Updates on Liquid Biopsy and ctDNA in Transplant Oncology
Simple Summary
Abstract
1. Introduction
1.1. Transplant Oncology
1.2. Liquid Biopsy and ctDNA
1.2.1. Tumor Informed vs. Agnostic
1.2.2. Current Status in Clinical Use
2. Advantages of Liquid Biopsy
2.1. Minimally Invasive
2.2. Improved Capture of Tumor Heterogeneity
2.3. Increased Sensitivity and Specificity Compared to Traditional Surveillance Methods
3. Proposed Applications of ctDNA in Transplant Oncology (Figure 1)
3.1. Earlier Detection and Diagnosis
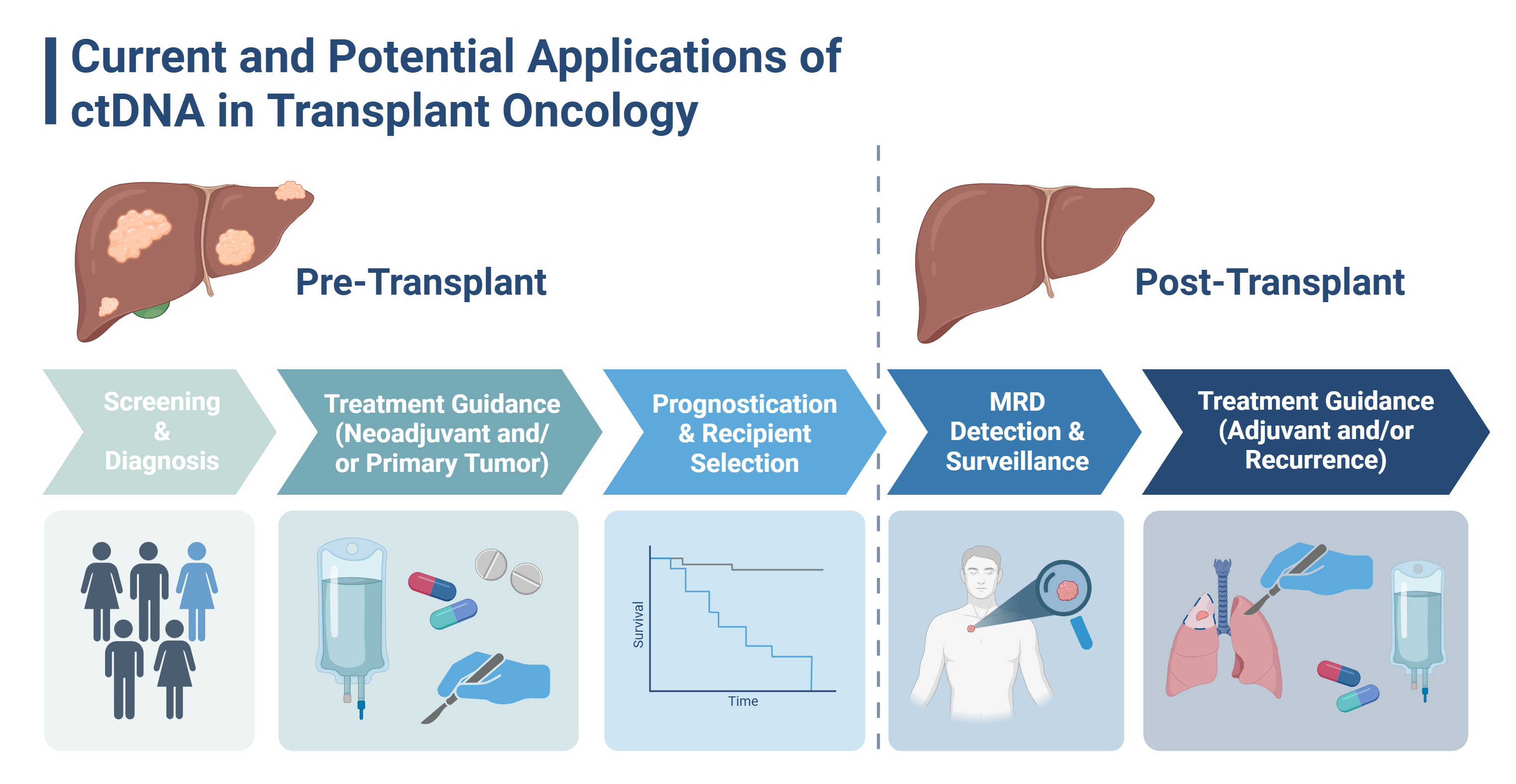
3.2. Risk Stratification and Patient Selection
3.3. Post-Transplant MRD Detection, Surveillance, and Recurrence Monitoring
3.4. Obstacles to Clinical Implementation
4. Current Evidence
4.1. Hepatocellular Carcinoma (HCC)
4.1.1. Diagnosis and Screening
| Study (Country) | Cancer (n) | Surgical Procedure | Assay Type/ Biomarkers | NSG/ ddPCR | Tumor- Informed/ -Agnostic | Time Point(s) | Relevant Findings |
|---|---|---|---|---|---|---|---|
| Abdelrahim et al., 2024 * [82] (USA) | HCC (111) | Resection Transplant | SignateraTM (16 bespoke variants) | NGS | Informed | Post-surgery | Among 36 LT patients with available MRD testing, all were ctDNA(−) In resection cohort, ctDNA(+) prognostic for poor RFS (HR: 12, p < 0.0001) |
| Wehrle et al., 2024 [83] (USA) | HCC (47) | Resection Transplant | Guardant 360® (83-gene panel) | NGS | Agnostic | Post-surgery | Identifiable TMB on postoperative ctDNA predicts HCC recurrence and outperformed AFP |
| Jiang et al., 2022 * [49] (China) | HCC (45) | Transplant | 328-gene panel | -- | -- | Pre-surgery | ctDNA(+) before LT strongly associated with a remarkably augmented RR (48.6% vs 0%) and decreased DFS |
| Zhao et al., 2022 [84] (China) | HCC (66) | Resection Transplant | Universal panel, 15 bespoke variants | NGS | Agnostic and Informed | Post-surgery | ctDNA(+) after resection/LT strongly associated with worse RFS CTC status was complementary to ctDNA status, and the combination improved MRD detection and recurrence prediction |
| Xu et al., 2023 [85] (China) | HCC (20) | Resection | 13-gene panel | NGS | Agnostic | Post-surgery | MRD positivity had sensitivity of 75% and specificity of 100% for early recurrence RD positivity was independent predictor of poor RFS (HR: 13.00, p = 0.002) |
| Fu et al., 2022 [86] (China) | HCC (258) | Resection | 150-gene panel + high-risk genes | NGS | Agnostic | Pre-surgery | Total mutant genes in ctDNA associated with early relapse (HR: 2.2, p < 0.001) High-risk patients had worse RFS (HR: 13.0, p < 0.001) |
4.1.2. Correlation to Clinicopathologic Variables
4.1.3. Prognosis and Surveillance
4.1.4. Comparison to Traditional Surveillance
4.2. Cholangiocarcinoma (CCA)
4.2.1. Correlation to Clinicopathologic Variables
4.2.2. Prognosis and Surveillance
4.2.3. Comparison to Traditional Surveillance
| Study (Country) | Cancer (n) | Surgical Procedure | Assay Type/ Biomarkers | NSG/ ddPCR | Tumor- Informed/ -Agnostic | Time Point(s) | Relevant Findings |
|---|---|---|---|---|---|---|---|
| Hong et al., 2024 [88] (USA) | HCC (9) CRLM (8) CCA (3) cHCC/CCA (1) | Transplant | Guardant 360® (83-gene panel) [All] Guardant 360 CDx® [HCC only] Guardant RevealTM [CRLM only] | NGS | Agnostic | Pre-nACT, Pre- and Post-surgery | ctDNA clearance (+/−) occurred in 50% of patients after LT Higher absolute RR in patients who had ctDNA(+) after LT (50%) compared to those who did not (25%) (p = 0.367) |
| Huang et al., 2024 [48] (China) | HCC (67) iCCA (7) | Transplant | Somatic mutations (SNV & CNV) | NGS | Informed | Pre- and Post- surgery | ctDNA(+) status pre- and post-LT associated with higher RR, shorter RFS ctDNA increased upon recurrence while AFP, DCP remained negative in 2 patients |
| Yoo et al., 2025 [98] (Korea) | eCCA (89) | Resection | SignateraTM (16 bespoke variants) | NGS | Informed | Post-surgery (Pre-, During, and Post-ACT) | 45.5% (5/11) had ctDNA turn (+) before imaging (mean lead time: 174d) while CA19-9 remained normal 27.3% (3/11) had ctDNA turn (+) before imaging (mean lead time: 222d), while CEA remained normal ctDNA(+) any time post-surgery had worse DFS (HR: 3.81, p < 0.001), higher RR (95.7% vs. 47.6%), inferior OS |
| Kim et al., 2023 [101] (Korea) | eCCA (14) iCCA (4) | Resection | 118-gene panel | NGS | Informed | Pre- and Post- surgery | Postoperative plasma mutations detected recurrence or metastasis with 44% sensitivity and 45% specificity Postoperative ctDNA from 50% (8/16) was (+) for new somatic mutations not present in resection specimen |
4.3. Colorectal Liver Metastases (CRLM)
4.3.1. Correlation to Clinicopathologic Variables
4.3.2. Prognosis and Surveillance
| Author, Year (Country) | Cancer (n) | Surgical Procedure | Assay Type/ Biomarkers | NSG/ ddPCR | Tumor- Informed/ -Agnostic | Time Point(s) | Relevant Findings |
|---|---|---|---|---|---|---|---|
| Wehrle et al., 2023 [120] (USA) | CRLM (29) | Resection Transplant | Guardant360® (83-gene panel) | NGS | Agnostic | Pre- and Post- surgery | Resection and LT associated with cleared ctDNA in patients who were ctDNA(+) before surgery (p = 0.009) Postoperative ctDNA associated with higher risk of recurrence (p = 0.042) |
| Kataoka et al., 2024 [58] (Japan) | CRLM (190) | Resection | Signatera™ (16 bespoke variants) | NGS | Informed | Pre- and Post- surgery | ctDNA positivity in the MRD window was 32.1% (61/190) ACT administered to 25.1% (48/190) In MRD-positive group, 24-month DFS was higher for patients treated with ACT (HR: 0.07, p < 0.0001) |
| Liu et al., 2024 [107] (China) | CRLM (114) | Resection | 620-gene panel | NGS | Agnostic | Pre-nACT, Pre- and Post-surgery | ctDNA(+) at baseline and (−) after nACT had longer RFS (p = 0.001) and HRFS (p < 0.001) than those with ctDNA(+) persistently after nACT RFS (all p < 0.05) improved in patients ctDNA(−) after nACT (HR: 0.51, 95% CI 0.28–0.93), major pathologic response (HR: 0.34, 95% CI 0.19–0.62) and surgery combined with radiofrequency ablation (HR: 2.62, 95% CI 1.38–5.00) |
| Li et al., 2024 [57] (China) | CRLM (60) | Resection | Signatera™ (16 bespoke variants) | NGS | Informed | Pre- and Post- surgery, Post-ACT | Higher risk of recurrence in those with ctDNA(+) post-resection (HR: 4.8), post-ACT (HR, 6.0), (both, p < 0.001) Post-resection ctDNA(+) was only independent prognostic marker in multivariant analysis (HR: 5.1, p < 0.001) |
| Wang et al., 2023 [108] (China) | CRLM (34) | Resection | 61-gene panel | NGS | Agnostic | Pre-nACT, Pre- and Post-surgery | Early changes in ctDNA but not CEA or CA19-9 were an independent indicator of RFS (HR: 4.0, p = 0.023) |
| Liu et al., 2023 [119] (China) | CRLM (134) | Resection | 25-gene (J25) and 642-gene panels | NGS | Informed | Post-surgery | ctDNA(+) subgroup had shorter RFS (HR: 2.96, p < 0.05) ctDNA(+) patients who received ACT >2 months had longer RFS than those who received ≤2 months (HR: 0.377; 95% CI, 0.189–0.751; p < 0.05) |
| Newhook et al., 2023 [109] (USA) | CRLM (48) | Resection | Guardant® variant classifier (23-gene panel) | NGS | Agnostic and Informed (for 38 patients) | Pre- and post-surgery | ctDNA(+) before and after surgery (+/+) associated with worse RFS (p = 0.001) ctDNA(+/−) associated with improved RFS and OS over ctDNA(+/+) |
| Marmorino et al., 2022 [118] (Italy) | CRLM (76) | Resection | 24-gene panel | ddPCR | Informed | Post-surgery | ctDNA(+) patients had shorter RFS than ctDNA(−) (median RFS 12.7 vs. 27.4, HR: 2.09, p = 0.008). |
| Nishioka et al., 2022 [117] (USA) | CRLM (105) | Resection | 70-gene panel | NGS | Agnostic | Post-surgery | ctDNA(+) within 180 days was the only independent risk factor on multivariate analysis for recurrence at 1 year (94% vs. 49%; HR: 11.8, p = 0.003) |
| Ogaard et al., 2022 [110] (Denmark) | CRLM (96) | Resection | Methylation profile/C9orf50, CLIP4, KCNQ5 | ddPCR | Agnostic | Pre- and Post-surgery, Post-ACT | Patients with ctDNA(+) postoperatively or post-ACT had lower RFS than patients with ctDNA(−) (HR: 4.5, p < 0.0001, HR: 8.4, p < 0.0001) ctDNA(+) detected before radiological recurrence in 55.6% of ctDNA(+) patients, with median 3.1-month lead time. ctDNA status at the time of inconclusive imaging predicted recurrence with PPV and NPV of 100%, and 75%, respectively (p = 0.0003). |
4.3.3. Comparison to Traditional Surveillance
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFP | alpha-fetoprotein |
| BEAMing | Beads, emulsion, amplification, and magnetics |
| CA19-9 | carbohydrate antigen 19-9 |
| CCA | cholangiocarcinoma |
| ccfRNA | circulating cell-free ribonucleic acid |
| CEA | carcinoembryonic antigen |
| cfDNA | cell-free deoxyribonucleic acid |
| CRLM | colorectal liver metastases |
| CT | computed tomograph |
| CTC | circulating tumor cells |
| ctDNA | circulating tumor deoxyribonucleic acid |
| ddPCR | digital droplet polymerase chain reaction |
| DFS | disease-free survival |
| FDA | Food and Drug Administration |
| HCC | hepatocellular carcinoma |
| LT | liver transplantation |
| MRD | minimal residual disease detection |
| MRI | magnetic resonance imaging |
| NGS | next-generation sequencing |
| OS | overall survival |
| PCR | polymerase chain reaction |
| PET | positron emission tomograph |
| PFS | progression-free survival |
| RFS | recurrence-free survival |
| TMB | tumor mutational burden |
| VAF | variant allele fraction |
References
- Abdelrahim, M.; Esmail, A.; Abudayyeh, A.; Murakami, N.; Saharia, A.; McMillan, R.; Victor, D.; Kodali, S.; Shetty, A.; Nolte Fong, J.V.; et al. Transplant Oncology: An Evolving Field in Cancer Care. Cancers 2021, 13, 4911. [Google Scholar] [CrossRef] [PubMed]
- Gorji, L.; Brown, Z.J.; Limkemann, A.; Schenk, A.D.; Pawlik, T.M. Liver Transplant as a Treatment of Primary and Secondary Liver Neoplasms. JAMA Surg. 2024, 159, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M.M.; Bekki, Y.; Chávez-Villa, M.; Hernandez-Alejandro, R. Recipient Prioritization and Graft Choice in Liver Transplantation for Colorectal Liver Metastasis. Curr. Opin. Organ Transplant. 2025, 30, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Fici, P. Cell-Free DNA in the Liquid Biopsy Context: Role and Differences Between ctDNA and CTC Marker in Cancer Management. Methods Mol. Biol. 2019, 1909, 47–73. [Google Scholar] [CrossRef]
- Neumann, M.H.D.; Bender, S.; Krahn, T.; Schlange, T. ctDNA and CTCs in Liquid Biopsy—Current Status and Where We Need to Progress. Comput. Struct. Biotechnol. J. 2018, 16, 190–195. [Google Scholar] [CrossRef]
- Lone, S.N.; Nisar, S.; Masoodi, T.; Singh, M.; Rizwan, A.; Hashem, S.; El-Rifai, W.; Bedognetti, D.; Batra, S.K.; Haris, M.; et al. Liquid Biopsy: A Step Closer to Transform Diagnosis, Prognosis and Future of Cancer Treatments. Mol. Cancer 2022, 21, 79. [Google Scholar] [CrossRef]
- Speicher, M.R.; Pantel, K. Tumor Signatures in the Blood. Nat. Biotechnol. 2014, 32, 441–443. [Google Scholar] [CrossRef]
- Stroun, M.; Maurice, P.; Vasioukhin, V.; Lyautey, J.; Lederrey, C.; Lefort, F.; Rossier, A.; Chen, X.Q.; Anker, P. The Origin and Mechanism of Circulating DNA. Ann. N. Y. Acad. Sci. 2000, 906, 161–168. [Google Scholar] [CrossRef]
- Thierry, A.R.; El Messaoudi, S.; Gahan, P.B.; Anker, P.; Stroun, M. Origins, Structures, and Functions of Circulating DNA in Oncology. Cancer Metastasis Rev. 2016, 35, 347–376. [Google Scholar] [CrossRef]
- Gobbini, E.; Swalduz, A.; Giaj Levra, M.; Ortiz-Cuaran, S.; Toffart, A.-C.; Pérol, M.; Moro-Sibilot, D.; Saintigny, P. Implementing ctDNA Analysis in the Clinic: Challenges and Opportunities in Non-Small Cell Lung Cancer. Cancers 2020, 12, 3112. [Google Scholar] [CrossRef]
- Biglari, N.; Soltani-Zangbar, M.S.; Mohammadian, J.; Mehdizadeh, A.; Abbasi, K. ctDNA as a Novel and Promising Approach for Cancer Diagnosis: A Focus on Hepatocellular Carcinoma. EXCLI J. 2023, 22, 752–780. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Castedo, B.; Camblor, D.G.; Martín-Arana, J.; Carbonell-Asins, J.A.; García-Micó, B.; Gambardella, V.; Huerta, M.; Roselló, S.; Roda, D.; Gimeno-Valiente, F.; et al. Minimal Residual Disease in Colorectal Cancer. Tumor-Informed versus Tumor-Agnostic Approaches: Unraveling the Optimal Strategy. Ann. Oncol. 2025, 36, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Chen, C.; Hu, Y.; Zhang, W.; Yang, X.; Qi, Y.; Zhu, C.; Chen, X.; Shen, X.; Ji, W. Clinical Application of Molecular Residual Disease Detection by Circulation Tumor DNA in Solid Cancers and a Comparison of Technologies: Review Article. Cancer Biol. Ther. 2023, 24, 2274123. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.T.; Nagayama, S.; Otaki, M.; Chin, Y.M.; Fukunaga, Y.; Ueno, M.; Nakamura, Y.; Low, S.-K. Tumor-Informed or Tumor-Agnostic Circulating Tumor DNA as a Biomarker for Risk of Recurrence in Resected Colorectal Cancer Patients. Front. Oncol. 2022, 12, 1055968. [Google Scholar] [CrossRef]
- Moosavi, S.H.; Eide, P.W.; Eilertsen, I.A.; Brunsell, T.H.; Berg, K.C.G.; Røsok, B.I.; Brudvik, K.W.; Bjørnbeth, B.A.; Guren, M.G.; Nesbakken, A.; et al. De Novo Transcriptomic Subtyping of Colorectal Cancer Liver Metastases in the Context of Tumor Heterogeneity. Genome Med. 2021, 13, 143. [Google Scholar] [CrossRef]
- Commissioner, O. of the FDA Approves First Liquid Biopsy Next-Generation Sequencing Companion Diagnostic Test. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-liquid-biopsy-ngs-companion-diagnostic-test-multiple-cancers-and-biomarkers (accessed on 16 April 2025).
- Research, C. for D.E. and FDA Approves Liquid Biopsy NGS Companion Diagnostic Test for Multiple Cancers and Biomarkers. FDA: Silver Spring, MD, USA, 2024; Available online: https://www.prnewswire.com/news-releases/fda-approves-first-liquid-biopsy-next-generation-sequencing-companion-diagnostic-test-301108536.html (accessed on 16 April 2025).
- Guardant Health’s ShieldTM Blood Test Approved by FDA as a Primary Screening Option, Clearing Path for Medicare Reimbursement and a New Era of Colorectal Cancer Screening. Available online: https://investors.guardanthealth.com/press-releases/press-releases/2024/Guardant-Healths-Shield-Blood-Test-Approved-by-FDA-as-a-Primary-Screening-Option-Clearing-Path-for-Medicare-Reimbursement-and-a-New-Era-of-Colorectal-Cancer-Screening/default.aspx (accessed on 3 June 2025).
- Health, C. for D. and R. Shield—P230009. FDA: Silver Spring, MD, USA, 2024. Available online: https://www.fda.gov/medical-devices/recently-approved-devices/shield-p230009 (accessed on 3 June 2025).
- Chung, D.C.; Gray, D.M.; Singh, H.; Issaka, R.B.; Raymond, V.M.; Eagle, C.; Hu, S.; Chudova, D.I.; Talasaz, A.; Greenson, J.K.; et al. A Cell-Free DNA Blood-Based Test for Colorectal Cancer Screening. N. Engl. J. Med. 2024, 390, 973–983. [Google Scholar] [CrossRef]
- Chen, K.; Shields, M.D.; Chauhan, P.S.; Ramirez, R.J.; Harris, P.K.; Reimers, M.A.; Zevallos, J.P.; Davis, A.A.; Pellini, B.; Chaudhuri, A.A. Commercial ctDNA Assays for Minimal Residual Disease Detection of Solid Tumors. Mol. Diagn. Ther. 2021, 25, 757–774. [Google Scholar] [CrossRef]
- FDA Grants Breakthrough Device Designation to ctDNA Monitoring Assay for Early-Stage Cancer. Available online: https://www.cancernetwork.com/view/fda-grants-breakthrough-device-designation-to-ctdna-monitoring-assay-for-early-stage-cancer (accessed on 16 April 2025).
- Chan, H.T.; Chin, Y.M.; Low, S.-K. Circulating Tumor DNA-Based Genomic Profiling Assays in Adult Solid Tumors for Precision Oncology: Recent Advancements and Future Challenges. Cancers 2022, 14, 3275. [Google Scholar] [CrossRef]
- Guardant Health Introduces Major Smart Liquid Biopsy Upgrade to Market-Leading Guardant360® Test, Further Extending Its Best-in-Class Performance. Available online: https://investors.guardanthealth.com/press-releases/press-releases/2024/Guardant-Health-Introduces-Major-Smart-Liquid-Biopsy-Upgrade-to-Market-Leading-Guardant360-Test-Further-Extending-Its-Best-in-Class-Performance/default.aspx (accessed on 3 June 2025).
- Verner, E.L.; Jackson, J.B.; Maddox, C.; Valkenburg, K.C.; White, J.R.; Occean, J.; Morris, L.; Karandikar, A.; Gerding, K.M.R.; Sausen, M.; et al. Analytical Validation of the Labcorp Plasma Complete Test, a Cell-Free DNA Comprehensive Genomic Profiling Tool for Precision Oncology. J. Mol. Diagn. 2025, 27, 216–231. [Google Scholar] [CrossRef]
- Nault, J.-C.; Villanueva, A. Intratumor Molecular and Phenotypic Diversity in Hepatocellular Carcinoma. Clin. Cancer Res. 2015, 21, 1786–1788. [Google Scholar] [CrossRef]
- Bedard, P.L.; Hansen, A.R.; Ratain, M.J.; Siu, L.L. Tumour Heterogeneity in the Clinic. Nature 2013, 501, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Zhang, X.; Zhou, S.-L.; Cao, Y.; Huang, X.-W.; Fan, J.; Yang, X.-R.; Zhou, J. Detecting Circulating Tumor DNA in Hepatocellular Carcinoma Patients Using Droplet Digital PCR Is Feasible and Reflects Intratumoral Heterogeneity. J. Cancer 2016, 7, 1907–1914. [Google Scholar] [CrossRef] [PubMed]
- Moldogazieva, N.T.; Zavadskiy, S.P.; Terentiev, A.A. Genomic Landscape of Liquid Biopsy for Hepatocellular Carcinoma Personalized Medicine. Cancer Genom. Proteom. 2021, 18, 369–383. [Google Scholar] [CrossRef]
- Varghese, A.M.; Patel, J.; Janjigian, Y.Y.; Meng, F.; Selcuklu, S.D.; Iyer, G.; Houck-Loomis, B.; Harding, J.J.; O’Reilly, E.M.; Abou-Alfa, G.K.; et al. Noninvasive Detection of Polyclonal Acquired Resistance to FGFR Inhibition in Patients with Cholangiocarcinoma Harboring FGFR2 Alterations. JCO Precis. Oncol. 2021, 5, 44–50. [Google Scholar] [CrossRef]
- Byrne, M.M.; Dunne, R.F.; Melaragno, J.I.; Chávez-Villa, M.; Hezel, A.; Liao, X.; Ertreo, M.; Al-Judaibi, B.; Orloff, M.; Hernandez-Alejandro, R.; et al. Neoadjuvant Pemigatinib as a Bridge to Living Donor Liver Transplantation for Intrahepatic Cholangiocarcinoma with FGFR2 Gene Rearrangement. Am. J. Transplant. 2025, 25, 623–627. [Google Scholar] [CrossRef]
- Pelizzaro, F.; Ferrarese, A.; Gambato, M.; Zanetto, A.; Russo, F.P.; Germani, G.; Senzolo, M.; Rizzato, M.D.; Soldà, C.; Vitale, A.; et al. Hepatocellular Carcinoma Recurrence after Liver Transplantation: Risk Factors, Targeted Surveillance and Management Options. Hepatoma Res. 2024, 10. [Google Scholar] [CrossRef]
- Aggarwal, A.; Te, H.S.; Verna, E.C.; Desai, A.P. A National Survey of Hepatocellular Carcinoma Surveillance Practices Following Liver Transplantation. Transplant. Direct 2021, 7, e638. [Google Scholar] [CrossRef]
- Martin, J.; Petrillo, A.; Smyth, E.C.; Shaida, N.; Khwaja, S.; Cheow, H.; Duckworth, A.; Heister, P.; Praseedom, R.; Jah, A.; et al. Colorectal Liver Metastases: Current Management and Future Perspectives. World J. Clin. Oncol. 2020, 11, 761–808. [Google Scholar] [CrossRef]
- Rahnemai-Azar, A.A.; Pandey, P.; Kamel, I.; Pawlik, T.M. Monitoring Outcomes in Intrahepatic Cholangiocarcinoma Patients Following Hepatic Resection. Hepatic Oncol. 2016, 3, 223–239. [Google Scholar] [CrossRef]
- Kim, S.; Park, B.K.; Seo, J.H.; Choi, J.; Choi, J.W.; Lee, C.K.; Chung, J.B.; Park, Y.; Kim, D.W. Carbohydrate Antigen 19-9 Elevation without Evidence of Malignant or Pancreatobiliary Diseases. Sci. Rep. 2020, 10, 8820. [Google Scholar] [CrossRef]
- Pericleous, S.; Bhogal, R.H.; Mavroeidis, V.K. The Role of Circulating Biomarkers in the Early Detection of Recurrent Colorectal Cancer Following Resection of Liver Metastases. Front. Biosci. 2022, 27, 189. [Google Scholar] [CrossRef] [PubMed]
- Kopystecka, A.; Patryn, R.; Leśniewska, M.; Budzyńska, J.; Kozioł, I. The Use of ctDNA in the Diagnosis and Monitoring of Hepatocellular Carcinoma—Literature Review. Int. J. Mol. Sci. 2023, 24, 9342. [Google Scholar] [CrossRef] [PubMed]
- Tocci, N.X.; Wehrle, C.J.; Sun, K.; Jiao, C.; Hong, H.; Gross, A.; Allkushi, E.; Uysal, M.; Linganna, M.W.; Stackhouse, K.; et al. Circulating Tumor DNA in Management of Primary Liver Malignancy: A Review of the Literature and Future Directions. J. Surg. Oncol. 2024, 131, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Diehl, F.; Li, M.; Dressman, D.; He, Y.; Shen, D.; Szabo, S.; Diaz, L.A.; Goodman, S.N.; David, K.A.; Juhl, H.; et al. Detection and Quantification of Mutations in the Plasma of Patients with Colorectal Tumors. Proc. Natl. Acad. Sci. USA 2005, 102, 16368–16373. [Google Scholar] [CrossRef]
- Diehl, F.; Schmidt, K.; Choti, M.A.; Romans, K.; Goodman, S.; Li, M.; Thornton, K.; Agrawal, N.; Sokoll, L.; Szabo, S.A.; et al. Circulating Mutant DNA to Assess Tumor Dynamics. Nat. Med. 2008, 14, 985–990. [Google Scholar] [CrossRef]
- Cai, Z.; Chen, G.; Zeng, Y.; Dong, X.; Li, Z.; Huang, Y.; Xin, F.; Qiu, L.; Xu, H.; Zhang, W.; et al. Comprehensive Liquid Profiling of Circulating Tumor DNA and Protein Biomarkers in Long-Term Follow-Up Patients with Hepatocellular Carcinoma. Clin. Cancer Res. 2019, 25, 5284–5294. [Google Scholar] [CrossRef]
- Tsukamoto, M.; Nitta, H.; Imai, K.; Higashi, T.; Nakagawa, S.; Okabe, H.; Arima, K.; Kaida, T.; Taki, K.; Hashimoto, D.; et al. Clinical Significance of Half-Lives of Tumor Markers α-Fetoprotein and Des-γ-Carboxy Prothrombin after Hepatectomy for Hepatocellular Carcinoma. Hepatol. Res. 2018, 48, E183–E193. [Google Scholar] [CrossRef]
- Trung, N.T.; Hoan, N.X.; Trung, P.Q.; Binh, M.T.; Van Tong, H.; Toan, N.L.; Bang, M.H.; Song, L.H. Clinical Significance of Combined Circulating TERT Promoter Mutations and miR-122 Expression for Screening HBV-Related Hepatocellular Carcinoma. Sci. Rep. 2020, 10, 8181. [Google Scholar] [CrossRef]
- White, T.; Algeri, S. Estimating the Lifetime Risk of a False Positive Screening Test Result. PLoS ONE 2023, 18, e0281153. [Google Scholar] [CrossRef]
- Ma, L.; Guo, H.; Zhao, Y.; Liu, Z.; Wang, C.; Bu, J.; Sun, T.; Wei, J. Liquid Biopsy in Cancer: Current Status, Challenges and Future Prospects. Signal Transduct. Target. Ther. 2024, 9, 336. [Google Scholar] [CrossRef]
- Wang, J.; Huang, A.; Wang, Y.-P.; Yin, Y.; Fu, P.-Y.; Zhang, X.; Zhou, J. Circulating Tumor DNA Correlates with Microvascular Invasion and Predicts Tumor Recurrence of Hepatocellular Carcinoma. Ann. Transl. Med. 2020, 8, 237. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Guo, D.-Z.; Zhang, X.; Sun, Y.; Zhang, S.-Y.; Zhang, X.; Fu, X.-T.; Wang, Y.-P.; Yang, G.-H.; Sun, Q.-M.; et al. Serial Circulating Tumor DNA Profiling Predicts Tumor Recurrence after Liver Transplantation for Liver Cancer. Hepatol. Int. 2024, 18, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Zeng, X.; Tang, J.; Liang, Z.; Qi, X.; Huang, L.; Pang, F. Circulating Tumor DNA Is a Potential Prognostic Risk Factor of Recurrence in Patients with Hepatocellular Carcinoma Treated by Liver Transplantation. J. Clin. Oncol. 2022, 40, e16196. [Google Scholar] [CrossRef]
- Liao, W.; Yang, H.; Xu, H.; Wang, Y.; Ge, P.; Ren, J.; Xu, W.; Lu, X.; Sang, X.; Zhong, S.; et al. Noninvasive Detection of Tumor-Associated Mutations from Circulating Cell-Free DNA in Hepatocellular Carcinoma Patients by Targeted Deep Sequencing. Oncotarget 2016, 7, 40481–40490. [Google Scholar] [CrossRef]
- Ono, A.; Fujimoto, A.; Yamamoto, Y.; Akamatsu, S.; Hiraga, N.; Imamura, M.; Kawaoka, T.; Tsuge, M.; Abe, H.; Hayes, C.N.; et al. Circulating Tumor DNA Analysis for Liver Cancers and Its Usefulness as a Liquid Biopsy. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 516–534. [Google Scholar] [CrossRef]
- Zhu, G.-Q.; Liu, W.-R.; Tang, Z.; Qu, W.-F.; Fang, Y.; Jiang, X.-F.; Song, S.-S.; Wang, H.; Tao, C.-Y.; Zhou, P.-Y.; et al. Serial Circulating Tumor DNA to Predict Early Recurrence in Patients with Hepatocellular Carcinoma: A Prospective Study. Mol. Oncol. 2022, 16, 549–561. [Google Scholar] [CrossRef]
- He, Y.; Ma, X.; Chen, K.; Liu, F.; Cai, S.; Han-Zhang, H.; Hou, T.; Xiang, J.; Peng, J. Perioperative Circulating Tumor DNA in Colorectal Liver Metastases: Concordance with Metastatic Tissue and Predictive Value for Tumor Burden and Prognosis. Cancer Manag. Res. 2020, 12, 1621–1630. [Google Scholar] [CrossRef]
- Wang, D.-S.; Yang, H.; Liu, X.-Y.; Chen, Z.-G.; Wang, Y.; Fong, W.P.; Hu, M.-T.; Zheng, Y.-C.; Zheng, Y.; Li, B.-K.; et al. Dynamic Monitoring of Circulating Tumor DNA to Predict Prognosis and Efficacy of Adjuvant Chemotherapy after Resection of Colorectal Liver Metastases. Theranostics 2021, 11, 7018–7028. [Google Scholar] [CrossRef]
- Narayan, R.R.; Goldman, D.A.; Gonen, M.; Reichel, J.; Huberman, K.H.; Raj, S.; Viale, A.; Kemeny, N.E.; Allen, P.J.; Balachandran, V.P.; et al. Peripheral Circulating Tumor DNA Detection Predicts Poor Outcomes After Liver Resection for Metastatic Colorectal Cancer. Ann. Surg. Oncol. 2019, 26, 1824–1832. [Google Scholar] [CrossRef]
- Tie, J.; Wang, Y.; Cohen, J.; Li, L.; Hong, W.; Christie, M.; Wong, H.L.; Kosmider, S.; Wong, R.; Thomson, B.; et al. Circulating Tumor DNA Dynamics and Recurrence Risk in Patients Undergoing Curative Intent Resection of Colorectal Cancer Liver Metastases: A Prospective Cohort Study. PLoS Med. 2021, 18, e1003620. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.; Hu, X.; Chen, Y.; Liu, F.; Chen, Y.; Ma, X.; Dong, Q.; Sun, L.; Mo, S.; et al. Personalized Circulating Tumor DNA Monitoring Improves Recurrence Surveillance and Management after Curative Resection of Colorectal Liver Metastases: A Prospective Cohort Study. Int. J. Surg. 2024, 110, 2776–2787. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Mori, K.; Nakamura, Y.; Watanabe, J.; Akazawa, N.; Hirata, K.; Yokota, M.; Kato, K.; Kotaka, M.; Yamazaki, K.; et al. Survival Benefit of Adjuvant Chemotherapy Based on Molecular Residual Disease Detection in Resected Colorectal Liver Metastases: Subgroup Analysis from CIRCULATE-Japan GALAXY. Ann. Oncol. 2024, 35, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Piedvache, C.; Chiche, L.; Adam, J.P.; Salamé, E.; Bucur, P.; Cherqui, D.; Scatton, O.; Granger, V.; Ducreux, M.; et al. Liver Transplantation plus Chemotherapy versus Chemotherapy Alone in Patients with Permanently Unresectable Colorectal Liver Metastases (TransMet): Results from a Multicentre, Open-Label, Prospective, Randomised Controlled Trial. Lancet 2024, 404, 1107–1118. [Google Scholar] [CrossRef]
- Byrne, M.M.; Chávez-Villa, M.; Ruffolo, L.I.; Loria, A.; Endo, Y.; Niewiemski, A.; Jimenez-Soto, C.; Melaragno, J.I.; Ramaraju, G.A.; Farooq, P.D.; et al. The Rochester Protocol for Living Donor Liver Transplantation of Unresectable Colorectal Liver Metastasis: A 5-Year Report on Selection, Approval, and Outcomes. Am. J. Transplant. 2025, 25, 780–792. [Google Scholar] [CrossRef]
- Dueland, S.; Smedman, T.M.; Syversveen, T.; Grut, H.; Hagness, M.; Line, P.-D. Long-Term Survival, Prognostic Factors, and Selection of Patients with Colorectal Cancer for Liver Transplant: A Nonrandomized Controlled Trial. JAMA Surg. 2023, 158, e232932. [Google Scholar] [CrossRef]
- Tabrizian, P.; Abdelrahim, M.; Schwartz, M. Immunotherapy and Transplantation for Hepatocellular Carcinoma. J. Hepatol. 2024, 80, 822–825. [Google Scholar] [CrossRef]
- Connor, A.A.; Kodali, S.; Abdelrahim, M.; Javle, M.M.; Brombosz, E.W.; Ghobrial, R.M. Intrahepatic Cholangiocarcinoma: The Role of Liver Transplantation, Adjunctive Treatments, and Prognostic Biomarkers. Front. Oncol. 2022, 12, 996710. [Google Scholar] [CrossRef]
- Kayali, S.; Pasta, A.; Plaz Torres, M.C.; Jaffe, A.; Strazzabosco, M.; Marenco, S.; Giannini, E.G. Immune Checkpoint Inhibitors in Malignancies after Liver Transplantation: A Systematic Review and Pooled Analysis. Liver Int. 2023, 43, 8–17. [Google Scholar] [CrossRef]
- Tovoli, F.; Pallotta, D.P.; Sansone, V.; Iavarone, M.; De Giorgio, M.; Ielasi, L.; Di Costanzo, G.G.; Giuffrida, P.; Sacco, R.; Pressiani, T.; et al. Outcomes of Sorafenib for Recurrent Hepatocellular Carcinoma After Liver Transplantation in the Era of Combined and Sequential Treatments. Transplantation 2023, 107, 156–161. [Google Scholar] [CrossRef]
- Rashid, S.; Sun, Y.; Ali Khan Saddozai, U.; Hayyat, S.; Munir, M.U.; Akbar, M.U.; Khawar, M.B.; Ren, Z.; Ji, X.; Ihsan Ullah Khan, M. Circulating Tumor DNA and Its Role in Detection, Prognosis and Therapeutics of Hepatocellular Carcinoma. Chin. J. Cancer Res. 2024, 36, 195–214. [Google Scholar] [CrossRef]
- Wang, D.; Xu, Y.; Goldstein, J.B.; Ye, K.; Hu, X.; Xiao, L.; Li, L.; Chang, L.; Guan, Y.; Long, G.; et al. Preoperative Evaluation of Microvascular Invasion with Circulating Tumour DNA in Operable Hepatocellular Carcinoma. Liver Int. 2020, 40, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Sun, K.; Tong, Y.K.; Cheng, S.H.; Cheng, T.H.T.; Heung, M.M.S.; Wong, J.; Wong, V.W.S.; Chan, H.L.Y.; Chan, K.C.A.; et al. Preferred End Coordinates and Somatic Variants as Signatures of Circulating Tumor DNA Associated with Hepatocellular Carcinoma. Proc. Natl. Acad. Sci. USA 2018, 115, E10925–E10933. [Google Scholar] [CrossRef] [PubMed]
- Labgaa, I.; Villanueva, A.; Dormond, O.; Demartines, N.; Melloul, E. The Role of Liquid Biopsy in Hepatocellular Carcinoma Prognostication. Cancers 2021, 13, 659. [Google Scholar] [CrossRef] [PubMed]
- Green, S.F. The Cost of Poor Blood Specimen Quality and Errors in Preanalytical Processes. Clin. Biochem. 2013, 46, 1175–1179. [Google Scholar] [CrossRef]
- Pascual, J.; Attard, G.; Bidard, F.-C.; Curigliano, G.; De Mattos-Arruda, L.; Diehn, M.; Italiano, A.; Lindberg, J.; Merker, J.D.; Montagut, C.; et al. ESMO Recommendations on the Use of Circulating Tumour DNA Assays for Patients with Cancer: A Report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2022, 33, 750–768. [Google Scholar] [CrossRef]
- Turabi, K.; Klute, K.; Radhakrishnan, P. Decoding the Dynamics of Circulating Tumor DNA in Liquid Biopsies. Cancers 2024, 16, 2432. [Google Scholar] [CrossRef]
- Henriksen, T.V.; Reinert, T.; Christensen, E.; Sethi, H.; Birkenkamp-Demtröder, K.; Gögenur, M.; Gögenur, I.; Zimmermann, B.G.; Dyrskjøt, L.; Andersen, C.L. The Effect of Surgical Trauma on Circulating Free DNA Levels in Cancer Patients—Implications for Studies of Circulating Tumor DNA. Mol. Oncol. 2020, 14, 1670–1679. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Rumgay, H.; Ferlay, J.; de Martel, C.; Georges, D.; Ibrahim, A.S.; Zheng, R.; Wei, W.; Lemmens, V.E.P.P.; Soerjomataram, I. Global, Regional and National Burden of Primary Liver Cancer by Subtype. Eur. J. Cancer 2022, 161, 108–118. [Google Scholar] [CrossRef]
- Kwong, A.J.; Kim, W.R.; Lake, J.R.; Schladt, D.P.; Handarova, D.; Howell, J.; Schumacher, B.; Weiss, S.; Snyder, J.J.; Israni, A.K. OPTN/SRTR 2023 Annual Data Report: Liver. Am. J. Transplant. 2025, 25, S193–S287. [Google Scholar] [CrossRef]
- Berenguer, M.; Burra, P.; Ghobrial, M.; Hibi, T.; Metselaar, H.; Sapisochin, G.; Bhoori, S.; Kwan Man, N.; Mas, V.; Ohira, M.; et al. Posttransplant Management of Recipients Undergoing Liver Transplantation for Hepatocellular Carcinoma. Working Group Report From the ILTS Transplant Oncology Consensus Conference. Transplantation 2020, 104, 1143. [Google Scholar] [CrossRef] [PubMed]
- Trinchet, J.-C.; Chaffaut, C.; Bourcier, V.; Degos, F.; Henrion, J.; Fontaine, H.; Roulot, D.; Mallat, A.; Hillaire, S.; Cales, P.; et al. Ultrasonographic Surveillance of Hepatocellular Carcinoma in Cirrhosis: A Randomized Trial Comparing 3- and 6-Month Periodicities. Hepatology 2011, 54, 1987–1997. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, F.; Singal, A.G. Surveillance for Hepatocellular Carcinoma: Current Best Practice and Future Direction. Gastroenterology 2019, 157, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Llovet, J.M.; Yarchoan, M.; Mehta, N.; Heimbach, J.K.; Dawson, L.A.; Jou, J.H.; Kulik, L.M.; Agopian, V.G.; Marrero, J.A.; et al. AASLD Practice Guidance on Prevention, Diagnosis, and Treatment of Hepatocellular Carcinoma. Hepatology 2023, 78, 1922. [Google Scholar] [CrossRef]
- Sangro, B.; Argemi, J.; Ronot, M.; Paradis, V.; Meyer, T.; Mazzaferro, V.; Jepsen, P.; Golfieri, R.; Galle, P.; Dawson, L.; et al. EASL Clinical Practice Guidelines on the Management of Hepatocellular Carcinoma. J. Hepatol. 2025, 82, 315–374. [Google Scholar] [CrossRef]
- Abdelrahim, M.; Mejia, A.; Esmail, A.; Ouf, M.; Franses, J.W.; Bhan, I.; Sudha, K.; Brdiges, C.; Tin, T.; Brewer, C.; et al. 181P Circulating Tumor DNA (ctDNA) Testing for Recurrence and Treatment Response Monitoring in Hepatocellular Carcinoma (HCC). Ann. Oncol. 2024, 35, S82. [Google Scholar] [CrossRef]
- Wehrle, C.J.; Hong, H.; Kamath, S.; Schlegel, A.; Fujiki, M.; Hashimoto, K.; Kwon, D.C.H.; Miller, C.; Walsh, R.M.; Aucejo, F. Tumor Mutational Burden From Circulating Tumor DNA Predicts Recurrence of Hepatocellular Carcinoma After Resection: An Emerging Biomarker for Surveillance. Ann. Surg. 2024, 280, 504–513. [Google Scholar] [CrossRef]
- Zhao, L.; Jiang, L.; Liu, Y.; Wang, X.; Song, J.; Sun, Y.; Bai, Y.; Dong, X.; Sun, L.; Wu, J.; et al. Integrated Analysis of Circulating Tumour Cells and Circulating Tumour DNA to Detect Minimal Residual Disease in Hepatocellular Carcinoma. Clin. Transl. Med. 2022, 12, e793. [Google Scholar] [CrossRef]
- Xu, Y.; Cai, J.; Zhong, K.; Wen, Y.; Cai, L.; He, G.; Liao, H.; Zhang, C.; Fu, S.; Chen, T.; et al. Plasma-Only Circulating Tumor DNA Analysis Detects Minimal Residual Disease and Predicts Early Relapse in Hepatocellular Carcinoma Patients Undergoing Curative Resection. Front. Oncol. 2023, 13, 1119744. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, Z.; Hu, Z.; Yang, Z.; Pan, Y.; Chen, J.; Wang, J.; Hu, D.; Zhou, Z.; Xu, L.; et al. Preoperative Serum ctDNA Predicts Early Hepatocellular Carcinoma Recurrence and Response to Systemic Therapies. Hepatol. Int. 2022, 16, 868–878. [Google Scholar] [CrossRef]
- Lin, J.; Zhao, S.; Wang, D.; Song, Y.; Che, Y.; Yang, X.; Mao, J.; Xie, F.; Long, J.; Bai, Y.; et al. Targeted Next-Generation Sequencing Combined with Circulating-Free DNA Deciphers Spatial Heterogeneity of Resected Multifocal Hepatocellular Carcinoma. Front. Immunol. 2021, 12, 673248. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Wehrle, C.J.; Zhang, M.; Fares, S.; Stitzel, H.; Garib, D.; Estfan, B.; Kamath, S.; Krishnamurthi, S.; Ma, W.W.; et al. Circulating Tumor DNA Profiling in Liver Transplant for Hepatocellular Carcinoma, Cholangiocarcinoma, and Colorectal Liver Metastases: A Programmatic Proof of Concept. Cancers 2024, 16, 927. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.D.; Lucey, M.R. Management of Hepatocellular Carcinoma Recurrence after Liver Transplantation. Ann. Hepatol. 2022, 27, 100654. [Google Scholar] [CrossRef]
- Bretthauer, M.; Løberg, M.; Holme, Ø.; Adami, H.-O.; Kalager, M. Deep Learning and Cancer Biomarkers: Recognising Lead-Time Bias. Lancet 2021, 397, 194. [Google Scholar] [CrossRef]
- Galle, P.R.; Foerster, F.; Kudo, M.; Chan, S.L.; Llovet, J.M.; Qin, S.; Schelman, W.R.; Chintharlapalli, S.; Abada, P.B.; Sherman, M.; et al. Biology and Significance of Alpha-Fetoprotein in Hepatocellular Carcinoma. Liver Int. 2019, 39, 2214–2229. [Google Scholar] [CrossRef]
- Lee, W.-C. Value of Alpha-Fetoprotein in Hepatocellular Carcinoma. Transl. Gastroenterol. Hepatol. 2021, 6, 52. [Google Scholar] [CrossRef]
- Marrero, J.A.; Feng, Z.; Wang, Y.; Nguyen, M.H.; Befeler, A.S.; Roberts, L.R.; Reddy, K.R.; Harnois, D.; Llovet, J.M.; Normolle, D.; et al. Alpha-Fetoprotein, Des-Gamma Carboxyprothrombin, and Lectin-Bound Alpha-Fetoprotein in Early Hepatocellular Carcinoma. Gastroenterology 2009, 137, 110–118. [Google Scholar] [CrossRef]
- Abdelrahim, M.; Esmail, A.; Saharia, A.; He, A.; Dhani, H.; Aushev, V.; Jurdi, A.; Starr, J.; Gauthier, P. P-165 The Feasibility of Tumor Recurrence Detection in Liver Post-Transplantation for Patients with Hepatocellular Carcinoma via Personalized, Tumor-Informed ctDNA Testing. Ann. Oncol. 2022, 33, S308–S309. [Google Scholar] [CrossRef]
- Alvaro, D.; Gores, G.J.; Walicki, J.; Hassan, C.; Sapisochin, G.; Komuta, M.; Forner, A.; Valle, J.W.; Laghi, A.; Ilyas, S.I.; et al. European Association for the Study of the Liver EASL-ILCA Clinical Practice Guidelines on the Management of Intrahepatic Cholangiocarcinoma. J. Hepatol. 2023, 79, 181–208. [Google Scholar] [CrossRef]
- Rea, D.J.; Heimbach, J.K.; Rosen, C.B.; Haddock, M.G.; Alberts, S.R.; Kremers, W.K.; Gores, G.J.; Nagorney, D.M. Liver Transplantation with Neoadjuvant Chemoradiation Is More Effective than Resection for Hilar Cholangiocarcinoma. Ann. Surg. 2005, 242, 451–458. [Google Scholar] [CrossRef]
- Wintachai, P.; Lim, J.Q.; Techasen, A.; Lert-itthiporn, W.; Kongpetch, S.; Loilome, W.; Chindaprasirt, J.; Titapun, A.; Namwat, N.; Khuntikeo, N.; et al. Diagnostic and Prognostic Value of Circulating Cell-Free DNA for Cholangiocarcinoma. Diagnostics 2021, 11, 999. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.; Jeong, H.; Jeong, J.H.; Kim, K.-P.; Lee, S.; Ryoo, B.-Y.; Hwang, D.W.; Lee, J.H.; Moon, D.-B.; Kim, K.-H.; et al. Circulating Tumor DNA Status and Dynamics Predict Recurrence in Patients with Resected Extrahepatic Cholangiocarcinoma. J. Hepatol. 2025, 82, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Monroe, G.; Malla, M. Post-Operative Surveillance and Management of Intrahepatic Cholangiocarcinoma Using Circulating Tumor DNA: A Case Report. Cureus 2024, 16, e55914. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Avriett, T.A.; Ray, C.M.; Kim, R.D. Circulating Tumor DNA Analysis Guiding Adjuvant Treatment in Resected Stage III Cholangiocarcinoma: A Case Report. J. Gastrointest. Oncol. 2024, 15, 485–490. [Google Scholar] [CrossRef]
- Kim, K.-H.; Yi, H.-S.; Lee, H.; Bae, G.-E.; Yeo, M.-K. Targeting the Sequences of Circulating Tumor DNA of Cholangiocarcinomas and Its Applications and Limitations in Clinical Practice. Int. J. Mol. Sci. 2023, 24, 7512. [Google Scholar] [CrossRef]
- Biller, L.H.; Schrag, D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef]
- REACCT Collaborative; Zaborowski, A.M.; Abdile, A.; Adamina, M.; Aigner, F.; d’Allens, L.; Allmer, C.; Álvarez, A.; Anula, R.; Andric, M.; et al. Characteristics of Early-Onset vs Late-Onset Colorectal Cancer: A Review. JAMA Surg. 2021, 156, 865–874. [Google Scholar] [CrossRef]
- Kobayashi, S.; Nakamura, Y.; Taniguchi, H.; Odegaard, J.I.; Nomura, S.; Kojima, M.; Sugimoto, M.; Konishi, M.; Gotohda, N.; Takahashi, S.; et al. Impact of Preoperative Circulating Tumor DNA Status on Survival Outcomes After Hepatectomy for Resectable Colorectal Liver Metastases. Ann. Surg. Oncol. 2021, 28, 4744–4755. [Google Scholar] [CrossRef]
- Bidard, F.-C.; Kiavue, N.; Ychou, M.; Cabel, L.; Stern, M.-H.; Madic, J.; Saliou, A.; Rampanou, A.; Decraene, C.; Bouché, O.; et al. Circulating Tumor Cells and Circulating Tumor DNA Detection in Potentially Resectable Metastatic Colorectal Cancer: A Prospective Ancillary Study to the Unicancer Prodige-14 Trial. Cells 2019, 8, 516. [Google Scholar] [CrossRef]
- Bolhuis, K.; van ’t Erve, I.; Mijnals, C.; Delis-Van Diemen, P.M.; Huiskens, J.; Komurcu, A.; Lopez-Yurda, M.; van den Broek, D.; Swijnenburg, R.-J.; Meijer, G.A.; et al. Postoperative Circulating Tumour DNA Is Associated with Pathologic Response and Recurrence-Free Survival after Resection of Colorectal Cancer Liver Metastases. EBioMedicine 2021, 70, 103498. [Google Scholar] [CrossRef]
- Liu, M.; Bao, Q.; Zhao, T.; Huang, L.; Zhang, D.; Wang, Y.; Yan, X.; Wang, H.; Jin, K.; Liu, W.; et al. Pre-Hepatectomy Dynamic Circulating Tumor DNA to Predict Pathologic Response to Preoperative Chemotherapy and Post-Hepatectomy Recurrence in Patients with Colorectal Liver Metastases. Hepatol. Int. 2024, 18, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhao, P.; Lu, T.; Ren, J.; Zhu, L.; Han, X.; Zhang, G.; Dong, X.; Ma, H.; Yu, M.; et al. ctDNA as a Prognostic Biomarker in Resectable CLM: Systematic Review and Meta-Analysis. Open Life Sci. 2023, 18, 20220615. [Google Scholar] [CrossRef] [PubMed]
- Newhook, T.E.; Overman, M.J.; Chun, Y.S.; Dasari, A.; Tzeng, C.-W.D.; Cao, H.S.T.; Raymond, V.; Parseghian, C.; Johnson, B.; Nishioka, Y.; et al. Prospective Study of Perioperative Circulating Tumor DNA Dynamics in Patients Undergoing Hepatectomy for Colorectal Liver Metastases. Ann. Surg. 2023, 277, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Øgaard, N.; Reinert, T.; Henriksen, T.V.; Frydendahl, A.; Aagaard, E.; Ørntoft, M.-B.W.; Larsen, M.Ø.; Knudsen, A.R.; Mortensen, F.V.; Andersen, C.L. Tumour-Agnostic Circulating Tumour DNA Analysis for Improved Recurrence Surveillance after Resection of Colorectal Liver Metastases: A Prospective Cohort Study. Eur. J. Cancer 2022, 163, 163–176. [Google Scholar] [CrossRef]
- Dueland, S.; Grut, H.; Syversveen, T.; Hagness, M.; Line, P.-D. Selection Criteria Related to Long-Term Survival Following Liver Transplantation for Colorectal Liver Metastasis. Am. J. Transplant. 2020, 20, 530–537. [Google Scholar] [CrossRef]
- Byrne, M.M.; Chávez-Villa, M.; Ruffolo, L.I.; Wanis, K.N.; Belt, B.; Tomiyama, K.; Hernandez-Alejandro, R. Progression-Free Survival for Liver Transplant vs Alternative Therapy in Unresectable Colorectal Liver Metastasis. JAMA Surg. 2024, 159, 1089–1091. [Google Scholar] [CrossRef]
- Adam, R.; Badrudin, D.; Chiche, L.; Bucur, P.; Scatton, O.; Granger, V.; Ducreux, M.; Cillo, U.; Cauchy, F.; Lesurtel, M.; et al. Safety and Feasibility of Chemotherapy Followed by Liver Transplantation for Patients with Definitely Unresectable Colorectal Liver Metastases: Insights from the TransMet Randomised Clinical Trial. EClinicalMedicine 2024, 72, 102608. [Google Scholar] [CrossRef]
- Loupakis, F.; Sharma, S.; Derouazi, M.; Murgioni, S.; Biason, P.; Rizzato, M.D.; Rasola, C.; Renner, D.; Shchegrova, S.; Koyen Malashevich, A.; et al. Detection of Molecular Residual Disease Using Personalized Circulating Tumor DNA Assay in Patients with Colorectal Cancer Undergoing Resection of Metastases. JCO Precis. Oncol. 2021, 5, 1166–1177. [Google Scholar] [CrossRef]
- Reinert, T.; Petersen, L.M.S.; Henriksen, T.V.; Larsen, M.Ø.; Rasmussen, M.H.; Johansen, A.F.B.; Øgaard, N.; Knudsen, M.; Nordentoft, I.; Vang, S.; et al. Circulating Tumor DNA for Prognosis Assessment and Postoperative Management after Curative-Intent Resection of Colorectal Liver Metastases. Int. J. Cancer 2022, 150, 1537–1548. [Google Scholar] [CrossRef]
- Schøler, L.V.; Reinert, T.; Ørntoft, M.-B.W.; Kassentoft, C.G.; Árnadóttir, S.S.; Vang, S.; Nordentoft, I.; Knudsen, M.; Lamy, P.; Andreasen, D.; et al. Clinical Implications of Monitoring Circulating Tumor DNA in Patients with Colorectal Cancer. Clin. Cancer Res. 2017, 23, 5437–5445. [Google Scholar] [CrossRef]
- Nishioka, Y.; Chun, Y.S.; Overman, M.J.; Cao, H.S.T.; Tzeng, C.-W.D.; Mason, M.C.; Kopetz, S.W.; Bauer, T.W.; Vauthey, J.-N.; Newhook, T.E.; et al. Effect of Co-Mutation of RAS and TP53 on Postoperative ctDNA Detection and Early Recurrence after Hepatectomy for Colorectal Liver Metastases. J. Am. Coll. Surg. 2022, 234, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Marmorino, F.; Prisciandaro, M.; Giordano, M.; Ortolan, E.; Crucitta, S.; Manca, P.; Antoniotti, C.; Valenti, M.M.; Danesi, R.; Conca, V.; et al. Circulating Tumor DNA as a Marker of Minimal Residual Disease After Radical Resection of Colorectal Liver Metastases. JCO Precis. Oncol. 2022, 6, e2200244. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Jin, K.-M.; Zhang, M.-H.; Bao, Q.; Liu, M.; Xu, D.; Wang, K.; Xing, B.-C. Recurrence Prediction by Circulating Tumor DNA in the Patient with Colorectal Liver Metastases After Hepatectomy: A Prospective Biomarker Study. Ann. Surg. Oncol. 2023, 30, 4916–4926. [Google Scholar] [CrossRef] [PubMed]
- Wehrle, C.J.; Raj, R.; Aykun, N.; Orabi, D.; Stackhouse, K.; Chang, J.; Estfan, B.; Kamath, S.; Krishnamurthi, S.; Walsh, R.M.; et al. Circulating Tumor DNA in Colorectal Cancer Liver Metastasis: Analysis of Patients Receiving Liver Resection and Transplant. JCO Clin. Cancer Inform. 2023, 7, e2300111. [Google Scholar] [CrossRef]
- Bando, H.; Nakamura, Y.; Taniguchi, H.; Shiozawa, M.; Yasui, H.; Esaki, T.; Kagawa, Y.; Denda, T.; Satoh, T.; Yamazaki, K.; et al. Effects of Metastatic Sites on Circulating Tumor DNA in Patients with Metastatic Colorectal Cancer. JCO Precis. Oncol. 2022, 6, e2100535. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loszko, A.; Byrne, M.M.; Jimenez-Soto, C.; Tomiyama, K.; Bekki, Y.; Hernandez-Alejandro, R. Updates on Liquid Biopsy and ctDNA in Transplant Oncology. Cancers 2025, 17, 1930. https://doi.org/10.3390/cancers17121930
Loszko A, Byrne MM, Jimenez-Soto C, Tomiyama K, Bekki Y, Hernandez-Alejandro R. Updates on Liquid Biopsy and ctDNA in Transplant Oncology. Cancers. 2025; 17(12):1930. https://doi.org/10.3390/cancers17121930
Chicago/Turabian StyleLoszko, Abigail, Matthew M. Byrne, Cristina Jimenez-Soto, Koji Tomiyama, Yuki Bekki, and Roberto Hernandez-Alejandro. 2025. "Updates on Liquid Biopsy and ctDNA in Transplant Oncology" Cancers 17, no. 12: 1930. https://doi.org/10.3390/cancers17121930
APA StyleLoszko, A., Byrne, M. M., Jimenez-Soto, C., Tomiyama, K., Bekki, Y., & Hernandez-Alejandro, R. (2025). Updates on Liquid Biopsy and ctDNA in Transplant Oncology. Cancers, 17(12), 1930. https://doi.org/10.3390/cancers17121930






