Clinico-Pathologic Profile of a Cohort of Patients with Actinic Keratosis in a Tertiary Center in Romania
Simple Summary
Abstract
1. Introduction
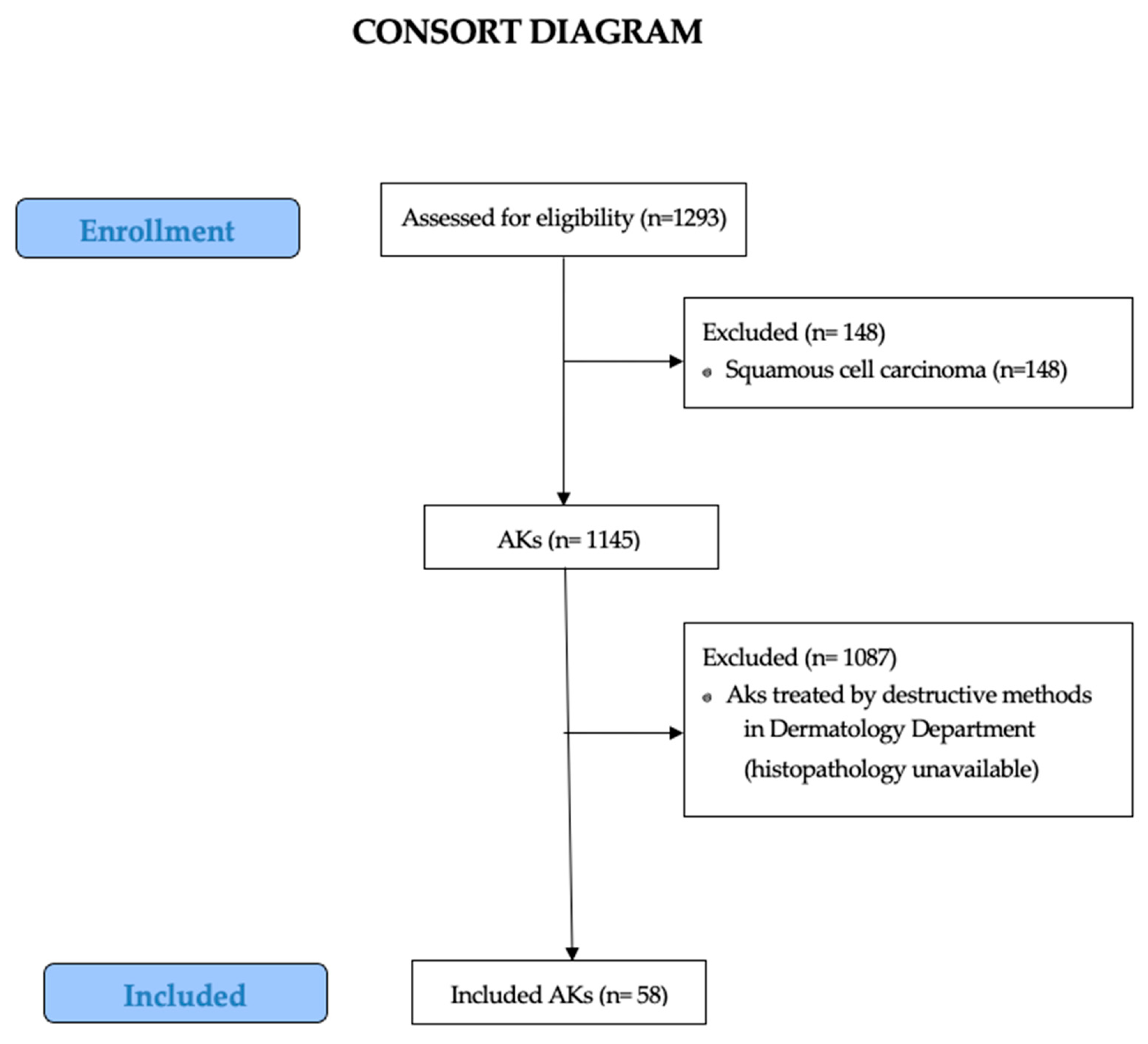
2. Materials and Methods
2.1. Study Design
2.2. Data Analysis
2.3. Ethical Aspects
3. Results
3.1. Demographic Data
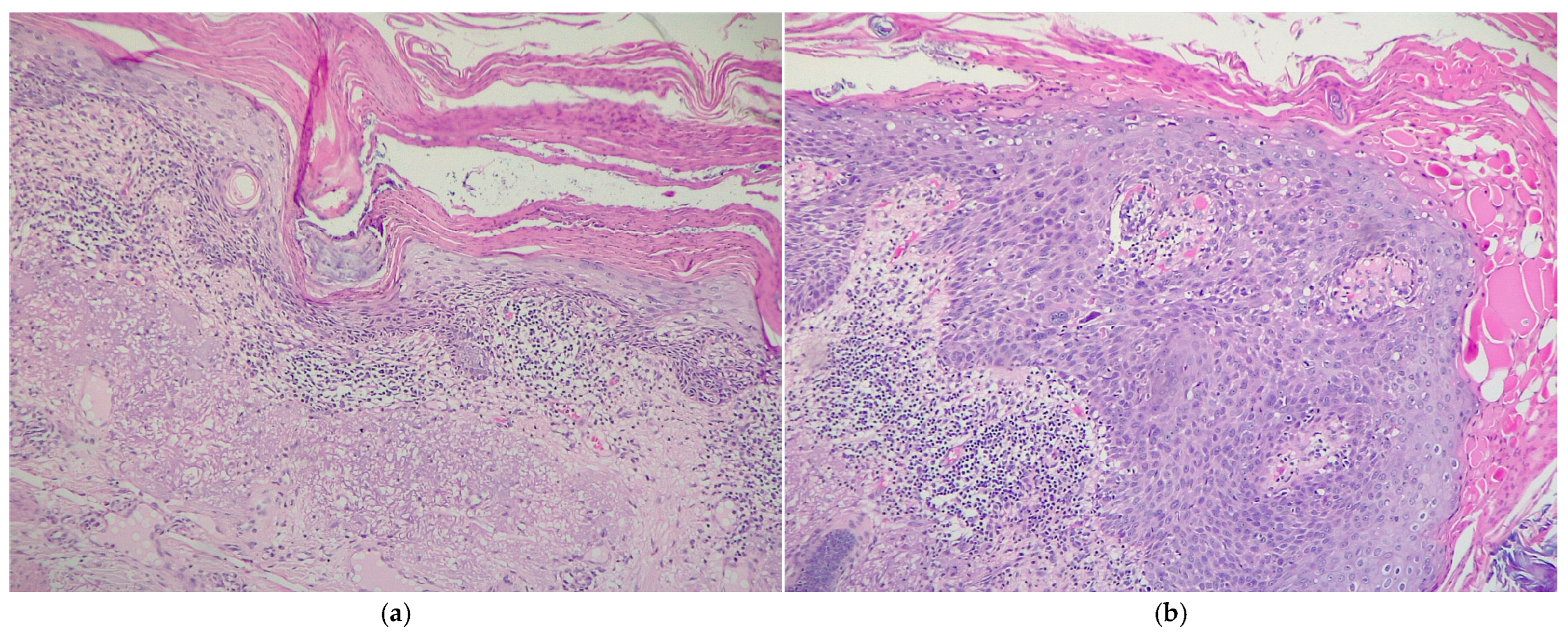
3.2. Histological Analysis
3.3. Associated Comorbidities Analysis
4. Discussion
4.1. Epidemiology-Related Aspects
4.2. Histopathology-Related Aspects
4.3. Associated Comorbidities
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quatresooz, P.; Piérard-Franchimont, C.; Paquet, P.; Hubert, P.; Delvenne, P.; Piérard, G.E. Crossroads between Actinic Keratosis and Squamous Cell Carcinoma, and Novel Pharmacological Issues. Eur. J. Dermatol. 2008, 18, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Röwert-Huber, J.; Patel, M.J.; Forschner, T.; Ulrich, C.; Eberle, J.; Kerl, H.; Sterry, W.; Stockfleth, E. Actinic Keratosis Is an Early in Situ Squamous Cell Carcinoma: A Proposal for Reclassification. Br. J. Dermatol. 2007, 156 (Suppl. S3), 8–12. [Google Scholar] [CrossRef] [PubMed]
- Salasche, S.J. Epidemiology of Actinic Keratoses and Squamous Cell Carcinoma. J. Am. Acad. Dermatol. 2000, 42 Pt 2, S4–S7. [Google Scholar] [CrossRef]
- Ackerman, A.B.; Mones, J.M. Solar (Actinic) Keratosis Is Squamous Cell Carcinoma. Br. J. Dermatol. 2006, 155, 9–22. [Google Scholar] [CrossRef]
- Stockfleth, E.; Ferrandiz, C.; Grob, J.J.; Leigh, I.; Pehamberger, H.; Kerl, H. Development of a Treatment Algorithm for Actinic Keratoses: A European Consensus. Eur. J. Dermatol. 2008, 18, 651–659. [Google Scholar] [PubMed]
- Glogau, R.G. The Risk of Progression to Invasive Disease. J. Am. Acad. Dermatol. 2000, 42 Pt 2, S23–S24. [Google Scholar] [CrossRef]
- Mittelbronn, M.A.; Mullins, D.L.; Ramos-Caro, F.A.; Flowers, F.P. Frequency of Pre-Existing Actinic Keratosis in Cutaneous Squamous Cell Carcinoma: Pre-Existing Actinic Keratosis in Cutaneous Squamous Cell carcinoma Report. Int. J. Dermatol. 1998, 37, 677–681. [Google Scholar] [CrossRef]
- Criscione, V.D.; Weinstock, M.A.; Naylor, M.F.; Luque, C.; Eide, M.J.; Bingham, S.F.; Department of Veteran Affairs Topical Tretinoin Chemoprevention Trial Group. Actinic Keratoses: Natural History and Risk of Malignant Transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer 2009, 115, 2523–2530. [Google Scholar] [CrossRef]
- Stockfleth, E.; Kerl, H.; Guideline Subcommittee of the European Dermatology Forum. Guidelines for the Management of Actinic Keratoses. Eur. J. Dermatol. 2006, 16, 599–606. [Google Scholar]
- Memon, A.A.; Tomenson, J.A.; Bothwell, J.; Friedmann, P.S. Prevalence of Solar Damage and Actinic Keratosis in a Merseyside Population. Br. J. Dermatol. 2000, 142, 1154–1159. [Google Scholar] [CrossRef]
- Frost, C.; Williams, G.; Green, A. High Incidence and Regression Rates of Solar Keratoses in a Queensland Community. J. Investig. Dermatol. 2000, 115, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, A.; Marmur, E. The Kinetics of Skin Cancer: Progression of Actinic Keratosis to Squamous Cell Carcinoma. Dermatol. Surg. 2007, 33, 1099–1101. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, A.; Shafi, R.; Nelson, J.; Cantrell, W.C.; Subhadarshani, S.; Andea, A.; Athar, M.; Elmets, C.A. The Clinical Course of Actinic Keratosis Correlates with Underlying Molecular Mechanisms. Br. J. Dermatol. 2020, 182, 995–1002. [Google Scholar] [CrossRef]
- Warszawik-Hendzel, O.; Olszewska, M.; Maj, M.; Rakowska, A.; Czuwara, J.; Rudnicka, L. Non-Invasive Diagnostic Techniques in the Diagnosis of Squamous Cell Carcinoma. J. Dermatol. Case Rep. 2015, 9, 89–97. [Google Scholar] [CrossRef]
- Que, S.K.T.; Zwald, F.O.; Schmults, C.D. Cutaneous Squamous Cell Carcinoma: Incidence, Risk Factors, Diagnosis, and Staging. J. Am. Acad. Dermatol. 2018, 78, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kozlow, J.H.; Mittal, B.; Moyer, J.; Olenecki, T.; Rodgers, P.; Alam, M.; Armstrong, A.; Baum, C.; Bordeaux, J.S.; et al. Guidelines of Care for the Management of Cutaneous Squamous Cell Carcinoma. J. Am. Acad. Dermatol. 2018, 78, 560–578. [Google Scholar] [CrossRef]
- Oppel, T.; Korting, H.C. Actinic Keratosis: The Key Event in the Evolution from Photoaged Skin to Squamous Cell Carcinoma. Therapy Based on Pathogenetic and Clinical Aspects. Skin Pharmacol. Physiol. 2004, 17, 67–76. [Google Scholar] [CrossRef]
- Rossi, R.; Mori, M.; Lotti, T. Actinic Keratosis. Int. J. Dermatol. 2007, 46, 895–904. [Google Scholar] [CrossRef]
- Ulrich, M.; Krueger-Corcoran, D.; Roewert-Huber, J.; Sterry, W.; Stockfleth, E.; Astner, S. Reflectance Confocal Microscopy for Noninvasive Monitoring of Therapy and Detection of Subclinical Actinic Keratoses. Dermatology 2010, 220, 15–24. [Google Scholar] [CrossRef]
- Slaughter, D.P.; Southwick, H.W.; Smejkal, W. Field Cancerization in Oral Stratified Squamous Epithelium; Clinical Implications of Multicentric Origin. Cancer 1953, 6, 963–968. [Google Scholar] [CrossRef]
- Willenbrink, T.J.; Ruiz, E.S.; Cornejo, C.M.; Schmults, C.D.; Arron, S.T.; Jambusaria-Pahlajani, A. Field Cancerization: Definition, Epidemiology, Risk Factors, and Outcomes. J. Am. Acad. Dermatol. 2020, 83, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, R.F.; Buffo, T.H.; Gonçalves, H.d.S.; Barcaui, C.B.; de Moraes, A.M. Field Cancerization in Dermatology. Rev. Assoc. Medica Bras. 2024, 70 (Suppl. S1), e2024S113. [Google Scholar] [CrossRef]
- Torezan, L.A.R.; Festa-Neto, C. Cutaneous Field Cancerization: Clinical, Histopathological and Therapeutic Aspects. An. Bras. Dermatol. 2013, 88, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Philipp-Dormston, W.G. Field Cancerization: From Molecular Basis to Selective Field-Directed Management of Actinic Keratosis. Curr. Probl. Dermatol. 2015, 46, 115–121. [Google Scholar] [CrossRef]
- Jetter, N.; Chandan, N.; Wang, S.; Tsoukas, M. Field Cancerization Therapies for Management of Actinic Keratosis: A Narrative Review. Am. J. Clin. Dermatol. 2018, 19, 543–557. [Google Scholar] [CrossRef]
- Roewert-Huber, J.; Stockfleth, E.; Kerl, H. Pathology and Pathobiology of Actinic (Solar) Keratosis—An Update. Br. J. Dermatol. 2007, 157 (Suppl. S2), 18–20. [Google Scholar] [CrossRef]
- Fernandez Figueras, M.T. From Actinic Keratosis to Squamous Cell Carcinoma: Pathophysiology Revisited. J. Eur. Acad. Dermatol. Venereol. 2017, 31 (Suppl. S2), 5–7. [Google Scholar] [CrossRef] [PubMed]
- Falkenberg, C.; Dirschka, T.; Gilbert, G.; Stockfleth, E.; Homey, B.; Schmitz, L. Basal Proliferation and Acantholysis May Represent Histological High-Risk Factors for Progression into Invasive Squamous Cell Carcinoma: A Comparison Study in Solid Organ Transplant Recipients and Matched Immunocompetent Patients. Cancers 2023, 15, 1765. [Google Scholar] [CrossRef]
- Quaedvlieg, P.J.F.; Tirsi, E.; Thissen, M.R.T.M.; Krekels, G.A. Actinic Keratosis: How to Differentiate the Good from the Bad Ones? Eur. J. Dermatol. 2006, 16, 335–339. [Google Scholar]
- Flohil, S.C.; van der Leest, R.J.T.; Dowlatshahi, E.A.; Hofman, A.; de Vries, E.; Nijsten, T. Prevalence of Actinic Keratosis and Its Risk Factors in the General Population: The Rotterdam Study. J. Investig. Dermatol. 2013, 133, 1971–1978. [Google Scholar] [CrossRef]
- Werner, R.N.; Sammain, A.; Erdmann, R.; Hartmann, V.; Stockfleth, E.; Nast, A. The Natural History of Actinic Keratosis: A Systematic Review. Br. J. Dermatol. 2013, 169, 502–518. [Google Scholar] [CrossRef] [PubMed]
- de Gálvez, M.V.; Aguilera, J.; Bernabó, J.-L.; Sánchez-Roldán, C.; Herrera-Ceballos, E. Human Hair as a Natural Sun Protection Agent: A Quantitative Study. Photochem. Photobiol. 2015, 91, 966–970. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Garbutcheon-Singh, K.B.; Dixit, S.; Brown, P.; Smith, S.D. The Influence of Age and Gender in Knowledge, Behaviors and Attitudes towards Sun Protection: A Cross-Sectional Survey of Australian Outpatient Clinic Attendees. Am. J. Clin. Dermatol. 2015, 16, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Cockerell, C.J. Histopathology of Incipient Intraepidermal Squamous Cell Carcinoma (“actinic Keratosis”). J. Am. Acad. Dermatol. 2000, 42 Pt 2, S11–S17. [Google Scholar] [CrossRef]
- Schmitz, L.; Oster-Schmidt, C.; Stockfleth, E. Nonmelanoma Skin Cancer—From Actinic Keratosis to Cutaneous Squamous Cell Carcinoma. J. Dtsch. Dermatol. Ges. 2018, 16, 1002–1013. [Google Scholar] [CrossRef]
- Zalaudek, I.; Giacomel, J.; Schmid, K.; Bondino, S.; Rosendahl, C.; Cavicchini, S.; Tourlaki, A.; Gasparini, S.; Bourne, P.; Keir, J.; et al. Dermatoscopy of Facial Actinic Keratosis, Intraepidermal Carcinoma, and Invasive Squamous Cell Carcinoma: A Progression Model. J. Am. Acad. Dermatol. 2012, 66, 589–597. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Shi, Y.; Wu, S.; Ding, Y.; Yao, G.; Chen, J. Advancements in Elucidating the Pathogenesis of Actinic Keratosis: Present State and Future Prospects. Front. Med. 2024, 11, 1330491. [Google Scholar] [CrossRef]
- Alam, M.; Ratner, D. Cutaneous Squamous-Cell Carcinoma. N. Engl. J. Med. 2001, 344, 975–983. [Google Scholar] [CrossRef]

| Clinicopathologic Parameters | Values |
|---|---|
| Age (years), median (IQR) | 77.0 (10.0) |
| Age groups | |
| 50–59 years, n (%) | 2 (3.4) |
| 60–69 years, n (%) | 9 (15.5) |
| 70–79 years, n (%) | 24 (41.4) |
| 80–89 years, n (%) | 22 (37.9) |
| >90 years, n (%) | 1 (1.7) |
| Gender | |
| Male, n (%) | 32 (55.2) |
| Female, n (%) | 26 (44.8) |
| Area of residence (urban vs. rural) | |
| Urban, n (%) | 38 (65.5) |
| Rural, n (%) | 20 (34.5) |
| Anatomical site | |
| Head and neck, n (%) | 51 (87.9) |
| Thorax, n (%) | 1 (1.7) |
| Upper and lower limbs, n (%) | 6 (10.3) |
| Lesion dimensions | |
| <1 cm, n (%) | 31 (53.4) |
| 1–2 cm, n (%) | 18 (31) |
| 2–3 cm, n (%) | 9 (15.5) |
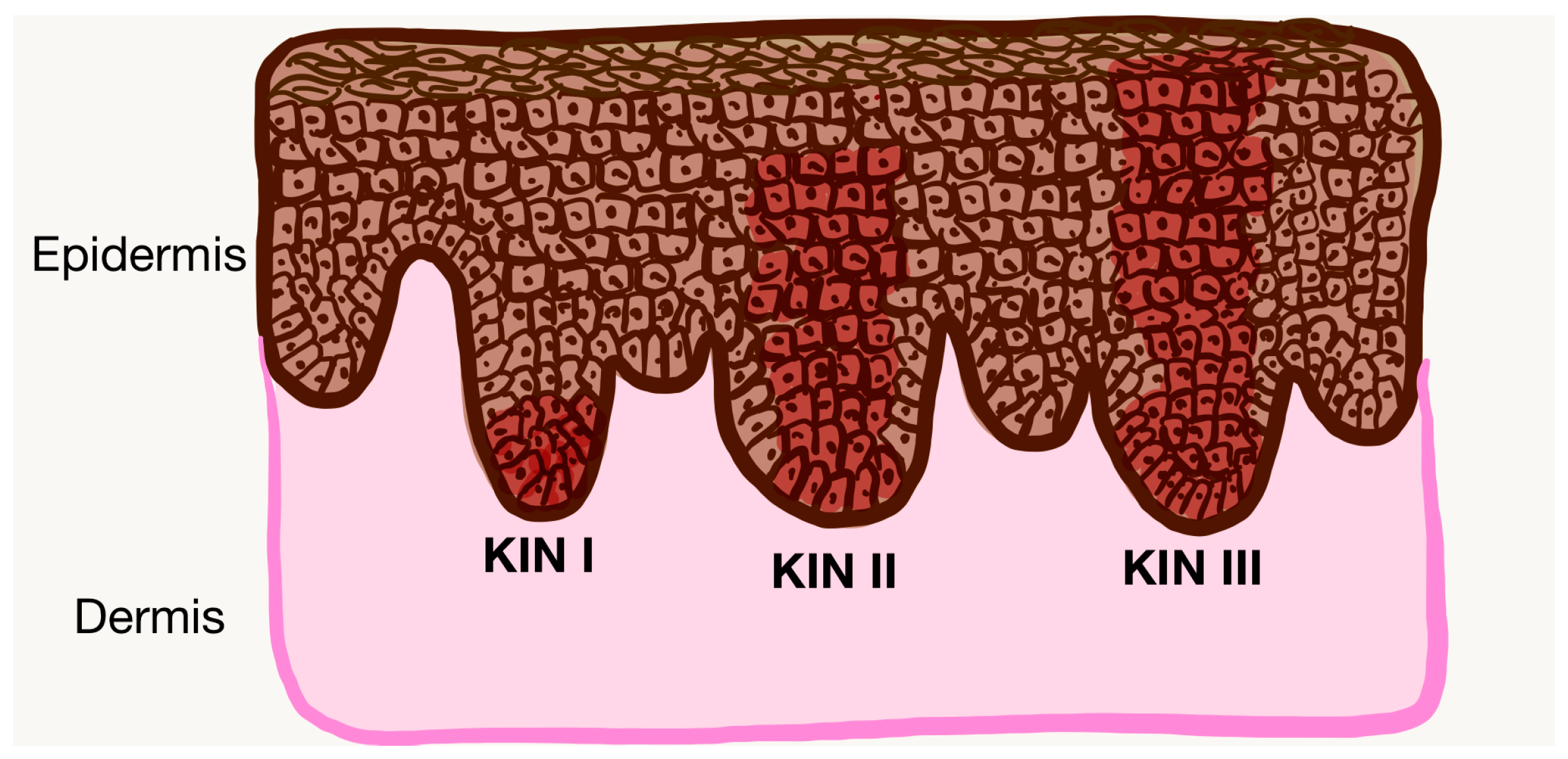
| KIN grade | |
| KIN I, n (%) | 20 (34.5) |
| KIN II, n (%) | 24 (41.4) |
| KIN III, n (%) | 14 (24.1) |
| Histopathological subtype | |
| Hypertrophic, n (%) | 35 (60.3) |
| Atrophic, n (%) | 16 (27.6) |
| Lichenoid, n (%) | 3 (5.2) |
| Bowenoid, n (%) | 4 (6.9) |
| Cytonuclear pleomorphism | |
| Mild, n (%) | 24 (41.4) |
| Moderate, n (%) | 28 (48.3) |
| Severe, n (%) | 6 (10.3) |
| Solar elastosis | |
| Mild, n (%) | 8 (13.8) |
| Moderate, n (%) | 22 (37.9) |
| Severe, n (%) | 28 (48.3) |
| Peritumoral inflammatory cell infiltrate severity | |
| Mild, n (%) | 8 (13.8) |
| Moderate, n (%) | 33 (56.9) |
| Severe, n (%) | 17 (29.3) |
| Peritumoral inflammatory cell infiltrate type | |
| Lymphoplasmacytic | 52 (89.7) |
| Polymorphic | 6 (10.3) |
| Ulceration | |
| Present, n (%) | 18 (31) |
| Absent, n (%) | 40 (69) |
| Comorbidities | |
| Smoking, n (%) | 8 (13.8) |
| Cardiovascular, n (%) | 35 (60.3) |
| Diabetes, n (%) | 8 (13.8) |
| Cancer, n (%) | 11 (19) |
| Clinicopathological Parameters | KIN Grade | p-Value | ||
|---|---|---|---|---|
| KIN I | KIN II | KIN III | ||
| Age group | ||||
| 50–59 years, n (%) | 0 (0) | 0 (0) | 2 (14.3) | 0.145 |
| 60–69 years, n (%) | 5 (25) | 3 (12.5) | 1 (7.1) | |
| 70–79 years, n (%) | 8 (40) | 10 (41.7) | 6 (42.9) | |
| 80–89 years, n (%) | 7 (35) | 11 (45.8) | 4 (37.9) | |
| >90 years, n (%) | 0 (0) | 0 (0) | 1 (7.1) | |
| Gender | ||||
| Male, n (%) | 12 (60) | 9 (37.5) | 11 (78.6) | 0.042 ** |
| Female, n (%) | 8 (40) | 15 (62.5) | 3 (21.4) | |
| Area of residence (urban vs. rural) | ||||
| Urban, n (%) | 13 (65) | 14 (58.3) | 38 (65.5) | 0.448 |
| Rural, n (%) | 7 (35) | 10 (41.7) | 20 (34.5) | |
| Anatomical site | ||||
| Head and neck, n (%) | 18 (90) | 20 (83.3) | 13 (92.9) | 0.229 |
| Thorax, n (%) | 0 (0) | 0 (0) | 1 (7.1) | |
| Upper and lower limbs, n (%) | 2 (10) | 4 (16.7) | 0 (0) | |
| Lesions dimensions | ||||
| <1 cm, n (%) | 15 (75) | 9 (37.5) | 7 (50) | 0.172 |
| 1–2 cm, n (%) | 3 (15) | 10 (41.7) | 5 (35.7) | |
| 2–3 cm, n (%) | 2 (10) | 5 (20.8) | 2 (14.3) | |
| Histopathological subtype | ||||
| Hypertrophic, n (%) | 12 (60) | 12 (50) | 11 (78.6) | 0.257 |
| Atrophic, n (%) | 7 (35) | 8 (33.3) | 1 (7.1) | |
| Lichenoid, n (%) | 1 (5) | 2 (8.3) | 0 (0) | |
| Bowenoid, n (%) | 0 (0) | 2 (8.3) | 2 (14.3) | |
| Cytonuclear pleomorphism | ||||
| Mild, n (%) | 19 (95) | 5 (20.8) | 0 (0) | <0.001 ** |
| Moderate, n (%) | 1 (5) | 19 (79.2) | 8 (57.1) | |
| Severe, n (%) | 0 (0) | 0 (0) | 6 (42.9) | |
| Solar elastosis | ||||
| Mild, n (%) | 8 (40) | 0 (0) | 0 (0) | <0.001 ** |
| Moderate, n (%) | 7 (35) | 14 (58.3) | 1 (7.1) | |
| Severe, n (%) | 5 (25) | 10 (41.7) | 13 (92.9) | |
| Peritumoral inflammatory cell infiltrate severity | ||||
| Mild, n (%) | 8 (40) | 0 (0) | 0 (0) | <0.001 ** |
| Moderate, n (%) | 10 (50) | 18 (75) | 5 (35.7) | |
| Severe, n (%) | 2 (10) | 6 (25) | 9 (64.3) | |
| Peritumoral inflammatory cell infiltrate type | ||||
| Lymphoplasmacytic | 18 (90) | 22 (91.7) | 12 (85.7) | 0.843 |
| Polymorphic | 2 (10) | 2 (8.3) | 2 (14.3) | |
| Ulceration | ||||
| Present, n (%) | 5 (25) | 7 (29.2) | 6 (42.9) | 0.524 |
| Absent, n (%) | 15 (75) | 17 (70.8) | 8 (57.1) | |
| Comorbidities | ||||
| Smoking, n (%) | 3 (15) | 2 (8.3) | 3 (21.4) | 0.519 |
| Cardiovascular, n (%) | 11 (55) | 15 (62.5) | 9 (64.3) | 0.829 |
| Diabetes, n (%) | 3 (15) | 2 (8.3) | 3 (21.4) | 0.519 |
| Cancer, n (%) | 6 (30) | 4 (16.7) | 1 (7.1) | 0.230 |
| Clinicopathological Parameters | Histopathological Subtype | p-Value | |||
|---|---|---|---|---|---|
| Hypertrophic | Atrophic | Lichenoid | Bowenoid | ||
| Age group | |||||
| 50–59 years, n (%) | 2 (5.7) | 0 (0) | 0 (0) | 0 (0) | 0.981 |
| 60–69 years, n (%) | 6 (17.1) | 3 (18.3) | 0 (0) | 0 (0) | |
| 70–79 years, n (%) | 13 (37.1) | 7 (43.8) | 2 (66.7) | 2 (50) | |
| 80–89 years, n (%) | 13 (37.1) | 6 (37.5) | 1 (33.3) | 2 (50) | |
| >90 years, n (%) | 1 (2.9) | 0 (0) | 0 (0) | 0 (0) | |
| Gender | |||||
| Male, n (%) | 21 (60) | 10 (62.5) | 0 (0) | 1 (25) | 0.120 |
| Female, n (%) | 14 (40) | 6 (37.5) | 3 (100) | 3 (75) | |
| Area of residence (urban vs. rural) | |||||
| Urban, n (%) | 23 (65.7) | 9 (56.3) | 2 (66.7) | 4 (100) | 0.438 |
| Rural, n (%) | 12 (34.3) | 7 (43.8) | 1 (33.3) | 0 (0) | |
| Anatomical site | |||||
| Head and neck, n (%) | 33 (94.3) | 13 (81.3) | 3 (100) | 2 (50) | 0.008 ** |
| Thorax, n (%) | 0 (0) | 0 (0) | 0 (0) | 1 (25) | |
| Upper and lower limbs, n (%) | 2 (5.7) | 3 (18.8) | 0 (0) | 1 (25) | |
| Lesion dimensions | |||||
| <1 cm, n (%) | 24 (68.6) | 5 (31.3) | 2 (66.7) | 0 (0) | 0.05 ** |
| 1–2 cm, n (%) | 6 (17.1) | 8 (50) | 1 (33) | 3 (75) | |
| 2–3 cm, n (%) | 5 (14.3) | 3 (18.8) | 0 (0) | 1 (25) | |
| KIN grade | |||||
| KIN I, n (%) | 12 (34.3) | 7 (43.8) | 1 (33.3) | 0 (0) | 0.257 |
| KIN II, n (%) | 12 (34.3) | 8 (50) | 2 (66.7) | 2 (50) | |
| KIN III, n (%) | 11 (31.4) | 1 (6.3) | 0 (0) | 2 (50) | |
| Cytonuclear polymoprhism | |||||
| Mild, n (%) | 5 (14.3) | 0 (0) | 0 (0) | 1 (25) | 0.299 |
| Moderate, n (%) | 17 (48.6) | 7 (43.8) | 1 (33.3) | 3 (75) | |
| Severe, n (%) | 13 (37.1) | 9 (56.3) | 2 (66.7) | 0 (0) | |
| Solar elastosis | |||||
| Mild, n (%) | 15 (42.9) | 7 (43.8) | 2 (66.7) | 4 (100) | 0.308 |
| Moderate, n (%) | 15 (42.9) | 7 (43.8) | 0 (0) | 0 (0) | |
| Severe, n (%) | 5 (14.3) | 2 (12.5) | 1 (33.3) | 0 (0) | |
| Peritumoral inflammatory cell infiltrate severity | |||||
| Mild, n (%) | 6 (17.1) | 2 (12.5) | 0 (0) | 0 (0) | 0.526 |
| Moderate, n (%) | 17 (48.6) | 12 (75) | 2 (66.7) | 2 (50) | |
| Severe, n (%) | 12 (34.3) | 2 (12.5) | 1 (33.3) | 2 (50) | |
| Peritumoral inflammatory cell infiltrate type | |||||
| Lymphoplasmacytic | 32 (91.4) | 14 (87.5) | 2 (66.7) | 4 (100) | 0.499 |
| Polymorphic | 3 (8.6) | 2 (12.5) | 1 (33.3) | 0 (0) | |
| Ulceration | |||||
| Present, n (%) | 10 (28.6) | 6 (37.5) | 1 (33.3) | 1 (25) | 0.922 |
| Absent, n (%) | 25 (71.4) | 10 (62.5) | 2 (66.7) | 3 (75) | |
| Comorbidities | |||||
| Smoking, n (%) | 5 (14.3) | 2 (12.5) | 0 (0) | 1 (25) | 0.818 |
| Cardiovascular, n (%) | 17 (48.6) | 13 (81.3) | 2 (66.7) | 3 (75) | 0.147 |
| Diabetes, n (%) | 6 (17.1) | 2 (12.5) | 0 (0) | 0 (0) | 0.689 |
| Cancer, n (%) | 7 (20) | 2 (12.5) | 1 (33.3) | 1 (25) | 0.812 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soare, C.; Cozma, E.C.; Poroșnicu, A.L.; Cristian, D.A.; Mandi, D.M.; Giurcăneanu, C.; Voiculescu, V.M. Clinico-Pathologic Profile of a Cohort of Patients with Actinic Keratosis in a Tertiary Center in Romania. Cancers 2025, 17, 1923. https://doi.org/10.3390/cancers17121923
Soare C, Cozma EC, Poroșnicu AL, Cristian DA, Mandi DM, Giurcăneanu C, Voiculescu VM. Clinico-Pathologic Profile of a Cohort of Patients with Actinic Keratosis in a Tertiary Center in Romania. Cancers. 2025; 17(12):1923. https://doi.org/10.3390/cancers17121923
Chicago/Turabian StyleSoare, Cristina, Elena Codruța Cozma, Andrei Ludovic Poroșnicu, Daniel Alin Cristian, Draga Maria Mandi, Călin Giurcăneanu, and Vlad Mihai Voiculescu. 2025. "Clinico-Pathologic Profile of a Cohort of Patients with Actinic Keratosis in a Tertiary Center in Romania" Cancers 17, no. 12: 1923. https://doi.org/10.3390/cancers17121923
APA StyleSoare, C., Cozma, E. C., Poroșnicu, A. L., Cristian, D. A., Mandi, D. M., Giurcăneanu, C., & Voiculescu, V. M. (2025). Clinico-Pathologic Profile of a Cohort of Patients with Actinic Keratosis in a Tertiary Center in Romania. Cancers, 17(12), 1923. https://doi.org/10.3390/cancers17121923







