Impaired Overall Survival of Melanoma Patients Due to Antibiotic Use Prior to Immune Checkpoint Inhibitor Therapy: Systematic Review and Meta-Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol
2.2. Search Strategies
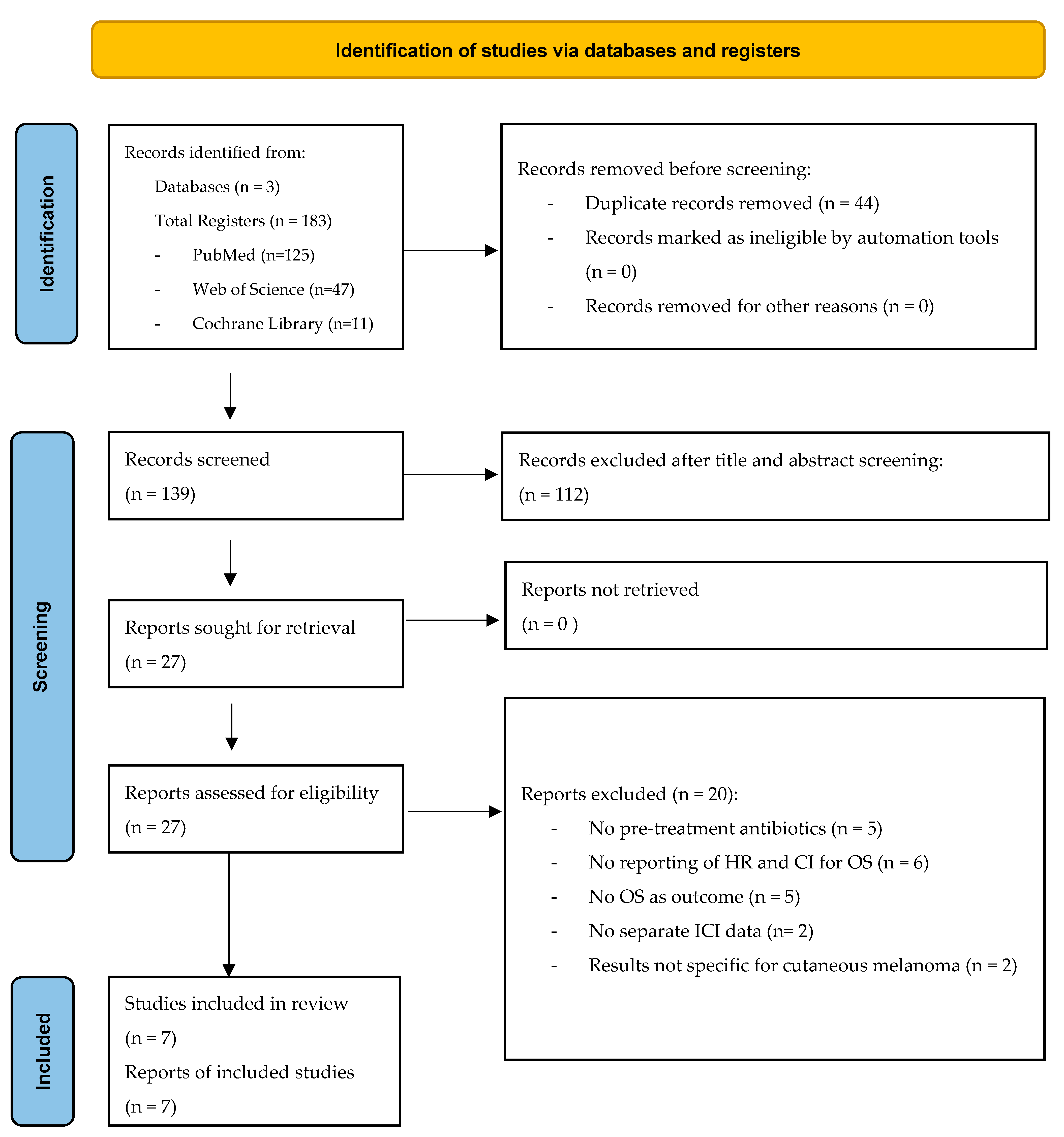
2.3. Selection of Studies and Data Extraction
2.4. Individual Assessment of Study Quality
2.5. Data Synthesis and Statistical Analysis
2.6. Primary Meta-Analysis and Forest Plot
2.7. Sensitivity Analyses
2.8. Subgroup Analyses
2.9. Meta-Regression
2.10. Publication Bias Assessment
3. Results
3.1. Extracted Data and Study Characteristics
3.2. NOS Assessment
3.3. Primary Meta-Analysis
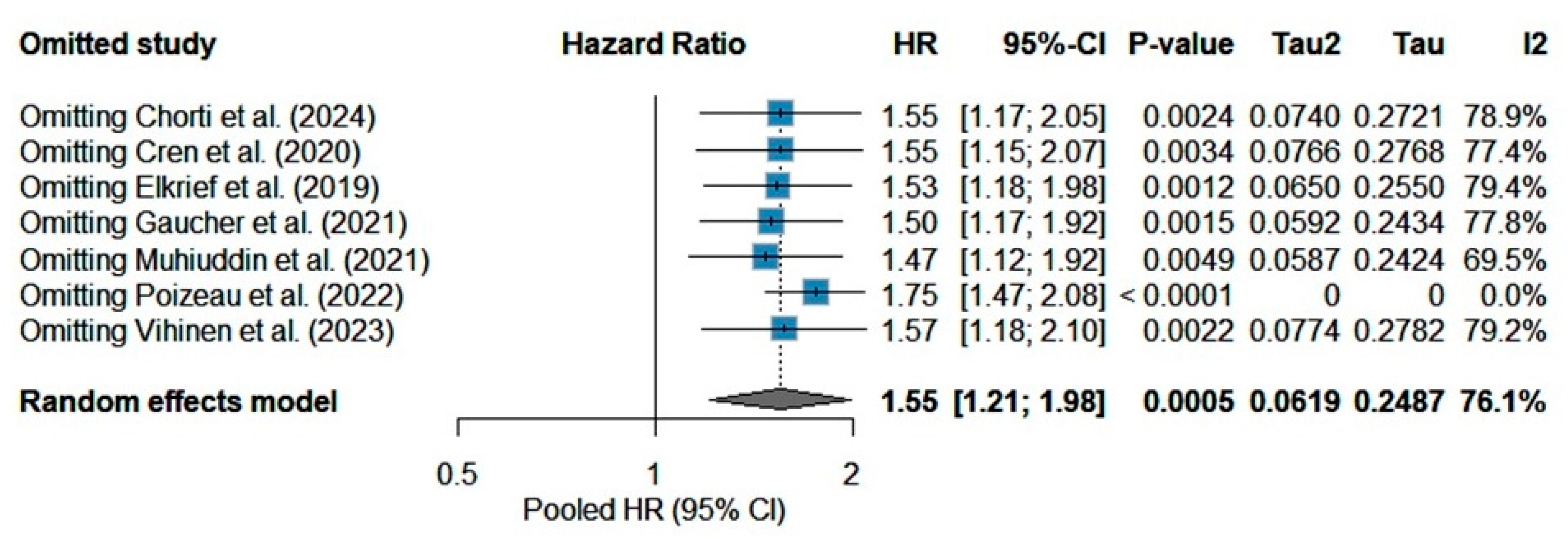
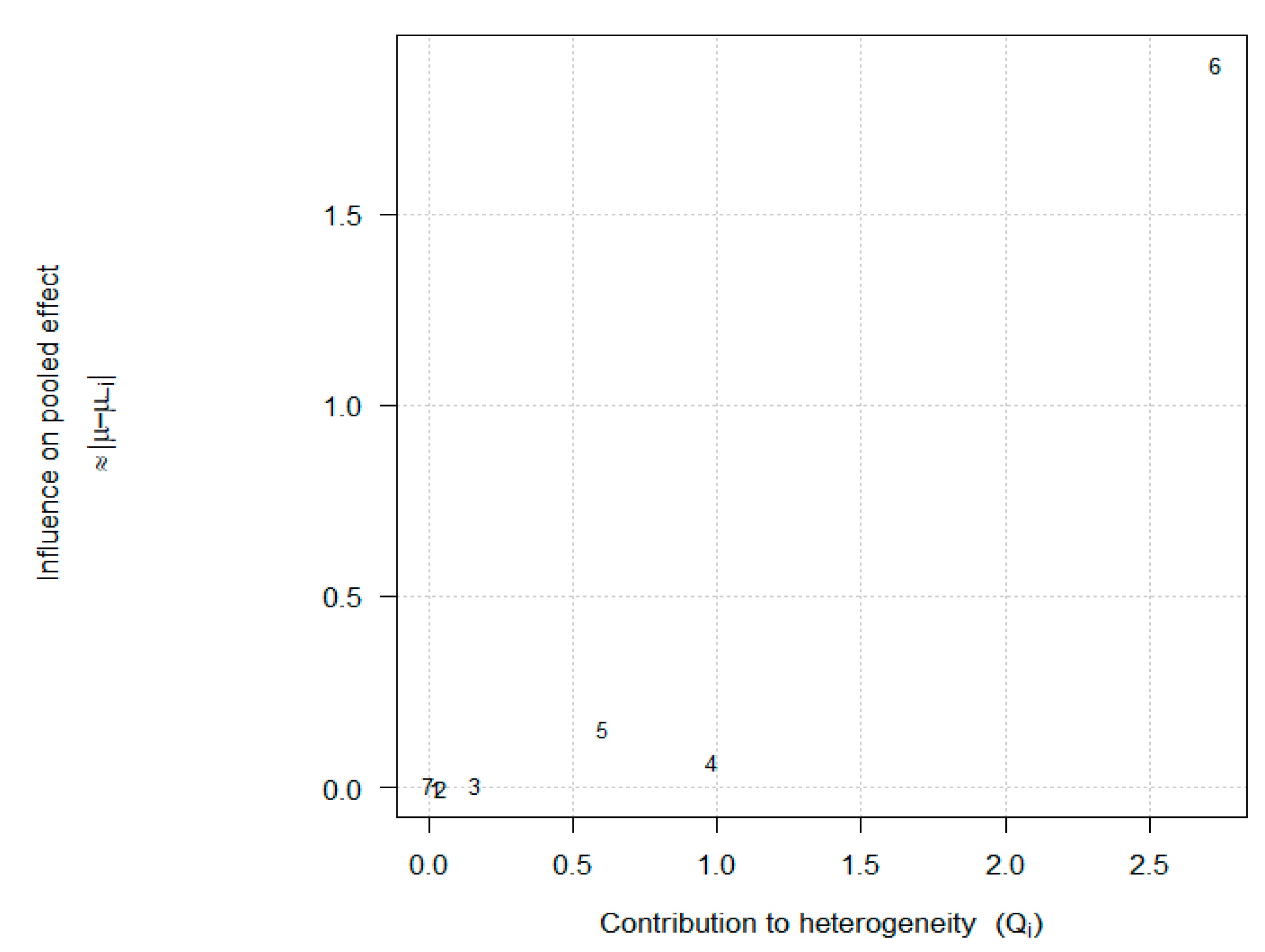
3.4. Sensitivity Analysis
3.5. Meta-Regression Subgroup Analyses
3.6. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maul, L.V.; Ramelyte, E.; Dummer, R.; Mangana, J. Management of metastatic melanoma with combinations including PD-1 inhibitors. Expert Opin. Biol. Ther. 2025, 25, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Reschke, R.; Enk, A.H.; Hassel, J.C. Prognostic Biomarkers in Evolving Melanoma Immunotherapy. Am. J. Clin. Dermatol. 2025, 26, 213–223. [Google Scholar] [CrossRef]
- Pekarek, L.; Sánchez Cedra, A.; Jaudenes, Y.D.Y.; Ospino, L.R.; Iglesias Pedrejón, B.; Bernier, L.; Roberts Cervantes, E.D.; Sánchez Cendra, C.; Cassinello, J.; Trasobares, L.; et al. Paradigm of biomarkers in metastatic melanoma (Review). Oncol. Lett. 2024, 29, 78. [Google Scholar] [CrossRef]
- Yan, J.; Yang, L.; Ren, Q.; Zhu, C.; Du, H.; Wang, Z.; Qi, Y.; Xian, X.; Chen, D. Gut microbiota as a biomarker and modulator of anti-tumor immunotherapy outcomes. Front. Immunol. 2024, 15, 1471273. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, J.; Mei, Y.; Zhang, W. Gut microbiota affects PD-L1 therapy and its mechanism in melanoma. Cancer Immunol. Immunother. 2025, 74, 169. [Google Scholar] [CrossRef]
- Dravillas, C.; Williams, N.; Husain, M.; Hoyd, R.; Hussein, A.; Meara, A.; Lynn, M.; Bibi, A.; Conrad, B.; Lepola, N.; et al. The Association of the Microbiome with Melanoma Tumor Response to Immune Checkpoint Inhibitor Treatment and Immune-Related Adverse Events (NCT05102773). medRxiv 2025. [Google Scholar] [CrossRef]
- Ciernikova, S.; Sevcikova, A.; Novisedlakova, M.; Mego, M. Insights into the Relationship Between the Gut Microbiome and Immune Checkpoint Inhibitors in Solid Tumors. Cancers 2024, 16, 4271. [Google Scholar] [CrossRef] [PubMed]
- Tsikala-Vafea, M.; Belani, N.; Vieira, K.; Khan, H.; Farmakiotis, D. Use of antibiotics is associated with worse clinical outcomes in patients with cancer treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Int. J. Infect. Dis. 2021, 106, 142–154. [Google Scholar] [CrossRef]
- Zhou, J.; Huang, G.; Wong, W.C.; Hu, D.H.; Zhu, J.W.; Li, R.; Zhou, H. The impact of antibiotic use on clinical features and survival outcomes of cancer patients treated with immune checkpoint inhibitors. Front. Immunol. 2022, 13, 968729. [Google Scholar] [CrossRef]
- Swami, U.; Chennamadhavuni, A.; Borcherding, N.; Bossler, A.D.; Mott, S.L.; Garje, R.; Zakharia, Y.; Milhem, M. Multivariable Analysis of 169 Cases of Advanced Cutaneous Melanoma to Evaluate Antibiotic Exposure as Predictor of Survival to Anti-PD-1 Based Immunotherapies. Antibiotics 2020, 9, 740. [Google Scholar] [CrossRef]
- Metselaar-Albers, M.; Meijerman, I.; Engels, F.; Haanen, J.; Beijnen, J.; Lalmohamed, A. No detrimental association between antibiotic use and immune checkpoint inhibitor therapy: An observational cohort study comparing patients with ICI-treated and TKI-treated melanoma and NSCLC. J. Immunother. Cancer 2024, 12, e008269. [Google Scholar] [CrossRef]
- Eng, L.; Sutradhar, R.; Niu, Y.; Liu, N.; Liu, Y.; Kaliwal, Y.; Powis, M.L.; Liu, G.; Peppercorn, J.M.; Bedard, P.L.; et al. Impact of Antibiotic Exposure Before Immune Checkpoint Inhibitor Treatment on Overall Survival in Older Adults with Cancer: A Population-Based Study. J. Clin. Oncol. 2023, 41, 3122–3134. [Google Scholar] [CrossRef]
- Spakowicz, D.; Hoyd, R.; Muniak, M.; Husain, M.; Bassett, J.S.; Wang, L.; Tinoco, G.; Patel, S.H.; Burkart, J.; Miah, A.; et al. Inferring the role of the microbiome on survival in patients treated with immune checkpoint inhibitors: Causal modeling, timing, and classes of concomitant medications. BMC Cancer 2020, 20, 383. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, N.; Zhou, C.; Tan, G.; Rack, S.; Lorigan, P.; Blackhall, F.; Krebs, M.; Carter, L.; Thistlethwaite, F.; Graham, D.; et al. Cumulative Antibiotic Use Significantly Decreases Efficacy of Checkpoint Inhibitors in Patients with Advanced Cancer. Oncologist 2020, 25, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Hirner, J.P.; Rajeh, A.; Semenov, Y.R.; Kwatra, S.G.; LeBoeuf, N.R. A retrospective cohort study of the time between prior antibiotics and checkpoint inhibitors and association with survival in melanoma patients. J. Am. Acad. Dermatol. 2025, 92, 878–879. [Google Scholar] [CrossRef] [PubMed]
- Angrish, M.D.; Agha, A.; Pezo, R.C. Association of Antibiotics and Other Drugs with Clinical Outcomes in Metastatic Melanoma Patients Treated with Immunotherapy. J. Skin. Cancer 2021, 2021, 9120162. [Google Scholar] [CrossRef]
- Pinato, D.J.; Howlett, S.; Ottaviani, D.; Urus, H.; Patel, A.; Mineo, T.; Brock, C.; Power, D.; Hatcher, O.; Falconer, A.; et al. Association of Prior Antibiotic Treatment with Survival and Response to Immune Checkpoint Inhibitor Therapy in Patients with Cancer. JAMA Oncol. 2019, 5, 1774–1778, Erratum in JAMA Oncol. 2020, 6, 302. [Google Scholar] [CrossRef]
- Jiang, S.; Geng, S.; Chen, Q.; Zhang, C.; Cheng, M.; Yu, Y.; Zhang, S.; Shi, N.; Dong, M. Effects of Concomitant Antibiotics Use on Immune Checkpoint Inhibitor Efficacy in Cancer Patients. Front. Oncol. 2022, 12, 823705. [Google Scholar] [CrossRef]
- Hong, S.; Lee, J.H.; Heo, J.Y.; Suh, K.J.; Kim, S.H.; Kim, Y.J.; Kim, J.H. Impact of concurrent medications on clinical outcomes of cancer patients treated with immune checkpoint inhibitors: Analysis of Health Insurance Review and Assessment data. J. Cancer Res. Clin. Oncol. 2024, 150, 186. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L.; Yang, F.; Zhao, R.; Li, X.; Li, H. Impact of concomitant medications on the efficacy of immune checkpoint inhibitors: An umbrella review. Front. Immunol. 2023, 14, 1218386. [Google Scholar] [CrossRef]
- Chorti, E.; Kowall, B.; Hassel, J.C.; Schilling, B.; Sachse, M.; Gutzmer, R.; Loquai, C.; Kähler, K.C.; Hüsing, A.; Gilde, C.; et al. Association of antibiotic treatment with survival outcomes in treatment-naïve melanoma patients receiving immune checkpoint blockade. Eur. J. Cancer 2024, 200, 113536. [Google Scholar] [CrossRef] [PubMed]
- Cren, P.Y.; Bertrand, N.; Le Deley, M.C.; Génin, M.; Mortier, L.; Odou, P.; Penel, N.; Chazard, E. Is the survival of patients treated with ipilimumab affected by antibiotics? An analysis of 1585 patients from the French National hospital discharge summary database (PMSI). Oncoimmunology 2020, 9, 1846914. [Google Scholar] [CrossRef]
- Elkrief, A.; El Raichani, L.; Richard, C.; Messaoudene, M.; Belkaid, W.; Malo, J.; Belanger, K.; Miller, W.; Jamal, R.; Letarte, N.; et al. Antibiotics are associated with decreased progression-free survival of advanced melanoma patients treated with immune checkpoint inhibitors. Oncoimmunology 2019, 8, e1568812. [Google Scholar] [CrossRef]
- Gaucher, L.; Adda, L.; Séjourné, A.; Joachim, C.; Guillaume, C.; Poulet, C.; Liabeuf, S.; Gras-Champel, V.; Masmoudi, K.; Houessinon, A.; et al. Associations between dysbiosis-inducing drugs, overall survival and tumor response in patients treated with immune checkpoint inhibitors. Ther. Adv. Med. Oncol. 2021, 13, 17588359211000591. [Google Scholar] [CrossRef]
- Mohiuddin, J.J.; Chu, B.; Facciabene, A.; Poirier, K.; Wang, X.; Doucette, A.; Zheng, C.; Xu, W.; Anstadt, E.J.; Amaravadi, R.K.; et al. Association of Antibiotic Exposure with Survival and Toxicity in Patients with Melanoma Receiving Immunotherapy. J. Natl. Cancer Inst. 2021, 113, 162–170. [Google Scholar] [CrossRef]
- Poizeau, F.; Kerbrat, S.; Balusson, F.; Tattevin, P.; Revest, M.; Cattoir, V.; Luque-Paz, D.; Lesimple, T.; Pracht, M.; Dinulescu, M.; et al. The Association Between Antibiotic Use and Outcome Among Metastatic Melanoma Patients Receiving Immunotherapy. J. Natl. Cancer Inst. 2022, 114, 686–694. [Google Scholar] [CrossRef]
- Vihinen, H.; Jokinen, A.; Laajala, T.D.; Wahid, N.; Peltola, L.; Kettunen, T.; Rönkä, A.; Tiainen, L.; Skyttä, T.; Kohtamäki, L.; et al. Antibiotic Treatment is an Independent Poor Risk Factor in NSCLC But Not in Melanoma Patients Who had Received Anti-PD-1/L1 Monotherapy. Clin. Lung Cancer 2023, 24, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Sawada, K.; Yamashita, R.; Sakai, S.A.; Horasawa, S.; Yoshikawa, A.; Fujisawa, T.; Kadowaki, S.; Kato, K.; Ueno, M.; Oki, E.; et al. Microbiome landscape and association with response to immune checkpoint inhibitors in advanced solid tumors: A SCRUM-Japan MONSTAR-SCREEN study. Cancer Res. Commun. 2025, 5, 857–870. [Google Scholar] [CrossRef]
- Elkrief, A.; Routy, B.; Derosa, L.; Bolte, L.; Wargo, J.A.; McQuade, J.L.; Zitvogel, L. Gut Microbiota in Immuno-Oncology: A Practical Guide for Medical Oncologists with a Focus on Antibiotics Stewardship. Am. Soc. Clin. Oncol. Educ. Book 2025, 45, e472902. [Google Scholar] [CrossRef] [PubMed]
- Almonte, A.A.; Thomas, S.; Zitvogel, L. Microbiota-centered interventions to boost immune checkpoint blockade therapies. J. Exp. Med. 2025, 222, e20250378. [Google Scholar] [CrossRef] [PubMed]
- Dean, N.J.; d’Arienzo, P.D.; Ibraheim, H.; Lee, K.A.; Olsson-Brown, A.C.; Pinato, D.J.; Powell, N. The role of the gut microbiome in regulating the response to immune checkpoint inhibitor therapy. Best. Pract. Res. Clin. Gastroenterol. 2024, 72, 101944. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. The Effect of the Microbiome on Immune Checkpoint Inhibitor Response in Melanoma Patients. Identifier NCT05102773. Available online: https://clinicaltrials.gov/ct2/show/NCT05102773 (accessed on 30 May 2025).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
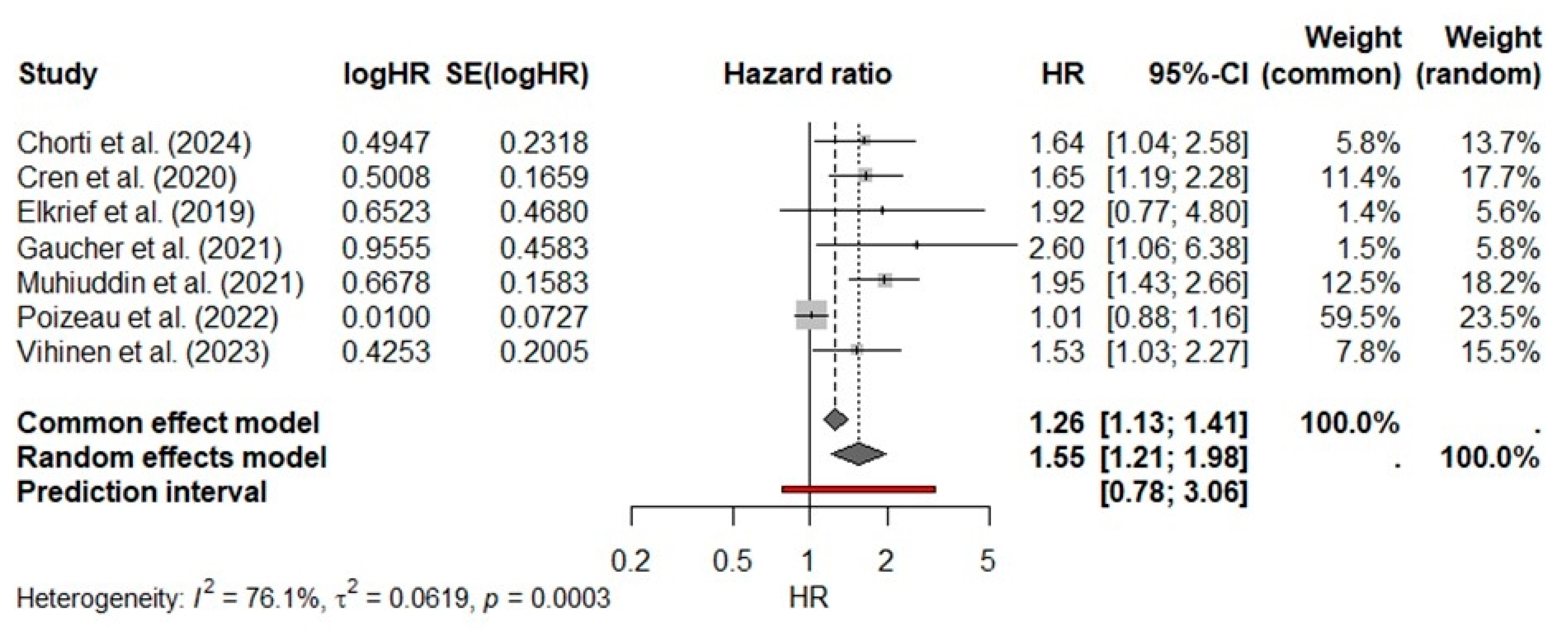

| Study | Year | Location | Study Design | Pabt No/Yes | ICI Type | ATB Prior ICI (Days) | HR for OS 95% CI |
|---|---|---|---|---|---|---|---|
| Chorti [21] | 2024 | Germany | observational, multi-center | 428/41 | PD1 PD1+CTLA-4 | 60 | 1.64 1.04–2.58 |
| Cren [22] | 2020 | France | observational, large database | 1514/71 | CTLA-4 | 60 | 1.65 1.19–2.28 |
| Elkrief [23] | 2019 | Canada | observational, oligo-center | 64/10 | PD1 PD1+CTLA-4 | 30 | 1.92 0.76–4.76 |
| Gaucher [24] | 2021 | France | observational, single-center | 94/16 | PD1 PD1+CTLA-4 CTLA-4 | 30–60 | 2.60 1.06–6.39 |
| Mohiuddin [25] | 2021 | USA | observational, large database | 454/114 | PD1 PD1+CTLA-4 CTLA-4 | 90 | 1.95 1.43–2.66 |
| Poizeau [26] | 2022 | France | observational, large database | 1856/749 | PD1 | 90 | 1.01 0.88–1.17 |
| Vihinen [27] | 2023 | Finland | observational, multi-center | 151/71 | PD1 | 30–90 | 1.53 1.03–2.26 |
| Study | Selection | Comparability | Outcome | |||||
|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | C | O1 | O2 | O3 | |
| Chorti et al. [21] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | |
| Cren et al. [22] | ★ | ★ | ★ | ★★ | ★ | ★ | ||
| Elkrief et al. [23] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ |
| Gaucher et al. [24] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ |
| Mohiuddin et al. [25] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ |
| Poizeau et al. [26] | ★ | ★ | ★ | ★★ | ★ | ★ | ||
| Vihinen et al. [7] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gambichler, T.; Weyer-Fahlbusch, S.S.; Overbeck, J.; Abu Rached, N.; Becker, J.C.; Susok, L. Impaired Overall Survival of Melanoma Patients Due to Antibiotic Use Prior to Immune Checkpoint Inhibitor Therapy: Systematic Review and Meta-Analysis. Cancers 2025, 17, 1872. https://doi.org/10.3390/cancers17111872
Gambichler T, Weyer-Fahlbusch SS, Overbeck J, Abu Rached N, Becker JC, Susok L. Impaired Overall Survival of Melanoma Patients Due to Antibiotic Use Prior to Immune Checkpoint Inhibitor Therapy: Systematic Review and Meta-Analysis. Cancers. 2025; 17(11):1872. https://doi.org/10.3390/cancers17111872
Chicago/Turabian StyleGambichler, Thilo, Sera S. Weyer-Fahlbusch, Jan Overbeck, Nessr Abu Rached, Jürgen C. Becker, and Laura Susok. 2025. "Impaired Overall Survival of Melanoma Patients Due to Antibiotic Use Prior to Immune Checkpoint Inhibitor Therapy: Systematic Review and Meta-Analysis" Cancers 17, no. 11: 1872. https://doi.org/10.3390/cancers17111872
APA StyleGambichler, T., Weyer-Fahlbusch, S. S., Overbeck, J., Abu Rached, N., Becker, J. C., & Susok, L. (2025). Impaired Overall Survival of Melanoma Patients Due to Antibiotic Use Prior to Immune Checkpoint Inhibitor Therapy: Systematic Review and Meta-Analysis. Cancers, 17(11), 1872. https://doi.org/10.3390/cancers17111872









