Towards Personalized Medicine: Microdevice-Assisted Evaluation of Cancer Stem Cell Dynamics and Treatment Response
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Fabrication of MDs
2.2. Cell Culture
2.3. CSC-Sphere Formation Assay
2.4. Immunofluorescence Assay into MD
2.5. Molecular Analysis by qPCR- RNA Purification from MD
2.6. Tumorsphere Isolation from Tumor Samples
2.7. Ethics Statement
2.8. Statistical Analysis
3. Results
3.1. MD Description
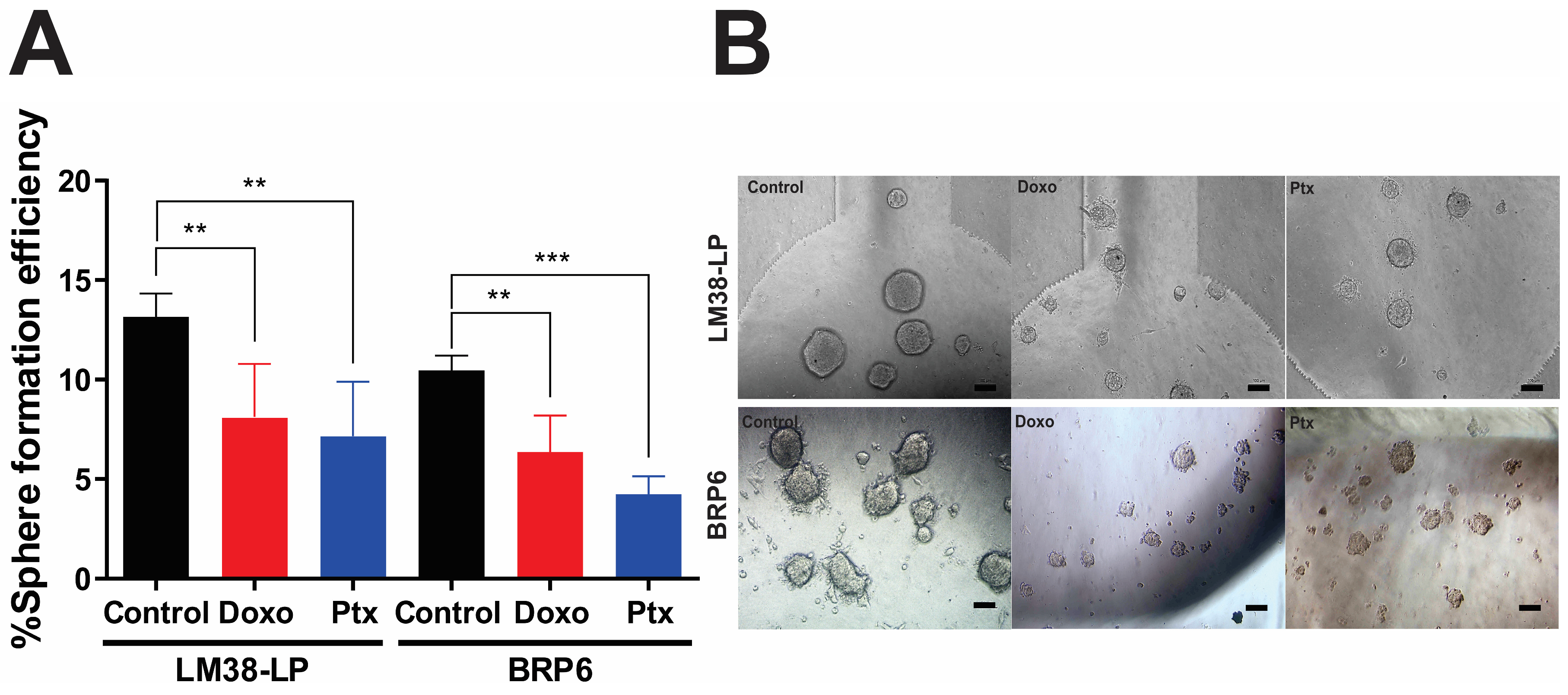
3.2. Sphere-Forming Efficiency and Diameter Under Chemotherapeutic Treatment
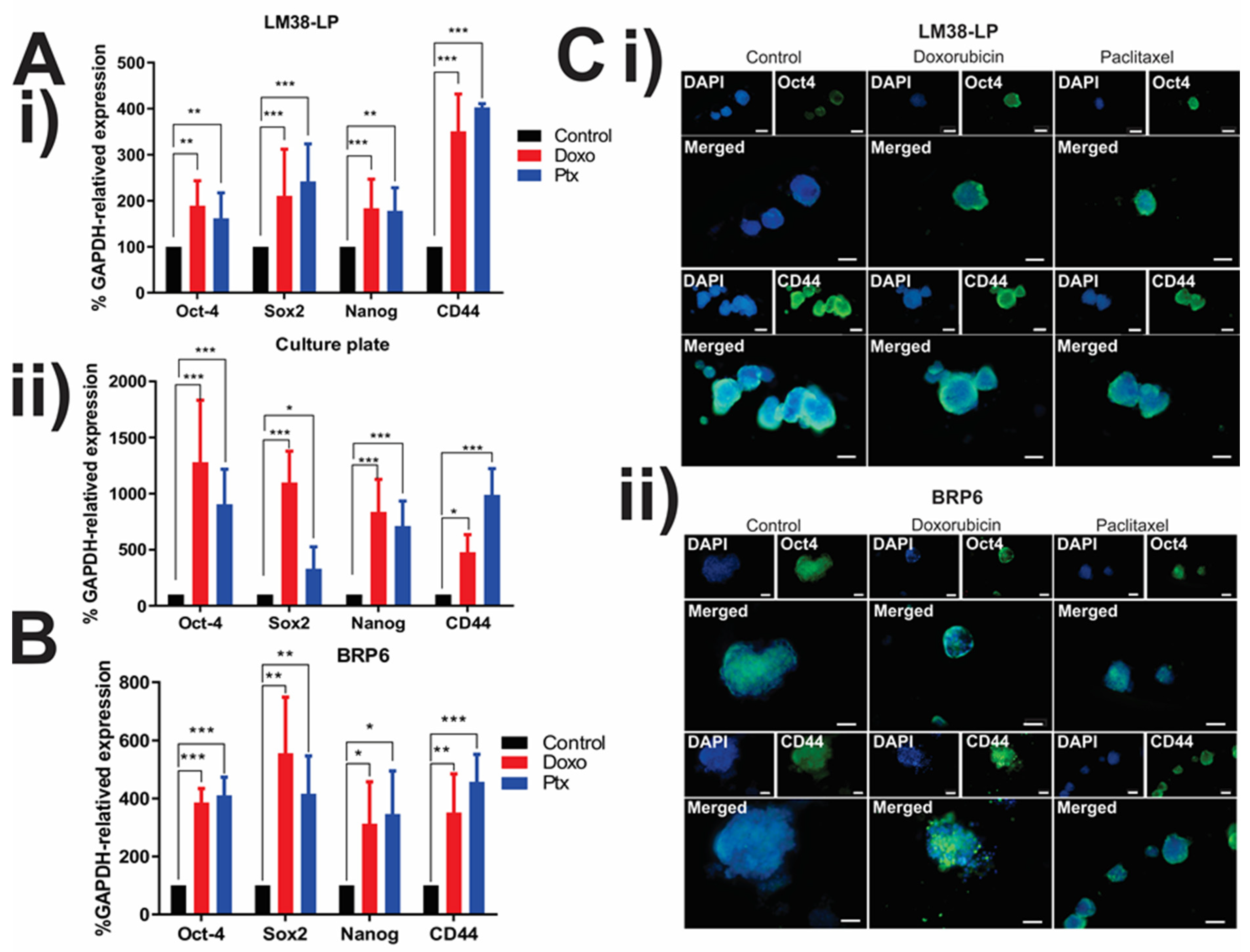
3.3. Expression of CSC-Associated Genes in LM38-LP and BRP6 Cell Lines
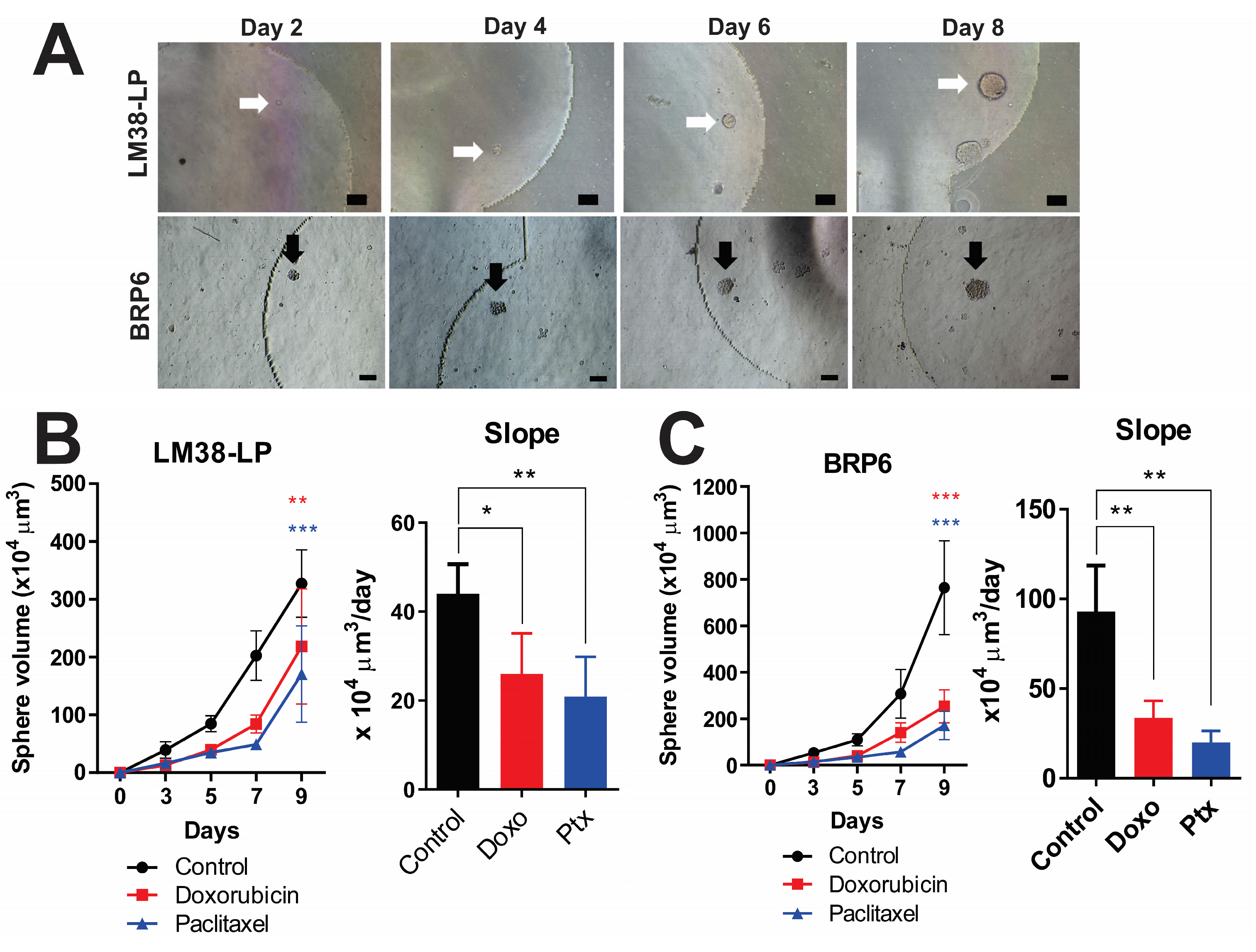
3.4. Tracking Individual Spheres—Setting Growth Rate
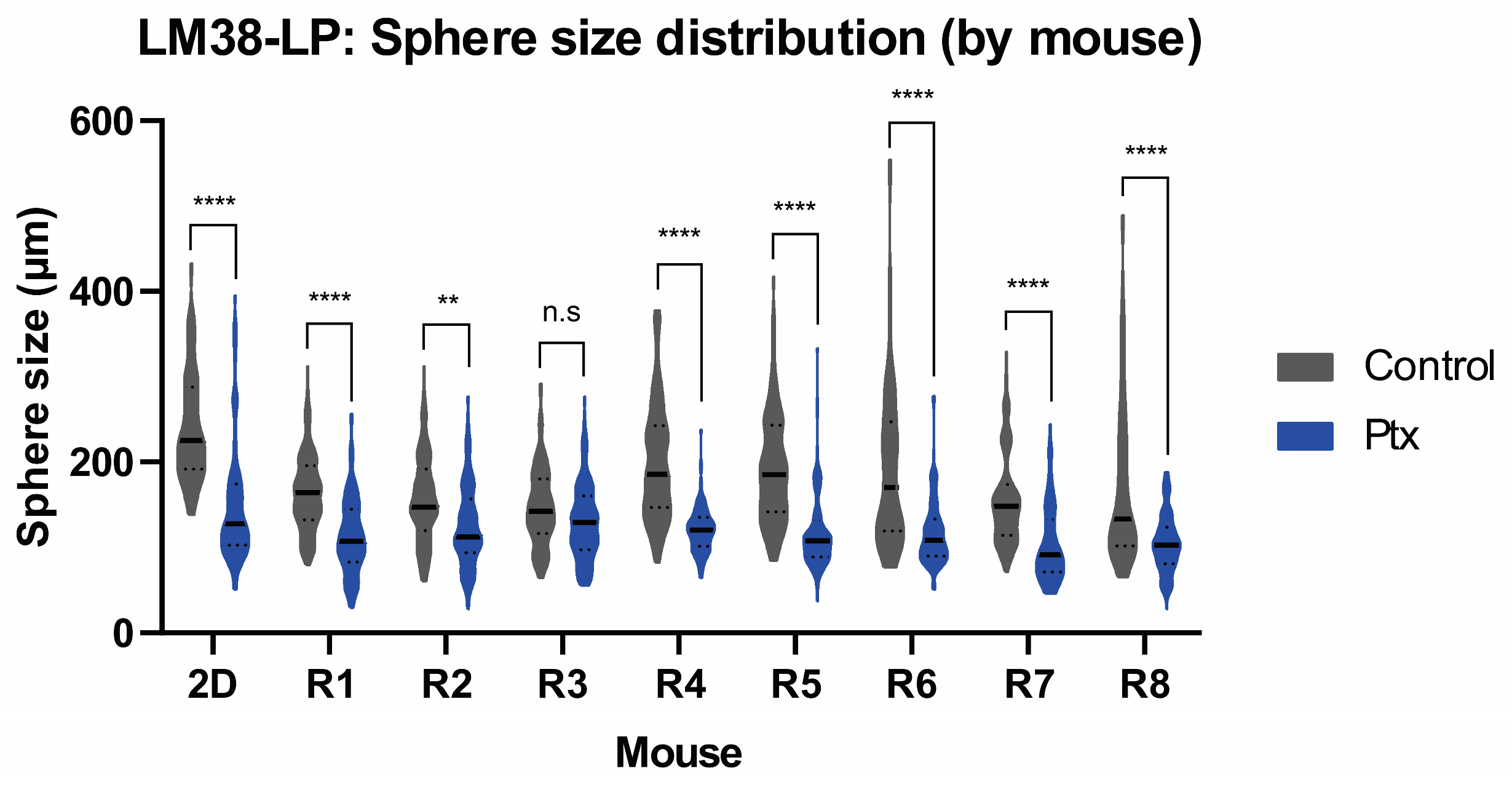
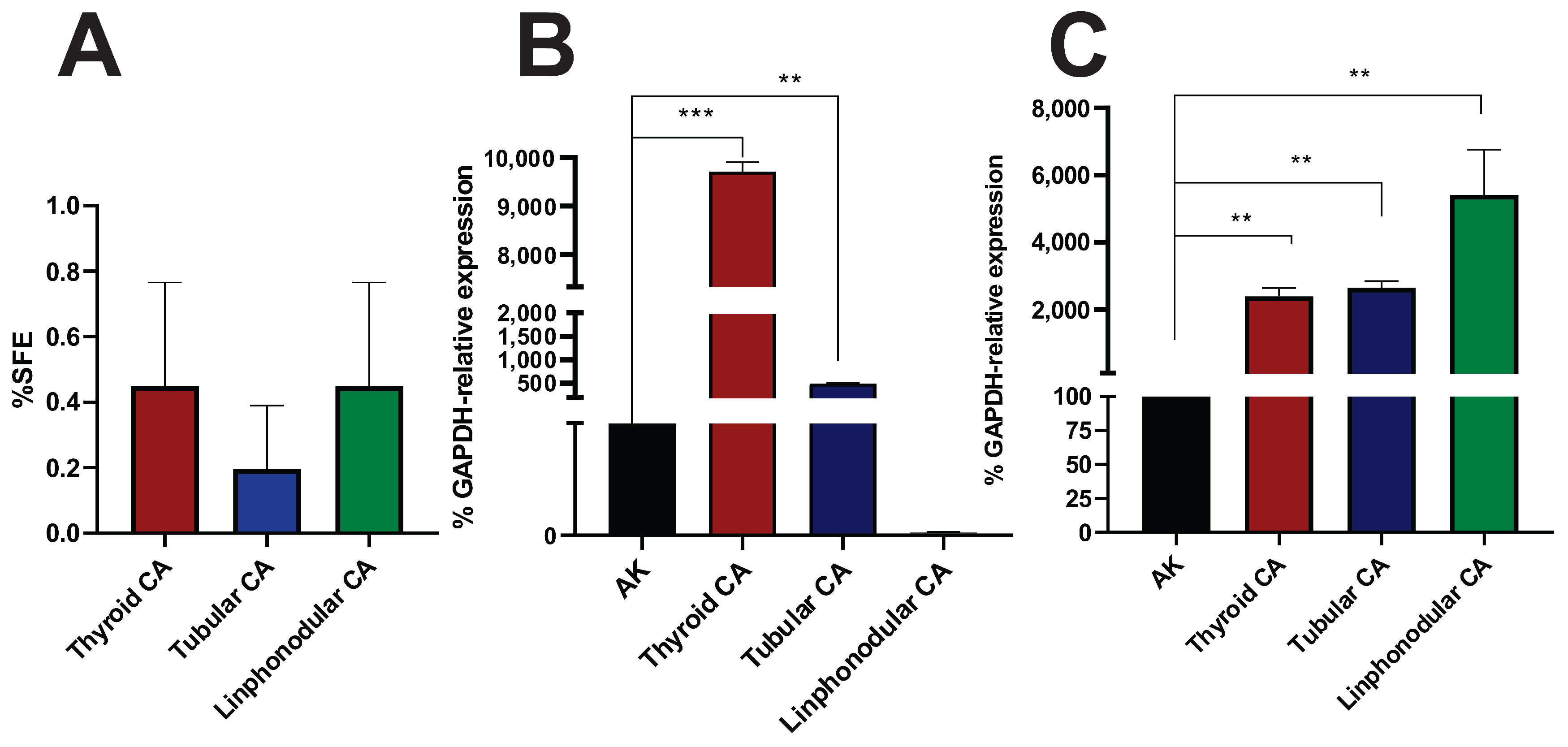
3.5. Sphere-Forming Ability of Murine and Canine Tumors Within the MD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CSCs | Cancer Stem Cells |
| MD | Microdevice |
| CT | Chemotherapy |
| SFE | Sphere-Forming Efficiency |
| LOC | Lab-On-A-Chip |
| IF | Immunofluorescence |
| CM | Complete Medium |
| Doxo | Doxorubicin |
| Ptx | Paclitaxel |
| PDMS | Polydimethylsiloxane |
| SFM | Serum-Free Medium |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.M.; Zhang, J.G.; Zhang, X.; Li, Q. Targeting cancer stem cells for reversing therapy resistance: Mechanism, signaling, and prospective agents. Signal Transduct. Target. Ther. 2021, 6, 62. [Google Scholar] [CrossRef] [PubMed]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef]
- Liu, X.; Li, G.; Liu, Q.; Guo, Z.; Li, B.; Zhang, Y.; Li, X.; Wang, J. Cancer stem cells and their niche in cancer progression and therapy. Cancer Cell Int. 2023, 23, 305. [Google Scholar] [CrossRef]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F.; et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target. Ther. 2020, 5, 8. [Google Scholar] [CrossRef]
- Vidal, S.J.; Rodriguez-Bravo, V.; Galsky, M.; Cordon-Cardo, C.; Domingo-Domenech, J. Targeting cancer stem cells to suppress acquired chemotherapy resistance. Oncogene 2014, 33, 4451–4463. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Qian, W.; Zhang, H.; Liang, Y.; Wu, M.; Zhang, Y.; Zhang, X.; Gao, Q.; Li, Y. SOX2 Is a Marker for Stem-like Tumor Cells in Bladder Cancer. Stem Cell Rep. 2017, 9, 429–437. [Google Scholar] [CrossRef]
- Chaudhary, A.; Raza, S.S.; Haque, R. Transcriptional factors targeting in cancer stem cells for tumor modulation. Semin. Cancer Biol. 2023, 88, 123–137. [Google Scholar] [CrossRef]
- Tsujii, M. [Cancer therapy targeting cancer stem cell]. Nihon Rinsho. 2014, 72, 35–41. [Google Scholar]
- Lytle, N.K.; Barber, A.G.; Reya, T. Stem cell fate in cancer growth, progression and therapy resistance. Nat. Rev. Cancer 2018, 18, 669–680. [Google Scholar] [CrossRef]
- Magee, J.A.; Piskounova, E.; Morrison, S.J. Cancer Stem Cells: Impact, Heterogeneity, and Uncertainty. Cancer Cell 2012, 21, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Belgorosky, D.; Fernández-Cabada, T.; Peñaherrera-Pazmiño, A.B.; Langle, Y.; Booth, R.; Bhansali, S.; Pérez, M.S.; Eiján, A.M.; Lerner, B. Analysis of tumoral spheres growing in a multichamber microfluidic device. J. Cell. Physiol. 2018, 233, 6327–6336. [Google Scholar] [CrossRef]
- Agüero, E.I.; Belgorosky, D.; García-Silva, J.I.; Booth, R.; Lerner, B.; Pérez, M.S.; Eiján, A.M. Correction to: Microdevices for cancer stem cell culture as a predictive chemotherapeutic response platform. J. Mol. Med. 2023, 101, 1477. [Google Scholar] [CrossRef] [PubMed]
- Bumaschny, V.; Urtreger, A.; Diament, M.; Krasnapolski, M.; Fiszman, G.; Klein, S.; Joffé, E.B.d.K. Malignant myoepithelial cells are associated with the differentiated papillary structure and metastatic ability of a syngeneic murine mammary adenocarcinoma model. Breast Cancer Res. 2004, 6, R116–R129. [Google Scholar] [CrossRef]
- Reyes-Moreno, C.; Girouard, J.; Marino, L.; Belgorosky, D.; Langle, Y.V.; Hamelin-Morrissete, J.; Agüero, E.I.; Malagrino, H.; Eiján, A.M. Relevance of iNOS expression in tumor growth and maintenance of cancer stem cells in a bladder cancer model. J. Mol. Med. 2020, 98, 1615–1627. [Google Scholar] [CrossRef]
- Herrera, M.; Berral-González, A.; López-Cade, I.; Galindo-Pumariño, C.; Bueno-Fortes, S.; Martín-Merino, M.; Carrato, A.; Ocaña, A.; De La Pinta, C.; López-Alfonso, A.; et al. Cancer-associated fibroblast-derived gene signatures determine prognosis in colon cancer patients. Mol. Cancer 2021, 20, 73. [Google Scholar] [CrossRef]
- Dankner, M.; Rose, A.A.N.; Rajkumar, S.; Siegel, P.M.; Watson, I.R. Classifying BRAF alterations in cancer: New rational therapeutic strategies for actionable mutations. Oncogene 2018, 37, 3183–3199. [Google Scholar] [CrossRef]
- Chu, X.; Tian, W.; Ning, J.; Xiao, G.; Zhou, Y.; Wang, Z.; Zhai, Z.; Tanzhu, G.; Yang, J.; Zhou, R. Cancer stem cells: Advances in knowledge and implications for cancer therapy. Signal Transduction and Targeted Therapy 2024, 9, 170. [Google Scholar] [CrossRef]
- Tannishtha, R.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar]
- Clevers, H. The cancer stem cell: Premises, promises and challenges. Nat. Med. 2011, 17, 313–319. [Google Scholar] [CrossRef] [PubMed]
- El-Tanani, M.; Rabbani, S.A.; Satyam, S.M.; Rangraze, I.R.; Wali, A.F.; El-Tanani, Y.; Aljabali, A.A.A. Deciphering the Role of Cancer Stem Cells: Drivers of Tumor Evolution, Therapeutic Resistance, and Precision Medicine Strategies. Cancers 2025, 17, 382. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, R.; Ouyang, Y.; Shen, Y.; Hu, L.; Xu, C. Cancer stem cells: A target for overcoming therapeutic resistance and relapse. Cancer Biol. Med. 2024, 20, 985–1020. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.A.; Letai, A.; Fisher, D.E.; Flaherty, K.T. Precision medicine for cancer with next-generation functional diagnostics. Nat. Rev. Cancer 2016, 15, 747–756. [Google Scholar] [CrossRef]
- Shibata, M.; Hoque, M.O. Targeting cancer stem cells: A strategy for effective eradication of cancer. Cancers 2019, 11, 732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Semenza, G.L.; Zhang, H.; Shimoda, L.A.; Lu, H.; Chen, I.; Park, Y.; Tran, L. Chemotherapy-Induced Ca2+ Release Stimulates Breast Cancer Stem Cell Enrichment. Cell Rep. 2017, 18, 1946–1957. [Google Scholar] [CrossRef]
- Guerra-Rebollo, M.; Garrido, C.; Sánchez-Cid, L.; Soler-Botija, C.; Meca-Cortés, O.; Rubio, N.; Blanco, J. Targeting of replicating CD133 and OCT4/SOX2 expressing glioma stem cells selects a cell population that reinitiates tumors upon release of therapeutic pressure. Sci. Rep. 2019, 9, 9549. [Google Scholar] [CrossRef]
- Bang, J.S.; Choi, N.Y.; Lee, M.; Ko, K.; Park, Y.S.; Ko, K. Reprogramming of cancer cells into induced pluripotent stem cells questioned. Int. J. Stem Cells 2019, 12, 430–439. [Google Scholar] [CrossRef]
- Navarro-Marchal, S.A.; GrinÌ án-Lisan, C.; Entrena, J.M.; Ruiz-Alcalá, G.; Tristán-Manzano, M.; Martin, F.; Pérez-Victoria, I.; Peula-Garciá, J.M.; Marchal, J.A. Anti-CD44-Conjugated Olive Oil Liquid Nanocapsules for Targeting Pancreatic Cancer Stem Cells. Biomacromolecules 2021, 22, 1374–1388. [Google Scholar] [CrossRef]
- Bocci, F.; Levine, H.; Onuchic, J.N.; Jolly, M.K. Deciphering the Dynamics of Epithelial-Mesenchymal Transition and Cancer Stem Cells in Tumor Progression. Curr. Stem Cell Rep. 2019, 5, 11–21. [Google Scholar] [CrossRef]
- Król, M.; Rybicka, A. Identification and characterization of cancer stem cells in canine mammary tumors. Acta Veter- Scand. 2016, 58, 86. [Google Scholar] [CrossRef]
- Pang, L.Y.; Cervantes-Arias, A.; Else, R.W.; Argyle, D.J. Canine mammary cancer stem cells are radio- and chemo- resistant and exhibit an epithelial-mesenchymal transition phenotype. Cancers 2011, 3, 1744–1762. [Google Scholar] [CrossRef] [PubMed]
- Michishita, M.; Akiyoshi, R.; Yoshimura, H.; Katsumoto, T.; Ichikawa, H.; Ohkusu-Tsukada, K.; Nakagawa, T.; Sasaki, N.; Takahashi, K. Characterization of spheres derived from canine mammary gland adenocarcinoma cell lines. Res. Veter.-Sci. 2011, 91, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, T.E.; Yashima, S.; Nakahira, R.; Onozawa, E.; Azakami, D.; Ujike, M.; Ochiai, K.; Ishiwata, T.; Takahashi, K.; Michishita, M. Identification of tumor-initiating cells derived from two canine rhabdomyosarcoma cell lines. J. Veter.-Med. Sci. 2017, 79, 1155–1162. [Google Scholar] [CrossRef][Green Version]
- Zhao, H.; Yan, C.; Hu, Y.; Mu, L.; Huang, K.; Li, Q.; Li, X.; Tao, D.; Qin, J. Sphere-forming assay vs. Organoid culture: Determining long-term stemness and the chemoresistant capacity of primary colorectal cancer cells. Int. J. Oncol. 2019, 54, 893–904. [Google Scholar] [CrossRef]

| Gene | Forward Primer (5′→3′) | Reverse Primer (5′→3′) | Amplicon Size (bp) |
|---|---|---|---|
| mOct4 | TCAGGTTGGACTGGGCCTAGT | GGAGGTTCCCTCTGAGTTGCTT | 100 |
| hOct4 | GTGGAGGAAGCTGACAACAATG | AATTCTCCAGGTTGCCTCTCATC | 121 |
| mSox2 | CCTCCGGGACATGATCAGCATGTA | GCAGTGTGCCGTTAATGGCCGTG | 131 |
| hSox2 | GAGCTTTGCAGGAAGTTTGC | GCAAGAAGCCTCTCCTTGAA | 190 |
| mNanog | GAAATCCCTTCCCTCGCCATC | CTCAGTAGCAGACCCTTGTAAGC | 161 |
| hNanog | ACAACTGGCCGAAGAATAGCA | GGTTCCCAGTCGGGTTCAC | 111 |
| mCD44 | AAAAAGCCATGCAGCAGCTC | TTGCCTCTTGGGTGGTGTTT | 145 |
| hCD44 | CGGACACCATGGACAAGTTT | GAAAGCCTTGCAGAGGTCAG | 176 |
| mGAPDH | CAAAATGGTGAAGGTCGGTG | CAATGAAGGGGTCGTTGATG | 113 |
| hGAPDH | ACCCACTCCTCCACCTTTGA | CTGTTGCTGAGCCAAATTCGT | 100 |
| cSox2 | AACCCCAGATGCACAACTC | CGGGGCCGGTATTTATAATC | 162 |
| cNanog | GAATAACCCGAATTGGAGCAG | AGCGATTCCTCTTCACAGTTG | 141 |
| cGAPDH | TGACACCCACTCTTCCACCTTC | CGGTTGCTGTAGCCAAATTCA | 105 |
| %SFE | Sphere Size (µm) | |||||
|---|---|---|---|---|---|---|
| LM38-LP (MW-12) | LM38-LP (MD) | BRP6 (MD) | LM38-LP (MW-12) | LM38-LP (MD) | BRP6 (MD) | |
| Control | 28.3 ± 1.42 | 13.2 ± 1.2 | 10.5 ± 0.8 | 133.3 ± 75.1 | 192.7 ± 67.3 | 182.1 ± 77.4 |
| Doxo | 16.7 ± 0.7 (***) | 8.1 ± 2.7 (**) | 6.4 ± 1.83 (**) | 98.9 ± 54.6 (**) | 103.1 ± 32.7 (****) | 133.7± 55.2 (***) |
| Ptx | 21.2 ± 1.91 (**) | 7.2 ± 2.7 (**) | 4.2 ± 0.89 (***) | 108.2 ± 55.2 (**) | 118.3 ± 39.9 (***) | 127.1 ± 49.3 (***) |
| #cells/mL (×103) | Total RNA (μg) | Oct4 | Sox2 | Nanog | CD44 | |||
|---|---|---|---|---|---|---|---|---|
| LM38-LP | MD | Control | 100 | 17.4 ± 1.9 | 100 | 100 | 100 | 100 |
| Doxo | 100 | 4.6 ± 0.8 | 189.1 ± 54.1 (**) | 210.7 ± 101.2 (***) | 183.5 ± 63.4 (***) | 350.9 ± 81.1 (***) | ||
| Ptx | 100 | 5.8 ± 2.1 | 161.9 ± 55.2 (**) | 242.1 ± 81.5 (***) | 177.9 ± 50.1 (**) | 403.1 ± 7.8 (***) | ||
| MW-12 | Control | 3 | 81.4 ± 17.2 | 100 | 100 | 100 | 100 | |
| Doxo | 3 | 53.7 ± 11.5 | 1308.1 ± 558.9 (***) | 1087.1 ± 356.9 (***) | 858.4 ± 342.9 (***) | 486.3 ± 199.9 (*) | ||
| Ptx | 3 | 51.8 ± 19.8 | 852.6 ± 452.8 (***) | 419.7 ± 183.30 (*) | 771.6 ± 287.3 (***) | 918.3 ± 263.6 (***) | ||
| BRP6 | MD | Control | 100 | 13.6 ± 1.5 | 100 | 100 | 100 | 100 |
| Doxo | 100 | 6.4 ± 1.1 | 386.17 ± 48.3 (***) | 556.3 ± 192.2 (**) | 313.2 ± 144.2 (*) | 351.5 ± 133.4 (**) | ||
| Ptx | 100 | 5.6 ± 0.9 | 410.84 ± 62.7 (***) | 416.2 ± 130.8 (**) | 346.2 ± 148.6 (*) | 457.7 ± 94.4 (***) |
| #spheres | Sphere Size (µm) | |||
|---|---|---|---|---|
| Cell Line | Tumor | Cell Line | Tumor | |
| Control | 140 ± 9 | 89 ± 18 | 243.2 ± 68.03 | 174.3 ± 72.85 |
| Ptx | 78 ± 29 (***) | 59 ± 20 (*) | 152.3 ± 73.76 (***) | 117 ± 42.90 (***) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agüero, E.I.; Gómez López, S.M.; Peñaherrera-Pazmiño, A.B.; Tellado, M.; Pérez, M.S.; Lerner, B.; Belgorosky, D.; Eiján, A.M. Towards Personalized Medicine: Microdevice-Assisted Evaluation of Cancer Stem Cell Dynamics and Treatment Response. Cancers 2025, 17, 1922. https://doi.org/10.3390/cancers17121922
Agüero EI, Gómez López SM, Peñaherrera-Pazmiño AB, Tellado M, Pérez MS, Lerner B, Belgorosky D, Eiján AM. Towards Personalized Medicine: Microdevice-Assisted Evaluation of Cancer Stem Cell Dynamics and Treatment Response. Cancers. 2025; 17(12):1922. https://doi.org/10.3390/cancers17121922
Chicago/Turabian StyleAgüero, Eduardo Imanol, Silvia María Gómez López, Ana Belén Peñaherrera-Pazmiño, Matías Tellado, Maximiliano Sebastián Pérez, Betiana Lerner, Denise Belgorosky, and Ana María Eiján. 2025. "Towards Personalized Medicine: Microdevice-Assisted Evaluation of Cancer Stem Cell Dynamics and Treatment Response" Cancers 17, no. 12: 1922. https://doi.org/10.3390/cancers17121922
APA StyleAgüero, E. I., Gómez López, S. M., Peñaherrera-Pazmiño, A. B., Tellado, M., Pérez, M. S., Lerner, B., Belgorosky, D., & Eiján, A. M. (2025). Towards Personalized Medicine: Microdevice-Assisted Evaluation of Cancer Stem Cell Dynamics and Treatment Response. Cancers, 17(12), 1922. https://doi.org/10.3390/cancers17121922







