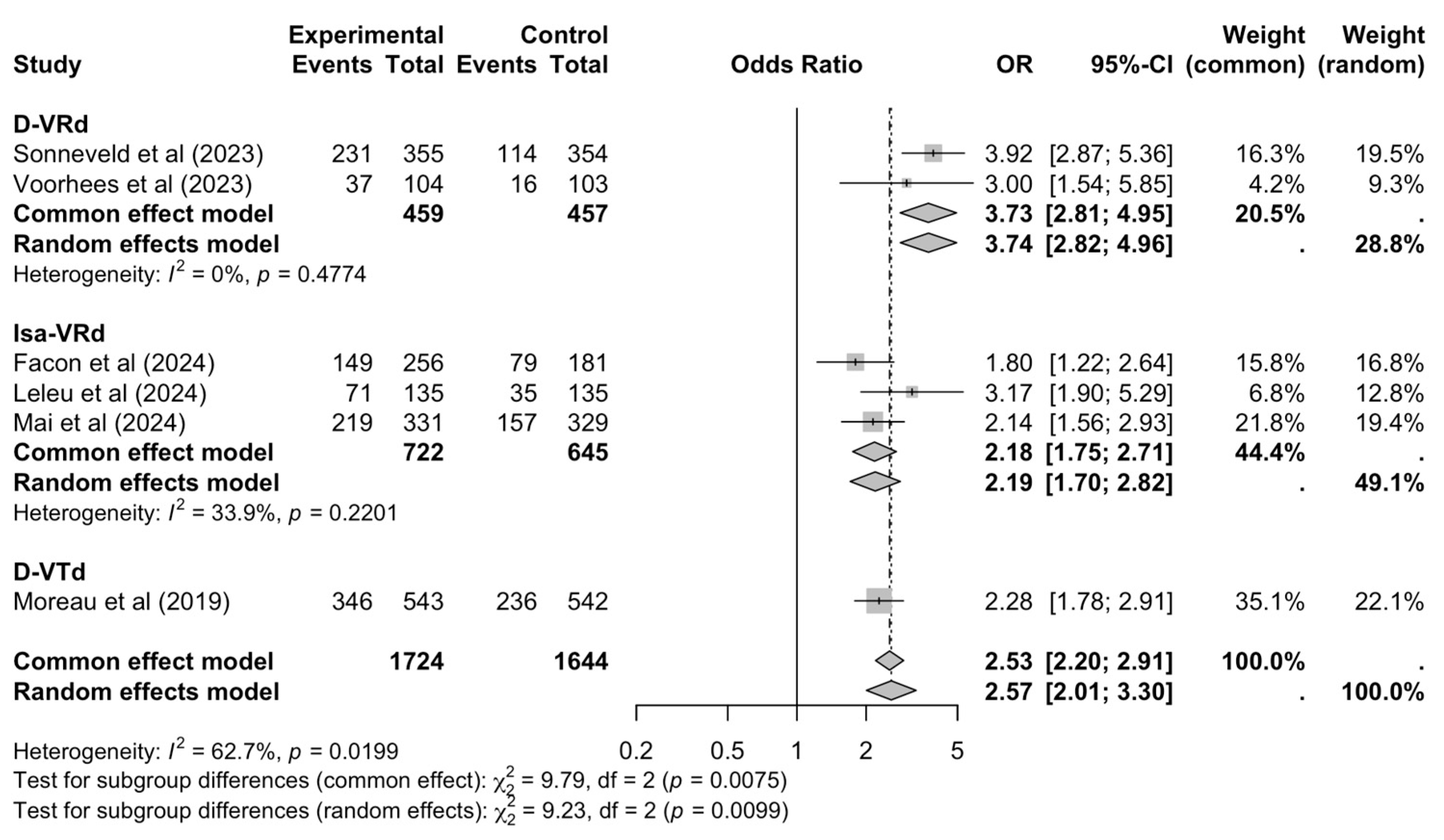
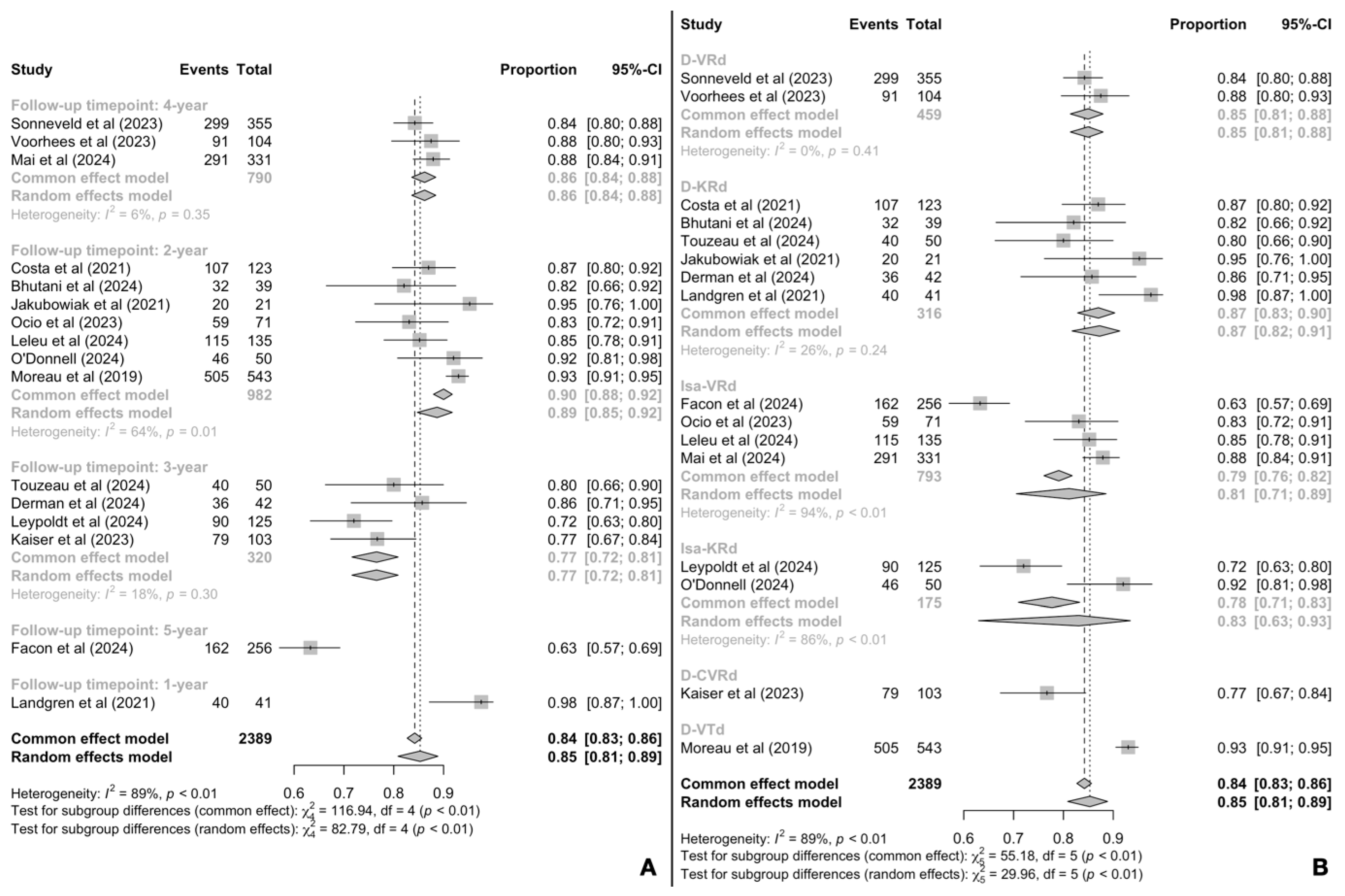
3.5. Complete Response Rates
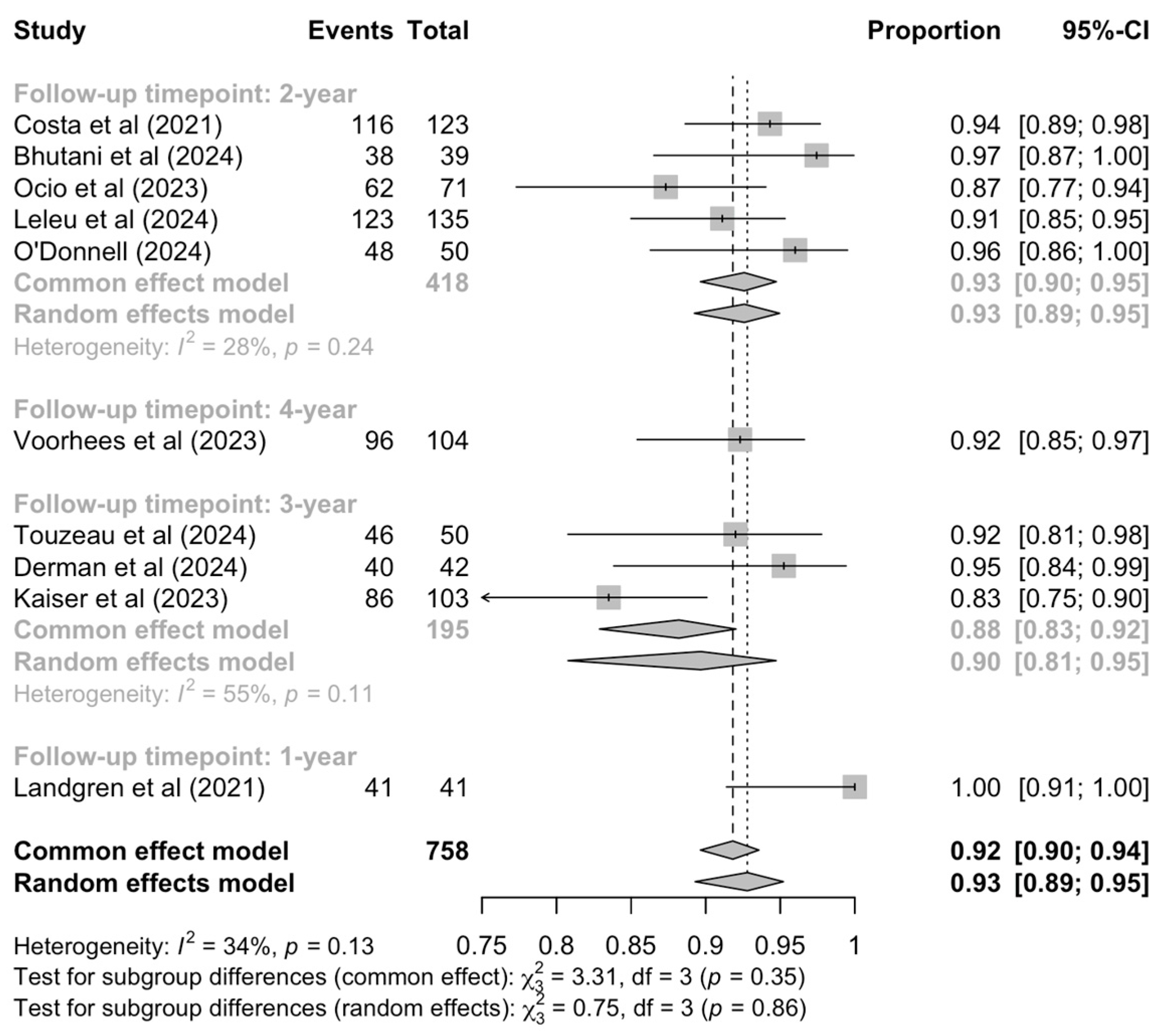
3.6. Minimal Residual Disease Negativity
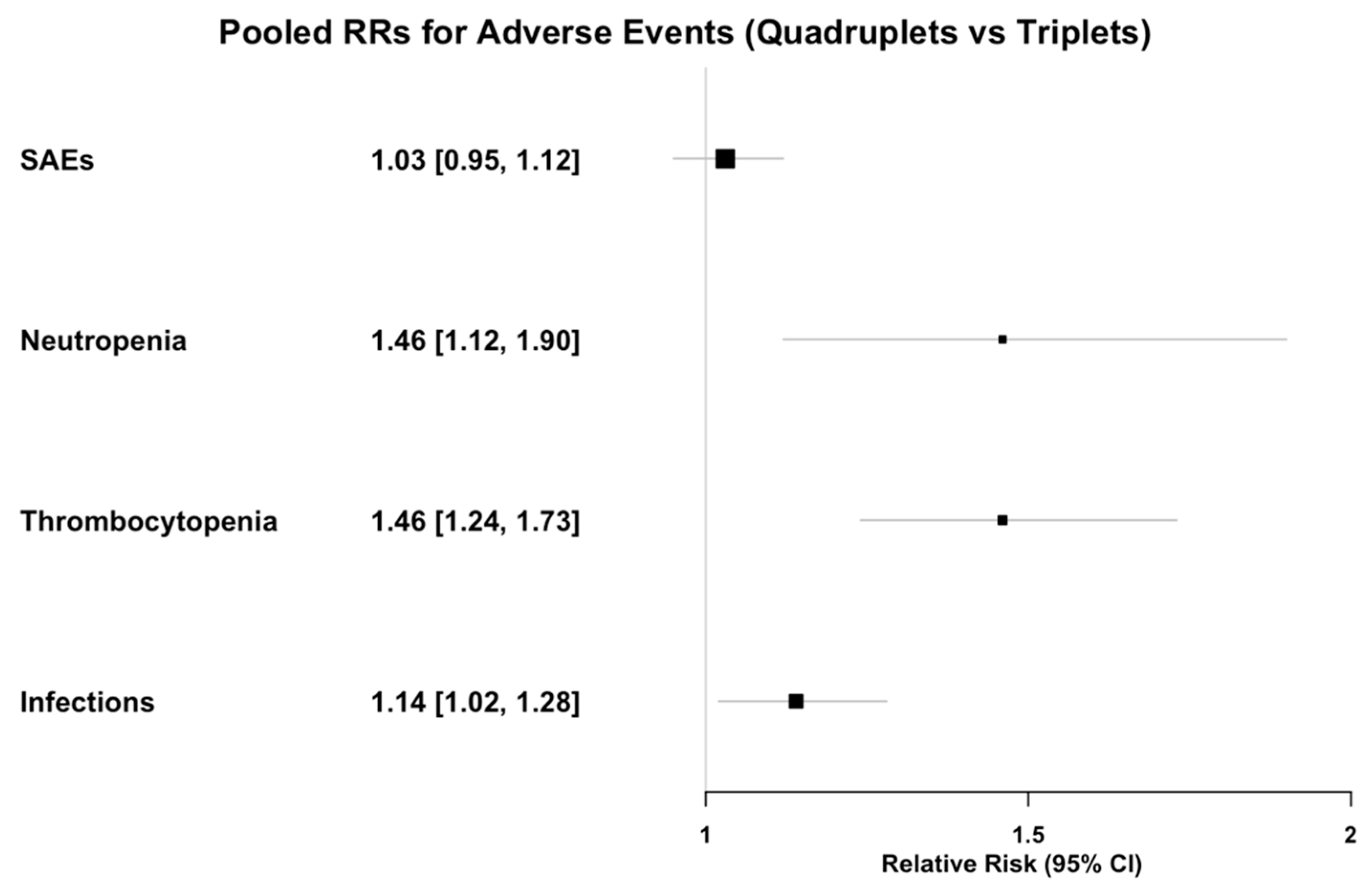
3.7. Safety
3.8. Risk of Bias Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sherbenou, D.W.; Mark, T.M.; Forsberg, P. Monoclonal Antibodies in Multiple Myeloma: A New Wave of the Future. Clin. Lymphoma Myeloma Leuk. 2017, 17, 545–554. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, M.; Gazzera, G.; Cetani, G.; Bringhen, S.; Boccadoro, M.; Gay, F. Clinical and Pharmacologic Features of Monoclonal Antibodies and Checkpoint Blockade Therapy in Multiple Myeloma. Curr. Med. Chem. 2019, 26, 5968–5981. [Google Scholar] [CrossRef] [PubMed]
- van de Donk, N.W.C.J.; Richardson, P.G.; Malavasi, F. CD38 antibodies in multiple myeloma: Back to the future. Blood 2018, 131, 13–29. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, M.; Mina, R.; Gay, F. Anti-CD38 monoclonal antibodies in multiple myeloma: Another cook in the kitchen? Lancet Haematol. 2020, 7, e355–e357. [Google Scholar] [CrossRef] [PubMed]
- van de Donk, N.W.C.J.; Usmani, S.Z. CD38 Antibodies in Multiple Myeloma: Mechanisms of Action and Modes of Resistance. Front. Immunol. 2018, 9, 2134. [Google Scholar] [CrossRef]
- McEllistrim, C.; Krawczyk, J.; O’Dwyer, M.E. New developments in the treatment of multiple myeloma-clinical utility of daratumumab. Biologics 2017, 11, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Phipps, C.; Chen, Y.; Gopalakrishnan, S.; Tan, D. Daratumumab and its potential in the treatment of multiple myeloma: Overview of the preclinical and clinical development. Ther. Adv. Hematol. 2015, 6, 120–127. [Google Scholar] [CrossRef]
- Offidani, M.; Corvatta, L.; Morè, S.; Nappi, D.; Martinelli, G.; Olivieri, A.; Cerchione, C. Daratumumab for the Management of Newly Diagnosed and Relapsed/Refractory Multiple Myeloma: Current and Emerging Treatments. Front. Oncol. 2021, 10, 624661. [Google Scholar] [CrossRef]
- Bonello, F.; Grasso, M.; D’Agostino, M.; Celeghini, I.; Castellino, A.; Boccadoro, M.; Bringhen, S. The Role of Monoclonal Antibodies in the First-Line Treatment of Transplant-Ineligible Patients with Newly Diagnosed Multiple Myeloma. Pharmaceuticals 2020, 14, 20. [Google Scholar] [CrossRef]
- Abdallah, N.; Kumar, S.K. Daratumumab in untreated newly diagnosed multiple myeloma. Ther. Adv. Hematol. 2019, 10, 2040620719894871. [Google Scholar] [CrossRef]
- Popat, R.; Chavda, S.J. Daratumumab combinations for patients with newly diagnosed and relapsed multiple myeloma. Lancet Haematol. 2023, 10, e788–e789. [Google Scholar] [CrossRef]
- Richardson, P.G.; Weller, E.; Lonial, S.; Jakubowiak, A.J.; Jagannath, S.; Raje, N.S.; Avigan, D.E.; Xie, W.; Ghobrial, I.M.; Schlossman, R.L.; et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood 2010, 116, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.G.; Jacobus, S.J.; Weller, E.A.; Hassoun, H.; Lonial, S.; Raje, N.S.; Medvedova, E.; McCarthy, P.L.; Libby, E.N.; Voorhees, P.M.; et al. Triplet Therapy, Transplantation, and Maintenance until Progression in Myeloma. N. Engl. J. Med. 2022, 387, 132–147. [Google Scholar] [CrossRef]
- Joseph, N.S.; Kaufman, J.L.; Dhodapkar, M.V.; Hofmeister, C.C.; Almaula, D.K.; Heffner, L.T.; Gupta, V.A.; Boise, L.H.; Lonial, S.; Nooka, A.K. Long-Term Follow-Up Results of Lenalidomide, Bortezomib, and Dexamethasone Induction Therapy and Risk-Adapted Maintenance Approach in Newly Diagnosed Multiple Myeloma. J. Clin. Oncol. 2020, 38, 1928–1937. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Hulin, C.; Perrot, A.; Arnulf, B.; Belhadj, K.; Benboubker, L.; Béné, M.C.; Zweegman, S.; Caillon, H.; Caillot, D.; et al. Maintenance with daratumumab or observation following treatment with bortezomib, thalidomide, and dexamethasone with or without daratumumab and autologous stem-cell transplant in patients with newly diagnosed multiple myeloma (CASSIOPEIA): An open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 1378–1390. [Google Scholar] [CrossRef] [PubMed]
- Voorhees, P.M.; Sborov, D.W.; Laubach, J.; Kaufman, J.L.; Reeves, B.; Rodriguez, C.; Chari, A.; Silbermann, R.; Costa, L.J.; Anderson, L.D., Jr.; et al. Addition of daratumumab to lenalidomide, bortezomib, and dexamethasone for transplantation-eligible patients with newly diagnosed multiple myeloma (GRIFFIN): Final analysis of an open-label, randomised, phase 2 trial. Lancet Haematol. 2023, 10, e825–e837. [Google Scholar] [CrossRef]
- Sonneveld, P.; Dimopoulos, M.A.; Boccadoro, M.; Quach, H.; Ho, P.J.; Beksac, M.; Hulin, C.; Antonioli, E.; Leleu, X.; Mangiacavalli, S.; et al. Daratumumab, Bortezomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2024, 390, 301–313. [Google Scholar] [CrossRef]
- Facon, T.; Dimopoulos, M.A.; Leleu, X.P.; Beksac, M.; Pour, L.; Hájek, R.; Liu, Z.; Minarik, J.; Moreau, P.; Romejko-Jarosinska, J.; et al. Isatuximab, Bortezomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2024, 391, 1597–1609. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Cheema, H.A.; Shahid, A.; Ehsan, M.; Ayyan, M. The misuse of funnel plots in meta-analyses of proportions: Are they really useful? Clin. Kidney J. 2022, 15, 1209–1210. [Google Scholar] [CrossRef]
- Hunter, J.P.; Saratzis, A.; Sutton, A.J.; Boucher, R.H.; Sayers, R.D.; Bown, M.J. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J. Clin. Epidemiol. 2014, 67, 897–903. [Google Scholar] [CrossRef]
- Lin, L.; Chu, H. Quantifying publication bias in meta-analysis. Biometrics 2018, 74, 785–794. [Google Scholar] [CrossRef]
- Sterne, J.A.; Egger, M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J. Clin. Epidemiol. 2001, 54, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.J.; Chhabra, S.; Medvedova, E.; Dholaria, B.R.; Schmidt, T.M.; Godby, K.N.; Silbermann, R.; Dhakal, B.; Bal, S.; Giri, S.; et al. Daratumumab, Carfilzomib, Lenalidomide, and Dexamethasone With Minimal Residual Disease Response-Adapted Therapy in Newly Diagnosed Multiple Myeloma. J. Clin. Oncol. 2022, 40, 2901–2912. [Google Scholar] [CrossRef] [PubMed]
- Bhutani, M.; Robinson, M.; Foureau, D.; Atrash, S.; Paul, B.A.; Guo, F.; Grayson, J.; Ivanina-Foureau, A.; Pineda-Roman, M.; Varga, C.; et al. MRD-driven phase 2 study of daratumumab, carfilzomib, lenalidomide and dexamethasone in newly diagnosed multiple myeloma. Blood Adv. 2025, 9, 507–519. [Google Scholar] [CrossRef]
- Touzeau, C.; Perrot, A.; Hulin, C.; Manier, S.; Macro, M.; Chretien, M.L.; Karlin, L.; Escoffre, M.; Jacquet, C.; Tiab, M.; et al. Daratumumab, carfilzomib, lenalidomide, and dexamethasone with tandem transplant for high-risk newly diagnosed myeloma. Blood 2024, 143, 2029–2036. [Google Scholar] [CrossRef]
- Chari, A.; Rodriguez-Otero, P.; McCarthy, H.; Suzuki, K.; Hungria, V.; Sureda Balari, A.; Perrot, A.; Hulin, C.; Magen, H.; Iida, S.; et al. Subcutaneous daratumumab plus standard treatment regimens in patients with multiple myeloma across lines of therapy (PLEIADES): An open-label Phase II study. Br. J. Haematol. 2021, 192, 869–878. [Google Scholar] [CrossRef]
- Jakubowiak, A.; Usmani, S.Z.; Krishnan, A.; Lonial, S.; Comenzo, R.L.; Wang, J.; de Boer, C.; Deraedt, W.; Weiss, B.M.; Schecter, J.M.; et al. Daratumumab Plus Carfilzomib, Lenalidomide, and Dexamethasone in Patients With Newly Diagnosed Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2021, 21, 701–710. [Google Scholar] [CrossRef]
- Derman, B.A.; Cooperrider, J.; Rosenblatt, J.; Avigan, D.E.; Rampurwala, M.; Barnidge, D.; Major, A.; Karrison, T.; Jiang, K.; Ramsland, A.; et al. Final analysis of a phase II trial of daratumumab, carfilzomib, lenalidomide, and dexamethasone in newly diagnosed multiple myeloma without transplant. Blood Cancer J. 2024, 14, 87. [Google Scholar] [CrossRef] [PubMed]
- Leypoldt, L.B.; Tichy, D.; Besemer, B.; Hänel, M.; Raab, M.S.; Mann, C.; Munder, M.; Reinhardt, H.C.; Nogai, A.; Görner, M.; et al. Isatuximab, Carfilzomib, Lenalidomide, and Dexamethasone for the Treatment of High-Risk Newly Diagnosed Multiple Myeloma. J. Clin. Oncol. 2024, 42, 26–37. [Google Scholar] [CrossRef]
- Kaiser, M.F.; Hall, A.; Walker, K.; Sherborne, A.; De Tute, R.M.; Newnham, N.; Roberts, S.; Ingleson, E.; Bowles, K.; Garg, M.; et al. Daratumumab, Cyclophosphamide, Bortezomib, Lenalidomide, and Dexamethasone as Induction and Extended Consolidation Improves Outcome in Ultra-High-Risk Multiple Myeloma. J. Clin. Oncol. 2023, 41, 3945–3955. [Google Scholar] [CrossRef]
- Mai, E.K.; Bertsch, U.; Pozek, E.; Fenk, R.; Besemer, B.; Hanoun, C.; Schroers, R.; von Metzler, I.; Hänel, M.; Mann, C.; et al. Isatuximab, Lenalidomide, Bortezomib, and Dexamethasone Induction Therapy for Transplant-Eligible Newly Diagnosed Multiple Myeloma: Final Part 1 Analysis of the GMMG-HD7 Trial. J Clin Oncol. 2025, 43, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Ocio, E.M.; Perrot, A.; Bories, P.; San-Miguel, J.F.; Blau, I.W.; Karlin, L.; Martinez-Lopez, J.; Wang, S.Y.; Bringhen, S.; Marcatti, M.; et al. Efficacy and safety of isatuximab plus bortezomib, lenalidomide, and dexamethasone in patients with newly diagnosed multiple myeloma ineligible/with no immediate intent for autologous stem cell transplantation. Leukemia 2023, 37, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Leleu, X.; Hulin, C.; Lambert, J.; Bobin, A.; Perrot, A.; Karlin, L.; Roussel, M.; Montes, L.; Cherel, B.; Chalopin, T.; et al. Isatuximab, lenalidomide, dexamethasone and bortezomib in transplant-ineligible multiple myeloma: The randomized phase 3 BENEFIT trial. Nat. Med. 2024, 30, 2235–2241. [Google Scholar] [CrossRef] [PubMed]
- Landgren, O.; Hultcrantz, M.; Diamond, B.; Lesokhin, A.M.; Mailankody, S.; Hassoun, H.; Tan, C.; Shah, U.A.; Lu, S.X.; Salcedo, M.; et al. Safety and Effectiveness of Weekly Carfilzomib, Lenalidomide, Dexamethasone, and Daratumumab Combination Therapy for Patients With Newly Diagnosed Multiple Myeloma: The MANHATTAN Nonrandomized Clinical Trial. JAMA Oncol. 2021, 7, 862–868. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, E.; Mo, C.; Yee, A.J.; Nadeem, O.; Laubach, J.; Rosenblatt, J.; Munshi, N.; Midha, S.; Cirstea, D.; Chrysafi, P.; et al. Isatuximab, carfilzomib, lenalidomide, and dexamethasone in patients with newly diagnosed, transplantation-eligible multiple myeloma (SKylaRk): A single-arm, phase 2 trial. Lancet Haematol. 2024, 11, e415–e424. [Google Scholar] [CrossRef]
- Leypoldt, L.B.; Besemer, B.; Asemissen, A.M.; Hänel, M.; Blau, I.W.; Görner, M.; Ko, Y.D.; Reinhardt, H.C.; Staib, P.; Mann, C.; et al. Isatuximab, carfilzomib, lenalidomide, and dexamethasone (Isa-KRd) in front-line treatment of high-risk multiple myeloma: Interim analysis of the GMMG-CONCEPT trial. Leukemia 2022, 36, 885–888. [Google Scholar] [CrossRef]
- Moreau, P.; Attal, M.; Hulin, C.; Arnulf, B.; Belhadj, K.; Benboubker, L.; Béné, M.C.; Broijl, A.; Caillon, H.; Caillot, D.; et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): A randomised, open-label, phase 3 study. Lancet 2019, 394, 29–38. [Google Scholar] [CrossRef]
- Richter, J.; Pan, D.; Salinardi, T.; Rice, M.S. Real-world multiple myeloma front-line treatment and outcomes by transplant in the United States. EJHaem 2023, 4, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Perrot, A.; Lauwers-Cances, V.; Cazaubiel, T.; Facon, T.; Caillot, D.; Clement-Filliatre, L.; Macro, M.; Decaux, O.; Belhadj, K.; Mohty, M.; et al. Early Versus Late Autologous Stem Cell Transplant in Newly Diagnosed Multiple Myeloma: Long-Term Follow-up Analysis of the IFM 2009 Trial. Blood 2020, 136 (Suppl. S1), 39. [Google Scholar] [CrossRef]
- Chari, A.; Ung, B.; Abouzaid, S.; Ni, Q.; Parikh, K.; Agarwal, A. Real-World Treatment Outcomes in Patients with Newly Diagnosed Multiple Myeloma Treated with Triplet Therapy without Stem Cell Transplant: An Enhanced Electronic Health Records Database. Blood 2017, 130 (Suppl. S1), 2190. [Google Scholar] [CrossRef]
- Usmani, S.Z.; Quach, H.; Mateos, M.V.; Landgren, O.; Leleu, X.; Siegel, D.; Weisel, K.; Shu, X.; Li, C.; Dimopoulos, M. Final analysis of carfilzomib, dexamethasone, and daratumumab vs carfilzomib and dexamethasone in the CANDOR study. Blood Adv. 2023, 7, 3739–3748. [Google Scholar] [CrossRef]
- Moreau, P.; Dimopoulos, M.A.; Mikhael, J.; Yong, K.; Capra, M.; Facon, T.; Hajek, R.; Špička, I.; Baker, R.; Kim, K.; et al. Isatuximab, carfilzomib, and dexamethasone in relapsed multiple myeloma (IKEMA): A multicentre, open-label, randomised phase 3 trial. Lancet 2021, 397, 2361–2371. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Oriol, A.; Nahi, H.; San-Miguel, J.; Bahlis, N.J.; Usmani, S.Z.; Rabin, N.; Orlowski, R.Z.; Komarnicki, M.; Suzuki, K.; et al. Daratumumab, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 375, 1319–1331. [Google Scholar] [CrossRef]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Gavriatopoulou, M.; Katodritou, E.; Hatjiharissi, E.; Malandrakis, P.; Verrou, E.; Golfinopoulos, S.; Migkou, M.; Manousou, K.; et al. Efficacy and safety of daratumumab with ixazomib and dexamethasone in lenalidomide-exposed patients after one prior line of therapy: Final results of the phase 2 study DARIA. Am. J. Hematol. 2024, 99, 396–407. [Google Scholar] [CrossRef]
- Raje, N.S.; Anaissie, E.; Kumar, S.K.; Lonial, S.; Martin, T.; Gertz, M.A.; Krishnan, A.; Hari, P.; Ludwig, H.; O’Donnell, E.; et al. Consensus guidelines and recommendations for infection prevention in multiple myeloma: A report from the International Myeloma Working Group. Lancet Haematol. 2022, 9, e143–e161. [Google Scholar] [CrossRef]
- Terpos, E.; Musto, P.; Engelhardt, M.; Delforge, M.; Cook, G.; Gay, F.; van de Donk, N.W.C.J.; Ntanasis-Stathopoulos, I.; Vangsted, A.J.; Driessen, C.; et al. Management of patients with multiple myeloma and COVID-19 in the post pandemic era: A consensus paper from the European Myeloma Network (EMN). Leukemia 2023, 37, 1175–1185. [Google Scholar] [CrossRef]
- Filippatos, C.; Ntanasis-Stathopoulos, I.; Sekeri, K.; Ntanasis-Stathopoulos, A.; Gavriatopoulou, M.; Psaltopoulou, T.; Dounias, G.; Sergentanis, T.N.; Terpos, E. Convalescent Plasma Therapy for COVID-19: A Systematic Review and Meta-Analysis on Randomized Controlled Trials. Viruses 2023, 15, 765. [Google Scholar] [CrossRef]
- Ntanasis-Stathopoulos, I.; Filippatos, C.; Gavriatopoulou, M.; Malandrakis, P.; Eleutherakis-Papaiakovou, E.; Spiliopoulou, V.; Syrigou, R.E.; Theodorakakou, F.; Fotiou, D.; Migkou, M.; et al. Tixagevimab/Cilgavimab as Pre-Exposure Prophylaxis against COVID-19 for Multiple Myeloma Patients: A Prospective Study in the Omicron Era. Diseases 2023, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Miguel, J.; Cavo, M.; Pawlyn, C.; Zweegman, S.; Facon, T.; Driessen, C.; Hajek, R.; Dimopoulos, M.A.; Gay, F.; Avet-Loiseau, H.; et al. Recommendations for vaccination in multiple myeloma: A consensus of the European Myeloma Network. Leukemia 2021, 35, 31–44. [Google Scholar] [CrossRef]
- Souto Filho, J.T.D.; Cantadori, L.O.; Crusoe, E.D.Q.; Hungria, V.; Maiolino, A. Daratumumab-Based Quadruplet Versus Triplet Induction Regimens in Frontline Transplant-Eligible Newly Diagnosed Multiple Myeloma: A Systematic Review and Meta-Analysis. Blood 2024, 144 (Suppl. S1), 258. [Google Scholar] [CrossRef]
- Ebraheem, M.S.; Chakraborty, R.; Rochwerg, B.; Visram, A.; Mohyuddin, G.R.; Venner, C.P.; Sandhu, I.; McCurdy, A.; Facon, T.; Mateos, M.V.; et al. Quadruplet regimens for patients with newly diagnosed multiple myeloma: A systematic review and meta-analysis. Blood Adv. 2024, 8, 5993–6002. [Google Scholar] [CrossRef]
- Gay, F.; Roeloffzen, W.; Dimopoulos, M.A.; Rosiñol, L.; van der Klift, M.; Mina, R.; Rocafiguera, A.O.; Katodritou, E.; Wu, K.L.; Otero, P.R.; et al. Results of the phase III randomized Iskia trial: Isatuximab-carfilzomib-lenalidomide-dexamethasone vs carfilzomib-lenalidomide-dexamethasone as pre-transplant induction and post-transplant consolidation in newly diagnosed multiple myeloma patients. Blood 2023, 142 (Suppl. S1), 4. [Google Scholar] [CrossRef]
- Mateos, M.-V.; Paiva, B.; Cedena Romero, M.T.; Puig, N.; Balari, A.M.S.; Oriol, A.; Ocio, E.M.; Rosiñol, L.; González-Montes, Y.; Bargay, J.J.; et al. GEM2017FIT trial: Induction therapy with bortezomib-melphalan and prednisone (VMP) followed by lenalidomide and dexamethasone (Rd) versus carfilzomib, lenalidomide and dexamethasone (KRd) plus/minus daratumumab (D), 18 cycles, followed by consolidation and maintenance therapy with lenalidomide and daratumumab: Phase III, multicenter, randomized trial for elderly fit newly diagnosed multiple myeloma (NDMM) patients aged between 65 and 80 years. Blood 2023, 142 (Suppl. S1), 209–211. [Google Scholar]
- Sonneveld, P.; Dimopoulos, M.A.; Boccadoro, M.; Quach, H.; Ho, P.J.; Beksac, M.; Hulin, C.; Antonioli, E.; Leleu, X.; Mangiacavalli, S. Phase 3 Randomized Study of Daratumumab (DARA) + Bortezomib, Lenalidomide and Dexamethasone (VRd) Versus Alone in Patients with Transplant-Ineligible Newly Diagnosed Multiple Myeloma or for Whom Transplant Is Not Planned As Initial Therapy: Analysis of Minimal Residual Disease in the Cepheus Trial. Blood 2024, 144 (Suppl. S1), 362. [Google Scholar] [CrossRef]
- Joseph, N.S.; Kaufman, J.L.; Gupta, V.A.; Hofmeister, C.C.; Dhodapkar, M.V.; Boise, L.H.; DiCamillo, S.M.; Roberts, D.; Nooka, A.K.; Lonial, S. Quadruplet therapy for newly diagnosed myeloma: Comparative analysis of sequential cohorts with triplet therapy lenalidomide, bortezomib and dexamethasone (RVd) versus daratumamab with RVD (DRVd) in transplant-eligible patients. Blood Cancer J. 2024, 14, 159. [Google Scholar] [CrossRef]
- Byun, J.M.; Park, S.S.; Yoon, S.S.; Ahn, A.; Kim, M.; Lee, J.Y.; Jeon, Y.W.; Shin, S.H.; Yahng, S.A.; Koh, Y.; et al. Advantage of achieving deep response following frontline daratumumab-VTd compared to VRd in transplant-eligible multiple myeloma: Multicenter study. Blood Res. 2023, 58, 83–90. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kirchhoff, D.C.; Murata, K.; Thoren, K.L. Use of a Daratumumab-Specific Immunofixation Assay to Assess Possible Immunotherapy Interference at a Major Cancer Center: Our Experience and Recommendations. J. Appl. Lab. Med. 2021, 6, 1476–1483. [Google Scholar] [CrossRef]
- Helene Caillon Alix Irimia Jason, S. Simon, Amy Axel, Kate Sasser, Matthew James Scullion, Thierry Ligneel, Georges Nouadje, Philippe Moreau, Thomas Dejoie; Overcoming the Interference of Daratumumab with Immunofixation Electrophoresis (IFE) Using an Industry-Developed Dira Test: Hydrashift 2/4 Daratumumab. Blood 2016, 128, 2063. [Google Scholar] [CrossRef]
- Landgren, O.; Prior, T.J.; Masterson, T.; Heuck, C.; Bueno, O.F.; Dash, A.B.; Einsele, H.; Goldschmidt, H.; Knop, S.; Li, C.; et al. EVIDENCE meta-analysis: Evaluating minimal residual disease as an intermediate clinical end point for multiple myeloma. Blood 2024, 144, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Ntanasis-Stathopoulos, I.; Filippatos, C.; Ntanasis-Stathopoulos, A.; Malandrakis, P.; Kastritis, E.; Tsitsilonis, O.E.; Dimopoulos, M.A.; Terpos, E.; Gavriatopoulou, M. Evaluating Minimal Residual Disease Negativity as a Surrogate Endpoint for Treatment Efficacy in Multiple Myeloma: A Meta-Analysis of Randomized Controlled Trials. Am. J. Hematol. 2025, 100, 427–438. [Google Scholar] [CrossRef]
- Szalat, R.; Anderson, K.; Munshi, N. Role of minimal residual disease assessment in multiple myeloma. Haematologica 2024, 109, 2049–2059. [Google Scholar] [CrossRef] [PubMed]
| Author (Year) | RCT § | Phase | ASCT § | HRMM § | Regimen | Age (yrs §) | Patients | FUP (mos §) |
|---|---|---|---|---|---|---|---|---|
| BENEFIT Leleu et al. (2024) [36] | Yes | 3 | TIE § | No | Isa-VRd § | 61 | 270 | 23.5 |
| CASSIOPEIA Moreau et al. (2019) [40] | Yes | 3 | TE § | No | D-VTd § | 59 | 1085 | 18.8 |
| GMGG-CONCEPT Leypoldt et al. (2024) [39] | No | 2 | Any | Yes | Isa-KRd § | 62 | 125 | 41.7 |
| GMGG-HD7 Mai et al. (2024) [34] | No | 3 | TE | Yes | Isa-VRd | 59 | 660 | 48 |
| GRIFFIN Voorhees et al. (2023) [16] | Yes | 2 | TE | No | D-VRd § | 59 | 207 | 49.6 |
| IFM 2018-04 Touzeau et al. (2024) [28] | No | 2 | TE | Yes | D-KRd § | 57 | 50 | 33 |
| IMROZ Facon et al. (2024) [18] | Yes | 3 | TIE | No | Isa-VRd | 72 | 437 | 59.7 |
| MANHATTAN Landgren et al. (2021) [37] | No | 2 | Any | No | D-KRd | 59 | 41 | 11 |
| MASTER Costa et al. (2021) [26] | No | 2 | TE | Yes | D-KRd | 60 | 123 | 25.1 |
| NCT01998971 Jakubowiak et al. (2021) [30] | No | 1 | TE | No | D-KRd | 59.5 | 21 | 23.3 |
| NCT02513186 Ocio et al. (2023) [35] | No | 1b | TIE | No | Isa-VRd | 71 | 71 | 24 |
| NCT03500445 Derman et al. (2024) [31] | No | 2 | Any | No | D-KRd | 58 | 42 | 27 |
| NCT04113018 Bhutani et al. (2024) [27] | No | 2 | Any | No | D-KRd | - | 39 | 30.1 |
| OPTIMUM Kaiser et al. (2023) [33] | No | 2 | TE | Yes | D-CVRd § | 60 | 103 | 30 |
| PERSEUS Sonneveld et al. (2023) [17] | Yes | 3 | TE | No | D-VRd | 61 | 709 | 47.5 |
| SKylaRk O’ Donnell et al. (2024) [38] | No | 2 | TE | No | Isa-KRd | 59 | 50 | 26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntanasis-Stathopoulos, I.; Filippatos, C.; Malandrakis, P.; Koutoulidis, V.; Kastritis, E.; Terpos, E.; Dimopoulos, M.-A.; Gavriatopoulou, M. Upfront Anti-CD38 Monoclonal Antibody-Based Quadruplet Therapy for Multiple Myeloma: A Systematic Review and Meta-Analysis of Clinical Trials. Cancers 2025, 17, 1943. https://doi.org/10.3390/cancers17121943
Ntanasis-Stathopoulos I, Filippatos C, Malandrakis P, Koutoulidis V, Kastritis E, Terpos E, Dimopoulos M-A, Gavriatopoulou M. Upfront Anti-CD38 Monoclonal Antibody-Based Quadruplet Therapy for Multiple Myeloma: A Systematic Review and Meta-Analysis of Clinical Trials. Cancers. 2025; 17(12):1943. https://doi.org/10.3390/cancers17121943
Chicago/Turabian StyleNtanasis-Stathopoulos, Ioannis, Charalampos Filippatos, Panagiotis Malandrakis, Vassilis Koutoulidis, Efstathios Kastritis, Evangelos Terpos, Meletios-Athanasios Dimopoulos, and Maria Gavriatopoulou. 2025. "Upfront Anti-CD38 Monoclonal Antibody-Based Quadruplet Therapy for Multiple Myeloma: A Systematic Review and Meta-Analysis of Clinical Trials" Cancers 17, no. 12: 1943. https://doi.org/10.3390/cancers17121943
APA StyleNtanasis-Stathopoulos, I., Filippatos, C., Malandrakis, P., Koutoulidis, V., Kastritis, E., Terpos, E., Dimopoulos, M.-A., & Gavriatopoulou, M. (2025). Upfront Anti-CD38 Monoclonal Antibody-Based Quadruplet Therapy for Multiple Myeloma: A Systematic Review and Meta-Analysis of Clinical Trials. Cancers, 17(12), 1943. https://doi.org/10.3390/cancers17121943