Clinical and Histological Predictors of Advanced Basal Cell Carcinoma Recurrence After Complete Response to Hedgehog Pathway Inhibitors: A Retrospective Multicenter Observational Study
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design and Data Collection
2.2. Statistical Analysis
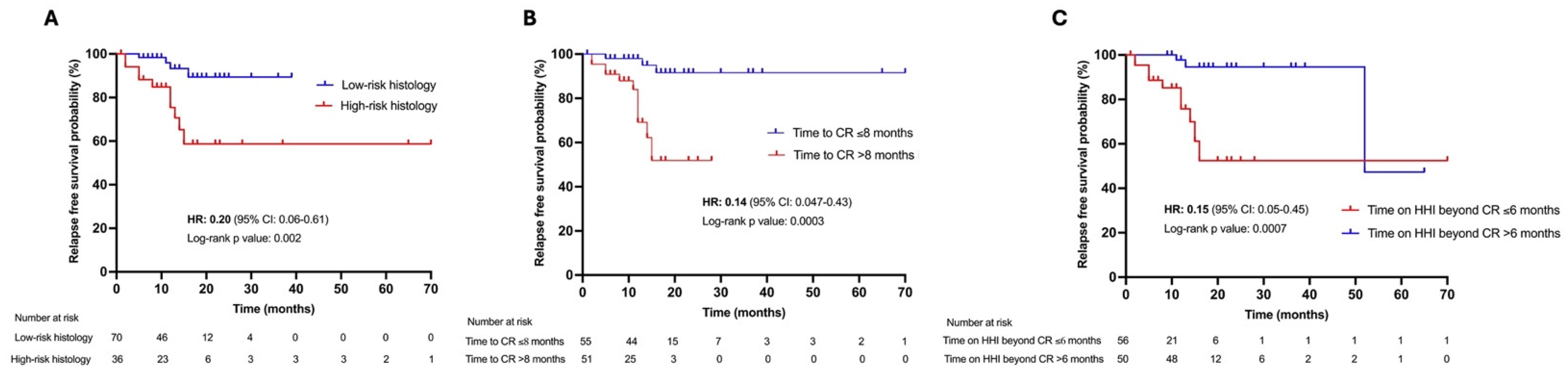
3. Results
3.1. Patient Population
3.2. Clinical and Histological Features Associated with Locally Advanced BCC Recurrence After CR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lomas, A.; Leonardi-Bee, J.; Bath-Hextall, F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br. J. Dermatol. 2012, 166, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Peris, K.; Fargnoli, M.C.; Kaufmann, R.; Arenberger, P.; Bastholt, L.; Seguin, N.B.; Bataille, V.; Brochez, L.; del Marmol, V.; Dummer, R.; et al. European consensus-based interdisciplinary guideline for diagnosis and treatment of basal cell carcinoma-update 2023. Eur. J. Cancer 2023, 192, 113254. [Google Scholar] [CrossRef]
- Grob, J.; Guminski, A.; Malvehy, J.; Basset-Seguin, N.; Bertrand, B.; Fernandez-Penas, P.; Kaufmann, R.; Zalaudek, I.; Gaudy-Marqueste, C.; Fargnoli, M.; et al. Position statement on classification of basal cell carcinomas. Part 1: Unsupervised clustering of experts as a way to build an operational classification of advanced basal cell carcinoma based on pattern recognition. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1949–1956. [Google Scholar] [CrossRef]
- Sekulic, A.; Migden, M.R.; Oro, A.E.; Dirix, L.; Lewis, K.D.; Hainsworth, J.D.; Solomon, J.A.; Yoo, S.; Arron, S.T.; Friedlander, P.A.; et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N. Engl. J. Med. 2012, 366, 2171–2179. [Google Scholar] [CrossRef]
- Migden, M.R.; Guminski, A.; Gutzmer, R.; Dirix, L.; Lewis, K.D.; Combemale, P.; Herd, R.M.; Kudchadkar, R.; Trefzer, U.; Gogov, S.; et al. Treatment with two different doses of sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): A multicentre, randomised, double-blind phase 2 trial. Lancet Oncol. 2015, 16, 716–728. [Google Scholar] [CrossRef] [PubMed]
- Axelson, M.; Liu, K.; Jiang, X.; He, K.; Wang, J.; Zhao, H.; Kufrin, D.; Palmby, T.; Dong, Z.; Russell, A.M.; et al. U.S. Food and Drug Administration approval: Vismodegib for recurrent, locally advanced, or metastatic basal cell carcinoma. Clin. Cancer Res. 2013, 19, 2289–2293. [Google Scholar] [CrossRef]
- Casey, D.; Demko, S.; Shord, S.; Zhao, H.; Chen, H.; He, K.; Putman, A.; Helms, W.S.; Keegan, P.; Pazdur, R. FDA Approval Summary: Sonidegib for Locally Advanced Basal Cell Carcinoma. Clin. Cancer Res. 2017, 23, 2377–2381. [Google Scholar] [CrossRef] [PubMed]
- Dummer, R.; Ascierto, P.; Basset-Seguin, N.; Dréno, B.; Garbe, C.; Gutzmer, R.; Hauschild, A.; Krattinger, R.; Lear, J.; Malvehy, J.; et al. Sonidegib and vismodegib in the treatment of patients with locally advanced basal cell carcinoma: A joint expert opinion. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1944–1956. [Google Scholar] [CrossRef]
- Sekulic, A.; Migden, M.R.; Basset-Seguin, N.; Garbe, C.; Gesierich, A.; Lao, C.D.; Miller, C.; Mortier, L.; Murrell, D.F.; Hamid, O.; et al. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: Final update of the pivotal ERIVANCE BCC study. BMC Cancer 2017, 17, 332, Erratum in BMC Cancer 2019, 19, 366. [Google Scholar] [CrossRef]
- Dummer, R.; Guminksi, A.; Gutzmer, R.; Lear, J.T.; Lewis, K.D.; Chang, A.L.S.; Combemale, P.; Dirix, L.; Kaatz, M.; Kudchadkar, R.; et al. Long-term efficacy and safety of sonidegib in patients with advanced basal cell carcinoma: 42-month analysis of the phase II randomized, double-blind BOLT study. Br. J. Dermatol. 2020, 182, 1369–1378. [Google Scholar] [CrossRef]
- Heppt, M.V.; Gebhardt, C.; Hassel, J.C.; Alter, M.; Gutzmer, R.; Leiter, U.; Berking, C. Long-Term Management of Advanced Basal Cell Carcinoma: Current Challenges and Future Perspectives. Cancers 2022, 14, 4547. [Google Scholar] [CrossRef] [PubMed]
- Mannino, M.; Piccerillo, A.; Fabbrocini, G.; Quaglino, P.; Argenziano, G.; Dika, E.; Ascierto, P.A.; Pellacani, G.; Longo, C.; Fargnoli, M.C.; et al. Clinical Characteristics of an Italian Patient Population with Advanced BCC and Real-Life Evaluation of Hedgehog Pathway Inhibitor Safety and Effectiveness. Dermatology 2023, 239, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Herms, F.; Lambert, J.; Grob, J.-J.; Haudebourg, L.; Bagot, M.; Dalac, S.; Dutriaux, C.; Guillot, B.; Jeudy, G.; Mateus, C.; et al. Follow-Up of Patients With Complete Remission of Locally Advanced Basal Cell Carcinoma After Vismodegib Discontinuation: A Multicenter French Study of 116 Patients. J. Clin. Oncol. 2019, 37, 3275–3282. [Google Scholar] [CrossRef]
- Bassompierre, A.; Dalac, S.; Dreno, B.; Neidhardt, E.; Maubec, E.; Capelle, C.; Andre, F.; Behal, H.; Dziwniel, V.; Bens, G.; et al. Efficacy of sonic hedgehog inhibitors rechallenge, after initial complete response in recurrent advanced basal cell carcinoma: A retrospective study from the CARADERM database. ESMO Open 2021, 6, 100284. [Google Scholar] [CrossRef]
- Alfieri, S.; Romanò, R.; Marceglia, S.; De Giorgi, V.; Peris, K.; Sollena, P.; Piccerillo, A.; Moro, R.; Gualdi, G.; Ascierto, P.A.; et al. Hedgehog Inhibitors Beyond Clinical Complete Response in Basal Cell Carcinoma: Should I Stop or Should I Go? Oncologist 2024, 29, e699–e707. [Google Scholar] [CrossRef] [PubMed]
- Scalvenzi, M.; Cappello, M.; Costa, C.; Fabbrocini, G.; Luciano, M.; Villani, A. Low-Dose Vismodegib as Maintenance Therapy After Locally Advanced Basal Cell Carcinoma Complete Remission: High Efficacy with Minimal Toxicity. Dermatol. Ther. 2020, 10, 465–468. [Google Scholar] [CrossRef]
- Carballo, G.B.; Honorato, J.R.; de Lopes, G.P.F.; Spohr, T.C.L.S.E. A highlight on Sonic hedgehog pathway. Cell Commun. Signal. 2018, 16, 11. [Google Scholar] [CrossRef]
- Biehs, B.; Dijkgraaf, G.J.P.; Piskol, R.; Alicke, B.; Boumahdi, S.; Peale, F.; Gould, S.E.; de Sauvage, F.J. A cell identity switch allows residual BCC to survive Hedgehog pathway inhibition. Nature 2018, 562, 429–433. [Google Scholar] [CrossRef]
- Saldanha, G.; Fletcher, A.; Slater, D.N. Basal cell carcinoma: A dermatopathological and molecular biological update. Br. J. Dermatol. 2003, 148, 195–202. [Google Scholar] [CrossRef]
- Fargnoli, M.; Pellegrini, C.; Piccerillo, A.; Spallone, G.; Rocco, T.; Ventura, A.; Necozione, S.; Bianchi, L.; Peris, K.; Cortellini, A. Clinical determinants of complete response to vismodegib in locally advanced basal cell carcinoma: A multicentre experience. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e923–e926. [Google Scholar] [CrossRef]
- Haensel, D.; Gaddam, S.; Li, N.Y.; Gonzalez, F.; Patel, T.; Cloutier, J.M.; Sarin, K.Y.; Tang, J.Y.; Rieger, K.E.; Aasi, S.Z.; et al. LY6D marks pre-existing resistant basosquamous tumor subpopulations. Nat. Commun. 2022, 13, 7520. [Google Scholar] [CrossRef] [PubMed]
| Variable | Overall Cohort (N = 106) | Tumor Recurrence After CR, Yes (N = 14) | Tumor Recurrence After CR, No (N = 92) | p Value |
|---|---|---|---|---|
| Age (years), median (range) | 78 (28–97) | 77 (51–97) | 78 (28–96) | 0.73 |
| Gender, N (%) | ||||
| Male | 68 (64.2) | 8 (57.1) | 60 (65.2) | 0.56 |
| Female | 38 (35.8) | 6 (42.9) | 32 (34.8) | |
| ECOG PS, N (%) | ||||
| 0–1 | 101 (95.3) | 12 (85.7) | 89 (96.7) | 0.12 |
| ≥2 | 5 (4.7) | 2 (14.3) | 3 (3.3) | |
| Immunosuppression, N (%) | ||||
| Yes | 12 (11.3) | 3 (21.4) | 9 (9.8) | 0.19 |
| No | 94 (88.7) | 11 (78.6) | 83 (90.2) | |
| LaBCC site, N (%) | ||||
| Head and neck | 79 (74.5) | 8 (57.1) | 71 (77.2) | 0.18 |
| Trunk and limbs | 27 (25.5) | 6 (42.9) | 21 (22.8) | |
| LaBCC histology, N (%) | ||||
| High risk * | 36 (44) | 10 (71.4) | 26 (28.3) | 0.004 |
| Low risk ± | 70 (66) | 4 (28.6) | 66 (71.7) | |
| Therapies prior to HHIs, N (%) | ||||
| Yes | 60 (56.6) | 9 (64.3) | 51 (55.4) | 0.57 |
| No | 46 (43.4) | 5 (35.7) | 41 (44.6) | |
| HHI duration (months), median (range) | 18 (1–37) | 15 (7–20) | 18 (1–37) | 0.06 |
| Time to CR (months), median (range) | 8 (1–34) | 12.5 (5–39) | 7 (1–32) | 0.04 |
| HHIs beyond CR, N (%) | ||||
| Yes | 73 (68.9) | 10 (71.4) | 63 (68.5) | 0.90 |
| No | 33 (31.2) | 4 (28.6) | 29 (31.5) | |
| HHI duration (months), median (range) | 6 (0–22) | 4.5 (0–16) | 6 (0–22) | 0.14 |
| Variable | Outcome: Tumor Recurrence After CR | p Value | |
|---|---|---|---|
| OR | 95% CI | ||
| Age (years) | 0.99 | 0.95–1.04 | 0.73 |
| Gender | |||
| Female | 1.40 | 0.42–4.39 | 0.55 |
| Male | |||
| ECOG PS | 1.35 | 0.50–3.29 | 0.51 |
| LaBCC location | |||
| Head and neck | 0.11 | 0.76–8.13 | 0.11 |
| Trunk and limbs | |||
| LaBCC histology | |||
| High risk * | 0.15 | 0.04–0.51 | 0.003 |
| Low risk ± | |||
| Therapies prior to HHI | |||
| Yes | 0.69 | 0.19–2.16 | 0.53 |
| No | |||
| HHI duration (months) | 0.94 | 0.86–1.01 | 0.11 |
| Time to CR (months) | 1.07 | 1.01–1.15 | 0.04 |
| Time on HHI beyond CR (months) | 0.91 | 0.81–1.00 | 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mannino, M.; Scalvenzi, M.; Di Stefani, A.; Costa, C.; Calzavara-Pinton, P.; Fargnoli, M.C.; Piccerillo, A.; Bocchino, E.; Zalaudek, I.; Ascierto, P.A.; et al. Clinical and Histological Predictors of Advanced Basal Cell Carcinoma Recurrence After Complete Response to Hedgehog Pathway Inhibitors: A Retrospective Multicenter Observational Study. Cancers 2025, 17, 1840. https://doi.org/10.3390/cancers17111840
Mannino M, Scalvenzi M, Di Stefani A, Costa C, Calzavara-Pinton P, Fargnoli MC, Piccerillo A, Bocchino E, Zalaudek I, Ascierto PA, et al. Clinical and Histological Predictors of Advanced Basal Cell Carcinoma Recurrence After Complete Response to Hedgehog Pathway Inhibitors: A Retrospective Multicenter Observational Study. Cancers. 2025; 17(11):1840. https://doi.org/10.3390/cancers17111840
Chicago/Turabian StyleMannino, Maria, Massimiliano Scalvenzi, Alessandro Di Stefani, Claudia Costa, Piergiacomo Calzavara-Pinton, Maria Concetta Fargnoli, Alfredo Piccerillo, Enrico Bocchino, Iris Zalaudek, Paolo Antonio Ascierto, and et al. 2025. "Clinical and Histological Predictors of Advanced Basal Cell Carcinoma Recurrence After Complete Response to Hedgehog Pathway Inhibitors: A Retrospective Multicenter Observational Study" Cancers 17, no. 11: 1840. https://doi.org/10.3390/cancers17111840
APA StyleMannino, M., Scalvenzi, M., Di Stefani, A., Costa, C., Calzavara-Pinton, P., Fargnoli, M. C., Piccerillo, A., Bocchino, E., Zalaudek, I., Ascierto, P. A., Quaglino, P., Queirolo, P., Dika, E., De Giorgi, V., Moscarella, E., Bianchi, L., Longo, C., Cantisani, C., Villani, A., ... Collaborators. (2025). Clinical and Histological Predictors of Advanced Basal Cell Carcinoma Recurrence After Complete Response to Hedgehog Pathway Inhibitors: A Retrospective Multicenter Observational Study. Cancers, 17(11), 1840. https://doi.org/10.3390/cancers17111840









