Simple Summary
Detecting cancer early can greatly improve a person’s chances of successful treatment. However, current screening methods have several drawbacks as they can be expensive, hard to access, and usually only check for one type of cancer at a time. Additionally, some of the deadliest cancers, such as pancreatic and ovarian cancer, still lack methods to identify them early. New non-invasive technologies, such as blood tests (liquid biopsies) and tests that analyze body scents based on volatile organic compounds (VOC), are showing promise. This review explores how these new methods, particularly those involving animal-assisted VOC detection and artificial intelligence, can be integrated into a more accessible and effective way to screen for several cancers at once. While challenges remain, ongoing research continues to move us closer toward a reliable system for early detection of many types of cancers.
Abstract
Early detection of cancer is crucial for improving patient outcomes. Traditional modalities such as mammography and low-dose computed tomography are effective but exhibit inherent limitations, including radiation exposure and accessibility challenges. This review explores innovative, non-invasive cancer screening methods, focusing on liquid biopsy and volatile organic compound (VOC)-based detection platforms. Liquid biopsy analyzes circulating tumor DNA and other biomarkers in bodily fluids, offering potential for early detection and monitoring of treatment response. VOC-based detection leverages unique metabolic signatures emitted by cancer cells, detectable in exhaled breath or other bodily emissions, providing a rapid and patient-friendly screening option. We provide a comprehensive overview of these advanced multi-cancer detection techniques to enhance diagnostic accuracy, accessibility, and patient adherence, and ultimately enhance survival rates and patient outcomes.
1. Introduction
Cancer is a leading cause of morbidity and mortality around the world. In 2022, an estimated 20 million new cancer cases were identified, and 9.7 million cancer deaths occurred [1]. Early cancer-detection schemes play a crucial role in identifying cancer at its initial, potentially treatable stages before it spreads locally or to distant organs. These schemes have proven highly effective in reducing mortality rates across numerous cancer types [2]. Notable examples include a reduction in breast cancer (BC) and lung cancer (LC) mortality rates. LC was the most frequently diagnosed cancer worldwide, with over 2.4 million newly diagnosed cases in 2022. LC was also the primary cause of cancer-related deaths (over 1.8 million) in the same year [1]. BC is the leading cancer diagnosis and cause of cancer-related mortality in females. Globally, 2.3 million new cases and 670,000 deaths from female BC occurred in 2022, with geographic variation in age-standardized incidence rate and age-standardized mortality rate [1]. Recommended surveillance schemes for BC and LC include annual mammography for all women aged 45–74 years [3] and low-dose computed tomography (LDCT) for high-risk individuals (i.e., heavy smokers or those exposed to asbestos) from age 50 years [4], respectively. These surveillance schemes have been adopted by national professional bodies (e.g., the National Comprehensive Cancer Network [NCCN], https://www.nccn.org/guidelines/category_2, accessed on 12 March 2025), and the UK National Screening Committee ([UKNSC], https://www.gov.uk/government/organisations/uk-national-screening-committee accessed on 12 March 2025), and are routinely revisited by independent bodies such as the US Preventive Services Task Force (USPSTF-https://www.uspreventiveservicestaskforce.org/uspstf/ accessed on 12 March 2025). Yet, despite their proven mortality-lowering effect, these surveillance schemes have drawbacks and caveats, including radiation exposure, false-positive results, incidental findings, accessibility issues, and low compliance due to distrust in the healthcare system, augmented by the COVID-19 pandemic and disinformation campaigns. These and other challenges may underlie the low rate of acceptance to LC screening in the USA, estimated at 18.1% of eligible individuals in 2022, with the lowest rates (6.7%) noted in the 50–54 age group [5]. Moreover, according to the American Lung Association, only 5.8% of high-risk individuals were screened for LC [6]. The adherence rates to the recommended early-detection scheme for BC in the USA in 2021 (mammography every 2 years for average-risk women aged 50–74 years) have been higher at 75.9% [7]. However, these rates do not reflect the new USPSTF recommendation to lower the age of mammography screening to age 40 [8], which coincides with a recent report showing an increase in BC incidence rates among young American women [9]. Interestingly, between 2015 and 2019, LC incidence rates remained higher among women than men in the 35–54 age group [10].
Unlike the proven efficacy of early-detection schemes for BC and LC, there are cancer types for which no effective surveillance is currently available. Notable examples include pancreatic ductal adenocarcinoma (PDAC) and ovarian cancer (OvC). These two cancers are relatively rare. PDAC accounts for almost 90% of all diagnosed pancreatic tumors with global variations across age, race, and ethnicity [11]. In 2022, there were 510,992 new cases of PDAC diagnosed globally, with 60,127 new cases in the USA, with an age-standardized rate (ASR) of 4.7/100,000 and 8.6/100,000, respectively [12]. OvC ranked as the 10th-most common cancer among women in 2022, with 324,603 new cases. However, it is the fifth-leading cause of cancer-related deaths in females; 206,956 deaths from OvC occurred in 2022 [1,13], Geographic and ethnic variations across age-standardized incidence rates and histological subtypes were observed for ovC [14]. In addition to their relative rarity, the vague and non-specific nature of early-stage cancer symptoms, and the anatomical location of both organs, have all contributed to the lack of any clinically proven effective early-detection schemes for these cancer types, despite the ongoing search for serum biomarkers, targeted imaging modalities, and other means that have been tested but failed to show any clinically relevant effect. Early detection of these cancers has a transformative potential to improve survival outcomes. Survival rates in early-stage PDAC are significantly higher than those diagnosed at advanced stages. Localized pancreatic cancer has a 5-year relative survival rate of 44%, compared to 17% for regional disease and 3% for metastatic disease [15]. Stage I OvC (i.e., the disease is confined to the ovaries) has a 5-year survival rate of 89%, and stage II OvC (i.e., the disease is confined to the pelvis) is associated with a 5-year survival of 70%. However, stage III and IV disease have a 5-year survival rate of 40% and 20% or less, respectively [16].
Over the past 2 decades, there has been renewed interest in early detection of cancer as part of the preventive personalized approach to healthcare (e.g., [17]). These advancements include various liquid biopsy (LB) technologies [18,19,20], the development of novel multiomics biomarkers [21], technologies for detecting volatile organic compounds (VOCs) [22], and other biomarkers including biomineral deposits, such as psammoma bodies and microcalcifications [23,24,25,26]. In breast cancer, mammography-detected microcalcifications remain one of the earliest signs of malignancy [24]. Similarly, fine punctate calcifications on thyroid ultrasound strongly correlate with papillary thyroid carcinoma, showing high specificity for malignancy [25]. Psammoma bodies in cytologic or histologic specimens are characteristic of papillary tumors (e.g., ovarian serous and thyroid carcinoma) and may aid in early diagnosis [26]. In lung nodules, calcifications usually indicate benign lesions, though certain patterns can signify early malignancy [23]. These mineral biomarkers improve diagnostic sensitivity and could augment early-detection strategies.
Together, these innovations, which are illustrated in Figure 1 and are further described below, aim to enhance multi-cancer early detection (MCED) by improving existing diagnostic methods and expanding early detection opportunities to cancers that currently lack effective screening strategies, ultimately aiming to reduce overall and cancer-specific mortality.
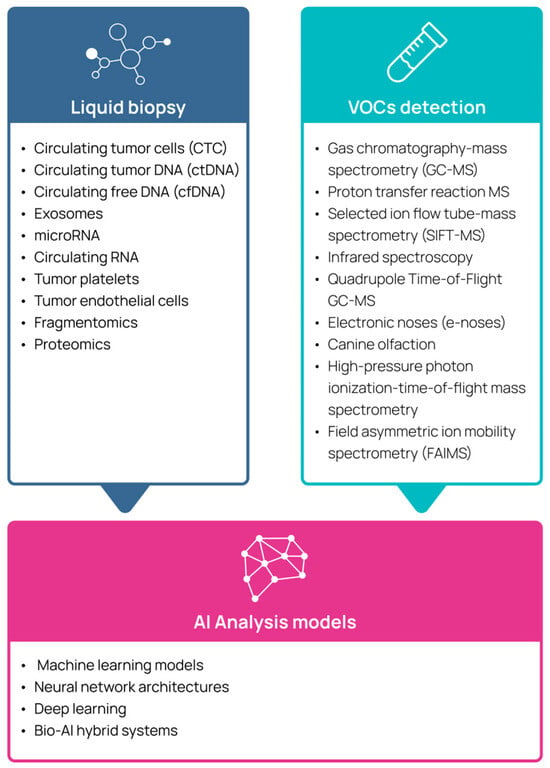
Figure 1.
Emerging technologies used for multi-cancer detection.
2. Liquid Biopsy
LB refers to the sampling and analysis of tumor-derived material from body fluids, offering a non-invasive alternative to surgical tissue biopsies. In practice, a LB usually involves a simple blood draw, from which various circulating biomarkers can be isolated and studied. LBs encompass several components: circulating tumor cells (CTC), circulating tumor DNA (ctDNA), circulating free DNA (cfDNA), exosomes, microRNA, circulating RNA, tumor platelets, and tumor endothelial cells [27,28,29]. Since somatic, genetic, and epigenetic alterations, and their protein products, are the hallmark of cancer tissue, and the rate of cellular turnover in cancerous lesions is rapid, levels of cfDNA and their genetic/epigenetic composition reflect the somatic tumor landscape. A variety of assays utilizing cfDNA analysis have been reported as tools in early cancer detection, predominantly including either cfDNA multigene panel mutation analysis or cancer-specific methylation patterns [30]. LB offers several advantages over early-detection techniques currently in use: it is less invasive, offers faster turnaround time, may detect more than one tumor type in a single assay, and can capture tumor heterogeneity. Clinical studies reviewed over the past 2 years (e.g., [20]) have focused on evaluating the effectiveness of various LB techniques in detecting cancer at an early stage. For example, the PATHFINDER study, which evaluated a methylation-based MCED assay as a potential screening tool in individuals aged 50 years or older with no signs or symptoms of cancer, reported a 99.1% specificity. However, 57 out of 92 individuals who received a positive cancer signal were later determined to be false positives after a comprehensive diagnostic workup [31]. A separate study evaluating the same MCED test in 5461 asymptomatic individuals detected a cancer signal in 323 participants, with 244 ultimately diagnosed with cancer after a comprehensive workup. The test demonstrated an overall specificity of 98.4% and a sensitivity of 66.3%, which was lower for early-stage disease (24.2%) compared to later-stage disease (95.3%) [32].
LBs have gained substantial attention in cancers for which effective early-detection methods are currently unavailable, such as PDAC and OvC. For example, Cohen et al. [33] reported a 30% sensitivity in detecting PDAC among 221 cases compared to 182 controls using KRAS detection as a single biomarker. The sensitivity increased to 64% with a specificity of 99.5% when KRAS mutation detection was combined with four protein biomarkers (CA 19-9, carcinoembryonic antigen, hepatocyte growth factor, and osteopontin). Reese et al. [34] emphasized the need for a multi-biomarker approach for a more effective early detection of PDAC compared to detection with single biomarkers. A meta-analysis of 14 studies examining multigene-based LB techniques, encompassing 369 patients with PDAC (57% stage I-II), reported an overall pooled sensitivity and specificity of 70% and 86%, respectively. Interestingly, the concordance rate of detected mutations was 31.9% [35]. A LB analysis of CA19-9, ctDNA, and CTCs in 68 patients with pancreatic tumors (58 PDAC, 10 benign) showed sensitivity and specificity of 78% and 91%, respectively [36]. Most recently, Montoya–Mira et al. [37] described a novel assay that detects cancer-associated serum protease activity in plasma. When combined with the clinical biomarker CA 19-9, the assay achieved sensitivity and specificity of 85% and 96%, respectively, in detecting stage I PDAC, albeit with a limited sample size.
The use of LB for early detection and management of OvC has been the focus of multiple studies over the past 2 decades, and several reviews on the efficacy and the clinical use of different techniques and methodologies have been published (e.g., [38,39]). A review of studies published between 2009 and 2020 examined the use of various LB assays for the early detection of OvC. These studies encompassed 21–216 OvC cases across all disease stages, with reported sensitivities ranging from 23–98% and specificities between 56–100% [40]. Asante et al. [41] reviewed studies from 2012 to 2019 that evaluated the ability to detect high-grade serous OvC early. These studies, which analyzed 3–97 cases, employed mutation analyses of either a single gene (TP53) or up to 500 genes, with sensitivities ranging from 27–97% and specificities between 60–100%. More recently, Kuo et al. [42] reported that by setting a threshold of 4.75 flow cytometry-enriched CTC/mL, they could distinguish between benign (n = 9) and malignant (n = 26) ovarian lesions. The Canadian consortium CHARM is now evaluating a variety of LB technologies as an additional tool for surveillance in genetically predisposed individuals [43].
Despite the clear potential of LB as a clinical tool for early cancer detection, several challenges must be addressed before it can be widely incorporated into clinical practice. Key issues include standardization of methodologies, large-scale longitudinal validation studies, cost-effectiveness analyses, and the integration of artificial intelligence (AI) and novel imaging modalities [18,19].
3. Volatile Organic Compounds
3.1. The Scientific Basis for the Use of Volatile Organic Compounds in Cancer Detection
VOCs are a diverse group of carbon-based molecules that are metabolic byproducts of cellular processes. They are released into the circulatory system and can be detected in various body fluids, as well as exhaled air. More than 2000 VOCs have been identified in different body fluids [44]. Since cancer cells exhibit altered metabolism compared to non-cancerous tissue, distinct VOC profiles associated with specific cancers could serve as potential biomarkers for cancer detection [45,46].
Research on cancer-related VOCs has involved collecting samples from various biological matrices, including body fluids and excretions such as blood, urine, saliva, sweat, and feces, as well as exhaled air [45,47,48,49,50,51,52]. Several studies have reported the feasibility and effectiveness of VOC analysis in detecting multiple cancer types, including BC, colon cancer, prostate cancer, gastric cancer, and melanoma [45].
VOCs can be detected using analytical instruments such as gas chromatography coupled with mass spectrometry (GC-MS), proton transfer reaction mass spectrometry, selected ion flow tube-mass spectrometry (SIFT-MS), infrared spectroscopy, or Quadrupole Time-of-Flight GC-MS (GC-MS QTOF) [53,54]. Yet, the clinical use of GS-MS and its associated technologies is expensive, time-consuming, and requires highly trained and skilled personnel, making it labor intensive for routine medical practice. As a result, its widespread clinical application is unlikely, remaining largely confined to highly specialized laboratory environments [54].
3.2. Use of Electronic Noses for Volatile Organic Compound Detection
Electronic noses (e-noses), which mimic the olfactory system, are sensor arrays designed to detect and differentiate between various odor compounds, offering a cheap, fast, portable, and user-friendly approach for VOC analyses. Research on the use of e-noses in oncology has increased significantly and has shown promise in identifying specific VOC patterns associated with LC [22,55,56]. Use of machine-based VOC detection, such as e-noses, has some inherent limitations, such as low sensitivity and/or specificity, as well as poor reproducibility [57]. In a systematic review of the performance of e-noses used for cancer detection among 3677 individuals in 52 studies, the sensitivity of e-noses ranged from 48.3% to 95.8%, and the specificity ranged from 10% to 100% [58]. VOC profiles are also affected by factors unrelated to cancer, such as genetics, sex, diet, age, and the environment. Moreover, there is a lack of universal thresholds for “a cancerous VOC profile”, and hitherto, a scarcity of real-world clinical data supporting the clinical utility of e-noses.
3.3. Use of Canines for Volatile Organic Compound Detection
An alternative method for detecting VOCs with artificial means is the use of canines [59]. Canines have up to 220–300 million olfactory receptors in their noses, compared to about 5–6 million in humans [60,61], and can detect odors at concentrations of parts per trillion [62]. These features contribute to their exceptional scent detection capability. Furthermore, their olfactory bulb, which is devoted to analyzing smells, is proportionally 40 times larger than that of humans, allowing dogs to maintain a heightened focus on olfactory stimuli. The structure of the canine’s nasal cavity, including the folds in the mucous membrane, increases the surface area that captures scent molecules [63]. Combined with unique breathing patterns that allow for improved odor sampling, canines can detect even the faintest traces of VOCs. This biological advantage enables canines to differentiate and remember a wide array of smells, which is crucial for tasks involving VOC detection [64].
While the detection capacity of trained canines is impressive, inherent challenges and limitations have been associated with this method for clinical use, including variability in training-related performance, handler expertise, specific dog species, and animal ethics [65].
3.4. Use of Volatile Organic Compound Analysis for Detection of Cancer
VOC profiling offers a promising, non-invasive avenue for detecting metabolic alterations associated with malignancy. Between 2002 and 2022, VOC analyses for cancer detection were examined in 301 clinical trials. The most studied cancers included LC (n = 127), colorectal cancer (n = 27), head and neck cancer (n = 22), BC (n = 16), prostate cancer (n = 15), gastric cancer (n = 13), and bladder cancer (n = 10). Notably, only 38 studies included more than 100 cancer cases, the majority of which focused on LC [66]. Only a small number of studies reported analyzing urinary VOCs via GC-MS as biomarkers for diagnosing BC (5 studies, 26–65 cases, 21–70 controls) and LC (4 studies, 10–20 cases, 20–30 controls) [67].
The feasibility and accuracy of using VOC analysis for early detection of cancer, either by sensors (e-noses), GC modalities, or animals, has been reviewed by several groups. Goertzen et al. [68] reported a combined sensitivity and specificity above 150% across 44 studies primarily focused on cancer-related urine-based VOC analyses of prostate, lung, breast, and bladder cancer. Sensitivities ranging from 19–99% and specificities between 60–99% were reported for cancer detection by canines across multiple cancers, including lung, ovarian, prostate, colorectal, and bladder cancers, though these figures reflect limited sample sizes per cancer type [65]. In a comprehensive meta-analysis of studies evaluating cancer detection by VOC analysis of breath and/or urine, the overall specificity and sensitivity were 88% and 89%, respectively [69]. Hintzen et al. [70] reviewed VOC detection as a diagnostic tool for gastrointestinal cancers. Among 23 studies (14 on gastro-esophageal cancer and 2 on PDAC), sensitivity ranged from 66.7–100%, and specificity varied between 48.1% and 97.9%. Hanna et al. [71] reviewed 63 studies involving 3554 patients that examined breath-sampled VOCs for cancer detection, reporting a combined sensitivity of 79% (95% confidence interval [CI] 77–81%) and a specificity of 89% (95% CI 88–90%). A bio-AI hybrid system composed of trained canines and machine-learning models for detecting cancer-associated VOCs in breath samples demonstrated a combined sensitivity of 93.9% (95% CI 90.3–96.2%) and a specificity of 94.3% (95% CI 92.7–95.5%) in detecting BC, LC, colorectal, and prostate cancer, with an early-stage cancer (stage 0–2) sensitivity of 94.8%. Moreover, 81.8% sensitivity was noted for 14 additional cancer types that the canines were not trained to detect [72].
In summary, current evidence from reviews and meta-analyses indicates that VOC detection represents a promising non-invasive approach for complementing established cancer screening modalities. However, integration into clinical practice remains contingent upon addressing critical methodological limitations, including variability in sampling procedures, detection technologies, and validation strategies.
4. Using Artificial Intelligence in the Analysis of Volatile Organic Compounds and Liquid Biopsy
The increasing complexity and volume of biomedical data have positioned AI as a powerful means to extract clinically meaningful patterns, especially in contexts where the signal is subtle or noisy. AI-driven tools are particularly relevant for early cancer detection, where traditional diagnostic methods, such as imaging, biopsies, and molecular assays, often fall short due to invasiveness, limited sensitivity at early stages, or high costs [19,73,74].
Unlike conventional approaches that focus on a small set of predefined metabolites, AI enables analysis of the entire compositional “fingerprint” of VOCs, capturing subtle changes that may not be apparent through univariate or rule-based methods. Vinhas et al. [75] exemplified this emerging trend by using gas chromatography-field asymmetric Ion mobility spectrometry (GC-FAIMS) to analyze breath samples from LC patients and healthy individuals. The study involved training several neural network architectures, including GoogLeNet and VGG-11, on breath sample spectrograms. The models demonstrated strong classification performance, with precision and recall metrics indicating the potential of breathomics as a viable tool for early LC detection, particularly when augmented by AI. Other reviews [76,77] further underscore AI’s critical role in advancing breath-based diagnostics across a range of cancers, highlighting the advantages of machine learning over conventional statistical classifiers in handling the high-dimensional and often non-linear data that characterize VOC analyses.
In the same vein, AI has become indispensable in overcoming the limitations of interpreting LB data, particularly for early-stage cancers where biomarker abundance is low. For instance, Kim et al. [78] demonstrated that a deep-learning model applied to cfDNA methylation and fragment-size profiles can distinguish between patients with LC and healthy individuals with an accuracy of 81.5% and an area under the receiver-operating characteristic curve of 0.87. Yan et al. [79] showed that circulating tumor cell-derived RNA (ctcRNA)-based tissue deconvolution using a deep-learning approach can identify the tissue of origin of metastatic tumors.
Beyond single-analyte models, AI is increasingly used to integrate multiple biomarker types into unified diagnostic frameworks [80]. Medina et al. [81] utilized machine learning to analyze whole-genome cfDNA fragmentome, CA-125, and human epididymis protein 4, detecting OvC with specificity exceeding >99% and sensitivities of 72%, 69%, 87%, and 100% for stages I to IV, respectively. Such integrative approaches exemplify the strengths of AI in navigating multimodal data, especially in a clinical landscape moving toward personalized, biomarker-driven medicine.
5. Schemes and Methods for Early Detection of Specific Cancers
5.1. Breast Cancer
5.1.1. Surveillance Schemes for Early Detection of Breast Cancer
The currently widely adopted screening scheme for early detection of BC in women at an average risk includes annual breast imaging, primarily by mammography, at ages 45–54 years. Starting at age 55 years, women can opt to either continue with annual mammograms or switch to a 2-year screening scheme [82,83]. This routine should be maintained for as long as the screened individual is in good health and expected to live at least 10 years. Recently, the USPSTF recommended that every average-risk woman aged 40–74 should obtain a mammogram every 2 years [8]. Notably, clinical breast exams, whether self-exams or physician-performed, have no clinically proven benefit in BC detection. For high-risk women, including those with BRCA1 or BRCA2 germline pathogenic variants, a history of breast irradiation during adolescence, or a 20% or higher lifetime risk for developing BC—determined by risk assessment algorithms such as BRCAPRO (https://projects.iq.harvard.edu/bayesmendel/brcapro accessed on 13 March 2025) or CanRisk (https://www.canrisk.org/ accessed on 13 March 2025)—it is recommended to begin BC screening at around age 30. The preferred imaging approach is magnetic resonance imaging (MRI), alternating with mammograms every 6 months [84].
The adoption of this screening methodology has been shown to positively impact early BC detection, improving survival rates and reducing the need for adjuvant chemotherapy [85]. However, this screening scheme is not without limitations. Challenges include overdiagnosis of slow-growing, non-invasive tumors (e.g., ductal carcinoma in situ), which may lead to overtreatment with surgery and irradiation; false-positive test results resulting in unnecessary biopsies and patient anxiety; and false-negative results leading to missed BC cases, particularly in young women and those with dense breasts. Additional concerns involve limited accessibility and fear of radiation exposure from mammography. To address these shortcomings, there is a need to enhance BC screening by incorporating additional methodologies. Noninvasive biomarkers, such as circulating cell-free tumor nucleic acids or microRNA, VOC detection in breath or urine, have been investigated as potential supplements to early-BC detection schemes [84].
5.1.2. Liquid Biopsy Analysis for Breast Cancer Detection
Biomarker detection via LB has emerged as an effective approach for diagnosing BC, with peripheral blood microRNA analysis being the most extensively studied method [86]. However, small, early-stage tumors may go undetected using current LB schemes. For example, a multi-cancer cfDNA methylation assay (Galleri) detected only ~30% of new BC cases (only 2.6% of stage I cases) at ~99.5% specificity [87]. Similarly, another blood test (CancerSEEK) identified only one-third of early BC cases compared to 98% of OvC cases [88]. These results may be attributed to the low rate of tumor DNA shedding in early-stage BC. These caveats are addressed by combining multiple biomarker types (ctDNA, circulating proteins, CTCs), as well as by pairing LB with imaging to increase detection [89]. Large-scale trials, such as STRIVE [90] and PATHFINDER [31], are underway to refine this approach.
5.1.3. VOC Analysis for Breast Cancer Detection
Several studies have examined VOC determination as a tool for early BC detection. Giró Benet et al. [91] reported 100% sensitivity but lower specificity (50–85.7%) in detecting BC in urine VOCs by an e-nose prototype (n = 90). A GC-based VOC analysis of breath samples from 54 women with BC and 124 cancer-free controls was associated with high negative and positive predictive values of 99.1% and 100%, respectively [92]. Using a high-throughput breathomics analysis by high-pressure photon ionization-time-of-flight mass spectrometry (HPPI-TOF-MS), Liu et al. [93] identified 10 optimal VOC markers to distinguish the breath samples of women with BC from those of women without cancer in a cohort of 5047 Chinese women undergoing BC screening. A diagnostic model (BreathBC-Plus) consisting of the 10 VOC markers and BC risk factors achieved detection rates of 96.97% for ductal carcinoma in situ, and 85.06%, 90.00%, 88.24%, and 100% for stages I, II, III, and IV BC, respectively. Phillips et al. [94] reported an accuracy of 83%, sensitivity of 82%, and specificity of 77.1% for BC detection in alveolar breath samples of 593 symptomatic women using a rapid point-of-care VOC detection method employing GC and surface acoustic wave detection. In another study, which examined a multi-omics approach combining metabolites in exhaled breath, ultrasound imaging, and basic clinical information, the breath samples of 1723 individuals were collected and analyzed using an automated breast volume scanner and HPPI-TOF-MS. This approach differentiated between benign (n = 168) and malignant (n = 82) breast masses, demonstrating sensitivity, specificity, and accuracy of 84.1% (95% CI 75.1–93.2%), 89.9% (95% CI 85.3–94.4%), and 88.0% (95% CI 84.0–92.0%), respectively [95].
A review of 32 studies examining various VOC detection methods using both in vivo and in vitro matrices for BC detection highlighted the potential of VOC analysis as a promising screening tool. However, the authors concluded that the findings are inconsistent, primarily due to methodological discrepancies [96]. Li et al. [84] also reviewed studies investigating noninvasive biomarkers, including VOCs, for BC detection. The studies, encompassing 10–54 BC cases and 10–543 controls, reported sensitivity rates ranging from 80–100% and specificity rates ranging from 40–77.1%.
5.2. Lung Cancer
5.2.1. Surveillance Schemes for Early Detection of Lung Cancer
The American Cancer Society recommends annual LC screening using LDCT for individuals aged 50–80 years who are current or former smokers who have at least a 20 pack-year smoking history [97]. This recommendation is an adoption of the USPSTF statement issued in 2021 [98]. This recommended surveillance scheme is based on studies that have demonstrated a 20% decrease in LC mortality [99,100,101,102] and a 6.7% decrease in all-cause mortality [99], as well as several meta-analyses confirming the benefits of this screening scheme for reducing both disease-specific and all-cause mortality [103,104]. Yet, despite its proven contribution to reducing mortality rates, the adoption rate among individuals who may benefit the most from this screening scheme is low. According to the United Kingdom Lung Cancer Screen (UKLS) trial, the response rate to a lung screening invitation among those at the highest risk for LC was 6.1% [105]. Common reasons for not attending LDCT screening include difficulty accessing services, cancer fear, fatalism, negative psychological impact of screening, and stigma associated with LC and smoking [106,107,108,109]. In addition, several controversial and unresolved issues related to LDCT should be addressed to potentially increase participation rates among eligible individuals. These include false positive rates, over-diagnosis, costs, disparities among minority populations, radiation exposure, and incidental findings [110].
Thus, despite its proven effectiveness in reducing both disease-specific and overall mortality rates, LDCT remains underutilized among high-risk individuals. This underuse has driven the continued search for alternative methods to improve early LC detection.
5.2.2. Liquid Biopsy Analysis for Lung Cancer Detection
Although lung tumors shed less DNA in early stages, ultrasensitive assays can detect tumor signals in many stage I patients [111]. For example, the multi-analyte blood test CancerSEEK demonstrated ~60% sensitivity for LC while maintaining ~99% specificity [88]. A cfDNA methylation assay from the Circulating Cell-Free Genome Atlas (CCGA) study achieved approximately 70% detection of LC across stages [112]. Although early detection of LC by LDCT has ~94% sensitivity [100], blood-based testing could complement imaging by using a two-step strategy: a reliable, valid LB could be used as an initial screening tool in high-risk smokers, identifying those who display an initial LC signal requiring a rapid and in-depth clinical analysis while reducing LDCT-based false positives [111]. Such a strategy may increase the overall detection rate and improve screening compliance. Ongoing studies, such as SUMMIT [113] and PATHFINDER [114], are currently evaluating the use of blood tests in real-world high-risk LC cohorts.
5.2.3. VOC Analysis for Early Detection of Lung Cancer
Tsou et al. [115] used SIFT-MS to quantitatively analyze 116 VOCs in breath samples from 148 patients with histologically confirmed LC and 168 healthy volunteers. Using eXtreme Gradient Boosting (XGBoost), a machine-learning method, and adjusting for confounders such as environmental VOCs, they constructed a LC prediction model with an accuracy, sensitivity, and specificity of 92%, 96%, and 88%, respectively. Gasparri et al. [116] analyzed VOCs in exhaled breath using a gas sensor array to generate a “breathprint” of 70 individuals with LC and 76 non-cancer controls. They found that breathprints of individuals with LC were distinct from those of healthy individuals, with a sensitivity of 81% and specificity of 91%. The highest sensitivity was observed in individuals with stage I LC, highlighting the potential of VOC analysis for early detection. Kort et al. [117] explored non-invasive LC detection using an e-nose-based VOC detection integrated into a prediction model incorporating clinical parameters. Their study included 376 cases in the training set and 199 cases in the validation set. The prediction model showed a sensitivity of 95%, a specificity of 49%, and a negative predictive value (NPV) of 94% in the validation set.
Several reviews have reported on the use of various e-nose methods for LC detection [118,119,120,121] or for differentiating between benign pulmonary nodules and LC [122]. Across all studies, exhaled VOCs successfully distinguished between individuals with LC and controls. A meta-analysis, which examined VOCs in exhaled breath as biomarkers for LC screening, found an integrated sensitivity and specificity of 85% and 86%, respectively [123].
A few studies have explored the ability of canines to detect VOCs as a means for distinguishing LC from non-cancerous conditions. Buszewski et al. [124] found that trained dogs could differentiate between breath samples of individuals diagnosed with LC (n = 29, including 18 with small cell lung cancer and 11 with non-small cell lung cancer) from non-cancer controls (n = 44), achieving sensitivity and specificity of 82.2% and 82.4%, respectively. False positive rates in healthy controls were 17.8%. A positive correlation was observed between dog indications and the presence of ethyl acetate and 2-pentanone in exhaled breath (r = 0.85 and r = 0.97, respectively). McCulloch et al. [125] found that five household dogs demonstrated 99% specificity and sensitivity in distinguishing LC cases (n = 55) from non-cancer controls (n = 83), regardless of disease stage. Biehl et al. [126] further highlighted that factors such as dog training level, breed, and the VOC collection system significantly impact the accuracy of canine-based VOC detection of LC.
5.3. Pancreatic Cancer
PDAC remains an intractable condition, ranking among the leading causes of global cancer-related deaths [127]. Five-year survival rates range from only 3% to 15% when the disease extends beyond the pancreas [128,129]. This poor prognosis is primarily due to late-stage diagnosis, often when the disease is already incurable, as well as high tumor chemoresistance [130].
Currently, no effective early-detection schemes for PDAC have been proven to reduce mortality rates or improve survival in individuals at average risk [131]. Due to the rarity of PDAC and its nonspecific symptoms in early stages of the disease, it is generally recommended not to offer routine screening for individuals at average risk [131]. Some form of screening may be considered annually from age 50 for individuals who are at high risk for developing pancreatic cancer (e.g., those carrying inherited pathogenic variants in pancreatic cancer susceptibility genes—BRCA2, PALB2, CDKN2A; individuals with two or more first and/or second degree relatives from the same parental lineage diagnosed with PDAC; individuals with a history of pancreatitis, those with mucinous pancreatic cysts; and elderly patients with new-onset diabetes). For these high-risk groups, annual screening using magnetic resonance cholangiopancreatography combined with endoscopic ultrasound has been suggested. However, the clinical impact of such a screening is not yet well established, and further research is needed to determine its effectiveness [132,133]. Notably, BRCA1 and BRCA2 germline pathogenic variant carriers have a relatively low lifetime risk of PDAC, estimated at less than 5% [134].
5.3.1. Liquid Biopsy for Pancreatic Cancer Detection
Since ctDNA is scarce in early-stage PDAC, LB aims to identify extracellular vesicles and microRNAs shed by pancreatic tumors [135,136,137]. Nakamura et al. [136] identified a panel of 15 exosomal microRNAs that, when combined with circulating free microRNAs, detected early-stage pancreatic cancer with an unprecedented 94% sensitivity and 97% specificity. The detection accuracy was further increased to ~97% for stage I–II disease when the classic CA19-9 protein marker was added in an independent cohort. While encouraging, these results need to be further independently validated. Other LB-based approaches, such as mutation-based ctDNA panels and methylation assays, are also being explored [138].
5.3.2. VOC Analysis for Pancreatic Cancer Detection
Martínez–Moral et al. [139] identified 433 VOCs in serum samples by applying a GC-based technique, with 40 VOCs showing statistically significant differences between PDAC cases (n = 20) and controls (n = 19). Of these, 11 VOCs were independently validated, and 4 (toluene, 2-ethyl-1-hexanol, pentylbenzene, and butoxymethylbenzene) were identified as potential biomarkers. Daulton et al. [140] used two GC techniques to identify VOCs in urine samples in PDAC screening. This method effectively distinguished PDAC cases (n = 55) from healthy controls (n = 33) but failed to differentiate PDAC cases from those diagnosed with chronic pancreatitis (n = 45). Nissinen et al. [141] demonstrated 79% sensitivity and specificity in distinguishing PDAC cases (n = 68) from non-pancreatic cancer conditions (n = 62) and healthy controls (n = 52) using FAIMS for detecting urinary VOCs. Markar et al. [142] reported that VOC detection in breath samples by GC-MS identified 12 VOCs that could distinguish between PDAC cases (n = 57) and non-cancer cases (n = 75), with a sensitivity of 81% and a specificity of 58%. Princivalle et al. [143] reported that 10 VOCs detected in exhaled breath by an MS-based technique could distinguish between PDAC cases (n = 65) and non-cancer controls (n = 102) with a sensitivity and specificity of 100% and 84%, respectively. These results were subsequently validated in a smaller case-control cohort. Arasaradnam et al. [144] reported that VOC detection in urine samples by ion mobility spectrometry achieved 91% sensitivity (95% CI 0.83–0.96%) and 83% specificity (95% CI 0.73–0.90%) in detecting PDAC cases (n = 81) compared to controls (n = 81). Moreover, when comparing early-stage disease (stages I/II) to advanced-stage disease (stage III/IV), this approach showed a sensitivity of 82% (95% CI 0.67–0.92%) and a specificity of 89% (95% CI 0.75–0.97%).
5.4. Ovarian Cancer
5.4.1. Ovarian Cancer Screening
OvC is a relatively rare cancer type, ranking as the 10th-most-common cancer among women. However, it is the fifth-leading cause of cancer-related deaths in females [13]. This high mortality rate is largely due to late-stage diagnosis, with nearly two-thirds of OvC cases diagnosed after the disease has already spread within the peritoneal cavity (stage III) or distant organs (stage IV) [145]. Indeed, the contrast in survival outcomes is stark: while the 5-year survival rate for localized OvC exceeds 90%, it drops to a mere 30% for metastatic disease [145].
Efforts at developing effective early-detection strategies for reducing OvC mortality have been ongoing since the 1980s [146]. Most large-scale studies have focused on serological markers, particularly CA-125, combined with ultrasonographic imaging of the ovaries and pelvic organs. While these methods have demonstrated the ability to detect OvC earlier and downstage the disease, they have consistently failed to show a significant reduction in mortality among women at average risk [147,148,149]. Notably, other screening modalities, including various serum markers and metabolic products, were studied over the years (e.g., ONCOVERYX-F [150]; CA-125:CA72-4 ratio [151]; reviewed by Davenport et al. [152]). However, none of these approaches have been validated as effective early-detection biomarkers, nor have they been widely adopted for clinical use.
Due to the lack of proven effect on mortality reduction and the relative rarity of OvC, many national health authorities advise against routine OvC screening for average-risk women [153,154]. For high-risk women, particularly those harboring germline pathogenic variants in OvC-susceptibility genes (e.g., BRCA1, BRCA2, PALB2), current recommendations emphasize active risk reduction through bilateral salpingo-oophorectomy at ages 35–45, depending on the specific mutated gene and family history, and after completing childbearing [153]. While this risk-reducing surgery significantly reduces OvC risk, it is associated with significant morbidity and negative effects on non-oncological health outcomes [155].
5.4.2. Liquid Biopsy for Ovarian Cancer Detection
As no routine OvC screening is currently available for women with average and/or high risk, LB analyzing OvC-associated biomarkers, such as ctDNA, tumor-secreted microRNAs, and exosomal vesicles [38,41] may offer hope. One multi-omics approach combining cfDNA fragment patterns with protein markers (DELFI-Pro) outperformed traditional CA-125 testing. This test detected 72% of stage I and 87% of stage III OvC cases with >99% specificity, whereas CA-125 alone detected only 34% and 63% cases at these stages [81]. Importantly, an approximately 90% detection rate was noted for high-grade serous OvC, which is the most lethal subtype. Emerging strategies integrate AI algorithms and multi-marker panels to increase detection accuracy [156]. If confirmed in large independent cohorts, a cost-effective blood test for mass screening of OvC could become clinically available in the near future.
5.4.3. VOCs for Ovarian Cancer Detection
In a study utilizing GC–MS and nanoarray-based VOC detection in breath samples of 48 women with OvC, 86 women with benign gynecological masses, and 48 healthy women, decanal, nonanal, styrene, 2-butanone, and hexadecane were identified as potential VOC markers for OvC. The sensitivity, specificity, and accuracy of this model were 79%, 100%, and 89%, respectively [128]. Raspagliesi et al. [157] demonstrated that an e-nose-based VOC analysis in breath samples collected from 86 women with OvC, 51 women with benign pelvic masses, and 114 controls, distinguished OvC cases from benign cases and controls with a sensitivity of 89% and specificity of 86%. In a proof-of-concept study involving four OvC cases, Horvath et al. [158] demonstrated the ability of canines to accurately detect OvC VOCs with a sensitivity of 100% and specificity of 97.5%. In a subsequent study by the same group, two trained dogs detected presymptomatic clinical recurrence of OvC in 42 women treated with chemotherapy with 97% sensitivity and 99% specificity [159]. Similarly, Kybert et al. [160] showed that three different dog breeds could distinguish among plasma and/or tissue samples from women with OvC (n = 10), women with benign gynecological conditions (n = 10), and plasma of healthy controls (n = 10). Detection rates varied between 93.3 ± 20.1% and 98.7 ± 5.1% depending on the breed.
Despite these promising findings, common limitations affecting VOC-based early detection of OvC include the small sample sizes, the variability in detection technologies and dog breeds, the lack of standardization across studies, and the failure to differentiate between sporadic OvC cases and those with an inherited predisposition, limiting the applicability of findings to high-risk populations.
6. Sex-Related Differences in Multicancer Early Detection for Pancreatic and Lung Cancer
Studies evaluating LB and VOC analyses for MCED have indicated minimal sex-related differences in detection rates for pancreatic and LC. LB technologies, such as the CancerSEEK [88] and Galleri [161] assays, reported no significant sex-based disparities in detection sensitivity. However, proteomics-based MCED approaches have shown subtle sex-specific performance variations. Budnik et al. [162] developed separate male- and female-optimized protein classifiers, achieving 90% sensitivity in men versus 85% in women at 99% specificity. VOC-based detection methods, such as the SpotitEarly bio-AI hybrid system [72], demonstrated equivalent high sensitivities (>90%) across both sexes for LC detection. Overall, while MCED technologies generally detect pancreatic and lung cancers effectively in both men and women, the most refined approaches are beginning to incorporate minor sex-based adjustments to further optimize accuracy.
7. Future Directions
Although current early-cancer-detection schemes are effective, they have inherent limitations that restrict their widespread adoption by the public. These challenges include targeting only a single cancer type, requiring specialized equipment and trained personnel, necessitating access to healthcare facilities, and, in some cases, involving exposure to ionizing radiation, albeit at low levels. Additionally, some prominent cancer types, such as PDAC and OvC, lack effective early-detection schemes, contributing to poor survival rates. Over the past 2 decades, basic and applied research has focused on developing non-invasive, accessible, easy-to-use, point-of-care, or at-home screening technologies that are also cost-effective. The ultimate goal is to establish an MCED approach that aligns with these criteria. Major clinical trials and studies utilizing these technologies are summarized in Table 1. Notably, several challenges must be considered when translating these technologies into clinical practice. Key limitations include the need for independent validation of findings in diverse populations with varying cancer prevalence, assessment of cost-effectiveness compared to developing alternatives (e.g., AI-assisted analyses of LDCT for LC and breast MRI for BC), and evaluation of assay scalability, accessibility, and regulatory compliance. Longitudinal studies are also essential to determine population-level impacts.

Table 1.
Summary of studies evaluating VOC and liquid biopsy methods for cancer detection.
VOC analysis is a non-invasive approach showing high sensitivity and specificity, particularly in early-stage LC. It may improve screening uptake and reduce false positives from LDCT scans. In BC, VOCs demonstrate high sensitivity but variable specificity, making them promising as supplemental tools to mammography, especially for women with dense breast tissue or radiation concerns. VOCs can distinguish PDAC cases from healthy individuals with moderate-to-high sensitivity, but they struggle to differentiate from benign conditions like chronic pancreatitis. Breath- and canine-based VOC studies show high accuracy in detecting OvC in small cohorts, but clinical application is limited by a lack of standardization and small sample sizes. In contrast to VOC technologies, LBs are more established and increasingly tested in clinical trials, though early-stage sensitivity remains limited due to low biomarker shedding. In LC, LB complements imaging and may enhance early detection in high-risk groups. Early detection of breast tumors is challenging, but ongoing studies are exploring multi-marker and multi-omics strategies. In PDAC, combining exosomal microRNAs with markers like CA19-9 improves early-stage accuracy, though further validation is needed. For OvC, LB, especially multi-omics platforms, shows strong early-detection performance, particularly in high-grade serous cases, and could become a viable, non-invasive screening option if validated in larger studies.
An integrated multi-modal approach that combines diverse detection strategies holds significant potential for improving early cancer detection. One promising method is LB-based multi-omics analysis, which allows for simultaneous assessment of circulating genomic markers, proteomic signatures, and metabolomic profiles, together offering a comprehensive molecular fingerprint of cancer [163]. In fact, multi-analyte blood tests have already demonstrated the ability to detect multiple cancer types in asymptomatic individuals with high specificity [161,164]. VOC detection methods also show strong potential as a non-invasive complementary approach. Recent studies have reported high diagnostic accuracy using VOC analyses [58,164]. Increasingly, AI algorithms are being applied to these platforms, enabling the integration of complex multi-omics data and VOC signatures for improved pattern recognition and predictive power. Notably, animal-assisted detection, particularly using canine olfaction, underscores the potential of VOC-based diagnosis. Trained dogs have detected cancer via breath or urine samples with remarkable sensitivity, inspiring the development of AI-based bio-mimetic detection systems [72].
8. Conclusions
Overall, integrating multi-omics LB with VOC detection, augmented by advanced AI and even insights from canine olfaction, represents a promising frontier in non-invasive early cancer screening (Table 2). Such an integrated strategy offers the greatest potential for developing a standardized, clinically implementable system. Although we have not fully achieved this vision, we are undoubtedly progressing toward it.

Table 2.
Integrative strategy for enhanced cancer detection: combining canine VOC detection with liquid biopsy.
Author Contributions
Writing—original draft preparation, R.E.A., E.F.; writing—review and editing, R.E.A., E.F., S.M., and S.F.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
Shlomi Madar is a SpotitEarly Inc. employee and Reef Einoch Amor is a SpotitEarly Ltd. employee. Sharon Furman-Assaf received funding from SpotitEarly Ltd. Eitan Friedman serves as a paid consultant for SpotitEarly Ltd.
Abbreviations
The following abbreviations are used in this manuscript:
| AI | Artificial Intelligence |
| ASR | Age-Standardized Rate |
| BC | Breast Cancer |
| CfDNA | Circulating Free DNA |
| CTC | Circulating Tumor Cells |
| CtDNA | Circulating Tumor DNA |
| GC | Gas Chromatography |
| HPPI-TOF-MS | High-Pressure Photon Ionization-Time-of-Flight Mass Spectrometry |
| LB | Liquid Biopsy |
| LC | Lung Cancer |
| MCED | Multi-Cancer Early Detection |
| MS | Mass Spectrometry |
| NCCN | National Comprehensive Cancer Network |
| OvC | Ovarian Cancer |
| PDAC | Pancreatic Ductal Adenocarcinoma |
| SIFT-MS | Selected Ion Flow Tube Mass Spectrometry |
| USPSTF | US Preventive Services Task Force |
| VOC | Volatile Organic Compounds |
References
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2024. [Google Scholar]
- Crosby, D.; Bhatia, S.; Brindle, K.M.; Coussens, L.M.; Dive, C.; Emberton, M.; Esener, S.; Fitzgerald, R.C.; Gambhir, S.S.; Kuhn, P.; et al. Early detection of cancer. Science 2022, 375, eaay9040. [Google Scholar] [CrossRef] [PubMed]
- Spear, G.; Lee, K.; DePersia, A.; Lienhoop, T.; Saha, P. Updates in Breast Cancer Screening and Diagnosis. Curr. Treat. Options Oncol. 2024, 25, 1451–1460. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.M.D.; Oeffinger, K.C.; Shih, T.Y.; Walter, L.C.; Church, T.R.; Fontham, E.T.H.; Elkin, E.B.; Etzioni, R.D.; Guerra, C.E.; Perkins, R.B.; et al. Screening for lung cancer: 2023 guideline update from the American Cancer Society. CA Cancer J. Clin. 2024, 74, 50–81. [Google Scholar] [CrossRef] [PubMed]
- Bandi, P.; Star, J.; Ashad-Bishop, K.; Kratzer, T.; Smith, R.; Jemal, A. Lung Cancer Screening in the US, 2022. JAMA Intern. Med. 2024, 184, 882–891. [Google Scholar] [CrossRef]
- State of Lung Cancer 2022; American Lung Association: Chicago, IL, USA, 2022.
- Cancer Trends Progress Report: Breast Cancer Screening. Available online: https://progressreport.cancer.gov/detection/breast_cancer (accessed on 6 March 2025).
- USPSTF. Final Recommendation Statement Breast Cancer: Screening. Available online: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/breast-cancer-screening (accessed on 6 March 2025).
- Xu, S.; Murtagh, S.; Han, Y.; Wan, F.; Toriola, A.T. Breast Cancer Incidence Among US Women Aged 20 to 49 Years by Race, Stage, and Hormone Receptor Status. JAMA Netw. Open 2024, 7, e2353331. [Google Scholar] [CrossRef]
- Jemal, A.; Schafer, E.J.; Sung, H.; Bandi, P.; Kratzer, T.; Islami, F.; Siegel, R.L. The Burden of Lung Cancer in Women Compared With Men in the US. JAMA Oncol. 2023, 9, 1727–1728. [Google Scholar] [CrossRef]
- Klein, A.P. Pancreatic cancer epidemiology: Understanding the role of lifestyle and inherited risk factors. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 493–502. [Google Scholar] [CrossRef]
- Pancreatic Cancer Statistics. Available online: https://www.wcrf.org/preventing-cancer/cancer-statistics/pancreatic-cancer-statistics/ (accessed on 6 March 2025).
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Wei, Y.-F.; Ning, L.; Xu, Y.-L.; Ma, J.; Li, D.-R.; Feng, Z.-F.; Liu, F.-H.; Li, Y.-Z.; Xu, H.-L.; Li, P.; et al. Worldwide patterns and trends in ovarian cancer incidence by histological subtype: A population-based analysis from 1988 to 2017. eClinicalMedicine 2025, 79, 102983. [Google Scholar] [CrossRef]
- SEER. Cancer Stat Facts: Pancreatic Cancer. Available online: https://seer.cancer.gov/statfacts/html/pancreas.html (accessed on 22 May 2025).
- Upadhyay, A.; Garg, V.; Mathur, S.; Singh Malik, P.; Bhatla, N.; Kumar, S.; Khurana, S.; Kumar, L. Early-Stage epithelial ovarian cancer: Predictors of survival. Gynecol. Oncol. Rep. 2022, 44, 101083. [Google Scholar] [CrossRef]
- Fu, S.W.; Tang, C.; Tan, X.; Srivastava, S. Liquid biopsy for early cancer detection: Technological revolutions and clinical dilemma. Expert. Rev. Mol. Diagn. 2024, 24, 937–955. [Google Scholar] [CrossRef] [PubMed]
- Batool, S.M.; Yekula, A.; Khanna, P.; Hsia, T.; Gamblin, A.S.; Ekanayake, E.; Escobedo, A.K.; You, D.G.; Castro, C.M.; Im, H.; et al. The Liquid Biopsy Consortium: Challenges and opportunities for early cancer detection and monitoring. Cell Rep. Med. 2023, 4, 101198. [Google Scholar] [CrossRef] [PubMed]
- Foser, S.; Maiese, K.; Digumarthy, S.R.; Puig-Butille, J.A.; Rebhan, C. Looking to the Future of Early Detection in Cancer: Liquid Biopsies, Imaging, and Artificial Intelligence. Clin. Chem. 2024, 70, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Guo, H.; Zhao, Y.; Liu, Z.; Wang, C.; Bu, J.; Sun, T.; Wei, J. Liquid biopsy in cancer current: Status, challenges and future prospects. Signal Transduct. Target. Ther. 2024, 9, 336. [Google Scholar] [CrossRef]
- Chakraborty, S.; Hosen, M.I.; Ahmed, M.; Shekhar, H.U. Onco-Multi-OMICS Approach: A New Frontier in Cancer Research. Biomed. Res. Int. 2018, 2018, 9836256. [Google Scholar] [CrossRef]
- Baldini, C.; Billeci, L.; Sansone, F.; Conte, R.; Domenici, C.; Tonacci, A. Electronic Nose as a Novel Method for Diagnosing Cancer: A Systematic Review. Biosensors 2020, 10, 84. [Google Scholar] [CrossRef]
- Baratella, E.; Carbi, M.; Minelli, P.; Segalotti, A.; Ruaro, B.; Salton, F.; Polverosi, R.; Cova, M.A. Calcified Lung Nodules: A Diagnostic Challenge in Clinical Daily Practice. Tomography 2025, 11, 28. [Google Scholar] [CrossRef]
- Brahimetaj, R.; Willekens, I.; Massart, A.; Forsyth, R.; Cornelis, J.; Mey, J.D.; Jansen, B. Improved automated early detection of breast cancer based on high resolution 3D micro-CT microcalcification images. BMC Cancer 2022, 22, 162. [Google Scholar] [CrossRef]
- O’Connor, E.; Mullins, M.; O’Connor, D.; Phelan, S.; Bruzzi, J. The relationship between ultrasound microcalcifications and psammoma bodies in thyroid tumours: A single-institution retrospective study. Clin. Radiol. 2022, 77, e48–e54. [Google Scholar] [CrossRef]
- Porcelli, F.; Verri, M.; De Santis, S.; Crescenzi, A.; Bianchi, A.; Felici, A.C.; Sotgiu, G.; Romano, S.; Orsini, M. Considerations on chemical composition of psammoma bodies: Automated detection strategy by infrared microspectroscopy in ovarian and thyroid cancer tissues. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 298, 122792. [Google Scholar] [CrossRef]
- Cena, B.; Melloul, E.; Demartines, N.; Dormond, O.; Labgaa, I. Basic Science with Preclinical Models to Investigate and Develop Liquid Biopsy: What Are the Available Data and Is It a Fruitful Approach? Int. J. Mol. Sci. 2022, 23, 5343. [Google Scholar] [CrossRef] [PubMed]
- Alix-Panabieres, C.; Marchetti, D.; Lang, J.E. Liquid biopsy: From concept to clinical application. Sci. Rep. 2023, 13, 21685. [Google Scholar] [CrossRef] [PubMed]
- Connal, S.; Cameron, J.M.; Sala, A.; Brennan, P.M.; Palmer, D.S.; Palmer, J.D.; Perlow, H.; Baker, M.J. Liquid biopsies: The future of cancer early detection. J. Transl. Med. 2023, 21, 118. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, C.; Zhou, Y.; Yao, Y.; Liu, J.; Wu, M.; Su, J. Liquid biopsies for cancer: From bench to clinic. MedComm (2020) 2023, 4, e329. [Google Scholar] [CrossRef]
- Schrag, D.; Beer, T.M.; McDonnell, C.H., 3rd; Nadauld, L.; Dilaveri, C.A.; Reid, R.; Marinac, C.R.; Chung, K.C.; Lopatin, M.; Fung, E.T.; et al. Blood-based tests for multicancer early detection (PATHFINDER): A prospective cohort study. Lancet 2023, 402, 1251–1260. [Google Scholar] [CrossRef]
- Nicholson, B.D.; Oke, J.; Virdee, P.S.; Harris, D.A.; O’Doherty, C.; Park, J.E.; Hamady, Z.; Sehgal, V.; Millar, A.; Medley, L.; et al. Multi-cancer early detection test in symptomatic patients referred for cancer investigation in England and Wales (SYMPLIFY): A large-scale, observational cohort study. Lancet Oncol. 2023, 24, 733–743. [Google Scholar] [CrossRef]
- Cohen, J.D.; Javed, A.A.; Thoburn, C.; Wong, F.; Tie, J.; Gibbs, P.; Schmidt, C.M.; Yip-Schneider, M.T.; Allen, P.J.; Schattner, M.; et al. Combined circulating tumor DNA and protein biomarker-based liquid biopsy for the earlier detection of pancreatic cancers. Proc. Natl. Acad. Sci. USA 2017, 114, 10202–10207. [Google Scholar] [CrossRef]
- Reese, K.L.; Pantel, K.; Smit, D.J. Multibiomarker panels in liquid biopsy for early detection of pancreatic cancer—A comprehensive review. J. Exp. Clin. Cancer Res. 2024, 43, 250. [Google Scholar] [CrossRef]
- Luchini, C.; Veronese, N.; Nottegar, A.; Cappelletti, V.; Daidone, M.G.; Smith, L.; Parris, C.; Brosens, L.A.A.; Caruso, M.G.; Cheng, L.; et al. Liquid Biopsy as Surrogate for Tissue for Molecular Profiling in Pancreatic Cancer: A Meta-Analysis Towards Precision Medicine. Cancers 2019, 11, 1152. [Google Scholar] [CrossRef]
- Sefrioui, D.; Blanchard, F.; Toure, E.; Basile, P.; Beaussire, L.; Dolfus, C.; Perdrix, A.; Paresy, M.; Antonietti, M.; Iwanicki-Caron, I.; et al. Diagnostic value of CA19.9, circulating tumour DNA and circulating tumour cells in patients with solid pancreatic tumours. Br. J. Cancer 2017, 117, 1017–1025. [Google Scholar] [CrossRef]
- Montoya Mira, J.L.; Quentel, A.; Patel, R.K.; Keith, D.; Sousa, M.; Minnier, J.; Kingston, B.R.; David, L.; Esener, S.C.; Sears, R.C.; et al. Early detection of pancreatic cancer by a high-throughput protease-activated nanosensor assay. Sci. Transl. Med. 2025, 17, eadq3110. [Google Scholar] [CrossRef] [PubMed]
- Giannopoulou, L.; Lianidou, E.S. Liquid biopsy in ovarian cancer. Adv. Clin. Chem. 2020, 97, 13–71. [Google Scholar] [CrossRef] [PubMed]
- Balla, A.; Bhak, J.; Biro, O. The application of circulating tumor cell and cell-free DNA liquid biopsies in ovarian cancer. Mol. Cell Probes 2022, 66, 101871. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.W.; Charkhchi, P.; Akbari, M.R. Potential clinical utility of liquid biopsies in ovarian cancer. Mol. Cancer 2022, 21, 114. [Google Scholar] [CrossRef]
- Asante, D.B.; Calapre, L.; Ziman, M.; Meniawy, T.M.; Gray, E.S. Liquid biopsy in ovarian cancer using circulating tumor DNA and cells: Ready for prime time? Cancer Lett. 2020, 468, 59–71. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Chuang, C.H.; Kuo, H.C.; Lin, C.T.; Chao, A.; Huang, H.J.; Wang, H.M.; Hsieh, J.C.; Chou, H.H. Circulating tumor cells help differentiate benign ovarian lesions from cancer before surgery: A literature review and proof of concept study using flow cytometry with fluorescence imaging. Oncol. Lett. 2024, 27, 234. [Google Scholar] [CrossRef]
- Farncombe, K.M.; Wong, D.; Norman, M.L.; Oldfield, L.E.; Sobotka, J.A.; Basik, M.; Bombard, Y.; Carile, V.; Dawson, L.; Foulkes, W.D.; et al. Current and new frontiers in hereditary cancer surveillance: Opportunities for liquid biopsy. Am. J. Hum. Genet. 2023, 110, 1616–1627. [Google Scholar] [CrossRef]
- Yao, M.; Amor, R.E.; Zheng, Y.; Haick, H.; Qian, Y.; Wu, W. Hybrid Volatilomics in Healthcare. In Volatile Biomarkers for Human Health: From Nature to Artificial Senses; Haick, H., Ed.; The Royal Society of Chemistry: London, UK, 2022. [Google Scholar]
- Allard-Coutu, A.; Singh, K.; David, D.; Dobson, V.; Dahmer, L.; Heller, B. Volatile organic compounds: A promising new frontier for cancer screening. Tumor Discov. 2024, 3, 2061. [Google Scholar] [CrossRef]
- Janfaza, S.; Khorsand, B.; Nikkhah, M.; Zahiri, J. Digging deeper into volatile organic compounds associated with cancer. Biol. Methods Protoc. 2019, 4, bpz014. [Google Scholar] [CrossRef]
- Sethi, S.; Nanda, R.; Chakraborty, T. Clinical application of volatile organic compound analysis for detecting infectious diseases. Clin. Microbiol. Rev. 2013, 26, 462–475. [Google Scholar] [CrossRef]
- Wang, C.; Li, P.; Lian, A.; Sun, B.; Wang, X.; Guo, L.; Chi, C.; Liu, S.; Zhao, W.; Luo, S.; et al. Blood volatile compounds as biomarkers for colorectal cancer. Cancer Biol. Ther. 2014, 15, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Cavaco, C.; Pereira, J.A.M.; Taunk, K.; Taware, R.; Rapole, S.; Nagarajaram, H.; Câmara, J.S. Screening of salivary volatiles for putative breast cancer discrimination: An exploratory study involving geographically distant populations. Anal. Bioanal. Chem. 2018, 410, 4459–4468. [Google Scholar] [CrossRef] [PubMed]
- Monedeiro, F.; Dos Reis, R.B.; Peria, F.M.; Sares, C.T.G.; De Martinis, B.S. Investigation of sweat VOC profiles in assessment of cancer biomarkers using HS-GC-MS. J. Breath. Res. 2020, 14, 026009. [Google Scholar] [CrossRef]
- Bajo-Fernandez, M.; Souza-Silva, E.A.; Barbas, C.; Rey-Stolle, M.F.; Garcia, A. GC-MS-based metabolomics of volatile organic compounds in exhaled breath: Applications in health and disease. A review. Front. Mol. Biosci. 2023, 10, 1295955. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Meng, S.; Arao, Y.; Saito, Y.; Inoue, K.; Alshammari, A.H.; Hatakeyama, H.; di Luccio, E.; Vecchione, A.; Hirotsu, T.; et al. Non-Invasive Detection of Tumors by Volatile Organic Compounds in Urine. Biomedicines 2025, 13, 109. [Google Scholar] [CrossRef]
- Kouremenos, K.A.; Johansson, M.; Marriott, P.J. Advances in gas chromatographic methods for the identification of biomarkers in cancer. J. Cancer 2012, 3, 404–420. [Google Scholar] [CrossRef]
- Wallace, M.A.G.; Pleil, J.D. Evolution of clinical and environmental health applications of exhaled breath research: Review of methods and instrumentation for gas-phase, condensate, and aerosols. Anal. Chim. Acta 2018, 1024, 18–38. [Google Scholar] [CrossRef]
- Einoch Amor, R.; Nakhleh, M.K.; Barash, O.; Haick, H. Breath analysis of cancer in the present and the future. Eur. Respir. Rev. 2019, 28, 190002. [Google Scholar] [CrossRef]
- Vassilenko, V.; Moura, P.C.; Raposo, M. Diagnosis of Carcinogenic Pathologies through Breath Biomarkers: Present and Future Trends. Biomedicines 2023, 11, 3029. [Google Scholar] [CrossRef]
- Karakaya, D.; Ulucan, O.; Turkan, M. Electronic Nose and Its Applications: A Survey. Int. J. Autom. Comput. 2020, 17, 179–209. [Google Scholar] [CrossRef]
- Scheepers, M.; Al-Difaie, Z.; Brandts, L.; Peeters, A.; van Grinsven, B.; Bouvy, N.D. Diagnostic Performance of Electronic Noses in Cancer Diagnoses Using Exhaled Breath: A Systematic Review and Meta-analysis. JAMA Netw. Open 2022, 5, e2219372. [Google Scholar] [CrossRef] [PubMed]
- Pirrone, F.; Albertini, M. Olfactory detection of cancer by trained sniffer dogs: A systematic review of the literature. J. Vet. Behav. 2017, 19, 105–117. [Google Scholar] [CrossRef]
- Lippi, G.; Heaney, L.M. The “olfactory fingerprint”: Can diagnostics be improved by combining canine and digital noses? Clin. Chem. Lab. Med. 2020, 58, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Padodara, R. Olfactory sense in different animals. Indian J. Vet. Sci. 2014, 2, 1–14. [Google Scholar]
- Angle, C.; Waggoner, L.P.; Ferrando, A.; Haney, P.; Passler, T. Canine Detection of the Volatilome: A Review of Implications for Pathogen and Disease Detection. Front. Vet. Sci. 2016, 3, 47. [Google Scholar] [CrossRef]
- Singletary, M.; Hagerty, S. Peripheral Olfactory Pathway Anatomy, Physiology, and Genetics. In Olfactory Research in Dogs; Lazarowski, L., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 3–37. [Google Scholar]
- Furton, K.G.; Caraballo, N.I.; Cerreta, M.M.; Holness, H.K. Advances in the use of odour as forensic evidence through optimizing and standardizing instruments and canines. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140262. [Google Scholar] [CrossRef]
- Jendrny, P.; Twele, F.; Meller, S.; Osterhaus, A.; Schalke, E.; Volk, H.A. Canine olfactory detection and its relevance to medical detection. BMC Infect. Dis. 2021, 21, 838. [Google Scholar] [CrossRef]
- Tan, S.Y.; Ma, Q.; Li, F.; Jiang, H.; Peng, X.Y.; Dong, J.; Ye, X.; Wang, Q.L.; You, F.M.; Fu, X.; et al. Does the last 20 years paradigm of clinical research using volatile organic compounds to non-invasively diagnose cancer need to change? Challenges and future direction. J. Cancer Res. Clin. Oncol. 2023, 149, 10377–10386. [Google Scholar] [CrossRef]
- da Costa, B.R.B.; De Martinis, B.S. Analysis of urinary VOCs using mass spectrometric methods to diagnose cancer: A review. Clin. Mass. Spectrom. 2020, 18, 27–37. [Google Scholar] [CrossRef]
- Goertzen, A.; Kidane, B.; Ahmed, N.; Aliani, M. Potential urinary volatile organic compounds as screening markers in cancer—A review. Front. Oncol. 2024, 14, 1448760. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, Q.; Lu, X.; Zhang, P.; Yang, R.; Chen, Y.; Xia, J.; Chen, D. Exhaled breath and urinary volatile organic compounds (VOCs) for cancer diagnoses, and microbial-related VOC metabolic pathway analysis: A systematic review and meta-analysis. Int. J. Surg. 2024, 110, 1755–1769. [Google Scholar] [CrossRef] [PubMed]
- Hintzen, K.F.H.; Grote, J.; Wintjens, A.; Lubbers, T.; Eussen, M.M.M.; van Schooten, F.J.; Bouvy, N.D.; Peeters, A. Breath analysis for the detection of digestive tract malignancies: Systematic review. BJS Open 2021, 5, zrab013. [Google Scholar] [CrossRef] [PubMed]
- Hanna, G.B.; Boshier, P.R.; Markar, S.R.; Romano, A. Accuracy and Methodologic Challenges of Volatile Organic Compound-Based Exhaled Breath Tests for Cancer Diagnosis: A Systematic Review and Meta-analysis. JAMA Oncol. 2019, 5, e182815. [Google Scholar] [CrossRef] [PubMed]
- Half, E.; Ovcharenko, A.; Shmuel, R.; Furman-Assaf, S.; Avdalimov, M.; Rabinowicz, A.; Arber, N. Non-invasive multiple cancer screening using trained detection canines and artificial intelligence: A prospective double-blind study. Sci. Rep. 2024, 14, 28204. [Google Scholar] [CrossRef]
- Haripriya, P.; Rangarajan, M.; Pandya, H.J. Breath VOC analysis and machine learning approaches for disease screening: A review. J. Breath. Res. 2023, 17, 024001. [Google Scholar] [CrossRef]
- Hunter, B.; Hindocha, S.; Lee, R.W. The Role of Artificial Intelligence in Early Cancer Diagnosis. Cancers 2022, 14, 1524. [Google Scholar] [CrossRef]
- Vinhas, M.; Leitão, P.M.; Raimundo, B.S.; Gil, N.; Vaz, P.D.; Luis-Ferreira, F. AI Applied to Volatile Organic Compound (VOC) Profiles from Exhaled Breath Air for Early Detection of Lung Cancer. Cancers 2024, 16, 2200. [Google Scholar] [CrossRef]
- Gallos, I.K.; Tryfonopoulos, D.; Shani, G.; Amditis, A.; Haick, H.; Dionysiou, D.D. Advancing Colorectal Cancer Diagnosis with AI-Powered Breathomics: Navigating Challenges and Future Directions. Diagnostics 2023, 13. [Google Scholar] [CrossRef]
- Grasso, A.; Altomare, V.; Fiorini, G.; Zompanti, A.; Pennazza, G.; Santonico, M. Innovative Methodologies for the Early Detection of Breast Cancer: A Review Categorized by Target Biological Samples. Biosensors 2025, 15, 257. [Google Scholar] [CrossRef]
- Kim, M.; Park, J.; Oh, S.; Jeong, B.-H.; Byun, Y.; Shin, S.H.; Im, Y.; Cho, J.H.; Cho, E.-H. Deep learning model integrating cfDNA methylation and fragment size profiles for lung cancer diagnosis. Sci. Rep. 2024, 14, 14797. [Google Scholar] [CrossRef]
- Yan, F.; Jiang, L.; Ye, F.; Ping, J.; Bowley, T.Y.; Ness, S.A.; Li, C.I.; Marchetti, D.; Tang, J.; Guo, Y. Deep neural network based tissue deconvolution of circulating tumor cell RNA. J. Transl. Med. 2023, 21, 783. [Google Scholar] [CrossRef] [PubMed]
- Moser, T.; Kühberger, S.; Lazzeri, I.; Vlachos, G.; Heitzer, E. Bridging biological cfDNA features and machine learning approaches. Trends Genet. 2023, 39, 285–307. [Google Scholar] [CrossRef] [PubMed]
- Medina, J.E.; Annapragada, A.V.; Lof, P.; Short, S.; Bartolomucci, A.L.; Mathios, D.; Koul, S.; Niknafs, N.; Noë, M.; Foda, Z.H.; et al. Early Detection of Ovarian Cancer Using Cell-Free DNA Fragmentomes and Protein Biomarkers. Cancer Discov. 2025, 15, 105–118. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society Recommendations for the Early Detection of Breast Cancer. Available online: https://www.cancer.org/cancer/types/breast-cancer/screening-tests-and-early-detection/american-cancer-society-recommendations-for-the-early-detection-of-breast-cancer.html (accessed on 11 March 2025).
- NCCN Guidelines Breast Cancer; National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2025.
- Li, J.; Guan, X.; Fan, Z.; Ching, L.M.; Li, Y.; Wang, X.; Cao, W.M.; Liu, D.X. Non-Invasive Biomarkers for Early Detection of Breast Cancer. Cancers 2020, 12, 2767. [Google Scholar] [CrossRef]
- Ginsburg, O.; Yip, C.H.; Brooks, A.; Cabanes, A.; Caleffi, M.; Dunstan Yataco, J.A.; Gyawali, B.; McCormack, V.; McLaughlin de Anderson, M.; Mehrotra, R.; et al. Breast cancer early detection: A phased approach to implementation. Cancer 2020, 126 (Suppl. S10), 2379–2393. [Google Scholar] [CrossRef]
- Duque, G.; Manterola, C.; Otzen, T.; Arias, C.; Palacios, D.; Mora, M.; Galindo, B.; Holguín, J.P.; Albarracín, L. Cancer Biomarkers in Liquid Biopsy for Early Detection of Breast Cancer: A Systematic Review. Clin. Med. Insights Oncol. 2022, 16, 11795549221134831. [Google Scholar] [CrossRef]
- Panet, F.; Papakonstantinou, A.; Borrell, M.; Vivancos, J.; Vivancos, A.; Oliveira, M. Use of ctDNA in early breast cancer: Analytical validity and clinical potential. Npj Breast Cancer 2024, 10, 50. [Google Scholar] [CrossRef]
- Cohen, J.D.; Li, L.; Wang, Y.; Thoburn, C.; Afsari, B.; Danilova, L.; Douville, C.; Javed, A.A.; Wong, F.; Mattox, A.; et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 2018, 359, 926–930. [Google Scholar] [CrossRef]
- Brito-Rocha, T.; Constâncio, V.; Henrique, R.; Jerónimo, C. Shifting the Cancer Screening Paradigm: The Rising Potential of Blood-Based Multi-Cancer Early Detection Tests. Cells 2023, 12, 935. [Google Scholar] [CrossRef]
- Liu, M.C.; Oxnard, G.R.; Klein, E.A.; Swanton, C.; Seiden, M.V. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann. Oncol. 2020, 31, 745–759. [Google Scholar] [CrossRef]
- Giro Benet, J.; Seo, M.; Khine, M.; Guma Padro, J.; Pardo Martnez, A.; Kurdahi, F. Breast cancer detection by analyzing the volatile organic compound (VOC) signature in human urine. Sci. Rep. 2022, 12, 14873. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.; Cataneo, R.N.; Cruz-Ramos, J.A.; Huston, J.; Ornelas, O.; Pappas, N.; Pathak, S. Prediction of breast cancer risk with volatile biomarkers in breath. Breast Cancer Res. Treat. 2018, 170, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, H.; Li, Y.; Fang, Y.; Guo, Y.; Li, S.; Xu, J.; Jia, Z.; Zou, J.; Liu, G.; et al. A novel non-invasive exhaled breath biopsy for the diagnosis and screening of breast cancer. J. Hematol. Oncol. 2023, 16, 63. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.; Bevers, T.B.; Larsen, L.H.; Pappas, N.; Pathak, S. Rapid point-of-care breath test predicts breast cancer and abnormal mammograms in symptomatic women. J. Breath. Res. 2024, 18, 046011. [Google Scholar] [CrossRef]
- Yang, Y.; Long, H.; Feng, Y.; Tian, S.; Chen, H.; Zhou, P. A multi-omics method for breast cancer diagnosis based on metabolites in exhaled breath, ultrasound imaging, and basic clinical information. Heliyon 2024, 10, e32115. [Google Scholar] [CrossRef]
- Leemans, M.; Bauer, P.; Cuzuel, V.; Audureau, E.; Fromantin, I. Volatile Organic Compounds Analysis as a Potential Novel Screening Tool for Breast Cancer: A Systematic Review. Biomark. Insights 2022, 17, 11772719221100709. [Google Scholar] [CrossRef]
- Can Lung Cancer Be Found Early? Available online: https://www.cancer.org/cancer/types/lung-cancer/detection-diagnosis-staging/detection.html#:~:text=LDCT%20scans%20can%20help%20find,of%20dying%20from%20lung%20cancer (accessed on 11 March 2025).
- USPSTF. Final Recommendation Statement: Lung Cancer: Screening. Available online: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening#fullrecommendationstart (accessed on 11 March 2025).
- Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; Sicks, J.D. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef]
- de Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef]
- Sadate, A.; Occean, B.V.; Beregi, J.P.; Hamard, A.; Addala, T.; de Forges, H.; Fabbro-Peray, P.; Frandon, J. Systematic review and meta-analysis on the impact of lung cancer screening by low-dose computed tomography. Eur. J. Cancer 2020, 134, 107–114. [Google Scholar] [CrossRef]
- Field, J.K.; Vulkan, D.; Davies, M.P.A.; Baldwin, D.R.; Brain, K.E.; Devaraj, A.; Eisen, T.; Gosney, J.; Green, B.A.; Holemans, J.A.; et al. Lung cancer mortality reduction by LDCT screening: UKLS randomised trial results and international meta-analysis. Lancet Reg. Health Eur. 2021, 10, 100179. [Google Scholar] [CrossRef]
- Adamek, M.; Wachuła, E.; Szabłowska-Siwik, S.; Boratyn-Nowicka, A.; Czyżewski, D. Risk factors assessment and risk prediction models in lung cancer screening candidates. Ann. Transl. Med. 2016, 4, 151. [Google Scholar] [CrossRef] [PubMed]
- Hunger, T.; Wanka-Pail, E.; Brix, G.; Griebel, J. Lung Cancer Screening with Low-Dose CT in Smokers: A Systematic Review and Meta-Analysis. Diagnostics 2021, 11, 1040. [Google Scholar] [CrossRef] [PubMed]
- McRonald, F.E.; Yadegarfar, G.; Baldwin, D.R.; Devaraj, A.; Brain, K.E.; Eisen, T.; Holemans, J.A.; Ledson, M.; Screaton, N.; Rintoul, R.C.; et al. The UK Lung Screen (UKLS): Demographic profile of first 88,897 approaches provides recommendations for population screening. Cancer Prev. Res. 2014, 7, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Lifford, K.J.; Carter, B.; McRonald, F.; Yadegarfar, G.; Baldwin, D.R.; Weller, D.; Hansell, D.M.; Duffy, S.W.; Field, J.K.; et al. Barriers to uptake among high-risk individuals declining participation in lung cancer screening: A mixed methods analysis of the UK Lung Cancer Screening (UKLS) trial. BMJ Open 2015, 5, e008254. [Google Scholar] [CrossRef]
- Quaife, S.L.; Marlow, L.A.V.; McEwen, A.; Janes, S.M.; Wardle, J. Attitudes towards lung cancer screening in socioeconomically deprived and heavy smoking communities: Informing screening communication. Health Expect. 2017, 20, 563–573. [Google Scholar] [CrossRef]
- Quaife, S.L.; Waller, J.; Dickson, J.L.; Brain, K.E.; Kurtidu, C.; McCabe, J.; Hackshaw, A.; Duffy, S.W.; Janes, S.M. Psychological Targets for Lung Cancer Screening Uptake: A Prospective Longitudinal Cohort Study. J. Thorac. Oncol. 2021, 16, 2016–2028. [Google Scholar] [CrossRef]
- O’Dowd, E.L.; Lee, R.W.; Akram, A.R.; Bartlett, E.C.; Bradley, S.H.; Brain, K.; Callister, M.E.J.; Chen, Y.; Devaraj, A.; Eccles, S.R.; et al. Defining the road map to a UK national lung cancer screening programme. Lancet Oncol. 2023, 24, e207–e218. [Google Scholar] [CrossRef]
- Rampariag, R.; Chernyavskiy, I.; Al-Ajam, M.; Tsay, J.J. Controversies and challenges in lung cancer screening. Semin. Oncol. 2022, 49, 191–197. [Google Scholar] [CrossRef]
- Chabon, J.J.; Hamilton, E.G.; Kurtz, D.M.; Esfahani, M.S.; Moding, E.J.; Stehr, H.; Schroers-Martin, J.; Nabet, B.Y.; Chen, B.; Chaudhuri, A.A.; et al. Integrating genomic features for non-invasive early lung cancer detection. Nature 2020, 580, 245–251. [Google Scholar] [CrossRef]
- Liu, M.C.; Klein, E.; Hubbell, E.; Maddala, T.; Aravanis, A.M.; Beausang, J.F.; Filippova, D.; Gross, S.; Jamshidi, A.; Kurtzman, K.; et al. Plasma cell-free DNA (cfDNA) assays for early multi-cancer detection: The circulating cell-free genome atlas (CCGA) study. Ann. Oncol. 2018, 29, viii14. [Google Scholar] [CrossRef]
- Bhamani, A.; Creamer, A.; Verghese, P.; Prendecki, R.; Horst, C.; Tisi, S.; Hall, H.; Khaw, C.R.; Mullin, M.; McCabe, J.; et al. Low-dose CT for lung cancer screening in a high-risk population (SUMMIT): A prospective, longitudinal cohort study. Lancet Oncol. 2025, 26, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Nadauld, L.D.; McDonnell, C.H., 3rd; Beer, T.M.; Liu, M.C.; Klein, E.A.; Hudnut, A.; Whittington, R.A.; Taylor, B.; Oxnard, G.R.; Lipson, J.; et al. The PATHFINDER Study: Assessment of the Implementation of an Investigational Multi-Cancer Early Detection Test into Clinical Practice. Cancers 2021, 13, 3501. [Google Scholar] [CrossRef] [PubMed]
- Tsou, P.H.; Lin, Z.L.; Pan, Y.C.; Yang, H.C.; Chang, C.J.; Liang, S.K.; Wen, Y.F.; Chang, C.H.; Chang, L.Y.; Yu, K.L.; et al. Exploring Volatile Organic Compounds in Breath for High-Accuracy Prediction of Lung Cancer. Cancers 2021, 13, 1431. [Google Scholar] [CrossRef] [PubMed]
- Gasparri, R.; Santonico, M.; Valentini, C.; Sedda, G.; Borri, A.; Petrella, F.; Maisonneuve, P.; Pennazza, G.; D’Amico, A.; Di Natale, C.; et al. Volatile signature for the early diagnosis of lung cancer. J. Breath. Res. 2016, 10, 016007. [Google Scholar] [CrossRef]
- Kort, S.; Brusse-Keizer, M.; Schouwink, H.; Citgez, E.; de Jongh, F.H.; van Putten, J.W.G.; van den Borne, B.; Kastelijn, E.A.; Stolz, D.; Schuurbiers, M.; et al. Diagnosing Non-Small Cell Lung Cancer by Exhaled Breath Profiling Using an Electronic Nose: A Multicenter Validation Study. Chest 2023, 163, 697–706. [Google Scholar] [CrossRef]
- Janssens, E.; van Meerbeeck, J.P.; Lamote, K. Volatile organic compounds in human matrices as lung cancer biomarkers: A systematic review. Crit. Rev. Oncol. Hematol. 2020, 153, 103037. [Google Scholar] [CrossRef]
- Keogh, R.J.; Riches, J.C. The Use of Breath Analysis in the Management of Lung Cancer: Is It Ready for Primetime? Curr. Oncol. 2022, 29, 7355–7378. [Google Scholar] [CrossRef]
- Vadala, R.; Pattnaik, B.; Bangaru, S.; Rai, D.; Tak, J.; Kashyap, S.; Verma, U.; Yadav, G.; Dhaliwal, R.S.; Mittal, S.; et al. A review on electronic nose for diagnosis and monitoring treatment response in lung cancer. J. Breath. Res. 2023, 17, 024002. [Google Scholar] [CrossRef]
- Chaudhary, V.; Taha, B.A.; Lucky; Rustagi, S.; Khosla, A.; Papakonstantinou, P.; Bhalla, N. Nose-on-Chip Nanobiosensors for Early Detection of Lung Cancer Breath Biomarkers. ACS Sens. 2024, 9, 4469–4494. [Google Scholar] [CrossRef]
- Su, Z.; Yu, X.; He, Y.; Sha, T.; Guo, H.; Tao, Y.; Liao, L.; Zhang, Y.; Lu, G.; Lu, G.; et al. Inconsistencies in predictive models based on exhaled volatile organic compounds for distinguishing between benign pulmonary nodules and lung cancer: A systematic review. BMC Pulm. Med. 2024, 24, 551. [Google Scholar] [CrossRef]
- Fan, X.; Zhong, R.; Liang, H.; Zhong, Q.; Huang, H.; He, J.; Chen, Y.; Wang, Z.; Xie, S.; Jiang, Y.; et al. Exhaled VOC detection in lung cancer screening: A comprehensive meta-analysis. BMC Cancer 2024, 24, 775. [Google Scholar] [CrossRef] [PubMed]
- Buszewski, B.; Ligor, T.; Jezierski, T.; Wenda-Piesik, A.; Walczak, M.; Rudnicka, J. Identification of volatile lung cancer markers by gas chromatography-mass spectrometry: Comparison with discrimination by canines. Anal. Bioanal. Chem. 2012, 404, 141–146. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, M.; Jezierski, T.; Broffman, M.; Hubbard, A.; Turner, K.; Janecki, T. Diagnostic accuracy of canine scent detection in early- and late-stage lung and breast cancers. Integr. Cancer Ther. 2006, 5, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Biehl, W.; Hattesohl, A.; Jörres, R.A.; Duell, T.; Althöhn, U.; Koczulla, A.R.; Schmetzer, H. VOC pattern recognition of lung cancer: A comparative evaluation of different dog- and eNose-based strategies using different sampling materials. Acta Oncologica 2019, 58, 1216–1224. [Google Scholar] [CrossRef]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Survival Rates for Pancreatic Cancer. Available online: https://www.cancer.org/cancer/types/pancreatic-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 12 March 2025).
- Kamisawa, T.; Wood, L.D.; Itoi, T.; Takaori, K. Pancreatic cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef]
- Can Pancreatic Cancer Be Found Early? Available online: https://www.cancer.org/cancer/types/pancreatic-cancer/detection-diagnosis-staging/detection.html (accessed on 12 March 2025).
- Sawhney, M.S.; Calderwood, A.H.; Thosani, N.C.; Rebbeck, T.R.; Wani, S.; Canto, M.I.; Fishman, D.S.; Golan, T.; Hidalgo, M.; Kwon, R.S.; et al. ASGE guideline on screening for pancreatic cancer in individuals with genetic susceptibility: Summary and recommendations. Gastrointest. Endosc. 2022, 95, 817–826. [Google Scholar] [CrossRef]
- Laish, I.; Schechter, M.; Dancour, A.; Lieberman, S.; Levi, Z.; Goldberg, Y.; Kedar, I.; Hasnis, E.; Half, E.; Levi, G.R.; et al. The benefit of pancreatic cancer surveillance in carriers of germline BRCA1/2 pathogenic variants. Cancer 2024, 130, 256–266. [Google Scholar] [CrossRef]
- Katona, B.W.; Lubinski, J.; Pal, T.; Huzarski, T.; Foulkes, W.D.; Moller, P.; Eisen, A.; Randall Armel, S.; Neuhausen, S.L.; Raj, R.; et al. The incidence of pancreatic cancer in women with a BRCA1 or BRCA2 mutation. Cancer 2025, 131, e35666. [Google Scholar] [CrossRef]
- Wang, K.; Wang, X.; Pan, Q.; Zhao, B. Liquid biopsy techniques and pancreatic cancer: Diagnosis, monitoring, and evaluation. Mol. Cancer 2023, 22, 167. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Zhu, Z.; Roy, S.; Jun, E.; Han, H.; Munoz, R.M.; Nishiwada, S.; Sharma, G.; Cridebring, D.; Zenhausern, F.; et al. An Exosome-based Transcriptomic Signature for Noninvasive, Early Detection of Patients with Pancreatic Ductal Adenocarcinoma: A Multicenter Cohort Study. Gastroenterology 2022, 163, 1252–1266.e1252. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Li, T.; Lin, C.; Zhao, B.; Li, Z.; Zhao, Y.; Wang, W. The systematic role of pancreatic cancer exosomes: Distant communication, liquid biopsy and future therapy. Cancer Cell Int. 2024, 24, 264. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hu, X.; Liu, T. Development of liquid biopsy in detection and screening of pancreatic cancer. Front. Oncol. 2024, 14, 1415260. [Google Scholar] [CrossRef]
- Martinez-Moral, M.P.; Tena, M.T.; Martin-Carnicero, A.; Martinez, A. Highly sensitive serum volatolomic biomarkers for pancreatic cancer diagnosis. Clin. Chim. Acta 2024, 557, 117895. [Google Scholar] [CrossRef]
- Daulton, E.; Wicaksono, A.N.; Tiele, A.; Kocher, H.M.; Debernardi, S.; Crnogorac-Jurcevic, T.; Covington, J.A. Volatile organic compounds (VOCs) for the non-invasive detection of pancreatic cancer from urine. Talanta 2021, 221, 121604. [Google Scholar] [CrossRef]
- Nissinen, S.I.; Roine, A.; Hokkinen, L.; Karjalainen, M.; Venalainen, M.; Helminen, H.; Niemi, R.; Lehtimaki, T.; Rantanen, T.; Oksala, N. Detection of Pancreatic Cancer by Urine Volatile Organic Compound Analysis. Anticancer Res. 2019, 39, 73–79. [Google Scholar] [CrossRef]
- Markar, S.R.; Brodie, B.; Chin, S.T.; Romano, A.; Spalding, D.; Hanna, G.B. Profile of exhaled-breath volatile organic compounds to diagnose pancreatic cancer. Br. J. Surg. 2018, 105, 1493–1500. [Google Scholar] [CrossRef]
- Princivalle, A.; Monasta, L.; Butturini, G.; Bassi, C.; Perbellini, L. Pancreatic ductal adenocarcinoma can be detected by analysis of volatile organic compounds (VOCs) in alveolar air. BMC Cancer 2018, 18, 529. [Google Scholar] [CrossRef]
- Arasaradnam, R.P.; Wicaksono, A.; O’Brien, H.; Kocher, H.M.; Covington, J.A.; Crnogorac-Jurcevic, T. Noninvasive Diagnosis of Pancreatic Cancer Through Detection of Volatile Organic Compounds in Urine. Gastroenterology 2018, 154, 485–487.e481. [Google Scholar] [CrossRef]
- All Cancer Sites Combined: Recent Trends in SEER Age-Adjusted Incidence Rates, 2000–2021. Available online: https://seer.cancer.gov/statistics-network/explorer/. (accessed on 12 March 2025).
- Jacobs, I.; Stabile, I.; Bridges, J.; Kemsley, P.; Reynolds, C.; Grudzinskas, J.; Oram, D. Multimodal approach to screening for ovarian cancer. Lancet 1988, 1, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Yamada, Y.; Sado, T.; Sakata, M.; Yoshida, S.; Kawaguchi, R.; Kanayama, S.; Shigetomi, H.; Haruta, S.; Tsuji, Y.; et al. A randomized study of screening for ovarian cancer: A multicenter study in Japan. Int. J. Gynecol. Cancer 2008, 18, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Menon, U.; Gentry-Maharaj, A.; Burnell, M.; Singh, N.; Ryan, A.; Karpinskyj, C.; Carlino, G.; Taylor, J.; Massingham, S.K.; Raikou, M.; et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): A randomised controlled trial. Lancet 2021, 397, 2182–2193. [Google Scholar] [CrossRef] [PubMed]
- Buys, S.S.; Partridge, E.; Black, A.; Johnson, C.C.; Lamerato, L.; Isaacs, C.; Reding, D.J.; Greenlee, R.T.; Yokochi, L.A.; Kessel, B.; et al. Effect of screening on ovarian cancer mortality: The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA 2011, 305, 2295–2303. [Google Scholar] [CrossRef]
- Nagarkar, R.; Gopichand, M.; Pal, S.K.; Gupta, A.; Saquib, N.M.; Sagar, G.; Rao, K.V.S.; Siddiqui, Z.; Longkumer, I. The High Sensitivity of the Multi-Cancer Detection Test ONCOVERYX-F Offers a Promising Platform for Ovarian Cancer Screening. Int. J. Womens Health 2024, 16, 1–7. [Google Scholar] [CrossRef]
- Margoni, A.; Gargalionis, A.N.; Papavassiliou, A.G. CA-125:CA72-4 ratio—Towards a promising cost-effective tool in ovarian cancer diagnosis and monitoring of post-menopausal women under hormone treatment. J. Ovarian Res. 2024, 17, 164. [Google Scholar] [CrossRef]
- Davenport, C.; Rai, N.; Sharma, P.; Deeks, J.J.; Berhane, S.; Mallett, S.; Saha, P.; Champaneria, R.; Bayliss, S.E.; Snell, K.I.; et al. Menopausal status, ultrasound and biomarker tests in combination for the diagnosis of ovarian cancer in symptomatic women. Cochrane Database Syst. Rev. 2022, 7, CD011964. [Google Scholar] [CrossRef]
- Sideris, M.; Menon, U.; Manchanda, R. Screening and prevention of ovarian cancer. Med. J. Aust. 2024, 220, 264–274. [Google Scholar] [CrossRef]
- Grossman, D.C.; Curry, S.J.; Owens, D.K.; Barry, M.J.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W., Jr.; Kemper, A.R.; Krist, A.H.; Kurth, A.E.; et al. Screening for Ovarian Cancer: US Preventive Services Task Force Recommendation Statement. Jama 2018, 319, 588–594. [Google Scholar] [CrossRef]
- Eleje, G.U.; Eke, A.C.; Ezebialu, I.U.; Ikechebelu, J.I.; Ugwu, E.O.; Okonkwo, O.O. Risk-reducing bilateral salpingo-oophorectomy in women with BRCA1 or BRCA2 mutations. Cochrane Database Syst. Rev. 2018, 8, CD012464. [Google Scholar] [CrossRef]
- Hong, M.K.; Ding, D.C. Early Diagnosis of Ovarian Cancer: A Comprehensive Review of the Advances, Challenges, and Future Directions. Diagnostics 2025, 15, 406. [Google Scholar] [CrossRef] [PubMed]
- Raspagliesi, F.; Bogani, G.; Benedetti, S.; Grassi, S.; Ferla, S.; Buratti, S. Detection of Ovarian Cancer through Exhaled Breath by Electronic Nose: A Prospective Study. Cancers 2020, 12, 2408. [Google Scholar] [CrossRef] [PubMed]
- Horvath, G.; Jarverud, G.A.; Jarverud, S.; Horvath, I. Human ovarian carcinomas detected by specific odor. Integr. Cancer Ther. 2008, 7, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Horvath, G.; Andersson, H.; Nemes, S. Cancer odor in the blood of ovarian cancer patients: A retrospective study of detection by dogs during treatment, 3 and 6 months afterward. BMC Cancer 2013, 13, 396. [Google Scholar] [CrossRef]
- Kybert, N.; Prigge, K.; Otto, C.; Ramirez, L.; Joffe, E.; Tanyi, J.; Piltz-Seymour, J.; Johnson, A.T.C.; Preti, G. Exploring ovarian cancer screening using a combined sensor approach: A pilot study. AIP Adv. 2020, 10, 035213. [Google Scholar] [CrossRef]
- Klein, E.A.; Richards, D.; Cohn, A.; Tummala, M.; Lapham, R.; Cosgrove, D.; Chung, G.; Clement, J.; Gao, J.; Hunkapiller, N.; et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann. Oncol. 2021, 32, 1167–1177. [Google Scholar] [CrossRef]
- Budnik, B.; Amirkhani, H.; Forouzanfar, M.H.; Afshin, A. Novel proteomics-based plasma test for early detection of multiple cancers in the general population. BMJ Oncol. 2024, 3, e000073. [Google Scholar] [CrossRef]
- Milner, D.A.; Lennerz, J.K. Technology and Future of Multi-Cancer Early Detection. Life 2024, 14, 833. [Google Scholar] [CrossRef]
- Lennon, A.M.; Buchanan, A.H.; Kinde, I.; Warren, A.; Honushefsky, A.; Cohain, A.T.; Ledbetter, D.H.; Sanfilippo, F.; Sheridan, K.; Rosica, D.; et al. Feasibility of blood testing combined with PET-CT to screen for cancer and guide intervention. Science 2020, 369, eabb9601. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).