The Impact of Prehabilitation on Patient Outcomes in Oesophagogastric Cancer Surgery: Combined Data from Four Prospective Clinical Trials Performed Across the UK and Ireland
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
| London | Southampton | Guildford | Dublin | |
|---|---|---|---|---|
| Randomised | No | Yes | Yes | Yes |
| Duration | Diagnosis to adjuvant chemo (26 weeks) | Diagnosis to post-op (15+/−2 weeks) | Diagnosis to post-op (15 weeks) | Diagnosis to post-op (18+/−2.4 weeks) |
| Aerobic training | Yes * | Yes * | Yes * | Yes * |
| Resistance training | Yes Whole body resistance band circuit (targeting 15–20 reps) involving 12 exercises and repeat 3 times. | No | Yes 12 repetitions of 2 sets of exercises using free weights and resistance bands involving six major muscle groups. | Yes Circuit of six to ten stations for alternating upper and lower body exercises. |
| Dietetic input | Yes—both groups | Yes—both groups | Yes—both groups | Yes—both groups |
| Psychological input | Yes—as required | No | Yes—prehab only | No |
| Fitness assessment | CPET | CPET | CPET | 6 MWT |
| Face-to-face/remote/hybrid | Hybrid | Face-to-face | Hybrid | Hybrid |
| Standard/personal | Personalised Need-based and used FITT criteria | Personalised Need-based and used FITT criteria | Personalised Need-based and used FITT criteria | Personalised Need-based and used FITT criteria |
| Delivered by | Exercise physiologist | Exercise physiologist | Exercise physiologist | Exercise physiologist |
| Outcome measures | 1. Fitness (CPET—AT, VO2 peak, physical activity) 2. Complications 3. Chemotherapy completion and toxicity 4. Body composition (skeletal muscle mass) 5. HRQL 6. Length of stay 7. Tumour regression | 1. Fitness (CPET—AT, VO2 peak, physical activity) 2. Complications 3. Chemotherapy completion and toxicity. 4. Sarcopenia 5. Disability adjusted survival (WHODAS) 6. Tumour regression 7. Overall survival. | 1. Fitness (CPET—AT, VO2 peak, weekly step count, hand grip strength) 2. Complications 3. Chemotherapy completion and toxicity 4. Body composition (skeletal muscle mass) 5. Insulin resistance 6. HRQL 7. Length of stay 8. Tumour regression. | 1. Fitness (6 MWT, sit-to-stand, Handgrip, Physical Activity) 2. Complications, Post-Operative Morbidity and Pathological Data. 3. Chemotherapy treatment completion, rates, Toxicity, tolerance. 4. BMI 5. HRQL (LOT-R, EQ-5D-5L, FACT-E) |
| London | Southampton | Guildford | Dublin | |
|---|---|---|---|---|
| Frequency | 5 exercise sessions per week | 3 exercise sessions per week (2 sessions per week if on cancer therapies) | 2 exercise session per week (supervised) 3 exercise sessions per week (home) | 3 exercise sessions per week (2–3 exercise sessions per week if on cancer therapies) |
| Intensity | Moderate to high | Moderate to high | Moderate to high | Interval (moderate to high); continuous (moderate) |
| Time | 5 × 30 min sessions per week | 3 × 40 min sessions per week or 2 × 30 min sessions per week if on cancer therapies | 5 × 60 min sessions per week | Pre-op: First interval and continuous exercise session is 30 min duration. Second and subsequent sessions are 40 min in duration. Post-op: Initially for 20 min sessions and increase the duration of exercise by 10 min per week. |
| Type | Walking program with some days steady and some days with intervals. | Sitting on the stationary bicycle and pedalling at a cadence of 60–65 revolutions per minute. | 5 min warm-up followed by 25 min cycling | Centre-based: upright cycle ergometer, recumbent cycle ergometer, treadmill, elliptical ergometer, and rowing ergometer, depending on patient preference. Home-based: combination of walking, jogging or cycling |
| Volume | 150-min per week for 26 weeks | 60–120 min per week for 15+/−2 weeks | 300 min per week for 15 weeks | 120 min per week or 80–120 min per week if on cancer therapies for 18 +/−2.4 weeks. |
2.1. Prehabilitation Program (Exposure)
2.2. Combined Study Outcomes
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Survival
3.3. Complications
3.4. Fitness
3.5. Chemotherapy Completion
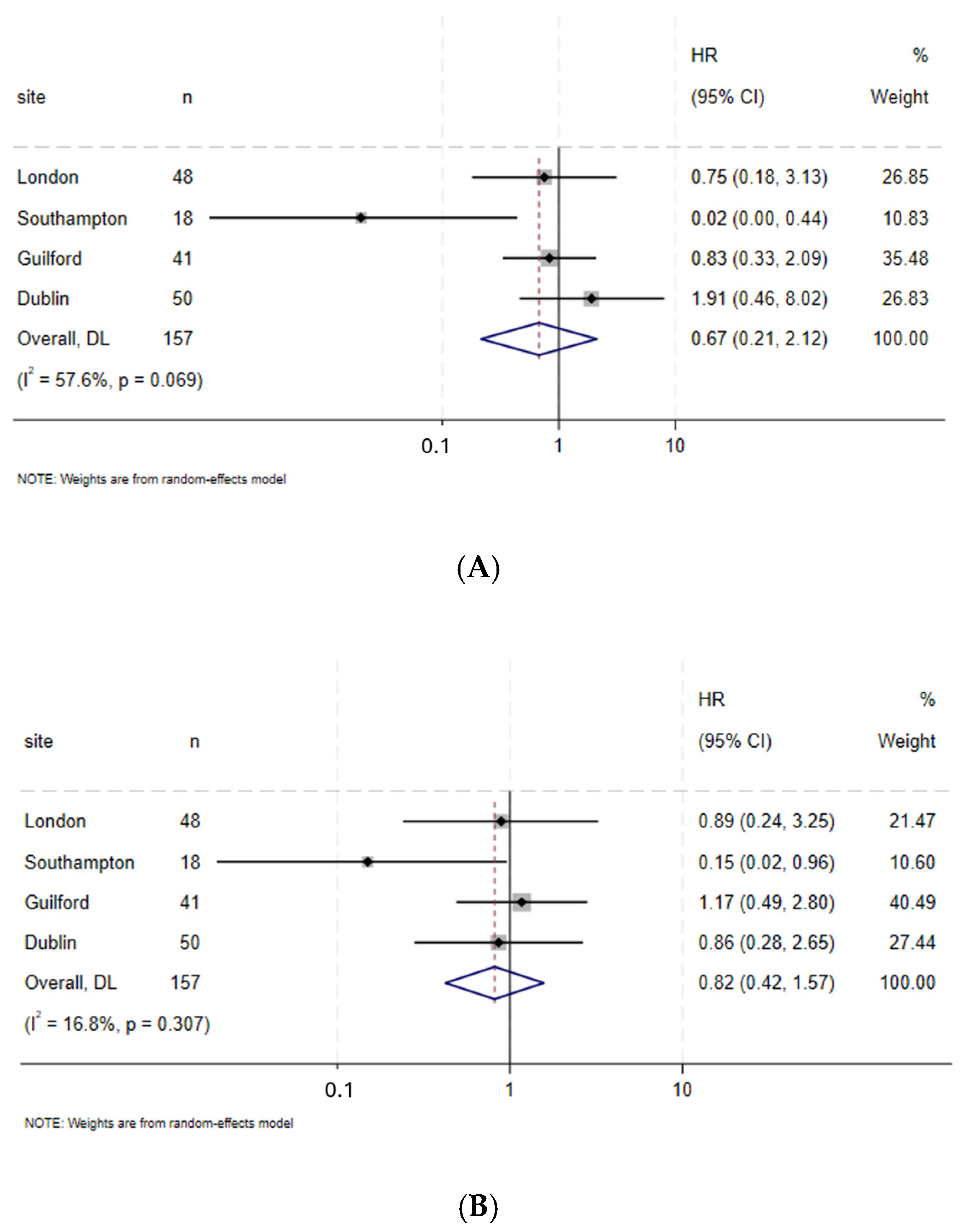
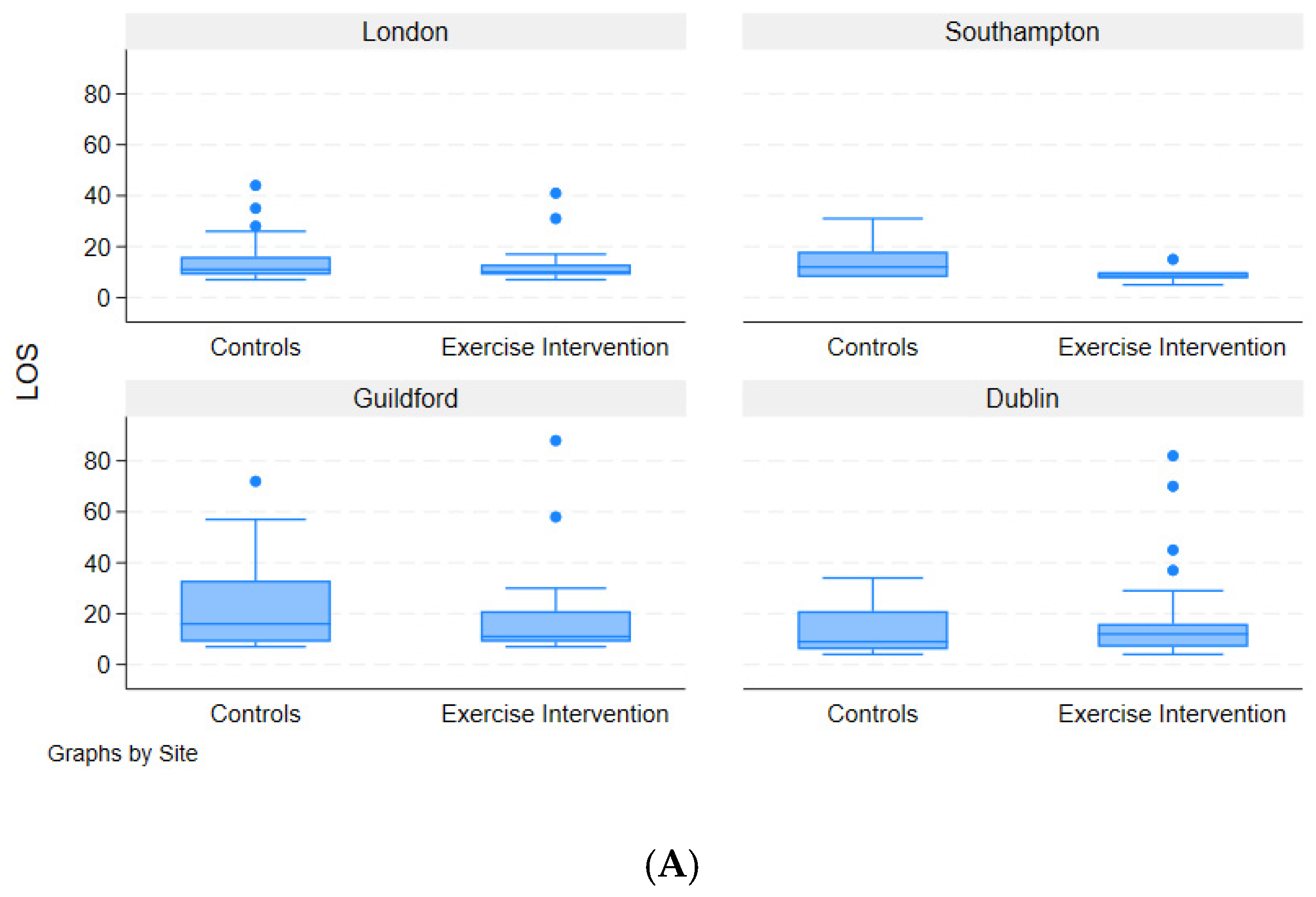
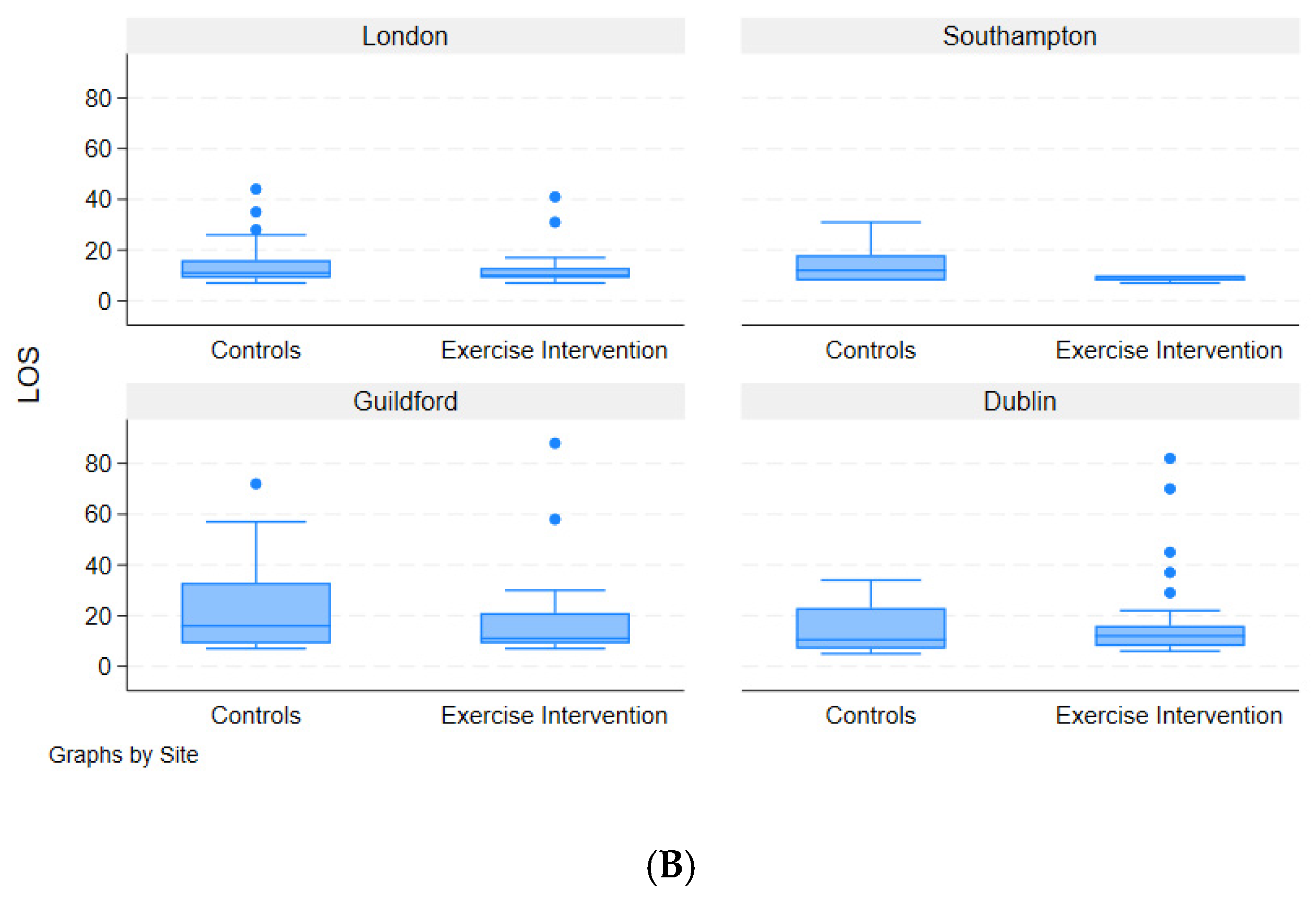
3.6. Length of Hospital Stay
3.7. Changes in Body Mass Index
3.8. Tumour Regression
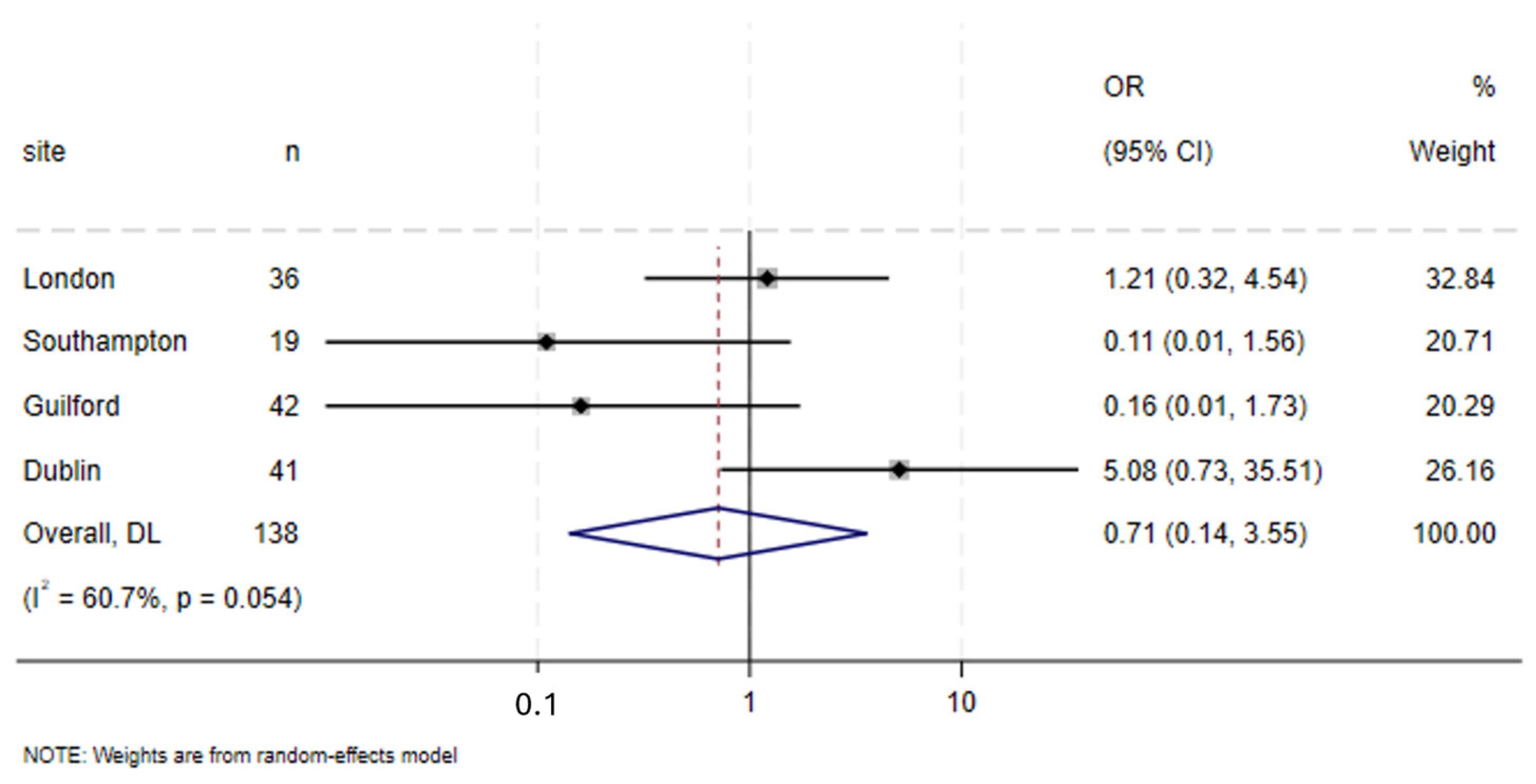
3.9. Specific Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paul, S.; Altorki, N. Outcomes in the Management of Esophageal Cancer. J. Surg. Oncol. 2014, 110, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Allum, W.H.; Blazeby, J.M.; Griffin, S.M.; Cunningham, D.; Jankowski, J.A.; Wong, R. Guidelines for the Management of Oesophageal and Gastric Cancer. Gut 2011, 60, 1449–1472. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, G.; Varagunam, M.; Groene, O.; Cromwell, D.; Hardwick, R.; Maynard, N.; Riley, S.; Crosby, T.; Greenaway, K. National Oesophago-Gastric Cancer Audit 2015. Available online: https://www.researchgate.net/publication/287201659_National_Oesophago-Gastric_Cancer_Audit_2015?channel=doi&linkId=5672d02c08ae04d9b099ac7a&showFulltext=true (accessed on 28 March 2025). [CrossRef]
- Low, D.E.; Kuppusamy, M.K.; Alderson, D.; Cecconello, I.; Chang, A.C.; Darling, G.; Davies, A.; D’Journo, X.B.; Gisbertz, S.S.; Griffin, S.M.; et al. Benchmarking Complications Associated with Esophagectomy. Ann. Surg. 2019, 269, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, J.; van Lanschot, J.J.B.; Hulshof, M.C.C.M.; van Hagen, P.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; van Laarhoven, H.W.M.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Neoadjuvant Chemoradiotherapy plus Surgery versus Surgery Alone for Oesophageal or Junctional Cancer (CROSS): Long-Term Results of a Randomised Controlled Trial. Lancet. Oncol. 2015, 16, 1090–1098. [Google Scholar] [CrossRef]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van de Velde, C.J.H.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative Chemotherapy versus Surgery Alone for Resectable Gastroesophageal Cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef]
- Al-Batran, S.-E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.-G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative Chemotherapy with Fluorouracil plus Leucovorin, Oxaliplatin, and Docetaxel versus Fluorouracil or Capecitabine plus Cisplatin and Epirubicin for Locally Advanced, Resectable Gastric or Gastro-Oesophageal Junction Adenocarcinoma (FLOT4): A Randomised, Phase 2/3 Trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef]
- Thomson, I.G.; Wallen, M.P.; Hall, A.; Ferris, R.; Gotley, D.C.; Barbour, A.P.; Lee, A.; Thomas, J.; Smithers, B.M. Neoadjuvant Therapy Reduces Cardiopulmunary Function in Patients Undegoing Oesophagectomy. Int. J. Surg. 2018, 53, 86–92. [Google Scholar] [CrossRef]
- Bloom, E. Prehabilitation Evidence and Insight Review. 2017. Available online: https://www.macmillan.org.uk/_images/prehabilitation-evidence-and-insight-review_tcm9-335025.pdf (accessed on 24 September 2024).
- Silver, J.K.; Baima, J. Cancer Prehabilitation. Am. J. Phys. Med. Rehabil. 2013, 92, 715–727. [Google Scholar] [CrossRef]
- Allen, S.K.; Brown, V.; White, D.; King, D.; Hunt, J.; Wainwright, J.; Emery, A.; Hodge, E.; Kehinde, A.; Prabhu, P.; et al. Multimodal Prehabilitation during Neoadjuvant Therapy prior to Esophagogastric Cancer Resection: Effect on Cardiopulmonary Exercise Test Performance, Muscle Mass and Quality of Life-A Pilot Randomized Clinical Trial. Ann. Surg. Oncol. 2022, 29, 1839–1850. [Google Scholar] [CrossRef]
- Walker, R.C.; Pezeshki, P.; Barman, S.; Ngan, S.; Whyte, G.; Lagergren, J.; Gossage, J.; Kelly, M.; Baker, C.; Knight, W.; et al. Exercise during Chemotherapy for Cancer: A Systematic Review. J. Surg. Oncol. 2024, 130, 1725–1736. [Google Scholar] [CrossRef]
- Walker, R.C.; Barman, S.; Pucher, P.H.; Singh, P.; Whyte, G.; Moore, J.; Huddy, F.; Evans, O.; Tham, G.; Zainab Noor, Z.; et al. Association of Upper Gastrointestinal Surgery of Great Britain and Ireland (AUGIS)/Perioperative Quality Initiative (POQI) Consensus Statement on Prehabilitation in Oesophagogastric Surgery. Br. J. Surg. 2024, 111, znae223. [Google Scholar] [CrossRef] [PubMed]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.-M.; Nieman, D.C.; Swain, D.P. Quantity and Quality of Exercise for Developing and Maintaining Cardiorespiratory, Musculoskeletal, and Neuromotor Fitness in Apparently Healthy Adults. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef] [PubMed]
- Montoye, H.J. Introduction: Evaluation of Some Measurements of Physical Activity and Energy Expenditure. Med. Sci. Sports Exerc. 2000, 32, S439–S441. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van Der Werf, L.R.; Busweiler, L.A.; Van Sandick, J.W.; van Berge Henegouwen, M.I.; Wijnhoven, B.P. Reporting National Outcomes after Esophagectomy and Gastrectomy according to the Esophageal Complications Consensus Group (ECCG). Ann. Surg. 2020, 271, 1095–1101. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo Classification of Surgical Complications. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Zylstra, J.; Whyte, G.P.; Beckmann, K.; Pate, J.; Santaolalla, A.; Gervais-Andre, L.; Russell, B.; Maisey, N.; Waters, J.; Tham, G.; et al. Exercise Prehabilitation during Neoadjuvant Chemotherapy May Enhance Tumour Regression in Oesophageal Cancer: Results from a Prospective Non-Randomised Trial. Br. J. Sports Med. 2022, 56, 402–409. [Google Scholar] [CrossRef]
- Tully, R.; Loughney, L.; Bolger, J.; Sorensen, J.; McAnena, O.; Collins, C.G.; Carroll, P.A.; Arumugasamy, M.; Murphy, T.J.; Robb, W.B. The Effect of a Pre- and Post-Operative Exercise Programme versus Standard Care on Physical Fitness of Patients with Oesophageal and Gastric Cancer Undergoing Neoadjuvant Treatment prior to Surgery (the PERIOP-OG Trial): Study Protocol for a Randomised Controlled Trial. Trials 2020, 21, 638. [Google Scholar] [CrossRef]
- van Rooijen, S.; Carli, F.; Dalton, S.; Thomas, G.; Bojesen, R.; Le Guen, M.; Barizien, N.; Awasthi, R.; Minnella, E.; Beijer, S.; et al. Multimodal Prehabilitation in Colorectal Cancer Patients to Improve Functional Capacity and Reduce Postoperative Complications: The First International Randomized Controlled Trial for Multimodal Prehabilitation. BMC Cancer 2019, 19, 98. [Google Scholar] [CrossRef]
- Gillis, C.; Li, C.; Lee, L.; Awasthi, R.; Augustin, B.; Gamsa, A.; Liberman, A.S.; Stein, B.; Charlebois, P.; Feldman, L.S.; et al. Prehabilitation versus Rehabilitation. Anesthesiology 2014, 121, 937–947. [Google Scholar] [CrossRef]
- Carli, F.; Bousquet-Dion, G.; Awasthi, R.; Elsherbini, N.; Liberman, S.; Boutros, M.; Stein, B.; Charlebois, P.; Ghitulescu, G.; Morin, N.; et al. Effect of Multimodal Prehabilitation vs Postoperative Rehabilitation on 30-Day Postoperative Complications for Frail Patients Undergoing Resection of Colorectal Cancer. JAMA Surg. 2020, 155, 233–242. [Google Scholar] [CrossRef]
- Zhou, N.; Ripley-Gonzalez, J.W.; Zhang, W.; Xie, K.; You, B.; Shen, Y.; Cao, Z.; Qiu, L.; Li, C.; Fu, S.; et al. Preoperative Exercise Training Decreases Complications of Minimally Invasive Lung Cancer Surgery-a Randomized Controlled Trial. J. Thorac. Cardiovasc. Surg. 2025, 169, 516–528.e10. [Google Scholar] [CrossRef] [PubMed]
- Sebio-Garcia, R.; Celada-Castro, C.; Arguis, M.J.; Sisó, M.; Torné, A.; Tena, B.; Díaz-Feijoo, B.; Martinez-Palli, G. Multimodal Prehabilitation Improves Functional Capacity in Patients with Advanced Ovarian Cancer Undergoing Cytoreductive Surgery. Int. J. Gynecol. Cancer 2025, 101858. [Google Scholar] [CrossRef] [PubMed]
- Lavery, J.A.; Boutros, P.C.; Scott, J.M.; Tammela, T.; Moskowitz, C.S.; Jones, L.W. Pan-Cancer Analysis of Postdiagnosis Exercise and Mortality. J. Clin. Oncol. 2023, 41, 4982–4992. [Google Scholar] [CrossRef] [PubMed]
- Knight, W.; Moore, J.L.; Whyte, G.P.; Zylstra, J.; Lane, A.M.; Pate, J.; Gervais-Andre, L.; Maisey, N.; Hill, M.; Tham, G.; et al. Prehabilitation Exercise before Oesophagectomy: Long-Term Follow-up of Patients Declining/Withdrawing from the Program. Br. J. Surg. 2023, 110, 1668–1672. [Google Scholar] [CrossRef]
- Rundqvist, H.; Rietz, M.; Mijwel, S.; Lindström, L.S.; Wengström, Y. 232O Effects of a High-Intensity Exercise Intervention on Recurrence and Survival: The OptiTrain Breast Cancer Trial. Ann. Oncol. 2024, 35, S309. [Google Scholar] [CrossRef]
- Tukanova, K.H.; Chidambaram, S.; Guidozzi, N.; Hanna, G.B.; McGregor, A.H.; Markar, S.R. Physiotherapy Regimens in Esophagectomy and Gastrectomy: A Systematic Review and Meta-Analysis. Ann. Surg. Oncol. 2022, 29, 3148–3167. [Google Scholar] [CrossRef]
- Leach, H.J.; Danyluk, J.M.; Nishimura, K.C.; Culos-Reed, S.N. Benefits of 24 versus 12 Weeks of Exercise and Wellness Programming for Women Undergoing Treatment for Breast Cancer. Support. Care Cancer 2016, 24, 4597–4606. [Google Scholar] [CrossRef]
- Halliday, L.J.; Doganay, E.; Wynter-Blyth, V.A.; Hanna, G.B.; Moorthy, K. The Impact of Prehabilitation on Post-Operative Outcomes in Oesophageal Cancer Surgery: A Propensity Score Matched Comparison. J. Gastrointest. Surg. 2021, 25, 2733–2741. [Google Scholar] [CrossRef]
- Lambert, J.E.; Hayes, L.D.; Keegan, T.J.; Subar, D.A.; Gaffney, C.J. The Impact of Prehabilitation on Patient Outcomes in Hepatobiliary, Colorectal, and Upper Gastrointestinal Cancer Surgery: A PRISMA-Accordant Meta-Analysis. Ann. Surg. 2021, 274, 70–77. [Google Scholar] [CrossRef]
- Sanft, T.; Harrigan, M.; McGowan, C.; Cartmel, B.; Zupa, M.; Li, F.Y.; Ferrucci, L.M.; Puklin, L.; Cao, A.; Nguyen, T.H.; et al. Randomized Trial of Exercise and Nutrition on Chemotherapy Completion and Pathologic Complete Response in Women with Breast Cancer: The Lifestyle, Exercise, and Nutrition Early after Diagnosis Study. J. Clin. Oncol. 2023, 41, 5285–5295. [Google Scholar] [CrossRef]
- West, M.A.; Astin, R.; Moyses, H.E.; Cave, J.; White, D.; Levett, D.Z.; Bates, A.; Brown, G.; Grocott, M.P.; Jack, S. Exercise Prehabilitation May Lead to Augmented Tumor Regression Following Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer. Acta Oncol. 2019, 58, 588–595. [Google Scholar] [CrossRef] [PubMed]
- West, M.; Bates, A.; Grimmett, C.; Allen, C.; Green, R.; Hawkins, L.; Moyses, H.; Leggett, S.; Levett, D.Z.; Rickard, S.; et al. The Wessex Fit-4-Cancer Surgery Trial (WesFit): A Protocol for a Factorial-Design, Pragmatic Randomised-Controlled Trial Investigating the Effects of a Multi-Modal Prehabilitation Programme in Patients Undergoing Elective Major Intra-Cavity Cancer Surgery. F1000Research 2021, 10, 952. [Google Scholar] [CrossRef] [PubMed]
- Grimmett, C.; Bates, A.; West, M.; Leggett, S.; Varkonyi-Sepp, J.; Campbell, A.; Davis, J.; Wootton, S.; Shaw, C.; Barlow, R.; et al. SafeFit Trial: Virtual Clinics to Deliver a Multimodal Intervention to Improve Psychological and Physical Well-Being in People with Cancer. Protocol of a COVID-19 Targeted Non-Randomised Phase III Trial. BMJ Open 2021, 11, e048175. [Google Scholar] [CrossRef] [PubMed]
- Barman, S.; Walker, R.; Pucher, P.; Jack, S.; Whyte, G.; Grocott, M.; West, M.; Maynard, N.; Underwood, T.; Gossage, J.; et al. A National Survey of the Provision of Prehabilitation for Oesophagogastric Cancer Patients in the UK. Ann. R. Coll. Surg. Engl. 2024, 107, 300–306. [Google Scholar] [CrossRef]

| All Sites Combined | |||||
|---|---|---|---|---|---|
| Prehabilitation Group (N = 88) | Control Group (N = 77) | p-Value | |||
| n | % | n | % | ||
| Age, years (mean (SD)) | 63.25 (9.34) | 61.69 (8.50) | 0.269 b | ||
| Sex | |||||
| Male | 69 | 78.4 | 63 | 81.8 | 0.585 a |
| Female | 19 | 21.6 | 14 | 18.2 | |
| Baseline BMI, kg/m2 (mean (SD)) | |||||
| Underweight | 0 | 0 | 0 | 0 | 0.756 a |
| Healthy | 27 | 30.7 | 23 | 29.9 | |
| Overweight | 32 | 36.4 | 22 | 28.6 | |
| Obese | 24 | 27.3 | 22 | 28.6 | |
| Missing | 5 | 5.7 | 10 | 13 | |
| Post-NAT BMI, kg/m2 (mean (SD)) | |||||
| Underweight | 1 | 1.1 | 0 | 0 | 0.818 a |
| Healthy | 27 | 30.7 | 22 | 28.6 | |
| Overweight | 31 | 35.2 | 28 | 36.4 | |
| Obese | 17 | 19.3 | 14 | 18.2 | |
| Missing | 12 | 13.6 | 13 | 16.9 | |
| VO2 peak, mean (SD), mL/kg/min | |||||
| At baseline | 22.19 (5.54) | 22.18 (3.89) | |||
| Post-neoadjuvant treatment | 20.92 (3.99) | 19.23 (3.25) | |||
| Delta/change in VO2 peak | −1.07 (4.47) | −2.74 (2.91) | 0.035 b | ||
| AT, mean (SD), mL/kg/min | |||||
| At baseline | 14.04 (3.69) | 14.48 (3.24) | |||
| Post-neoadjuvant treatment | 12.81 (2.62) | 11.81 (2.68) | |||
| Delta/change in anaerobic threshold | −0.96 (4.00) | −1.78 (5.72) | 0.385 b | ||
| ASA | |||||
| 2 | 58 | 65.9 | 51 | 66.2 | 0.965 a |
| 3 | 30 | 34.1 | 26 | 33.8 | |
| Treatment characteristics | |||||
| Neoadjuvant chemotherapy (NAC) | 55 | 62.5 | 58 | 75.3 | 0.077 a |
| Neoadjuvant chemoradiotherapy (NACRT) | 33 | 37.5 | 19 | 24.7 | |
| Chemotherapy type | |||||
| ECF/EOX/ECX | 32 | 36.4 | 33 | 42.9 | 0.357 a |
| FLOT | 24 | 27.3 | 24 | 31.2 | |
| Others-CROSS/CF/Ciscape | 32 | 36.4 | 20 | 26 | |
| Chemotherapy completion | |||||
| No | 9 | 10.2 | 18 | 23.4 | 0.016 a |
| Yes | 79 | 89.8 | 56 | 72.7 | |
| Missing | 0 | 0 | 3 | 3.9 | |
| Clavien–Dindo (CD) excluding CD 5 d | |||||
| CD 0–2 | 68 | 80 | 49 | 64 | |
| CD 3–4 | 17 | 20 | 27 | 36 | 0.034 c |
| Tumour regression grade (TRG) | |||||
| All patients | |||||
| Responder Mandard 1–3 | 49 | 56 | 35 | 45 | |
| Non-responder Mandard 4–5 | 38 | 43.2 | 42 | 55 | 0.211 c |
| Missing | 1 | 1.14 | |||
| Neoadjuvant chemotherapy | |||||
| Responder Mandard 1–3 | 22 | 41 | 20 | 35 | |
| Non-responder Mandard 4–5 | 32 | 59 | 38 | 65 | 0.494 a |
| Neoadjuvant chemoradiotherapy | |||||
| Responder Mandard 1–3 | 27 | 82 | 15 | 79 | |
| Non-responder Mandard 4–5 | 6 | 18 | 4 | 21 | 0.800 a |
| Anastomotic Leak | |||||
| No | 83 | 94.3 | 71 | 92.2 | 0.588 a |
| Yes | 5 | 5.7 | 6 | 7.8 | |
| Pneumonia | |||||
| No | 74 | 84.1 | 62 | 80.5 | 0.548 a |
| Yes | 14 | 15.9 | 15 | 19.5 | |
| Post-operative mortality | |||||
| Yes | 35 | 39.8 | 31 | 40.3 | 0.878 a |
| No | 51 | 58 | 43 | 55.8 | |
| Missing | 2 | 2.3 | 3 | 3.9 | |
| Recurrence | |||||
| No | 64 | 72 | 58 | 75 | |
| Yes | 24 | 28 | 18 | 23 | |
| Missing | 1 | 2 |
| Prehabilitation Group (N = 88) | Control Group (N = 77) | p-Value for t-Test | |
|---|---|---|---|
| Baseline VO2 peak (mean (s.d.)) | 22.19 (5.54) | 22.18 (3.89) | |
| Post-NAT VO2 peak(mean (s.d.)) | 20.92 (3.99) | 19.23 (3.25) | |
| Changes in VO2 peak(s.d.) | −1.07 (4.47) | −2.74 (2.91) | 0.035 |
| Prehabilitation Group (N = 88) | Control Group (N = 77) | p-Value for t-Test | |
|---|---|---|---|
| Baseline AT (mean (s.d.)) | 14.04 (3.69) | 14.48 (3.24) | |
| Post-NAT AT (mean (s.d.)) | 12.81 (2.62) | 11.81 (2.68) | |
| Changes in AT (s.d.) | −0.96 (4.00) | −1.78 (5.72) | 0.385 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barman, S.; Russell, B.; Walker, R.C.; Knight, W.; Baker, C.; Kelly, M.; Gossage, J.; Zylstra, J.; Whyte, G.; Pate, J.; et al. The Impact of Prehabilitation on Patient Outcomes in Oesophagogastric Cancer Surgery: Combined Data from Four Prospective Clinical Trials Performed Across the UK and Ireland. Cancers 2025, 17, 1836. https://doi.org/10.3390/cancers17111836
Barman S, Russell B, Walker RC, Knight W, Baker C, Kelly M, Gossage J, Zylstra J, Whyte G, Pate J, et al. The Impact of Prehabilitation on Patient Outcomes in Oesophagogastric Cancer Surgery: Combined Data from Four Prospective Clinical Trials Performed Across the UK and Ireland. Cancers. 2025; 17(11):1836. https://doi.org/10.3390/cancers17111836
Chicago/Turabian StyleBarman, Sowrav, Beth Russell, Robert C. Walker, William Knight, Cara Baker, Mark Kelly, James Gossage, Janine Zylstra, Greg Whyte, James Pate, and et al. 2025. "The Impact of Prehabilitation on Patient Outcomes in Oesophagogastric Cancer Surgery: Combined Data from Four Prospective Clinical Trials Performed Across the UK and Ireland" Cancers 17, no. 11: 1836. https://doi.org/10.3390/cancers17111836
APA StyleBarman, S., Russell, B., Walker, R. C., Knight, W., Baker, C., Kelly, M., Gossage, J., Zylstra, J., Whyte, G., Pate, J., Lagergren, J., Van Hemelrijck, M., Browning, M., Allen, S., Preston, S. R., Sultan, J., Singh, P., Rockall, T., Robb, W. B., ... Davies, A. R. (2025). The Impact of Prehabilitation on Patient Outcomes in Oesophagogastric Cancer Surgery: Combined Data from Four Prospective Clinical Trials Performed Across the UK and Ireland. Cancers, 17(11), 1836. https://doi.org/10.3390/cancers17111836









