Changes Connected to Early Chronic Pancreatitis and Early Pancreatic Cancer in Endoscopic Ultrasonography (EUS): Clinical Implications
Simple Summary
Abstract
1. Introduction
2. Chronic Pancreatitis
3. Imaging Modalities for Chronic Pancreatitis
3.1. Transabdominal Ultrasonography (US)
3.2. Computed Tomography (CT)
3.3. Magnetic Resonance Imaging (MRI)
3.4. Endoscopic Retrograde Cholangiopancreatography (ERCP)
3.5. Endoscopic Ultrasound
4. Rosemont Diagnostic Criteria
5. Japanese Diagnostic Criteria for Early Chronic Pancreatitis (ECP) 2009
6. Japanese Diagnostic Criteria for Early Chronic Pancreatitis (JDCECP) 2019
7. Modified JDCECP 2019
8. Pancreatic Cancer—Introduction
9. Screening in High-Risk Individuals
10. Imaging Modalities of PDAC
10.1. Transabdominal Ultrasonography (US)
10.2. Computed Tomography (CT)
10.3. Magnetic Resonance Imaging (MRI)
10.4. Endoscopic Ultrasound
11. EUS-Related Techniques
11.1. EUS Elastography
11.2. EUS-Fine Needle Aspiration (EUS-FNA)
11.3. EUS-Fine Needle Biopsy (EUS-FNB)
11.4. Contrast-Enhanced EUS (CE-EUS)
12. EUS and Biomarkers
13. Diabetes as an Early Indicator of PDAC
14. Why Early Diagnosis of CP and PDAC Should Be Implemented
15. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CP | chronic pancreatitis |
| ECP | early chronic pancreatitis |
| US | transabdominal ultrasonography |
| CT | computed tomography |
| MRI | magnetic resonance imaging |
| ERCP | endoscopic retrograde cholangiopancreatography |
| EUS | endoscopic ultrasound |
| RC | Rosemont criteria |
| ECP | early chronic pancreatitis |
| JPS | Japan Pancreas Society |
| JDCECP | Japanese Diagnostic Criteria for Early Chronic Pancreatitis |
| PDAC | pancreatic cancer |
| HRI | high-risk individuals |
| SR | strain ratio |
| EUS-FNA | EUS-fine needle aspiration |
| ROSE | rapid on-site cytologic evaluation |
| EUS-FNB | EUS-fine needle biopsy |
| MOSE | macroscopic on-site evaluation |
| VOSE | visual on-site evaluation |
| CE-EUS | contrast-enhanced EUS |
References
- Ge, Q.-C.; Dietrich, C.F.; Bhutani, M.S.; Zhang, B.-Z.; Zhang, Y.; Wang, Y.-D.; Zhang, J.-J.; Wu, Y.-F.; Sun, S.-Y.; Guo, J.-T. Comprehensive review of diagnostic modalities for early chronic pancreatitis. World J. Gastroenterol. 2021, 27, 4342–4357. [Google Scholar] [CrossRef]
- Sakamoto, H.; Kitano, M.; Kamata, K.; El-Masry, M.; Kudo, M. Diagnosis of pancreatic tumors by endoscopic ultrasonography. World J. Radiol. 2010, 2, 122–134. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, R.S.; Stoita, A. Biomarkers in the diagnosis of pancreatic cancer: Are we closer to finding the golden ticket? World J. Gastroenterol. 2021, 27, 4045–4087. [Google Scholar] [CrossRef]
- Sekine, M.; Tanaka, A.; Akimoto, M.; Miura, T.; Fujiwara, J.; Noda, H.; Rikiyama, T.; Ohnishi, H.; Mashima, H. A Comparative Study of Endoscopic Ultrasonography and Histopathology Images for the Diagnosis of Early Chronic Pancreatitis. Pancreas 2021, 50, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Ingraham, N.E.; King, S.; Proper, J.; Siegel, L.; Zolfaghari, E.J.; Murray, T.A.; Vakayil, V.; Sheka, A.; Feng, R.; Guzman, G.; et al. Morbidity and Mortality Trends of Pancreatitis: An Observational Study. Surg. Infect. 2021, 22, 1021–1030. [Google Scholar] [CrossRef]
- Löhr, J.M.; Dominguez-Munoz, E.; Rosendahl, J.; Besselink, M.; Mayerle, J.; Lerch, M.M.; Haas, S.; Akisik, F.; Kartalis, N.; Iglesi-as-Garcia, J.; et al. United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis (HaPanEU). United Eur. Gastroenterol. J. 2017, 5, 153–199. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.V.; Catalano, M.F. EUS in the diagnosis of early-stage chronic pancreatitis. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 243–249. [Google Scholar] [CrossRef]
- Bastati, N.; Kristic, A.; Poetter-Lang, S.; Messner, A.; Herold, A.; Hodge, J.C.; Schindl, M.; Ba-Ssalamah, A. Imaging of inflammatory disease of the pancreas. Br. J. Radiol. 2021, 94, 20201214. [Google Scholar] [CrossRef]
- Matsuda, Y. Age-related morphological changes in the pancreas and their association with pancreatic carcinogenesis. Pathol. Int. 2019, 69, 450–462. [Google Scholar] [CrossRef]
- Shimizu, K.; Ito, T.; Irisawa, A.; Ohtsuka, T.; Ohara, H.; Kanno, A.; Kida, M.; Sakagami, J.; Sata, N.; Takeyama, Y.; et al. Evidence-based clinical practice guidelines for chronic pancreatitis 2021. J. Gastroenterol. 2022, 57, 709–724. [Google Scholar] [CrossRef]
- Takasaki, Y.; Ishii, S.; Fujisawa, T.; Ushio, M.; Takahashi, S.; Yamagata, W.; Ito, K.; Suzuki, A.; Ochiai, K.; Tomishima, K.; et al. Endoscopic Ultrasonography Findings of Early and Suspected Early Chronic Pancreatitis. Diagnostics 2020, 10, 1018. [Google Scholar] [CrossRef]
- Barry, K. Chronic Pancreatitis: Diagnosis and Treatment. Am. Fam. Physician 2018, 97, 385–393. [Google Scholar] [PubMed]
- Kashima, K.; Yamamiya, A.; Abe, Y.; Nagashima, K.; Minaguchi, T.; Kunogi, Y.; Sakuma, F.; Fukushi, K.; Inaba, Y.; Sugaya, T.; et al. Proposal and Validation of New Diagnostic Criteria for Diagnostic Weights of Endoultrasonographic Findings for Early Chronic Pancreatitis. J. Clin. Med. 2023, 12, 5320. [Google Scholar] [CrossRef] [PubMed]
- Małecka-Panas, E.; Juszyński, A.; Wilamski, E. Acute alcoholic pancreatitis does not lead to complete recovery. Mater. Med. Pol. 1996, 28, 64–68. [Google Scholar] [PubMed]
- Anaizi, A.; Hart, P.A.; Conwell, D.L. Diagnosing Chronic Pancreatitis. Dig. Dis. Sci. 2017, 62, 1713–1720. [Google Scholar] [CrossRef]
- Cieszanowski, A. Zastosowanie badania rezonansu magnetycznego w onkologii. The use of magnetic resonance imaging in oncology. Onkol. W Prakt. Klin. 2013, 9, 60–69. [Google Scholar]
- Beyer, G.; Habtezion, A.; Werner, J.; Lerch, M.M.; Mayerle, J. Chronic pancreatitis. Lancet 2020, 396, 499–512. [Google Scholar] [CrossRef]
- Issa, Y.; Kempeneers, M.A.; van Santvoort, H.C.; Bollen, T.L.; Bipat, S.; Boermeester, M.A. Diagnostic performance of imaging modalities in chronic pancreatitis: A systematic review and meta-analysis. Eur. Radiol. 2017, 27, 3820–3844. [Google Scholar] [CrossRef]
- Dimastromatteo, J.; Brentnall, T.; Kelly, K.A. Imaging in pancreatic disease. Nat. Rev. Gastroenterol. Hepatol. 2016, 14, 97–109. [Google Scholar] [CrossRef]
- Sugiyama, M.; Haradome, H.; Atomi, Y. Magnetic resonance imaging for diagnosing chronic pancreatitis. J. Gastroenterol. 2007, 42, 108–112. [Google Scholar] [CrossRef]
- Shah, J.; Chatterjee, A.; Kothari, T.H. The Role of Endoscopic Ultrasound in Early Chronic Pancreatitis. Diagnostics 2024, 14, 298. [Google Scholar] [CrossRef] [PubMed]
- Kitano, M.; Yoshida, T.; Itonaga, M.; Tamura, T.; Hatamaru, K.; Yamashita, Y. Impact of endoscopic ultrasonography on diagnosis of pancreatic cancer. J. Gastroenterol. 2018, 54, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Ueda, K.; Itonaga, M.; Yoshida, T.; Maeda, H.; Maekita, T.; Iguchi, M.; Tamai, H.; Ichinose, M.; Kato, J. Usefulness of Contrast-Enhanced Endoscopic Sonography for Discriminating Mural Nodules From Mucous Clots in Intraductal Papillary Mucinous Neoplasms: A single-center prospective study. J. Ultrasound Med. 2013, 32, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Wiersema, M.J.; Hawes, R.H.; Lehman, G.A.; Kochman, M.L.; Sherman, S.; Kopecky, K.K. Prospective Evaluation of Endoscopic Ultrasonography and Endoscopic Retrograde Cholangiopancreatography in Patients with Chronic Abdominal Pain of Suspected Pancreatic Origin. Endoscopy 1993, 25, 555–564. [Google Scholar] [CrossRef]
- Kahl, S.; Glasbrenner, B.; Leodolter, A.; Pross, M.; Schulz, H.-U.; Malfertheiner, P. EUS in the diagnosis of early chronic pancreatitis: A prospective follow-up study. Gastrointest. Endosc. 2002, 55, 507–511. [Google Scholar] [CrossRef]
- Rana, S.S.; Vilmann, P. Endoscopic ultrasound features of chronic pancreatitis: A pictorial review. Endosc. Ultrasound 2015, 4, 10–14. [Google Scholar] [CrossRef]
- Seicean, A.; Talukdar, R. Endoscopic ultrasound in chronic pancreatitis: Where are we now? World J. Gastroenterol. 2010, 16, 4253–4263. [Google Scholar] [CrossRef]
- Vullierme, M.-P.; Menassa, L.; Couvelard, A.; Rebours, V.; Maire, F.; Ibrahim, T.; Cros, J.; Ruszniewski, P.; Sauvanet, A.; Levy, P.; et al. Non-branched microcysts of the pancreas on MR imaging of patients with pancreatic tumors who had pancreatectomy may predict the presence of pancreatic intraepithelial neoplasia (PanIN): A preliminary study. Eur. Radiol. 2019, 29, 5731–5741. [Google Scholar] [CrossRef]
- Hollerbach, S.; Klamann, A.; Topalidis, T.; Schmiegel, W. Endoscopic Ultrasonography (EUS) and Fine-Needle Aspiration (FNA) Cytology for Diagnosis of Chronic Pancreatitis. Endoscopy 2001, 33, 824–831. [Google Scholar] [CrossRef]
- Engjom, T.; Sangnes, D.A.; Havre, R.F.; Erchinger, F.; Pham, K.D.; Haldorsen, I.S.; Gilja, O.H.; Dimcevski, G. Diagnostic Accuracy of Transabdominal Ultrasound in Chronic Pancreatitis. Ultrasound Med. Biol. 2017, 43, 735–743. [Google Scholar] [CrossRef]
- Javadi, S.; Menias, C.O.; Korivi, B.R.; Shaaban, A.M.; Patnana, M.; Alhalabi, K.; Elsayes, K.M. Pancreatic Calcifications and Calcified Pancreatic Masses: Pattern Recognition Approach on CT. Am. J. Roentgenol. 2017, 209, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Tirkes, T.; Fogel, E.L.; Sherman, S.; Lin, C.; Swensson, J.; Akisik, F.; Sandrasegaran, K. Detection of exocrine dysfunction by MRI in patients with early chronic pancreatitis. Abdom. Radiol. 2017, 42, 544–551. [Google Scholar] [CrossRef]
- Kalmin, B.; Hoffman, B.; Hawes, R.; Romagnuolo, J. Conventional Versus Rosemont Endoscopic Ultrasound Criteria for Chronic Pancreatitis: Comparing Interobserver Reliability and Intertest Agreement. Can. J. Gastroenterol. Hepatol. 2011, 25, 261–264. [Google Scholar] [CrossRef]
- Busireddy, K.K.; AlObaidy, M.; Ramalho, M.; Kalubowila, J.; Baodong, L.; Santagostino, I.; Semelka, R.C. Pancreatitis-imaging approach. World J. Gastrointest. Pathophysiol. 2014, 5, 252–270. [Google Scholar] [CrossRef] [PubMed]
- I Tornel-Avelar, A.; Ruiz-Velasco, J.A.V.; Pelaez-Luna, M. Pancreatic cancer, autoimmune or chronic pancreatitis, beyond tissue diagnosis: Collateral imaging and clinical characteristics may differentiate them. World J. Gastrointest. Oncol. 2023, 15, 925–942. [Google Scholar] [CrossRef] [PubMed]
- Pancreatic Cancer Statistics. Available online: https://www.wcrf.org/cancer-trends/pancreatic-cancer-statistics/ (accessed on 16 March 2024).
- Global Cancer Observatory. Available online: https://gco.iarc.who.int/media/globocan/factsheets/populations/616-poland-fact-sheet.pdf (accessed on 16 March 2024).
- Yang, J.; Xu, R.; Wang, C.; Qiu, J.; Ren, B.; You, L. Early screening and diagnosis strategies of pancreatic cancer: A comprehensive review. Cancer Commun. 2021, 41, 1257–1274. [Google Scholar] [CrossRef]
- Sugimoto, M.; Takagi, T.; Suzuki, R.; Konno, N.; Asama, H.; Watanabe, K.; Nakamura, J.; Kikuchi, H.; Waragai, Y.; Takasumi, M.; et al. Contrast-enhanced harmonic endoscopic ultrasonography in gallbladder cancer and pancreatic cancer. Fukushima J. Med Sci. 2017, 63, 39–45. [Google Scholar] [CrossRef]
- Michl, P.; Löhr, M.; Neoptolemos, J.P.; Capurso, G.; Rebours, V.; Malats, N.; Ollivier, M.; Ricciardiello, L. UEG position paper on pancreatic cancer. Bringing pancreatic cancer to the 21st century: Prevent, detect, and treat the disease earlier and better. United Eur. Gastroenterol. J. 2021, 9, 860–871. [Google Scholar] [CrossRef]
- Catalano, M.F.; Sahai, A.; Levy, M.; Romagnuolo, J.; Wiersema, M.; Brugge, W.; Freeman, M.; Yamao, K.; Canto, M.; Hernandez, L.V. EUS-based criteria for the diagnosis of chronic pancreatitis: The Rosemont classification. Gastrointest. Endosc. 2009, 69, 1251–1261. [Google Scholar] [CrossRef]
- Lorenzo, D.; Rebours, V.; Maire, F.; Palazzo, M.; Gonzalez, J.-M.; Vullierme, M.-P.; Aubert, A.; Hammel, P.; Lévy, P.; de Mestier, L. Role of endoscopic ultrasound in the screening and follow-up of high-risk individuals for familial pancreatic cancer. World J. Gastroenterol. 2019, 25, 5082–5096. [Google Scholar] [CrossRef]
- Zar, S.; Kohoutová, D.; Bureš, J. Pancreatic Adenocarcinoma: Epidemiology, Role of EUS in Diagnosis, Role of ERCP, Endoscopic Palliation. Acta Medica 2019, 62, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Trikudanathan, G.; Vega-Peralta, J.; Malli, A.; Munigala, S.; Han, Y.; Bellin, M.; Barlass, U.; Chinnakotla, S.; Dunn, T.; Pruett, T.; et al. Diagnostic Performance of Endoscopic Ultrasound (EUS) for Non-Calcific Chronic Pancreatitis (NCCP) Based on Histopathology. Am. J. Gastroenterol. 2016, 111, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, C.; Shridhar, R.; Huston, J.; Meredith, K. Correlation of tumor size and survival in pancreatic cancer. J. Gastrointest. Oncol. 2018, 9, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Singhi, A.D.; Koay, E.J.; Chari, S.T.; Maitra, A. Early Detection of Pancreatic Cancer: Opportunities and Challenges. Gastroenterology 2019, 156, 2024–2040. [Google Scholar] [CrossRef]
- Conroy, T.; Pfeiffer, P.; Vilgrain, V.; Lamarca, A.; Seufferlein, T.; O’Reilly, E.; Hackert, T.; Golan, T.; Prager, G.; Haustermans, K.; et al. Pancreatic cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 987–1002. [Google Scholar] [CrossRef]
- Survival Rates for Pancreatic Cancer. Available online: https://www.cancer.org/cancer/types/pancreatic-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 16 March 2024).
- Ariyama, J.; Suyama, M.; Ogawa, K.; Ikari, T.; Nagaiwa, J.; Fujii, D.; Tsuchida, A. The detection and prognosis of small pancreatic carcinoma. Int. J. Pancreatol. 1990, 7, 37–47. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yagi, S.; Kinoshita, H.; Sakamoto, Y.; Okada, K.; Uryuhara, K.; Morimoto, T.; Kaihara, S.; Hosotani, R. Long-term survival after resection of pancreatic cancer: A single-center retrospective analysis. World J. Gastroenterol. 2015, 21, 262–268. [Google Scholar] [CrossRef]
- Franko, J.; Hugec, V.; Lopes, T.L.; Goldman, C.D. Survival Among Pancreaticoduodenectomy Patients Treated for Pancreatic Head Cancer <1 or 2 cm. Ann. Surg. Oncol. 2012, 20, 357–361. [Google Scholar] [CrossRef]
- Nagakawa, T.; Konishi, I.; Ueno, K.; Ohta, T.; Akiyama, T.; Kayahara, M.; Miyazaki, I. Surgical treatment of pancreatic cancer. Int. J. Pancreatol. 1991, 9, 135–143. [Google Scholar] [CrossRef]
- Fortner, J.G.; Klimstra, D.S.; Senie, R.T.; Maclean, B.J. Tumor Size Is the Primary Prognosticator for Pancreatic Cancer After Regional Pancreatectomy. Ann. Surg. 1996, 223, 147–153. [Google Scholar] [CrossRef]
- Kaťuchová, J.; Bober, J.; Radoňak, J. Postoperative complications and survival rates for pancreatic cancer patients. Wien. Klin. Wochenschr. 2011, 123, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Kim, J.; Wang, Q.; Lee, A.; Babic, A.; PanScan/PanC4 I-III Consortium; Amundadottir, L.; Klein, A.; Li, D.; McCullough, M.; et al. The age-dependent association of risk factors with pancreatic cancer. Ann. Oncol. 2022, 33, 693–701. [Google Scholar] [CrossRef]
- Goggins, M.; Overbeek, K.A.; Brand, R.; Syngal, S.; Del Chiaro, M.; Bartsch, D.K.; Bassi, C.; Carrato, A.; Farrell, J.; Fishman, E.K.; et al. Management of patients with increased risk for familial pancreatic cancer: Updated recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium. Gut 2019, 69, 7–17. [Google Scholar] [CrossRef] [PubMed]
- NCCN Guidelines. Available online: https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf (accessed on 16 March 2024).
- Lee, E.S.; Lee, J.M. Imaging diagnosis of pancreatic cancer: A state-of-the-art review. World J. Gastroenterol. 2014, 20, 7864–7877. [Google Scholar] [CrossRef]
- Menke, A.; Yamaguchi, H.; Gress, T.; Adler, G. Extracellular matrix is reduced by inhibition of transforming growth factor beta1 in pancreatitis in the rat. Gastroenterology 1997, 113, 295–303. [Google Scholar] [CrossRef]
- Maguchi, H.; Takahashi, K.; Osanai, M.; Katanuma, A. Small pancreatic lesions: Is there need for EUS-FNA preoperatively? What to do with the incidental lesions? Endoscopy 2006, 38, 53–56. [Google Scholar] [CrossRef]
- Shin, E.J.; Canto, M.I. Pancreatic Cancer Screening. Gastroenterol. Clin. N. Am. 2012, 41, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.F.; Meyenberger, C.; Bertschinger, P.; Schaer, R.; Marincek, B. Pancreatic tumors: Evaluation with endoscopic US, CT, and MR imaging. Radiology 1994, 190, 745–751. [Google Scholar] [CrossRef]
- Sakamoto, H.; Kitano, M.; Suetomi, Y.; Maekawa, K.; Takeyama, Y.; Kudo, M. Utility of Contrast-Enhanced Endoscopic Ultrasonography for Diagnosis of Small Pancreatic Carcinomas. Ultrasound Med. Biol. 2008, 34, 525–532. [Google Scholar] [CrossRef]
- Kamata, K.; Kitano, M.; Kudo, M.; Sakamoto, H.; Kadosaka, K.; Miyata, T.; Imai, H.; Maekawa, K.; Chikugo, T.; Kumano, M.; et al. Value of EUS in early detection of pancreatic ductal adenocarcinomas in patients with intraductal papillary mucinous neoplasms. Endoscopy 2013, 46, 22–29. [Google Scholar] [CrossRef]
- Ugbarugba, E.E.M.; Grieco, C.; Hart, P.A.; Li, F.; Sklaw, B.; Cronley, K.; Oza, V.M.; Swanson, B.J.; Walker, J.P.; El-Dika, S.; et al. Diagnostic Accuracy of Preoperative Imaging for Differentiation of Branch Duct Versus Mixed Duct Intraductal Papillary Mucinous Neoplasms. Pancreas 2018, 47, 556–560. [Google Scholar] [CrossRef]
- Zakaria, A.; Al-Share, B.; Klapman, J.B.; Dam, A. The Role of Endoscopic Ultrasonography in the Diagnosis and Staging of Pancreatic Cancer. Cancers 2022, 14, 1373. [Google Scholar] [CrossRef] [PubMed]
- Brune, K.; Abe, T.; Canto, M.; O’Malley, L.; Klein, A.P.; Maitra, A.; Adsay, N.V.; Fishman, E.K.; Cameron, J.L.; Yeo, C.J.; et al. Multifocal neoplastic precursor lesions associated with lobular atrophy of the pancreas in patients having a strong family history of pancreatic cancer. Am. J. Surg. Pathol. 2006, 30, 1067–1076. [Google Scholar] [PubMed]
- Yamaguchi, K.; Okusaka, T.; Shimizu, K.; Furuse, J.; Ito, Y.; Hanada, K.; Shimosegawa, T.; Okazaki, K. Committee for Revision of Clinical Guidelines for Pancreatic Cancer of the Japan Pancreas Society. Clinical Practice Guidelines for Pancreatic Cancer 2016 From the Japan Pancreas Society: A Synopsis. Pancreas 2017, 46, 595–604. [Google Scholar] [CrossRef]
- Hunt, G.C.; Faigel, D.O. Assessment of EUS for diagnosing, staging, and determining resectability of pancreatic cancer: A review. Gastrointest. Endosc. 2002, 55, 232–237. [Google Scholar] [CrossRef]
- Li, J.-H.; He, R.; Li, Y.-M.; Cao, G.; Ma, Q.-Y.; Yang, W.-B. Endoscopic Ultrasonography for Tumor Node Staging and Vascular Invasion in Pancreatic Cancer: A Meta-Analysis. Dig. Surg. 2014, 31, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Săftoiu, A.; Vilmann, P.; Gorunescu, F.; Gheonea, D.I.; Gorunescu, M.; Ciurea, T.; Popescu, G.L.; Iordache, A.; Hassan, H.; Iordache, S. Neural network analysis of dynamic sequences of EUS elastography used for the differential diagnosis of chronic pancreatitis and pancreatic cancer. Gastrointest. Endosc. 2008, 68, 1086–1094. [Google Scholar] [CrossRef]
- Giovannini, M.; Botelberge, T.; Bories, E.; Pesenti, C.; Caillol, F.; Esterni, B.; Monges, G.; Arcidiacono, P.; Deprez, P.; Yeung, R.; et al. Endoscopic ultrasound elastography for evaluation of lymph nodes and pancreatic masses: A multicenter study. World J. Gastroenterol. 2009, 15, 1587–1593. [Google Scholar] [CrossRef]
- Yousaf, M.N.; Chaudhary, F.S.; Ehsan, A.; Suarez, A.L.; Muniraj, T.; Jamidar, P.; Aslanian, H.R.; Farrell, J.J. Endoscopic ultrasound (EUS) and the management of pancreatic cancer. BMJ Open Gastroenterol. 2020, 7, e000408. [Google Scholar] [CrossRef]
- Kim, S.Y.; Cho, J.H.; Kim, Y.J.; Kim, E.J.; Park, J.Y.; Jeon, T.J.; Kim, Y.S. Diagnostic efficacy of quantitative endoscopic ultrasound elastography for differentiating pancreatic disease. J. Gastroenterol. Hepatol. 2017, 32, 1115–1122. [Google Scholar] [CrossRef]
- Janssen, J.; Schlörer, E.; Greiner, L. EUS elastography of the pancreas: Feasibility and pattern description of the normal pancreas, chronic pancreatitis, and focal pancreatic lesions. Gastrointest. Endosc. 2007, 65, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Garcia, J.; Larino-Noia, J.; Abdulkader, I.; Forteza, J.; Dominguez-Munoz, J.E. EUS elastography for the characterization of solid pancreatic masses. Gastrointest. Endosc. 2009, 70, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, B.; Soares, J.B.; Bastos, P. Endoscopic Ultrasound in the Diagnosis and Staging of Pancreatic Cancer. GE Port. J. Gastroenterol. 2015, 22, 161–171. [Google Scholar] [CrossRef]
- DeWitt, J.; Devereaux, B.M.; Lehman, G.A.; Sherman, S.; Imperiale, T.F. Comparison of Endoscopic Ultrasound and Computed Tomography for the Preoperative Evaluation of Pancreatic Cancer: A Systematic Review. Clin. Gastroenterol. Hepatol. 2006, 4, 717–725. [Google Scholar] [CrossRef]
- Kataoka, K.; Ishikawa, T.; Ohno, E.; Iida, T.; Suzuki, H.; Uetsuki, K.; Furukawa, K.; Nakamura, M.; Honda, T.; Ishigami, M.; et al. Endoscopic ultrasound elastography for small solid pancreatic lesions with or without main pancreatic duct dilatation. Pancreatology 2021, 21, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Stasi, E.; Di Maso, M.; Serviddio, G.; Hussein, M.S.A.; Muscatiello, N. Endoscopic ultrasound-guided fine needle aspiration of pancreatic lesions with 22 versus 25 Gauge needles: A meta-analysis. United Eur. Gastroenterol. J. 2017, 5, 846–853. [Google Scholar] [CrossRef]
- Ak, C.; Sayar, S.; Kilic, E.T.; Kahraman, R.; Ozturk, O.; Zemheri, E.; Ozdil, K. EUS-FNA and ROSE in Solid Lesions of The Pancreas; Have The Same Diagnostic Efficacy Compared to Pancreatic Sites? North. Clin. Istanb. 2022, 9, 464–469. [Google Scholar] [CrossRef]
- Siddiqui, A.A.; Brown, L.J.; Hong, S.-K.S.; Draganova-Tacheva, R.A.; Korenblit, J.; Loren, D.E.; Kowalski, T.E.; Solomides, C. Relationship of Pancreatic Mass Size and Diagnostic Yield of Endoscopic Ultrasound-Guided Fine Needle Aspiration. Dig. Dis. Sci. 2011, 56, 3370–3375. [Google Scholar] [CrossRef]
- Haba, S.; Yamao, K.; Bhatia, V.; Mizuno, N.; Hara, K.; Hijioka, S.; Imaoka, H.; Niwa, Y.; Tajika, M.; Kondo, S.; et al. Diagnostic ability and factors affecting accuracy of endoscopic ultrasound-guided fine needle aspiration for pancreatic solid lesions: Japanese large single center experience. J. Gastroenterol. 2012, 48, 973–981. [Google Scholar] [CrossRef]
- Uehara, H.; Ikezawa, K.; Kawada, N.; Fukutake, N.; Katayama, K.; Takakura, R.; Takano, Y.; Ishikawa, O.; Takenaka, A. Diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration for suspected pancreatic malignancy in relation to the size of lesions. J. Gastroenterol. Hepatol. 2011, 26, 1256–1261. [Google Scholar] [CrossRef]
- Winter, K.; Talar-Wojnarowska, R.; Dąbrowski, A.; Degowska, M.; Durlik, M.; Gąsiorowska, A.; Głuszek, S.; Jurkowska, G.; Kaczka, A.; Lampe, P.; et al. Diagnostic and therapeutic recommendations in pancreatic ductal adenocarcinoma. Recommendations of the Working Group of the Polish Pancreatic Club. Gastroenterol. Rev. 2019, 14, 1–18. [Google Scholar] [CrossRef]
- Dumonceau, J.-M.; Deprez, P.H.; Jenssen, C.; Iglesias-Garcia, J.; Larghi, A.; Vanbiervliet, G.; Aithal, G.P.; Arcidiacono, P.G.; Bastos, P.; Carrara, S.; et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline—Updated January 2017. Endoscopy 2017, 49, 695–714. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.J.; Kim, J.; Park, S.W.; Cho, J.H.; Kim, E.J.; Lee, Y.N.; Lee, D.W.; Park, C.H.; Lee, S.S. Comparison between three types of needles for endoscopic ultrasound-guided tissue acquisition of pancreatic solid masses: A multicenter observational study. Sci. Rep. 2023, 13, 3677. [Google Scholar] [CrossRef]
- Facciorusso, A.; Crinò, S.F.; Ramai, D.; Madhu, D.; Fugazza, A.; Carrara, S.; Spadaccini, M.; Mangiavillano, B.; Gkolfakis, P.; Mohan, B.P.; et al. Comparative diagnostic performance of different techniques for EUS-guided fine-needle biopsy sampling of solid pancreatic masses: A network meta-analysis. Gastrointest. Endosc. 2023, 97, 839–848.e5. [Google Scholar] [CrossRef]
- Crinò, S.F.; Bellocchi, M.C.C.; Di Mitri, R.; Inzani, F.; Rimbaș, M.; Lisotti, A.; Manfredi, G.; Teoh, A.Y.B.; Mangiavillano, B.; Sendino, O.; et al. Wet-suction versus slow-pull technique for endoscopic ultrasound-guided fine-needle biopsy: A multicenter, randomized, crossover trial. Endoscopy 2022, 55, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Stigliano, S.; Balassone, V.; Biasutto, D.; Covotta, F.; Signoretti, M.; Di Matteo, F.M. Accuracy of visual on-site evaluation (Vose) In predicting the adequacy of Eus-guided fine needle biopsy: A single center prospective study. Pancreatology 2021, 21, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Pickartz, T.; Mayerle, J.; Lerch, M.M. Autoimmune pancreatitis. Nat. Clin. Pract. Gastroenterol. Hepatol. 2007, 4, 314–323. [Google Scholar] [CrossRef]
- Sharma, V.; Rana, S.S.; Kumar, A.; Bhasin, D.K. Pancreatic tuberculosis. J. Gastroenterol. Hepatol. 2016, 31, 310–318. [Google Scholar] [CrossRef]
- Kitano, M.; Kudo, M.; Yamao, K.; Takagi, T.; Sakamoto, H.; Komaki, T.; Kamata, K.; Imai, H.; Chiba, Y.; Okada, M.; et al. Characterization of Small Solid Tumors in the Pancreas: The Value of Contrast-Enhanced Harmonic Endoscopic Ultrasonography. Am. J. Gastroenterol. 2012, 107, 303–310. [Google Scholar] [CrossRef]
- Yamashita, Y.; Tanioka, K.; Kawaji, Y.; Tamura, T.; Nuta, J.; Hatamaru, K.; Itonaga, M.; Yoshida, T.; Ida, Y.; Maekita, T.; et al. Utility of Contrast-Enhanced Harmonic Endoscopic Ultrasonography for Early Diagnosis of Small Pancreatic Cancer. Diagnostics 2020, 10, 23. [Google Scholar] [CrossRef]
- Harima, H.; Kaino, S.; Shinoda, S.; Kawano, M.; Suenaga, S.; Sakaida, I. Differential diagnosis of benign and malignant branch duct intraductal papillary mucinous neoplasm using contrast-enhanced endoscopic ultrasonography. World J. Gastroenterol. 2015, 21, 6252–6260. [Google Scholar] [CrossRef]
- Zubarik, R.; Gordon, S.R.; Lidofsky, S.D.; Anderson, S.R.; Pipas, J.M.; Badger, G.; Ganguly, E.; Vecchio, J. Screening for pancreatic cancer in a high-risk population with serum CA 19-9 and targeted EUS: A feasibility study. Gastrointest. Endosc. 2011, 74, 87–95. [Google Scholar] [CrossRef]
- Kapszewicz, M.; Małecka-Wojciesko, E. Simple Serum Pancreatic Ductal Adenocarcinoma (PDAC) Protein Biomarkers—Is There Anything in Sight? J. Clin. Med. 2021, 10, 5463. [Google Scholar] [CrossRef] [PubMed]
- Bausch, D.; Thomas, S.; Mino-Kenudson, M.; Fernández-Del, C.C.; Bauer, T.W.; Williams, M.; Warshaw, A.L.; Thayer, S.P.; Kelly, K.A. Plectin-1 as a Novel Biomarker for Pancreatic Cancer. Clin. Cancer Res. 2011, 17, 302–309. [Google Scholar] [CrossRef]
- Kishikawa, T.; Otsuka, M.; Ohno, M.; Yoshikawa, T.; Takata, A.; Koike, K. Circulating RNAs as new biomarkers for detecting pancreatic cancer. World J. Gastroenterol. 2015, 21, 8527–8540. [Google Scholar] [CrossRef]
- Poulsen, V.V.; Hadi, A.; Werge, M.P.; Karstensen, J.G.; Novovic, S. Circulating Biomarkers Involved in the Development of and Progression to Chronic Pancreatitis—A Literature Review. Biomolecules 2024, 14, 239. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Sahoo, J.; Kamalanathan, S.; Naik, D.; Mohan, P.; Kalayarasan, R. Diabetes and pancreatic cancer: Exploring the two-way traffic. World J. Gastroenterol. 2021, 27, 4939–4962. [Google Scholar] [CrossRef] [PubMed]
- A Hart, P.; Bellin, M.D.; Andersen, D.K.; Bradley, D.; Cruz-Monserrate, Z.; E Forsmark, C.; O Goodarzi, M.; Habtezion, A.; Korc, M.; Kudva, Y.C.; et al. Type 3c (pancreatogenic) diabetes mellitus secondary to chronic pancreatitis and pancreatic cancer. Lancet Gastroenterol. Hepatol. 2016, 1, 226–237. [Google Scholar] [CrossRef]
- Aggarwal, G.; Kamada, P.; Chari, S.T. Prevalence of Diabetes Mellitus in Pancreatic Cancer Compared to Common Cancers. Pancreas 2013, 42, 198–201. [Google Scholar] [CrossRef]
- Pannala, R.; Leirness, J.B.; Bamlet, W.R.; Basu, A.; Petersen, G.M.; Chari, S.T. Prevalence and Clinical Profile of Pancreatic Cancer–Associated Diabetes Mellitus. Gastroenterology 2008, 134, 981–987. [Google Scholar] [CrossRef]
- A Neuschwander-Tetri, B.; Bridle, K.R.; Wells, L.D.; Marcu, M.; A Ramm, G. Repetitive Acute Pancreatic Injury in the Mouse Induces Procollagen α1(I) Expression Colocalized to Pancreatic Stellate Cells. Mod. Pathol. 2000, 80, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Vonlaufen, A.; Phillips, P.A.; Xu, Z.; Zhang, X.; Yang, L.; Pirola, R.C.; Wilson, J.S.; Apte, M.V. Withdrawal of alcohol promotes regression while continued alcohol intake promotes persistence of LPS-induced pancreatic injury in alcohol-fed rats. Gut 2010, 60, 238–246. [Google Scholar] [CrossRef]
- Shelton, C.; LaRusch, J.; Whitcomb, D.C. Pancreatitis Overview. In GeneReviews® [Internet]; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; [Updated 2020 Jul 2]; University of Washington: Seattle, WA, USA, 2014. Available online: https://www.ncbi.nlm.nih.gov/books/NBK190101/ (accessed on 28 October 2024).
- Uc, A.; Andersen, D.K.; Bellin, M.D.; Bruce, J.I.; Drewes, A.M.M.; Engelhardt, J.F.; Forsmark, C.E.; Lerch, M.M.; Lowe, M.E.; Neuschwander-Tetri, B.A.; et al. Chronic Pancreatitis in the 21st Century—Research Challenges and Opportunities. Pancreas 2016, 45, 1365–1375. [Google Scholar] [CrossRef]
- Kim, D.-S.; Scherer, P.E. Obesity, Diabetes, and Increased Cancer Progression. Diabetes Metab. J. 2021, 45, 799–812. [Google Scholar] [CrossRef]
- Lankisch, P.G.; Löhr-Happe, A.; Otto, J.; Creutzfeldt, W. Natural Course in Chronic Pancreatitis. Digestion 1993, 54, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Yusoff, I.; Sahai, A.V. A prospective, quantitative assessment of the effect of ethanol and other variables on the endosonographic appearance of the pancreas. Clin. Gastroenterol. Hepatol. 2004, 2, 405–409. [Google Scholar] [CrossRef]
- Apte, M.; Wilson, J. Mechanisms of Pancreatic Fibrosis. Dig. Dis. 2004, 22, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Pereda, J.; Sabater, L.; Cassinello, N.; Gómez-Cambronero, L.; Closa, D.; Folch-Puy, E.; Aparisi, L.; Calvete, J.; Cerdá, M.; Lledó, S.; et al. Effect of Simultaneous Inhibition of TNF-α Production and Xanthine Oxidase in Experimental Acute Pancreatitis: The role of mitogen activated protein kinases. Ann. Surg. 2004, 240, 108–116. [Google Scholar] [CrossRef]
- Vege, S.S.; Chari, S.T. Chronic Pancreatitis. N. Engl. J. Med. 2022, 386, 869–878. [Google Scholar] [CrossRef]
- Alexander, V.J.; Karwatowska-Prokopczuk, E.; Prohaska, T.A.; Li, L.; Geary, R.S.; Gouni-Berthold, I.; Oral, E.A.; Hegele, R.A.; Stroes, E.S.; Witztum, J.L.; et al. Volanesorsen to Prevent Acute Pancreatitis in Hypertriglyceridemia. N. Engl. J. Med. 2024, 390, 476–477. [Google Scholar] [CrossRef]
- Watts, G.F.; Rosenson, R.S.; Hegele, R.A.; Goldberg, I.J.; Gallo, A.; Mertens, A.; Baass, A.; Zhou, R.; Muhsin, M.; Hellawell, J.; et al. Plozasiran for Managing Persistent Chylomicronemia and Pancreatitis Risk. N. Engl. J. Med. 2025, 392, 127–137. [Google Scholar] [CrossRef]
- Pollice, P.F.; Rosier, R.N.; Looney, R.J.; Puzas, J.E.; Schwarz, E.M.; O’Keefe, R.J. Oral Pentoxifylline Inhibits Release of Tumor Necrosis Factor-Alpha from Human Peripheral Blood Monocytes: A potential treatment for aseptic loosening of total joint components. J. Bone Jt. Surg. 2001, 83, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Sareen, S.; Kumari, V.; Gajebasia, K.S.; Gajebasia, N.K. Yoga: A tool for improving the quality of life in chronic pancreatitis. World J. Gastroenterol. 2007, 13, 391–397. [Google Scholar] [CrossRef]
- Sareen, S. Yoga for rehabilitation in chronic pancreatitis. Gut 2006, 55, 1051. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Lu, G.; Huai, J.; Ding, J. Impact of Smoking on the Risk of Pancreatitis: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0124075. [Google Scholar] [CrossRef] [PubMed]
- Śliwińska-Mossoń, M.; Milnerowicz, H. The impact of smoking on the development of diabetes and its complications. Diabetes Vasc. Dis. Res. 2017, 14, 265–276. [Google Scholar] [CrossRef]
- Yadav, D.; Whitcomb, D.C. The role of alcohol and smoking in pancreatitis. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 131–145. [Google Scholar] [CrossRef]
- Vujasinovic, M.; Valente, R.; Del Chiaro, M.; Permert, J.; Löhr, J.-M. Pancreatic Exocrine Insufficiency in Pancreatic Cancer. Nutrients 2017, 9, 183. [Google Scholar] [CrossRef]
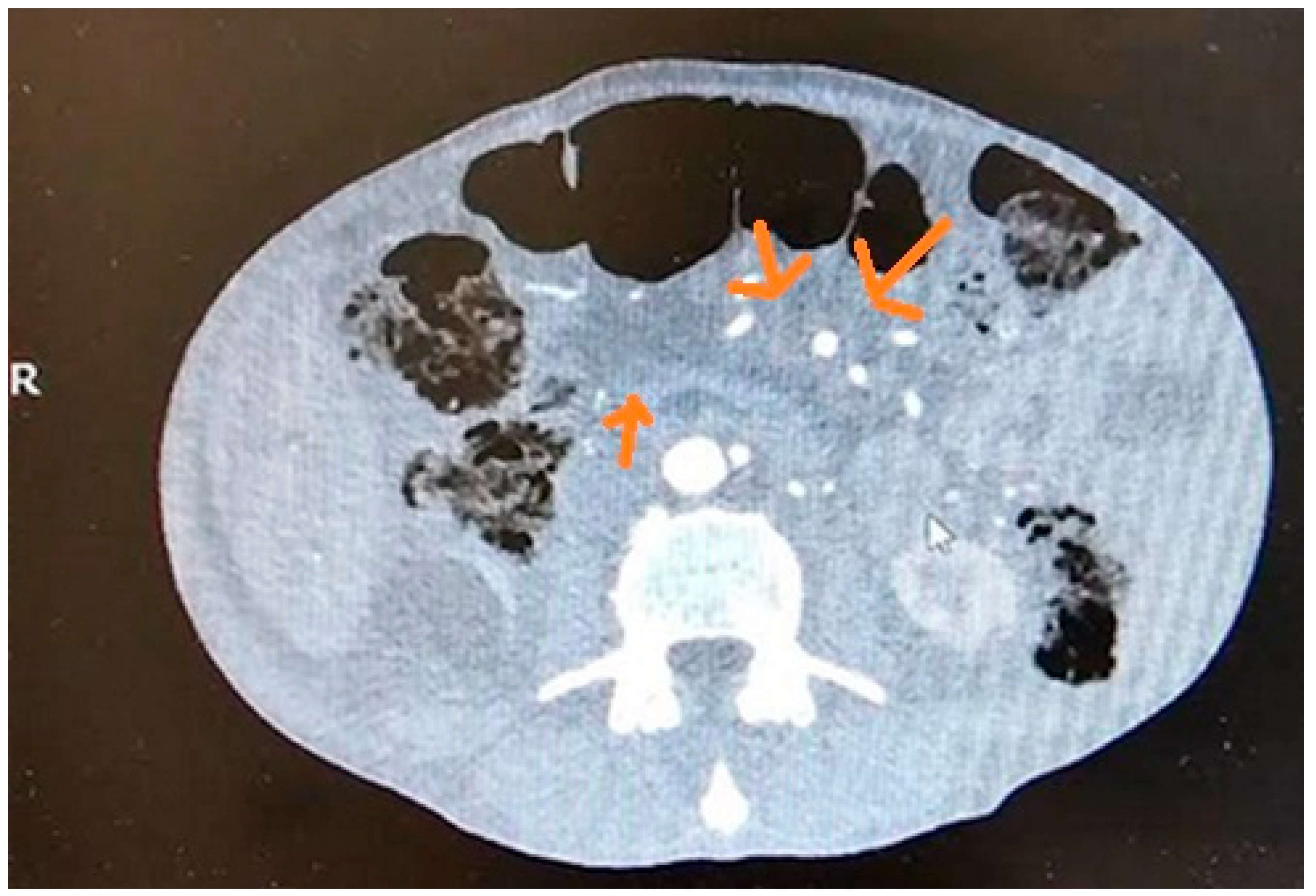
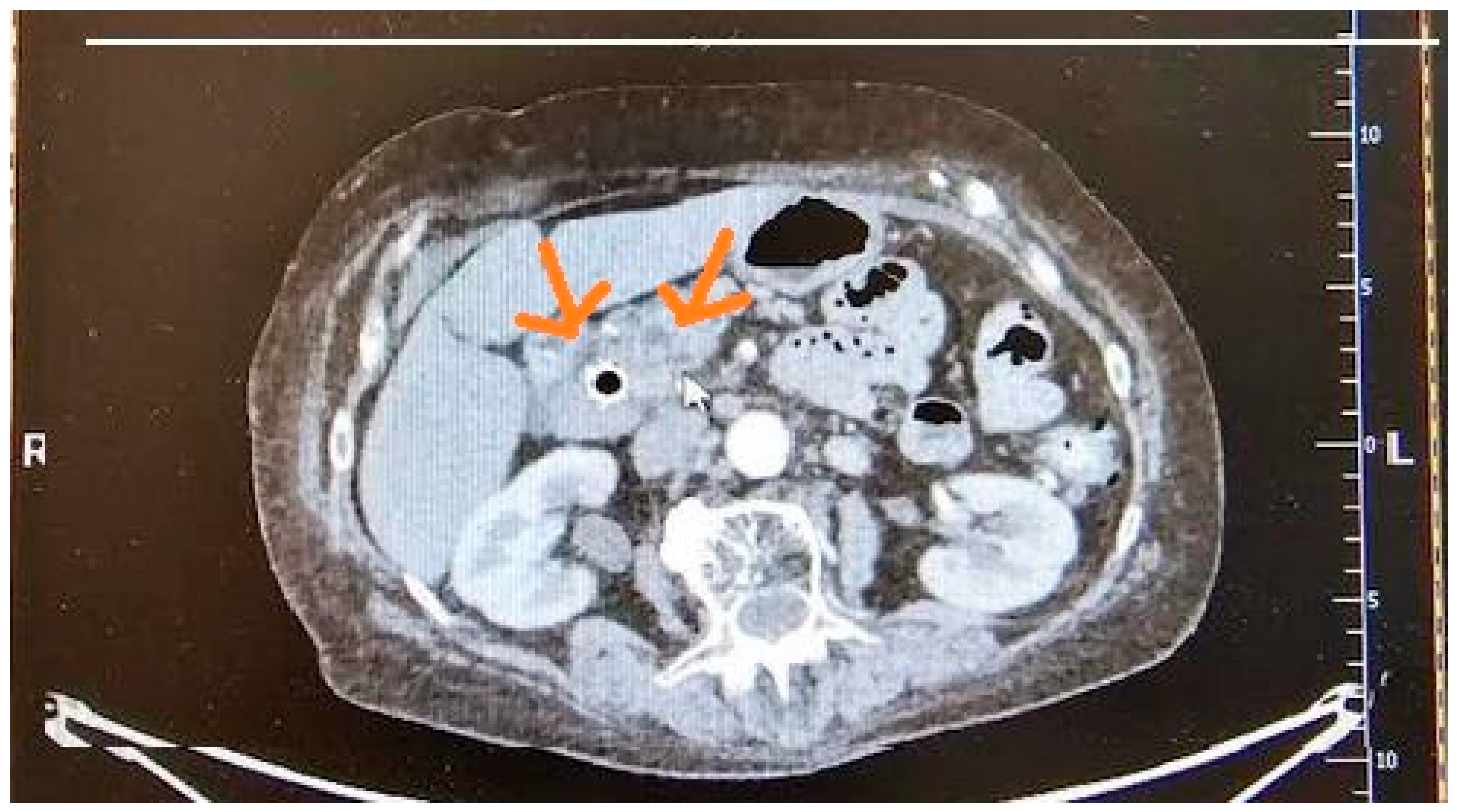
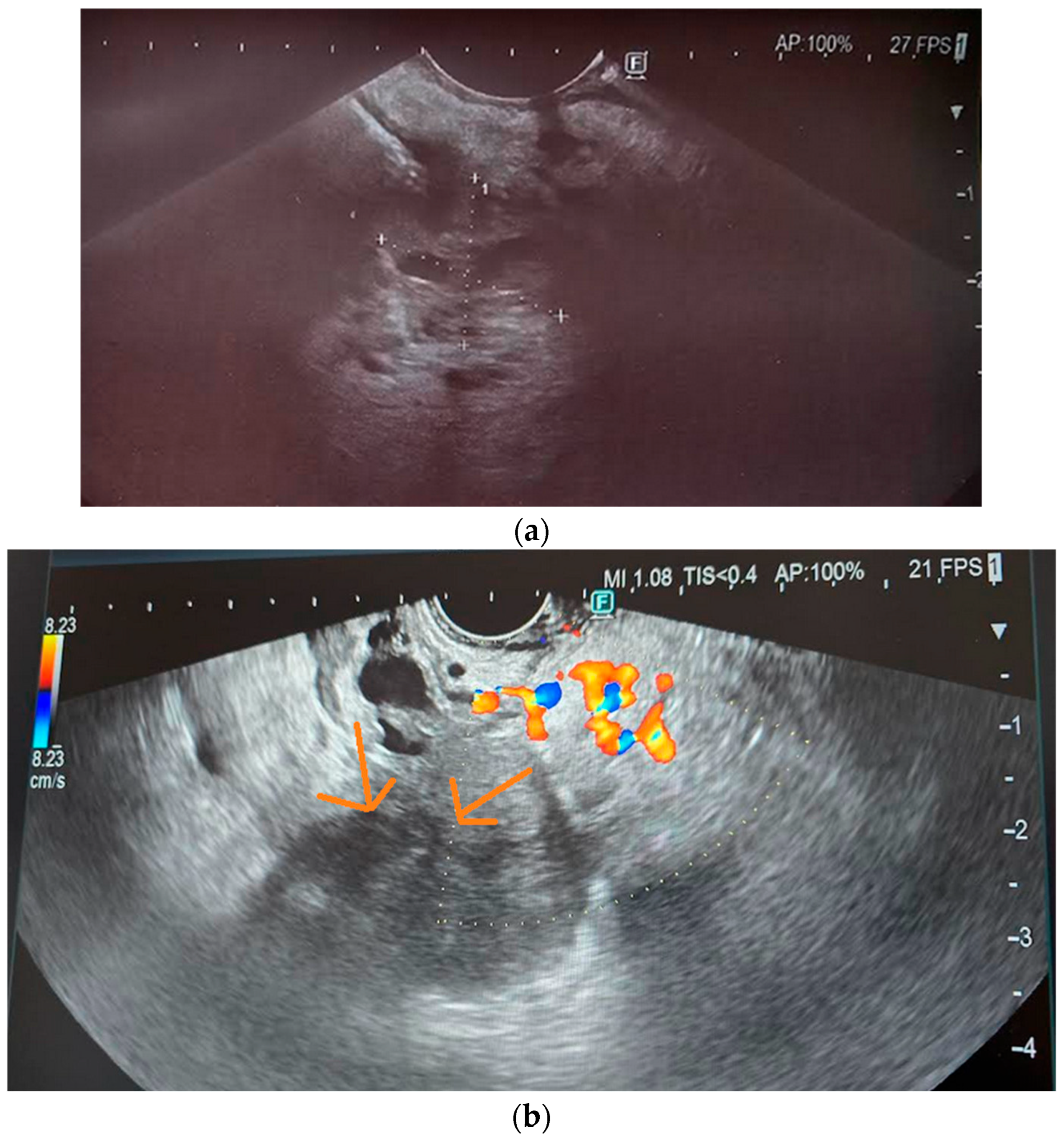
| Imaging Modalities | Sensitivity | Specificity | References |
|---|---|---|---|
| US | 67–69% | 90–98% | [18,30,31] |
| CT | 75% | 91% | [18] |
| MRI | 77–78% | 83–96% | [18,32] |
| EUS | 81–84% | 90–100% | [1,6,8] |
| Size | 1 Year | 3 Years | 5 Years | References |
|---|---|---|---|---|
| ≤2 cm | 66.7% | 66.7% | 26.4–69.9% | [50,51,52] |
| 2.1 cm–5 cm | 38.5–81.8% | 7.7–36.8% | 7.7–24.5% | [51,52,53,54] |
| >5 cm | 64.3% | 36.7% | 0–36.7% | [52,53] |
| Mutation | NCNN Guidelines Age at Screening Start (Years) | CAPS Guidelines Age at Screening Start (Years) |
|---|---|---|
| Peutz–Jeghers syndrome (STK11) | 30–35 | 40 |
| Familial pancreatic cancer (CDKN2A) | 40 | 40 |
| Familial pancreatic cancer (BRCA1/2), ataxia–telangiectasia (ATM), PALB2, Lynch syndrome (MLH1, MSH2, MSH6) | 50 | 45–50 |
| Lynch syndrome (EPCAM), Li–Fraumeni syndrome (TP53) | 50 | - |
| Imaging Modalities | Sensitivity | Specificity | References |
|---|---|---|---|
| US | 17–70% | - | [22,68] |
| CT | 33–80% | - | [22,60] |
| MRI | 52.3–93% | 77.1–89% | [28,35,69] |
| EUS | 72–100% | 90% | [60,68,69,70] |
| Pathology | Sensitivity | Specificity | Research |
|---|---|---|---|
| Detection of CP | 66–72% | 57–75.2% | [74,75] |
| Detection of PDAC | 95.6–100% | 67–96.3% | [72,74,76] |
| Differentiation of benign and malignant masses | 80–97% | 67–92.3% | [71,72,73,77,78] |
| Imaging Modality | Sensitivity | Specificity | Accuracy | References |
|---|---|---|---|---|
| EUS-FNA | 40–100% | 80–100% | 47–100% | [82,83,84] |
| CE-EUS | 70–95.1% | 83–100% | 77–100% | [63,93,94] |
| EUS elastography | 95% | 53% | - | [79] |
| CT | 20–70.6% | 91.9–100% | - | [93,94] |
| MRI | 50–73% | 33–100% | 62–68% | [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawelec, N.; Durko, Ł.; Małecka-Wojciesko, E. Changes Connected to Early Chronic Pancreatitis and Early Pancreatic Cancer in Endoscopic Ultrasonography (EUS): Clinical Implications. Cancers 2025, 17, 1891. https://doi.org/10.3390/cancers17111891
Pawelec N, Durko Ł, Małecka-Wojciesko E. Changes Connected to Early Chronic Pancreatitis and Early Pancreatic Cancer in Endoscopic Ultrasonography (EUS): Clinical Implications. Cancers. 2025; 17(11):1891. https://doi.org/10.3390/cancers17111891
Chicago/Turabian StylePawelec, Natalia, Łukasz Durko, and Ewa Małecka-Wojciesko. 2025. "Changes Connected to Early Chronic Pancreatitis and Early Pancreatic Cancer in Endoscopic Ultrasonography (EUS): Clinical Implications" Cancers 17, no. 11: 1891. https://doi.org/10.3390/cancers17111891
APA StylePawelec, N., Durko, Ł., & Małecka-Wojciesko, E. (2025). Changes Connected to Early Chronic Pancreatitis and Early Pancreatic Cancer in Endoscopic Ultrasonography (EUS): Clinical Implications. Cancers, 17(11), 1891. https://doi.org/10.3390/cancers17111891







