A Comprehensive Evaluation of Clinicopathologic Characteristics, Molecular Features and Prognosis in Lung Adenocarcinoma with an Acinar Component
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinicopathological Data
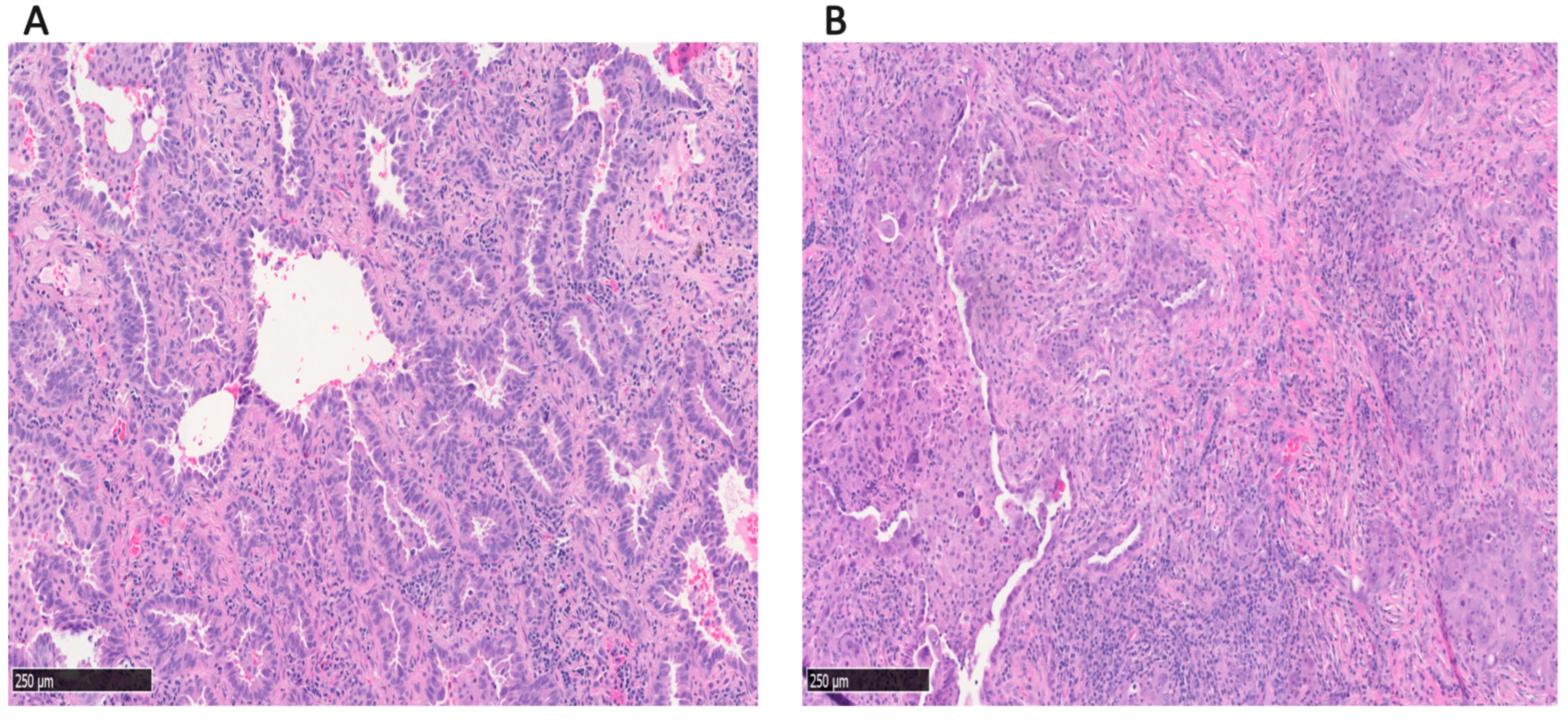
2.3. Histological Evaluation
2.4. Mutational Analysis
2.5. Statistical Analysis and Visualization
3. Results
3.1. Clinicopathologic Factors
3.2. Status of Common Driver Mutations
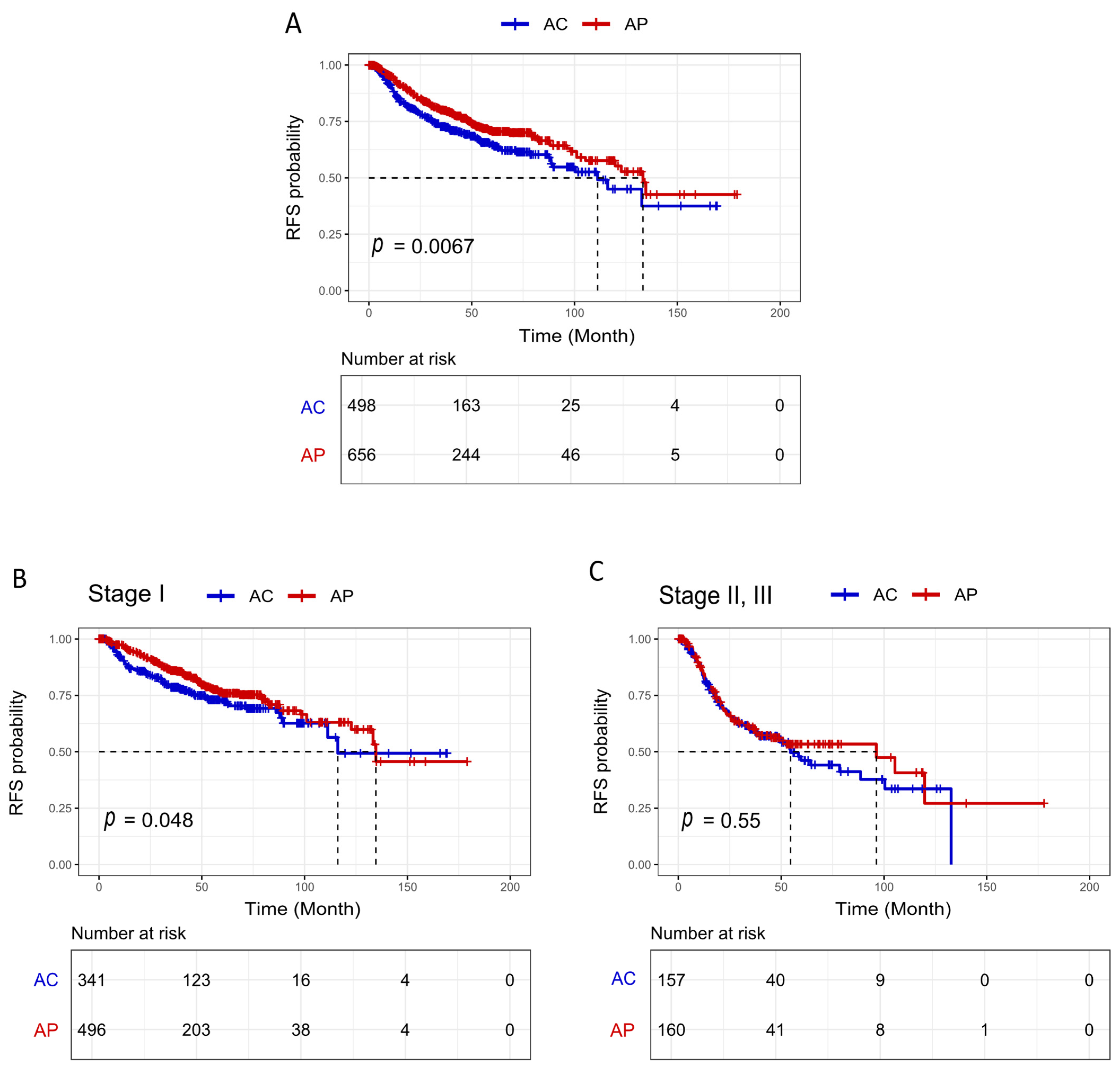
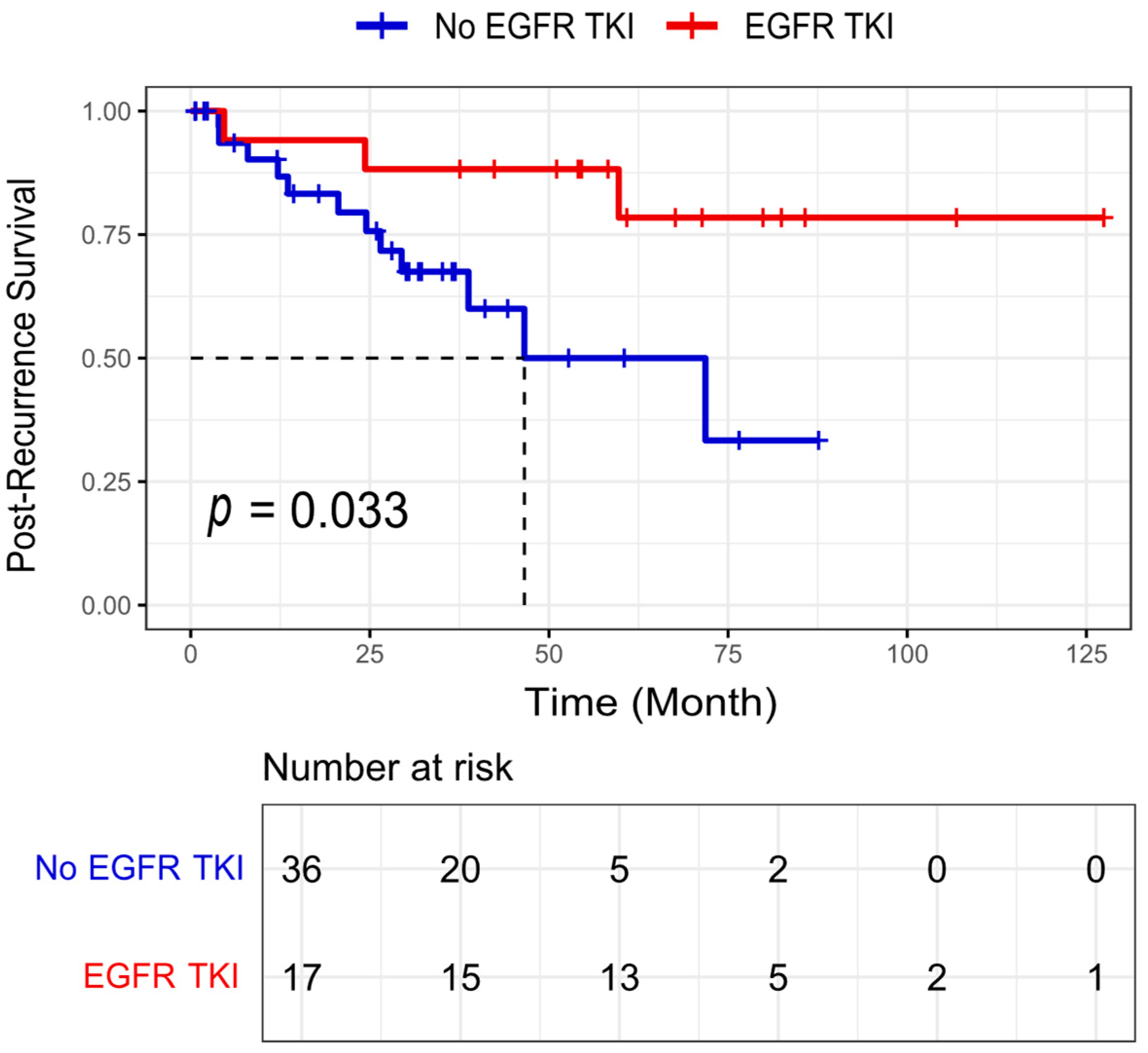
3.3. Survival Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Adjei, A.A. Lung Cancer Worldwide. J. Thorac. Oncol. 2019, 14, 956. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.L.; Ocampo, P.S.; Xia, Y.; Zhong, H.; Russell, P.A.; Minami, Y.; Cooper, W.A.; Yoshida, A.; Bubendorf, L.; Papotti, M.; et al. A Grading System for Invasive Pulmonary Adenocarcinoma: A Proposal From the International Association for the Study of Lung Cancer Pathology Committee. J. Thorac. Oncol. 2020, 15, 1599–1610. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Brambilla, E.; Noguchi, M.; Nicholson, A.G.; Geisinger, K.R.; Yatabe, Y.; Beer, D.G.; Powell, C.A.; Riely, G.J.; Van Schil, P.E.; et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma. J. Thorac. Oncol. 2011, 6, 244–285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, R.; Cai, D.; Li, Y.; Pan, Y.; Hu, H.; Wang, L.; Li, H.; Ye, T.; Luo, X.; et al. A comprehensive investigation of molecular features and prognosis of lung adenocarcinoma with micropapillary component. J. Thorac. Oncol. 2014, 9, 1772–1778. [Google Scholar] [CrossRef]
- Deng, C.; Zheng, Q.; Zhang, Y.; Jin, Y.; Shen, X.; Nie, X.; Fu, F.; Ma, X.; Ma, Z.; Wen, Z.; et al. Validation of the Novel International Association for the Study of Lung Cancer Grading System for Invasive Pulmonary Adenocarcinoma and Association with Common Driver Mutations. J. Thorac. Oncol. 2021, 16, 1684–1693. [Google Scholar] [CrossRef]
- Bossé, Y.; Gagné, A.; Althakfi, W.A.; Orain, M.; Couture, C.M.; Trahan, S.; Pagé, S.; Joubert, D.; Fiset, P.O.; Desmeules, P.M.; et al. A Simplified Version of the IASLC Grading System for Invasive Pulmonary Adenocarcinomas with Improved Prognosis Discrimination. Am. J. Surg. Pathol. 2023, 47, 686–693. [Google Scholar] [CrossRef]
- She, Y.; Zhong, Y.; Hou, L.; Zhao, S.; Zhang, L.; Xie, D.; Zhu, Y.; Wu, C.; Chen, C. Application of the Novel Grading System of Invasive Pulmonary Adenocarcinoma in a Real Diagnostic Scenario: A Brief Report of 9353 Cases. JTO Clin. Res. Rep. 2023, 4, 100465. [Google Scholar] [CrossRef]
- Borczuk, A.C. Updates in grading and invasion assessment in lung adenocarcinoma. Mod. Pathol. 2022, 35, 28–35. [Google Scholar] [CrossRef]
- Li, P.; Liu, L.; Wang, D.; Yang, R.; Xuan, Y.; Han, Y.; Wang, J.; Guo, L.; Zhang, L.; Zhang, S.; et al. Genomic and clinicopathological features of lung adenocarcinomas with micropapillary component. Front. Oncol. 2022, 12, 989349. [Google Scholar] [CrossRef]
- Bai, J.; Deng, C.; Zheng, Q.; Li, D.; Fu, F.; Li, Y.; Zhang, Y.; Chen, H. Comprehensive analysis of mutational profile and prognostic significance of complex glandular pattern in lung adenocarcinoma. Transl. Lung Cancer Res. 2022, 11, 1337–1347. [Google Scholar] [CrossRef]
- Lin, G.; Li, H.; Kuang, J.; Tang, K.; Guo, Y.; Han, A.; Xie, C. Acinar-Predominant Pattern Correlates with Poorer Prognosis in Invasive Mucinous Adenocarcinoma of the Lung. Am. J. Clin. Pathol. 2018, 149, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Tsubokawa, N.; Mimae, T.; Miyata, Y.; Sasada, S.; Yoshiya, T.; Kushitani, K.; Takeshima, Y.; Murakami, S.; Yokose, T.; Ito, H.; et al. Prognostic significance of vascular invasion in intermediate-grade subtype of lung adenocarcinoma. Ultrasound Med. Biol. 2016, 46, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.-C.; Wang, L.-D.; Peng, K.; Yang, J.; Li, X.; Zhong, Z.; Zhang, H.-M.; Ouyang, X.; Xue, Q. Development and Validation of a Nomogram for Predicting the 1-, 3-, and 5-year Survival in Patients with Acinar-predominant Lung Adenocarcinoma. Curr. Med. Sci. 2022, 42, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Chung, Y.S.; A Kim, K.; Shim, H.S. Prognostic factors of acinar- or papillary-predominant adenocarcinoma of the lung. Lung Cancer 2019, 137, 129–135. [Google Scholar] [CrossRef]
- Dong, Y.-J.; Cai, Y.-R.; Zhou, L.-J.; Su, D.; Mu, J.; Chen, X.-J.; Zhang, L. Association between the histological subtype of lung adenocarcinoma, EGFR/KRAS mutation status and the ALK rearrangement according to the novel IASLC/ATS/ERS classification. Oncol. Lett. 2016, 11, 2552–2558. [Google Scholar] [CrossRef]
- Kadota, K.; Sima, C.S.; Arcila, M.E.; Hedvat, C.; Kris, M.G.; Jones, D.R.; Adusumilli, P.S.M.; Travis, W.D. KRAS Mutation Is a Significant Prognostic Factor in Early-stage Lung Adenocarcinoma. Am. J. Surg. Pathol. 2016, 40, 1579–1590. [Google Scholar] [CrossRef]
- Russell, P.A.; Barnett, S.A.; Walkiewicz, M.; Wainer, Z.; Conron, M.; Wright, G.M.; Gooi, J.; Knight, S.; Wynne, R.; Liew, D.; et al. Correlation of Mutation Status and Survival with Predominant Histologic Subtype According to the New IASLC/ATS/ERS Lung Adenocarcinoma Classification in Stage III (N2) Patients. J. Thorac. Oncol. 2013, 8, 461–468. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Q.; Zhang, T.; Li, L.; Xu, C. Minor histological components predict the recurrence of patients with resected stage I acinar- or papillary-predominant lung adenocarcinoma. Front. Oncol. 2022, 12, 1090544. [Google Scholar] [CrossRef]
- Tang, E.R.; Schreiner, A.M.; Pua, B.B. Advances in lung adenocarcinoma classification: A summary of the new international multidisciplinary classification system (IASLC/ATS/ERS). J. Thorac. Dis. 2014, 6, S489–S501. [Google Scholar] [CrossRef]
- Lu, D.; Yang, J.; Liu, X.; Feng, S.; Dong, X.; Shi, X.; Zhai, J.; Mai, S.; Jiang, J.; Wang, Z.; et al. Clinicopathological features, survival outcomes, and appropriate surgical approaches for stage I acinar and papillary predominant lung adenocarcinoma. Cancer Med. 2020, 9, 3455–3462. [Google Scholar] [CrossRef]
- Rokutan-Kurata, M.; Yoshizawa, A.; Ueno, K.; Nakajima, N.; Terada, K.; Hamaji, M.; Sonobe, M.; Menju, T.; Date, H.; Morita, S.; et al. Validation Study of the International Association for the Study of Lung Cancer Histologic Grading System of Invasive Lung Adenocarcinoma. J. Thorac. Oncol. 2021, 16, 1753–1758. [Google Scholar] [CrossRef] [PubMed]
- Detterbeck, F.C.; Woodard, G.A.; Bader, A.S.; Dacic, S.; Grant, M.J.; Park, H.S.; Tanoue, L.T. The Proposed Ninth Edition TNM Classification of Lung Cancer. Chest 2024, 166, 882–895. [Google Scholar] [CrossRef] [PubMed]
- Melosky, B.; Kambartel, K.; Häntschel, M.; Bennetts, M.; Nickens, D.J.; Brinkmann, J.; Kayser, A.; Moran, M.; Cappuzzo, F. Worldwide Prevalence of Epidermal Growth Factor Receptor Mutations in Non-Small Cell Lung Cancer: A Meta-Analysis. Mol. Diagn. Ther. 2021, 26, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zheng, L.; Yang, Q.; Sun, J. The Evolution of BRAF Activation in Non-Small-Cell Lung Cancer. Front. Oncol. 2022, 12, 882940. [Google Scholar] [CrossRef]
- Fujino, T.; Suda, K.; Mitsudomi, T. Lung Cancer with MET exon 14 Skipping Mutation: Genetic Feature, Current Treatments, and Future Challenges. Lung Cancer Targets Ther. 2021, 12, 35–50. [Google Scholar] [CrossRef]
- Ghimessy, A.; Radeczky, P.; Laszlo, V.; Hegedus, B.; Renyi-Vamos, F.; Fillinger, J.; Klepetko, W.; Lang, C.; Dome, B.; Megyesfalvi, Z. Current therapy of KRAS-mutant lung cancer. Cancer Metastasis Rev. 2020, 39, 1159–1177. [Google Scholar] [CrossRef]
- Tibshirani, R. The lasso method for variable selection in the Cox model. Stat. Med. 1997, 16, 385–395. [Google Scholar] [CrossRef]
- Zugazagoitia, J.; Enguita, A.B.; Nuñez, J.A.; Iglesias, L.; Ponce, S. The new IASLC/ATS/ERS lung adenocarcinoma classification from a clinical perspective: Current concepts and future prospects. J. Thorac. Dis. 2014, 6, S526–S536. [Google Scholar] [CrossRef]
- Tsuta, K.; Kawago, M.; Inoue, E.; Yoshida, A.; Takahashi, F.; Sakurai, H.; Watanabe, S.-I.; Takeuchi, M.; Furuta, K.; Asamura, H.; et al. The utility of the proposed IASLC/ATS/ERS lung adenocarcinoma subtypes for disease prognosis and correlation of driver gene alterations. Lung Cancer 2013, 81, 371–376. [Google Scholar] [CrossRef]
- de Melo, A.C.; de Sá, V.K.; Sternberg, C.; Olivieri, E.R.; Werneck da Cunha, I.; Fabro, A.T.; Carraro, D.M.; de Barros e Silva, M.J.; Inada, H.K.P.; de Mello, E.S.; et al. Mutational Profile and New IASLC/ATS/ERS Classification Provide Additional Prognostic Information about Lung Adenocarcinoma: A Study of 125 Patients from Brazil. Oncology 2015, 89, 175–186. [Google Scholar] [CrossRef]
- Arrieta, O.; Cardona, A.F.; Corrales, L.; Campos-Parra, A.D.; Sánchez-Reyes, R.; Amieva-Rivera, E.; Rodríguez, J.; Vargas, C.; Carranza, H.; Otero, J.; et al. The impact of common and rare EGFR mutations in response to EGFR tyrosine kinase inhibitors and platinum-based chemotherapy in patients with non-small cell lung cancer. Lung Cancer 2014, 87, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Caso, R.; Sanchez-Vega, F.; Tan, K.S.; Mastrogiacomo, B.; Zhou, J.; Jones, G.D.; Nguyen, B.; Schultz, N.; Connolly, J.G.; Brandt, W.S.; et al. The Underlying Tumor Genomics of Predominant Histologic Subtypes in Lung Adenocarcinoma. J. Thorac. Oncol. 2020, 15, 1844–1856. [Google Scholar] [CrossRef] [PubMed]
- Renaud, S.; Seitlinger, J.; Falcoz, P.-E.; Schaeffer, M.; Voegeli, A.-C.; Legrain, M.; Beau-Faller, M.; Massard, G. Specific KRAS amino acid substitutions and EGFR mutations predict site-specific recurrence and metastasis following non-small-cell lung cancer surgery. Br. J. Cancer 2016, 115, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-Y.; Lee, S.-W.; Hsu, Y.-C. Drug Resistance in Late-Stage Epidermal Growth Factor Receptor (EGFR)-Mutant Non-Small Cell Lung Cancer Patients After First-Line Treatment with Tyrosine Kinase Inhibitors. Int. J. Mol. Sci. 2025, 26, 2042. [Google Scholar] [CrossRef]
- Nicholson, A.G.; Moreira, A.L.; Mino-Kenudson, M.; Popat, S. Grading in Lung Adenocarcinoma: Another New Normal. J. Thorac. Oncol. 2021, 16, 1601–1604. [Google Scholar] [CrossRef]

| Characteristics | Overall (N = 1263) | AP (N = 716) | AC (N = 547) | p-Value (AP vs. AC) |
|---|---|---|---|---|
| Age | ||||
| Median | 66 | 66 | 66 | 0.34 |
| Min–Max | 26–88 | 37–88 | 26–84 | |
| Sex | ||||
| Male | 478 (37.9%) | 255 (35.6%) | 225 (41.1%) | 0.045 |
| Female | 785 (62.1%) | 461 (64.4%) | 322 (58.9%) | |
| Smoking status | ||||
| Ever | 1194 (94.5%) | 675 (94.3%) | 519 (94.9%) | 0.637 |
| Never | 69 (5.5%) | 41 (5.7%) | 28 (5.1%) | |
| N status | ||||
| N0 | 497 (39.3%) | 270 (37.7%) | 227 (41.5%) | 0.171 |
| N1/2 | 766 (60.7%) | 446 (62.3%) | 320 (58.5%) | |
| Stage | 0.009 | |||
| I | 923 (73.1%) | 543 (75.9%) | 380 (69.5%) | |
| II | 219 (17.3%) | 119 (16.6%) | 100 (18.3%) | |
| III | 121 (9.6%) | 54 (7.5%) | 67 (12.2%) | |
| Tumor size (cm) | ||||
| Median | 2.3 | 2.3 | 2.5 | 0.0009 |
| Range | 1–15 | 1–15 | 1–12.5 | |
| Grade | <0.00001 | |||
| 1 | 136 (10.8%) | 0 (0.0%) | 136 (24.9%) | |
| 2 | 437 (34.6%) | 387 (54.1%) | 50 (9.1%) | |
| 3 | 690 (54.6%) | 329 (45.9%) | 361 (66.0%) | |
| Type of surgery | 0.961 | |||
| Lobectomy | 954 (75.6%) | 539 (75.3%) | 415 (75.9%) | |
| Segmentectomy | 132 (10.5%) | 75 (10.5%) | 57 (10.4%) | |
| Other | 177 (14.0%) | 102 (14.3%) | 75 (13.7%) | |
| Tumor localization | 0.045 | |||
| Left Upper Lobe | 323 (25.6%) | 186 (26.0%) | 137 (25.1%) | |
| Left Lower Lobe | 153 (12.1%) | 84 (11.7%) | 69 (12.6%) | |
| Right Upper Lobe | 486 (38.5%) | 295 (41.2%) | 189 (34.5%) | |
| Right Lower Lobe | 202 (16.0%) | 101 (14.1%) | 103 (18.8%) | |
| Other | 99 (7.8%) | 50 (7.0%) | 49 (9.0%) | |
| STAS | ||||
| Yes | 537 (42.5%) | 312 (43.6%) | 225 (41.1%) | 0.384 |
| No | 726 (57.5%) | 404 (56.4%) | 322 (58.9%) | |
| LVI | ||||
| Yes | 592 (46.9%) | 329 (46.0%) | 263 (48.1%) | 0.452 |
| No | 571 (45.2%) | 387 (54.0%) | 284 (51.9%) | |
| VPI | ||||
| Yes | 343 (27.2%) | 194 (27.1%) | 149 (27.2%) | 0.954 |
| No | 920 (72.8%) | 522 (72.9%) | 398 (72.8%) | |
| Mutational status | ||||
| KRAS-G12C | 264 (20.9%) | 149 (20.8%) | 115 (21.0%) | 0.981 |
| KRAS-G12V | 119 (9.4%) | 67 (9.4%) | 52 (9.5%) | 1.000 |
| KRAS-G12D | 62 (4.9%) | 33 (4.6%) | 29 (5.3%) | 0.664 |
| KRAS-G12A | 39 (3.1%) | 22 (3.1%) | 17 (3.1%) | 1.000 |
| KRAS-G12X | 24 (1.9%) | 12 (1.7%) | 12 (2.2%) | 0.645 |
| KRAS-G13X | 34 (2.7%) | 18 (2.5%) | 16 (2.9%) | 0.785 |
| KRAS-Q61H | 33 (2.6%) | 19 (2.7%) | 14 (2.6%) | 1.000 |
| KRAS-Q61L | 11 (0.9%) | 5 (0.7%) | 6 (1.1%) | 0.652 |
| EGFR-Del-19 | 59 (4.7%) | 43 (6.0%) | 16 (2.9%) | 0.014 |
| EGFR-L858R | 62 (4.9%) | 39 (5.4%) | 23 (4.2%) | 0.378 |
| EGFR-Ins 20 | 13 (1.0%) | 8 (1.1%) | 5 (0.9%) | 0.941 |
| EGFR-Other | 35 (2.8%) | 19 (2.7%) | 16 (2.9%) | 0.905 |
| MET-Exon 14 | 43 (3.4%) | 23 (3.2%) | 20 (3.7%) | 0.783 |
| BRAF-V600E | 13 (1.0%) | 8 (1.1%) | 5 (0.9%) | 0.941 |
| BRAF-Other | 41 (3.2%) | 25 (3.5%) | 16 (2.9%) | 0.687 |
| PIK3CA | 17 (1.3%) | 7 (1.0%) | 10 (1.8%) | 0.292 |
| Other | 38 (3.0%) | 24 (3.4%) | 14 (2.6%) | 0.515 |
| WT | 356 (28.2%) | 195 (27.2%) | 161 (29.4%) | 0.425 |
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| p-Value | OR (95% CI) | p-Value | OR (95% CI) | |
| Acinar: AP (vs. AC) | 0.011 | 1.187 (1.101–1.219) | 0.030 | 1.951 (1.701–2.205) |
| TNM stage: II, III (vs. I) | 0.791 | 0.922 (0.490–1.641) | ||
| Age | 0.821 | 1.003 (0.971–1.038) | ||
| Sex: female (vs. Male) | 0.146 | 1.538 (0.877–2.795) | ||
| Smoking: never (vs. ever) | 1.85 × 10−9 | 7.260 (6.107–8.004) | 1.39 × 10−7 | 6.22 (5.066–7.060) |
| Grade: 3 (vs. 1, 2) | 0.007 | 0.477 (0.324–0.551) | 0.773 | 0.905 (0.457–1.762) |
| LVI: presence (vs. absence) | 0.330 | 0.767 (0.446–1.229) | ||
| VPI: presence (vs. absence) | 0.231 | 0.673 (0.337–1.243) | ||
| STAS: presence (vs. absence) | 0.058 | 0.579 (0.331–1.004) | ||
| Lepidic-predominant: Yes (vs. No) | 0.002 | 2.198 (1.877–3.022) | 0.055 | 2.158 (1.019–5.021) |
| Papillary-predominant: Yes (vs. No) | 0.665 | 0.875 (0.695–0.978) | ||
| Micropapillary-predominant: Yes (vs. No) | 0.939 | 0.979 (0.576–1.688) | ||
| Solid-predominant: Yes (vs. No) | 0.019 | 0.520 (0.396–0.692) | 0.530 | 0.814 (0.424–1.537) |
| CGP-predominant: Yes (vs. No) | 0.785 | 0.910 (0.555–1.492) | ||
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Acinar: AC (vs. AP) | 1.358 (1.188–1.541) | 0.006 | 1.240 (1.103–1.312) | 0.04 |
| TNM stage: II, III (vs. I) | 2.481 (1.980–3.109) | 2.76 × 10−15 | 1.830 (1.533–2.118) | 1.25 × 10−6 |
| Age | 1.379 (1.265–0.422) | 0.002 | 1.293 (1.199–1.321) | 0.014 |
| Sex: Female (vs. Male) | 0.926 (0.738–1.162) | 0.506 | ||
| Smoking status: Never (vs. Ever) | 0.745 (0.427–1.299) | 0.300 | ||
| Grade: 3 (vs. 1/2) | 2.049 (1.816–2.401) | 3.38 × 10−9 | 1.281 (0.981–1.445) | 0.114 |
| LVI: Present (vs. Absent) | 1.935 (1.737–2.007) | 2 × 10−8 | 1.270 (0.974–1.356) | 0.076 |
| VPI: Present (vs. Absent) | 1.500 (1.391–1.689) | 0.0005 | 1.130 (0.985–1.343) | 0.324 |
| STAS: Present (vs. Absent) | 1.399 (1.279–1.549) | 0.003 | 1.119 (0.082–1.220) | 0.352 |
| Lepidic-predominant: Yes (vs. No) | 0.592 (0.473–0.660) | 4.1 × 10−6 | 0.859 (0.769–1.003) | 0.234 |
| Papillary-predominant: Yes (vs. No) | 0.927 (0.721–1.191) | 0.555 | ||
| Micropapillary-predominant: Yes (vs. No) | 1.383 (1.230–1.540) | 0.005 | 1.296 (1.107–1.367) | 0.043 |
| Solid-predominant: Yes (vs. No) | 1.551 (1.335–1.646) | 0.0001 | 1.008 (0.876–1.127) | 0.951 |
| CGP-predominant: Yes (vs. No) | 1.417 (1.297–1.513) | 0.005 | 1.146 (0.983–1.289) | 0.303 |
| KRAS: Mutant (vs. WT) | 1.151 (0.921–1.438) | 0.214 | ||
| EGFR: Mutant (vs. WT) | 0.997 (0.715–1.391) | 0.989 | ||
| BRAF: Mutant (vs. WT) | 1.101 (0.655–1.852) | 0.716 | ||
| MET: Mutant (vs. WT) | 0.615 (0.305–1.243) | 0.176 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abolfathi, H.; Kordahi, M.; Armero, V.S.; Gaudreault, N.; Boudreau, D.K.; Gagné, A.; Orain, M.; Fiset, P.O.; Desmeules, P.; Lamaze, F.C.; et al. A Comprehensive Evaluation of Clinicopathologic Characteristics, Molecular Features and Prognosis in Lung Adenocarcinoma with an Acinar Component. Cancers 2025, 17, 1825. https://doi.org/10.3390/cancers17111825
Abolfathi H, Kordahi M, Armero VS, Gaudreault N, Boudreau DK, Gagné A, Orain M, Fiset PO, Desmeules P, Lamaze FC, et al. A Comprehensive Evaluation of Clinicopathologic Characteristics, Molecular Features and Prognosis in Lung Adenocarcinoma with an Acinar Component. Cancers. 2025; 17(11):1825. https://doi.org/10.3390/cancers17111825
Chicago/Turabian StyleAbolfathi, Hanie, Manal Kordahi, Victoria Saavedra Armero, Nathalie Gaudreault, Dominique K. Boudreau, Andréanne Gagné, Michèle Orain, Pierre Oliver Fiset, Patrice Desmeules, Fabien Claude Lamaze, and et al. 2025. "A Comprehensive Evaluation of Clinicopathologic Characteristics, Molecular Features and Prognosis in Lung Adenocarcinoma with an Acinar Component" Cancers 17, no. 11: 1825. https://doi.org/10.3390/cancers17111825
APA StyleAbolfathi, H., Kordahi, M., Armero, V. S., Gaudreault, N., Boudreau, D. K., Gagné, A., Orain, M., Fiset, P. O., Desmeules, P., Lamaze, F. C., Bossé, Y., & Joubert, P. (2025). A Comprehensive Evaluation of Clinicopathologic Characteristics, Molecular Features and Prognosis in Lung Adenocarcinoma with an Acinar Component. Cancers, 17(11), 1825. https://doi.org/10.3390/cancers17111825







