The Increased Early Onset Colorectal Cancer in South East Scotland Is Indicative of a Wider UK Problem
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Data Collection
2.2. Data Analysis
3. Results
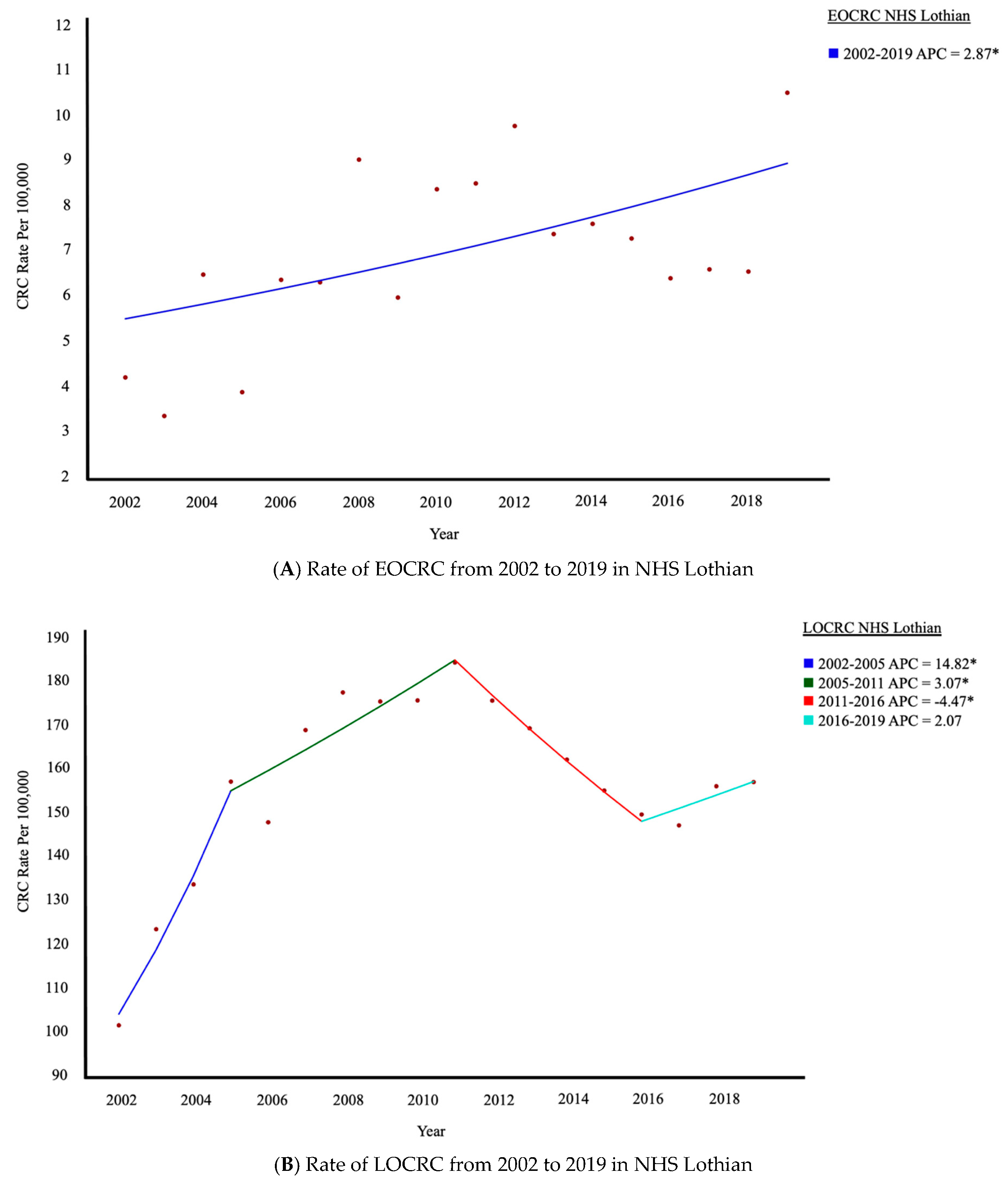
3.1. Trends in CRC Within NHS Lothian: Increase in EOCRC
3.2. Comparison of EOCRC Trends in NHS Lothian to Other Areas of UK
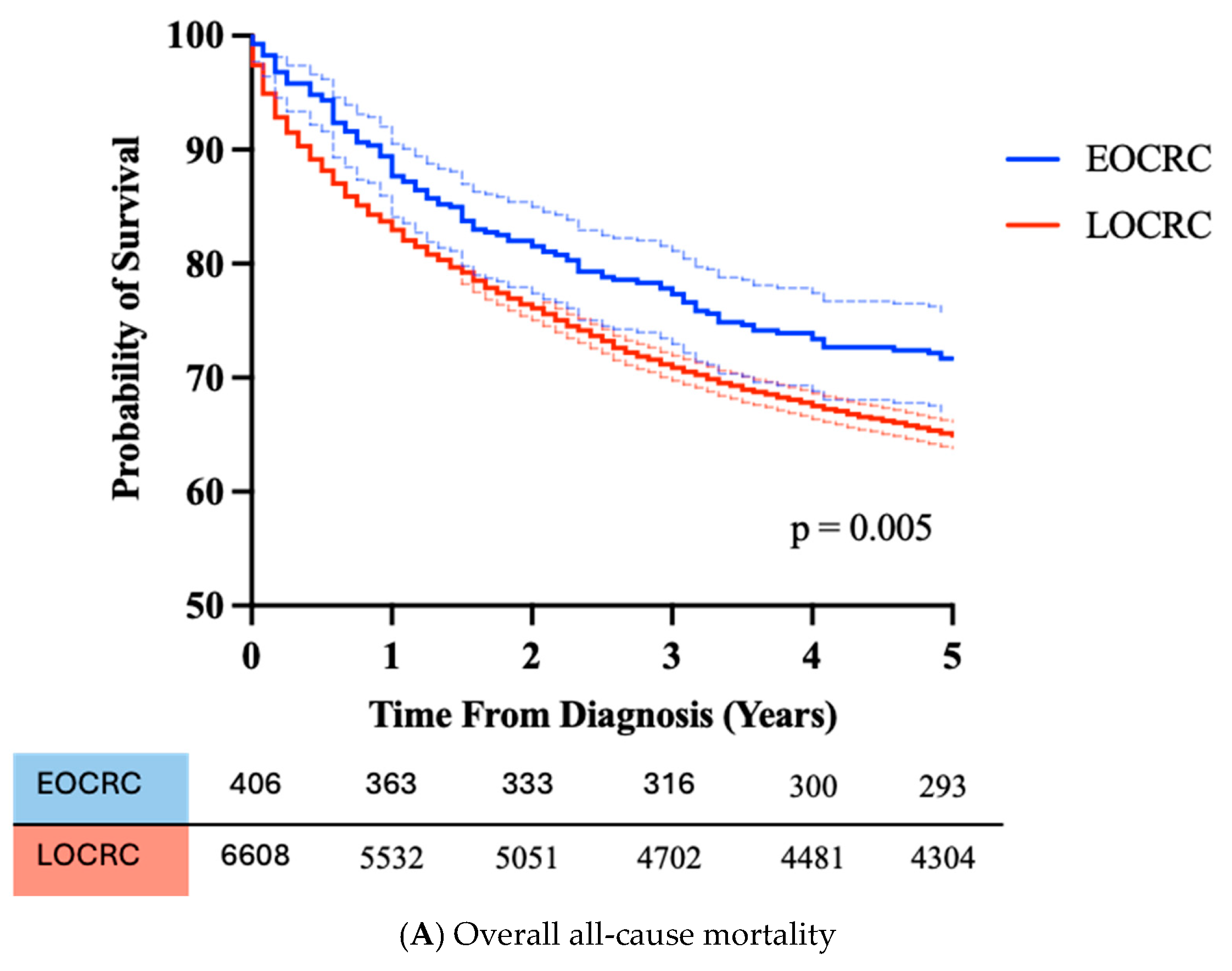
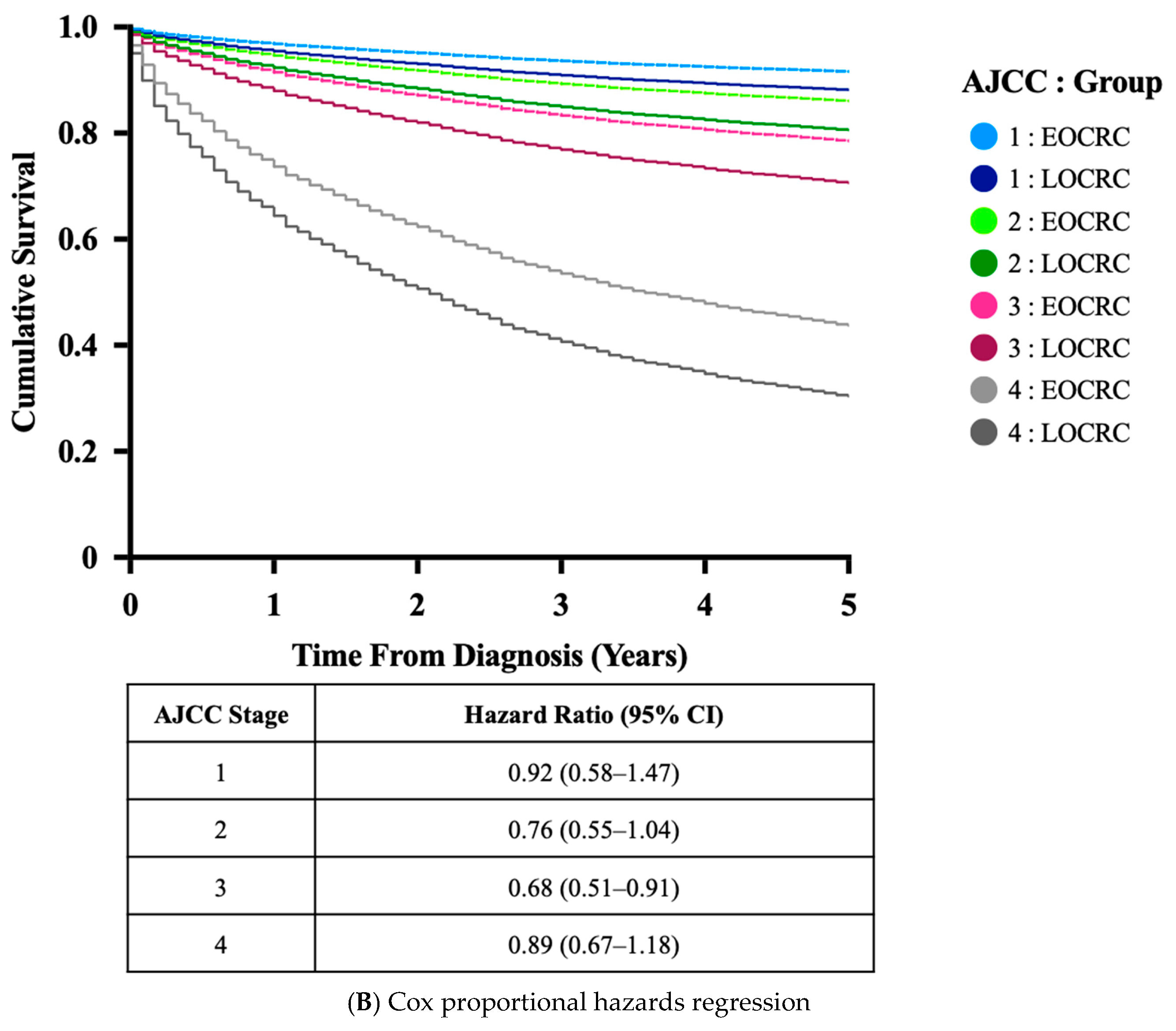
3.3. Clinical Features of EOCRC Diagnosed in NHS Lothian
3.4. FIT in Patients Under 50 Years Old
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Gastroenterol. Rev. 2019, 14, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Torre, L.A.; Soerjomataram, I.; Hayes, R.B.; Bray, F.; Weber, T.K.; Jemal, A. Global patterns and trends in colorectal cancer incidence in young adults. Gut 2019, 68, 2179–2185. [Google Scholar] [CrossRef] [PubMed]
- Sinicrope, F.A. Increasing Incidence of Early-Onset Colorectal Cancer. N. Engl. J. Med. 2022, 386, 1547–1558. [Google Scholar] [CrossRef]
- Wu, C.W.; Lui, R.N. Early-onset colorectal cancer: Current insights and future directions. World J. Gastrointest. Oncol. 2022, 14, 230–241. [Google Scholar] [CrossRef]
- Mauri, G.; Sartore-Bianchi, A.; Russo, A.G.; Marsoni, S.; Bardelli, A.; Siena, S. Early-onset colorectal cancer in young individuals. Mol. Oncol. 2019, 13, 109–131. [Google Scholar] [CrossRef]
- Zaborowski, A.M.; Abdile, A.; Adamina, M.; Aigner, F.; D’allens, L.; Allmer, C.; Álvarez, A.; Anula, R.; Andric, M.; REACCT Collaborative; et al. Characteristics of Early-Onset vs Late-Onset Colorectal Cancer. JAMA Surg. 2021, 156, 865. [Google Scholar] [CrossRef]
- Connell, L.C.; Mota, J.M.; Braghiroli, M.I.; Hoff, P.M. The Rising Incidence of Younger Patients With Colorectal Cancer: Questions About Screening, Biology, and Treatment. Curr. Treat. Options Oncol. 2017, 18, 23. [Google Scholar] [CrossRef]
- Scott, R.B.; Rangel, L.E.; Osler, T.M.; Hyman, N.H. Rectal cancer in patients under the age of 50 years: The delayed diagnosis. Am. J. Surg. 2016, 211, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, J.B.; Maggard, M.A.; Liu, J.H.; Etzioni, D.A.; Livingston, E.H.; Ko, C.Y. Rates of colon and rectal cancers are increasing in young adults. Am. Surg. 2003, 69, 866–872. [Google Scholar] [CrossRef]
- Khan, M.; Korphaisarn, K.; Saif, A.; Foo, W.C.; Kopetz, S. Early-Onset Signet-Ring Cell Adenocarcinoma of the Colon: A Case Report and Review of the Literature. Case Rep. Oncol. Med. 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- Kirzin, S.; Marisa, L.; Guimbaud, R.; De Reynies, A.; Legrain, M.; Laurent-Puig, P.; Cordelier, P.; Pradère, B.; Bonnet, D.; Meggetto, F.; et al. Sporadic early-onset colorectal cancer is a specific sub-type of cancer: A morphological, molecular and genetics study. PLoS ONE 2014, 9, e103159. [Google Scholar] [CrossRef] [PubMed]
- Hofseth, L.J.; Hebert, J.R.; Chanda, A.; Chen, H.; Love, B.L.; Pena, M.M.; Murphy, E.A.; Sajish, M.; Sheth, A.; Buckhaults, P.J.; et al. Early-onset colorectal cancer: Initial clues and current views. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, N.; Ugai, T.; Zhong, R.; Hamada, T.; Fujiyoshi, K.; Giannakis, M.; Wu, K.; Cao, Y.; Ng, K.; Ogino, S. Rising incidence of early-onset colorectal cancer—A call to action. Nat. Rev. Clin. Oncol. 2021, 18, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Low, E.E.; Demb, J.; Liu, L.; Earles, A.; Bustamante, R.; Williams, C.D.; Provenzale, D.; Kaltenbach, T.; Gawron, A.J.; Martinez, M.E.; et al. Risk Factors for Early-Onset Colorectal Cancer. Gastroenterology 2020, 159, 492–501.e7. [Google Scholar] [CrossRef]
- Yen, T.; Levin, T.R.; Patel, S.G. Strategies to Curb the Increasing Burden of Early Onset Colorectal Cancer. Tech. Innov. Gastrointest. Endosc. 2023, 25, 246–258. [Google Scholar] [CrossRef]
- Keane, M.G.; Johnson, G.J. Early diagnosis improves survival in colorectal cancer. Practitioner 2012, 256, 15–18. [Google Scholar]
- Force UPST. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021, 325, 1965–1977. [Google Scholar] [CrossRef]
- Garrett, C.; Steffens, D.; Solomon, M.; Koh, C. Early-onset colorectal cancer: Why it should be high on our list of differentials. ANZ J. Surg. 2022, 92, 1638–1643. [Google Scholar] [CrossRef]
- National Records of Scotland. Mid-2021 Population Estimates, Scotland, 2022. Available online: https://www.nrscotland.gov.uk/publications/rebased-population-estimates-scotland-mid-2011-to-mid-2021/ (accessed on 14 April 2023).
- Gerrard, A.D.; Maeda, Y.; Miller, J.; Gunn, F.; Theodoratou, E.; Noble, C.; Porteous, L.; Glancy, S.; MacLean, P.; Pattenden, R.; et al. Double faecal immunochemical testing in patients with symptoms suspicious of colorectal cancer. Br. J. Surg. 2023, 110, 471–480. [Google Scholar] [CrossRef]
- Public Health Scotland. Cancer Incidene in Scotland. 2022. Available online: https://publichealthscotland.scot/publications/cancer-incidence-in-scotland/cancer-incidence-in-scotland-to-december-2022/ (accessed on 10 April 2023).
- Office for National Statistics. Cancer Registration Statistics 2023. Available online: https://www.ons.gov.uk/ (accessed on 14 April 2023).
- Surveillance Research Program, National Cancer Institute. Joinpoint Regression Software, Version 5.4.0. April 2025. Available online: https://surveillance.cancer.gov/joinpoint (accessed on 14 April 2023).
- Kim, H.J.; Fay, M.P.; Feuer, E.J.; Midthune, D.N. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 2000, 19, 335–351. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.R-project.org/ (accessed on 14 April 2023).
- GraphPad Prism Version 8.0.0 for Windows GS, San Diego, California USA. Available online: www.graphpad.com (accessed on 14 April 2023).
- Scottish Government. Scottish Index of Multiple Deprivation 2020v2 Local and National Share Calculator; Scottish Government: Edinburgh, Scotland, 2020.
- Perrott, S.; Laurie, K.; Laws, K.; Johnes, A.; Miedzybrodzka, Z.; Samuel, L. Young-onset colorectal cancer in the North East of Scotland: Survival, clinico-pathological features and genetics. BMC Cancer 2020, 20, 108. [Google Scholar] [CrossRef] [PubMed]
- Chambers, A.C.; Dixon, S.W.; White, P.; Williams, A.C.; Thomas, M.G.; E Messenger, D. Demographic trends in the incidence of young-onset colorectal cancer: A population-based study. Br. J. Surg. 2020, 107, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, A.; Khakoo, S.; Edwards, P.; Minchom, A.; Kouvelakis, K.; Kalaitzaki, E.; Nobar, N.; Calamai, V.; Ifijen, M.; Husson, O.; et al. Outcomes of Patients with Early Onset Colorectal Cancer Treated in a UK Specialist Cancer Center. Cancers 2019, 11, 1558. [Google Scholar] [CrossRef]
- Rogers, J.E.; Johnson, B. The reality of early-onset colorectal cancer: Highlighting the needs in a unique but emerging population. Dig. Med. Res. 2021, 4, 63. [Google Scholar] [CrossRef]
- Silla, I.O.; Rueda, D.; Rodríguez, Y.; García, J.L.; Vigo, F.d.l.C.; Perea, J. Early-onset colorectal cancer: A separate subset of colorectal cancer. World J. Gastroenterol. 2014, 20, 17288–17296. [Google Scholar] [CrossRef] [PubMed]
- Lamprell, K.; Pulido, D.F.; Arnolda, G.; Easpaig, B.N.G.; Tran, Y.; Owais, S.S.; Liauw, W.; Braithwaite, J. People with early-onset colorectal cancer describe primary care barriers to timely diagnosis: A mixed-methods study of web-based patient reports in the United Kingdom, Australia and New Zealand. BMC Prim. Care 2023, 24, 12. [Google Scholar] [CrossRef]
- D’Souza, N.; Monahan, K.; Benton, S.C.; Wilde, L.; Abulafi, M.; The NICE FIT Steering Group. Finding the needle in the haystack: The diagnostic accuracy of the faecal immunochemical test for colorectal cancer in younger symptomatic patients. Color. Dis. 2021, 23, 2539–2549. [Google Scholar] [CrossRef]
- Cho, M.Y.; Siegel, D.A.; Demb, J.; Richardson, L.C.; Gupta, S. Increasing Colorectal Cancer Incidence Before and After Age 50: Implications for Screening Initiation and Promotion of “On-Time” Screening. Dig. Dis. Sci. 2022, 67, 4086–4091. [Google Scholar] [CrossRef]
- Fisher, D.A.; Saoud, L.; Finney Rutten, L.J.; Ozbay, A.B.; Brooks, D.; Limburg, P.J. Lowering the colorectal cancer screening age improves predicted outcomes in a microsimulation model. Curr. Med. Res. Opin. 2021, 37, 1005–1010. [Google Scholar] [CrossRef]
- Gausman, V.; Dornblaser, D.; Anand, S.; Hayes, R.B.; O’Connell, K.; Du, M.; Liang, P.S. Risk Factors Associated With Early-Onset Colorectal Cancer. Clin. Gastroenterol. Hepatol. 2020, 18, 2752–2759.e2. [Google Scholar] [CrossRef]
- Arif, A.A.; Chahal, D.; Ladua, G.K.; Bhang, E.; Salh, B.; Rosenfeld, G.; Loree, J.M.; Donnellan, F. Hereditary and Inflammatory Bowel Disease-Related Early Onset Colorectal Cancer Have Unique Characteristics and Clinical Course Compared with Sporadic Disease. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
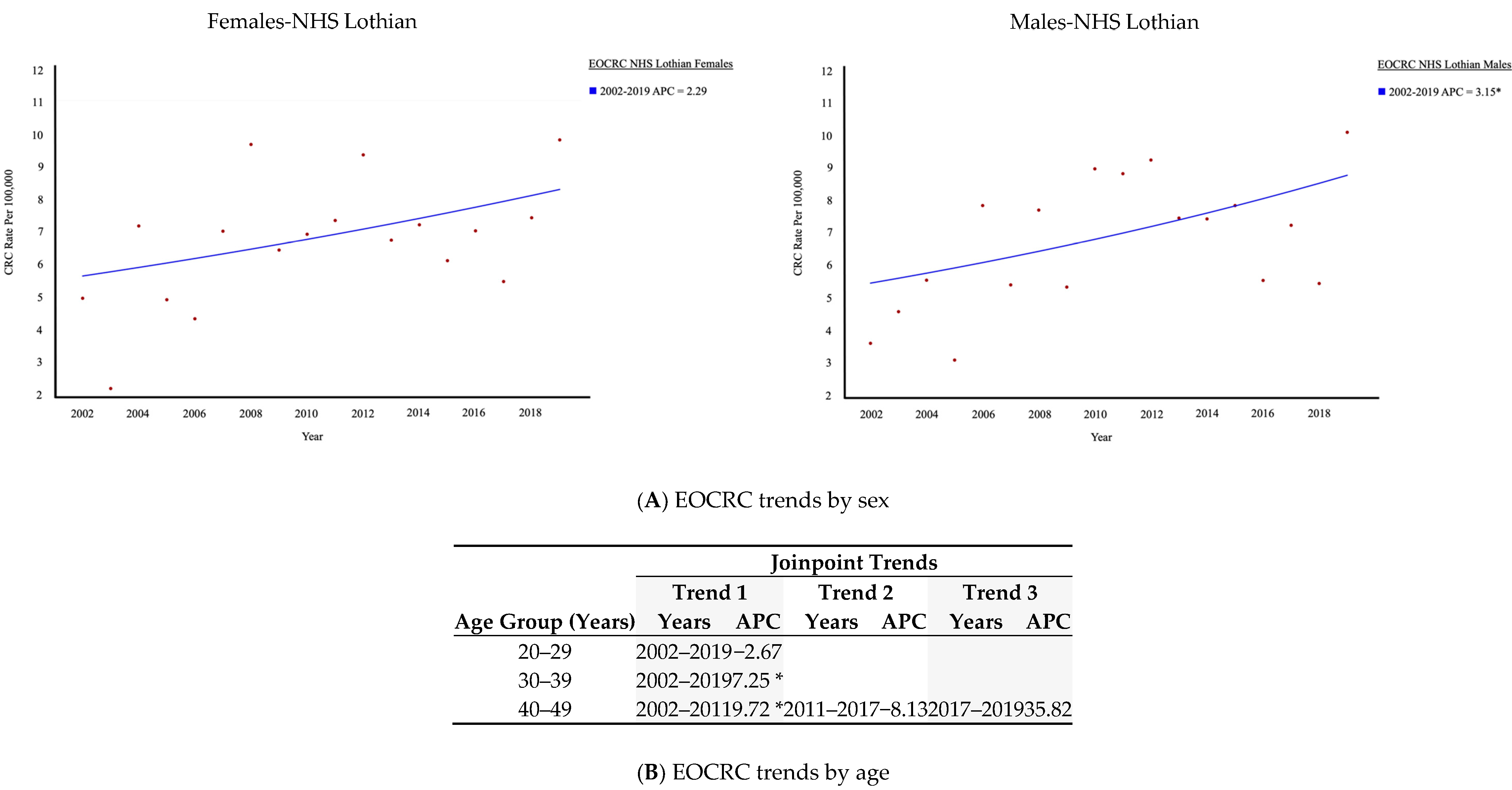

| Joinpoint Trends | |||||||
|---|---|---|---|---|---|---|---|
| Trend 1 | Trend 2 | Trend 3 | |||||
| Ages | Years | APC | Years | APC | Years | APC | |
| NHS Lothian | 45–49 | 2002–2019 | 2.71 | ||||
| 50–54 | 2002–2008 | 13.17 * | 2008–2019 | −1.42 | |||
| Scotland | 45–49 | 1993–2019 | 0.26 | ||||
| 50–54 | 1993–2019 | 0.71 * | |||||
| England | 45–49 | 1993–2019 | 0.46* | ||||
| 50–54 | 1993–1999 | 0.3 | 1999–2002 | −3.27 | 2002–2019 | 0.86 * | |
| Wales | 45–49 | 1993–1999 | 0.63 | ||||
| 50–54 | 1993–1999 | 0.32 | |||||
| EOCRC | LOCRC | p-Value | |
|---|---|---|---|
| Number of CRCs (%) | 406 (5.8) | 6608 (94.2) | |
| Median Age (IQR) | 45 (39–48) | 73 (64–80) | |
| Sex, F (%) | 195 (48.0) | 2906 (44.0) | 0.111 |
| Mean SIMD (SD) | 6.4 (3.0) | 6.3 (2.9) | 0.509 |
| Method of Diagnosis (%) | |||
| Primary Care | 212 (52.2) | 3011 (45.6) | 0.010 |
| Emergency Presentation | 115 (28.3) | 1156 (17.5) | 0.004 |
| Screening Service | 2 (0.4) | 1044 (15.8) | <0.001 |
| Incidental Finding | 41 (10.1) | 560 (8.5) | 0.272 |
| Other | 36 (8.9) | 837 (12.7) | 0.024 |
| Primary Care Referral Priority (%) | |||
| Routine | 49 (23.2) | 7 * (4.0) | <0.001 |
| Urgent | 92 (43.5) | 67 * (38.7) | 0.403 |
| USOC | 71 (33.3) | 99 * (57.2) | <0.001 |
| CRC Location (%) | |||
| Right Side | 123 (30.3) | 2480 (37.5) | 0.004 |
| Left or Rectal | 272 (67.0) | 4001 (60.6) | 0.010 |
| Left Side to Rectosigmoid | 135 (33.3) | 2126 (32.2) | 0.662 |
| Rectosigmoid or Rectum | 137 (33.7) | 1875 (28.4) | 0.020 |
| Information Not Available | 11 (2.7) | 127 (1.9) | |
| CRC Stage (%) | |||
| Early (AJCC 1 and 2) | 151 (37.2) | 2800 (42.4) | |
| Late (AJCC (3 and 4) | 255 (62.8) | 3096 (46.9) | <0.001 |
| Palliation Without Staging | 0 | 374 (5.7) | |
| Information Not Available | 0 | 338 (5.1) | |
| CRC Pathology (%) | |||
| Differentiation | |||
| Poor | 89 (21.9) | 53 * (13.1) | 0.001 |
| Moderate | 214 (52.7) | 213 * (52.5) | 0.999 |
| Well | 9 (2.2) | 8 * (2.0) | 0.999 |
| Information Not Available | 94 (23.2) | 132 * (32.5) | |
| Signet Cell Formation | 25 (6.2) | 8 (2.0) | 0.004 |
| 5 Year all-cause mortality | 28.3% | 35.0% | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerrard, A.D.; Garside, H.; Maeda, Y.; Theodoratou, E.; Dunlop, M.G.; Din, F.V.N. The Increased Early Onset Colorectal Cancer in South East Scotland Is Indicative of a Wider UK Problem. Cancers 2025, 17, 1913. https://doi.org/10.3390/cancers17121913
Gerrard AD, Garside H, Maeda Y, Theodoratou E, Dunlop MG, Din FVN. The Increased Early Onset Colorectal Cancer in South East Scotland Is Indicative of a Wider UK Problem. Cancers. 2025; 17(12):1913. https://doi.org/10.3390/cancers17121913
Chicago/Turabian StyleGerrard, Adam D., Hannah Garside, Yasuko Maeda, Evropi Theodoratou, Malcolm G. Dunlop, and Farhat V. N. Din. 2025. "The Increased Early Onset Colorectal Cancer in South East Scotland Is Indicative of a Wider UK Problem" Cancers 17, no. 12: 1913. https://doi.org/10.3390/cancers17121913
APA StyleGerrard, A. D., Garside, H., Maeda, Y., Theodoratou, E., Dunlop, M. G., & Din, F. V. N. (2025). The Increased Early Onset Colorectal Cancer in South East Scotland Is Indicative of a Wider UK Problem. Cancers, 17(12), 1913. https://doi.org/10.3390/cancers17121913






