Single-Center Cohort of Pediatric Patients with High-Risk Neuroblastoma Receiving Immunotherapy
Simple Summary
Abstract
1. Introduction
2. Material and Methods
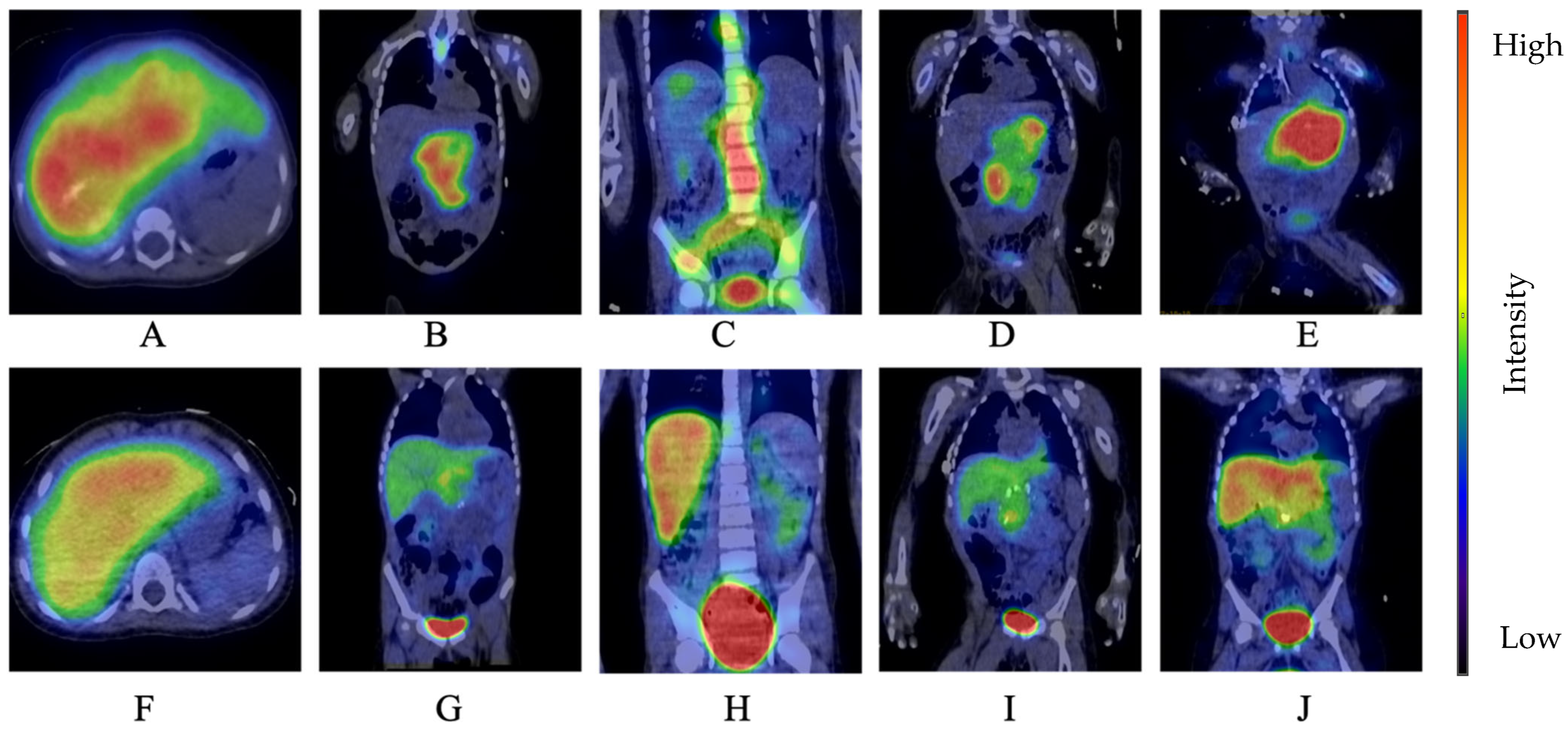
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campbell, K.; Siegel, D.A.; Umaretiya, P.J.; Dai, S.; Heczey, A.; Lupo, P.J.; Schraw, J.M.; Thompson, T.D.; Scheurer, M.E.; Foster, J.H. A comprehensive analysis of neuroblastoma incidence, survival, and racial and ethnic disparities from 2001 to 2019. Pediatr. Blood Cancer 2024, 71, e30732. [Google Scholar] [CrossRef] [PubMed]
- Nyári, T.A.; Kajtár, P.; Parker, L.; Hungarian Paediatric Oncology Group. Neuroblastoma in hungary. Eur. J. Cancer 2006, 42, 2350–2354. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.S.; Naranjo, A.; Zhang, F.F.; Cohn, S.L.; London, W.B.; Gastier-Foster, J.M.; Ramirez, N.C.; Pfau, R.; Reshmi, S.; Wagner, E.; et al. Revised Neuroblastoma Risk Classification System. J. Clin. Oncol. 2021, 39, 3229–3241. [Google Scholar] [CrossRef] [PubMed]
- Twist, C.J.; Schmidt, M.L.; Naranjo, A.; London, W.B.; Tenney, S.C.; Marachelian, A.; Shimada, H.; Collins, M.H.; Esiashvili, N.; Adkins, E.S.; et al. Maintaining outstanding outcomes using response- and biology-based therapy for intermediate-risk neuroblastoma: A report from the Children’s Oncology Group study ANBL0531. J. Clin. Oncol. 2019, 37, 3243–3255. [Google Scholar] [CrossRef]
- Qiu, B.; Matthay, K.K. Advancing therapy for neuroblastoma. Nat. Rev. Clin. Oncol. 2022, 19, 515–533. [Google Scholar] [CrossRef]
- Brodeur, G.M.; Pritchard, J.; Berthold, F.; Carlsen, N.L.; Castel, V.; Castelberry, R.P.; De Bernardi, B.; Evans, A.E.; Favrot, M.; Hedborg, F.; et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J. Clin. Oncol. 1993, 11, 1466–1477. [Google Scholar] [CrossRef]
- Wolden, S.L.; Gollamudi, S.V.; Kushner, B.H.; LaQuaglia, M.; Kramer, K.; Rosen, N.; Abramson, S.; Cheung, N.V. Local control with multimodality therapy for stage 4 neuroblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2000, 46, 969–974. [Google Scholar] [CrossRef]
- Haas-Kogan, D.A.; Swift, P.S.; Selch, M.; Haase, G.M.; Seeger, R.C.; Gerbing, R.B.; Stram, D.O.; Matthay, K.K. Impact of radiotherapy for high-risk neuroblastoma: A Children’s Cancer Group study. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 28–39. [Google Scholar] [CrossRef]
- McGinty, L.; Kolesar, J. Dinutuximab for maintenance therapy in pediatric neuroblastoma. Am. J. Health Syst. Pharm. 2017, 74, 563–567. [Google Scholar] [CrossRef]
- High-Risk Neuroblastoma Study 2 of SIOP-Europa-Neuroblastoma (SIOPEN) (HR-NBL2) ClinicalTrials.gov ID NCT04221035. Available online: https://clinicaltrials.gov/ct2/show/NCT04221035 (accessed on 25 May 2025).
- Del Bufalo, F.; De Angelis, B.; Caruana, I.; Del Baldo, G.; De Ioris, M.A.; Serra, A.; Mastronuzzi, A.; Cefalo, M.G.; Pagliara, D.; Amicucci, M.; et al. GD2-CART01 for Relapsed or Refractory High-Risk Neuroblastoma. N. Engl. J. Med. 2023, 388, 1284–1295. [Google Scholar] [CrossRef]
- Gray, J. A Phase I Study of 131-1 mIBG Followed by Nivolumab and Dinutuximab Beta Antibody in Children with Relapsed/Refractory Neuroblastoma. (The MINIVAN Trial). Available online: https://www.siopen.org/siopen-clinical-trials-1/a-phase-i-study-of-131-1-mibg-followed-by-nivolumab-and-dinutuximab-beta-antibody-in-children-with-relapsed/refractory-neuroblastoma-the-minivan-trial (accessed on 25 May 2025).
- Ehlert, K.; Hansjuergens, I.; Zinke, A.; Otto, S.; Siebert, N.; Henze, G.; Lode, H. Nivolumab and dinutuximab beta in two patients with refractory neuroblastoma. J. Immunother. Cancer 2020, 8, e000540. [Google Scholar] [CrossRef] [PubMed]
- Siebert, N.; Zumpe, M.; Schwencke, C.H.; Biskupski, S.; Troschke-Meurer, S.; Leopold, J.; Zikoridse, A.; Lode, H.N. Combined Blockade of TIGIT and PD-L1 Enhances Anti-Neuroblastoma Efficacy of GD2-Directed Immunotherapy with Dinutuximab Beta. Cancers 2023, 15, 3317. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.L.; Fox, E.; Isikwei, E.; Reid, J.M.; Liu, X.; Minard, C.G.; Voss, S.; Berg, S.L.; Weigel, B.J.; Mackall, C.L. A Phase I/II Trial of Nivolumab plus Ipilimumab in Children and Young Adults with Relapsed/Refractory Solid Tumors: A Children’s Oncology Group Study ADVL1412. Clin. Cancer Res. 2022, 28, 5088–5097. [Google Scholar] [CrossRef]
- Dhillon, S. Dinutuximab: First global approval. Drugs 2015, 75, 923–927. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.L.; Gilman, A.L.; Ozkaynak, M.F.; London, W.B.; Kreissman, S.G.; Chen, H.X.; Smith, M.; Anderson, B.; Villablanca, J.G.; Matthay, K.K.; et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med. 2010, 363, 1324–1334. [Google Scholar] [CrossRef]
- Ladenstein, R.; Pötschger, U.; Valteau-Couanet, D.; Luksch, R.; Castel, V.; Ash, S.; Laureys, G.; Brock, P.; Michon, J.M.; Owens, C.; et al. Investigation of the Role of Dinutuximab Beta-Based Immunotherapy in the SIOPEN High-Risk Neuroblastoma 1 Trial (HR-NBL1). Cancers 2020, 12, 309. [Google Scholar] [CrossRef]
- Keyel, M.E.; Reynolds, C.P. Spotlight on dinutuximab in the treatment of high-risk neuroblastoma: Development and place in therapy. Biologics 2019, 13, 1–12. [Google Scholar] [CrossRef]
- Gur, H.; Ozen, F.; Can Saylan, C.; Atasever Arslan, B. Dinutuximab in the treatment of high risk neuroblastoma in children. Clin. Med. Insights Ther. 2017, 9, 1179559X17719106. [Google Scholar] [CrossRef]
- Mohd, A.B.; Mohd, O.B.; Alabdallat, Y.J.; Al Dwairy, S.Y.; Ghannam, R.A.; Hanaqtah, B.M.; Albakri, K.A. Safety and efficacy of dinutuximab in the treatment of neuroblastoma: A review. J. Res. Med. Sci. 2023, 28, 71. [Google Scholar] [CrossRef]
- Wieczorek, A.; Żebrowska, U.; Ussowicz, M.; Sokół, A.; Stypińska, M.; Dembowska-Bagińska, B.; Pawińska-Wąsikowska, K.; Balwierz, W. Dinutuximab Beta Maintenance Therapy in Patients with High-Risk Neuroblastoma in First-Line and Refractory/Relapsed Settings-Real-World Data. J. Clin. Med. 2023, 12, 5252. [Google Scholar] [CrossRef]
- Wieczorek, A.; Zaniewska-Tekieli, A.; Ehlert, K.; Pawinska-Wasikowska, K.; Balwierz, W.; Lode, H. Dinutuximab beta combined with chemotherapy in patients with relapsed or refractory neuroblastoma. Front. Oncol. 2023, 13, 1082771. [Google Scholar] [CrossRef]
- Hoshi, Y.; Enokida, T.; Tamura, S.; Nakashima, T.; Okano, S.; Fujisawa, T.; Sato, M.; Wada, A.; Tanaka, H.; Takeshita, N.; et al. Efficacy of anti-PD-1 monotherapy for recurrent or metastatic olfactory neuroblastoma. Front. Oncol. 2024, 14, 1379013. [Google Scholar] [CrossRef]
- Pasqualini, C.; Rubino, J.; Brard, C.; Cassard, L.; André, N.; Rondof, W.; Scoazec, J.Y.; Marchais, A.; Nebchi, S.; Boselli, L.; et al. Phase II and biomarker study of programmed cell death protein 1 inhibitor nivolumab and metronomic cyclophosphamide in paediatric relapsed/refractory solid tumours: Arm G of AcSe-ESMART, a trial of the European Innovative Therapies for Children With Cancer Consortium. Eur. J. Cancer 2021, 150, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.L.; Fox, E.; Merchant, M.S.; Reid, J.M.; Kudgus, R.A.; Liu, X.; Minard, C.G.; Voss, S.; Berg, S.L.; Weigel, B.J.; et al. Nivolumab in children and young adults with relapsed or refractory solid tumours or lymphoma (ADVL1412): A multicentre, open-label, single-arm, phase 1-2 trial. Lancet Oncol. 2020, 21, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Wagner, L.M.; Adams, V.R. Targeting the PD-1 pathway in pediatric solid tumors and brain tumors. OncoTargets Ther. 2017, 10, 2097–2106. [Google Scholar] [CrossRef] [PubMed]
- Siebert, N.; Zumpe, M.; Jüttner, M.; Troschke-Meurer, S.; Lode, H.N. PD-1 blockade augments anti-neuroblastoma immune response induced by anti-GD(2) antibody ch14.18/CHO. Oncoimmunology 2017, 6, e1343775. [Google Scholar] [CrossRef]
- Lode, H.N.; Ladenstein, R.; Troschke-Meurer, S.; Struppe, L.; Siebert, N.; Zumpe, M.; Ehlert, K.; Huber, S.; Glogova, E.; Hundsdoerfer, P.; et al. Effect and Tolerance of N5 and N6 Chemotherapy Cycles in Combination with Dinutuximab Beta in Relapsed High-Risk Neuroblastoma Patients Who Failed at Least One Second-Line Therapy. Cancers 2023, 15, 3364. [Google Scholar] [CrossRef]
- Mora, J.; Chan, G.C.F.; Morgenstern, D.A.; Amoroso, L.; Nysom, K.; Faber, J.; Wingerter, A.; Bear, M.K.; Rubio-San-Simon, A.; de Las Heras, B.M.; et al. The anti-GD2 monoclonal antibody naxitamab plus GM-CSF for relapsed or refractory high-risk neuroblastoma: A phase 2 clinical trial. Nat. Commun. 2025, 16, 1636. [Google Scholar] [CrossRef]
- Troschke-Meurer, S.; Zumpe, M.; Meißner, L.; Siebert, N.; Grabarczyk, P.; Forkel, H.; Maletzki, C.; Bekeschus, S.; Lode, H.N. Chemotherapeutics Used for High-Risk Neuroblastoma Therapy Improve the Efficacy of Anti-GD2 Antibody Dinutuximab Beta in Preclinical Spheroid Models. Cancers 2023, 15, 904. [Google Scholar] [CrossRef]
- Xu, C.; Chen, Y.P.; Du, X.J.; Liu, J.Q.; Huang, C.L.; Chen, L.; Zhou, G.Q.; Li, W.F.; Mao, Y.P.; Hsu, C.; et al. Comparative safety of immune checkpoint inhibitors in cancer: Systematic review and network meta-analysis. BMJ 2018, 363, k4226. [Google Scholar] [CrossRef]
- Haanen, J.B.A.G.; Carbonnel, F.; Robert, C.; Kerr, K.M.; Peters, S.; Larkin, J.; Jordan, K.; ESMO Guidelines Committee. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28 (Suppl. S4), iv119–iv142. [Google Scholar] [CrossRef]
- Lozano, R.; Franco, M.E.; Bona, C. Severe autoimmune hemolytic anemia associated with glucose-6-phosphate dehydrogenase deficiency as a complication of nivolumab treatment: A case report. Int. J. Clin. Pharmacol. Ther. 2025. online ahead of print. [Google Scholar] [CrossRef]
| Characteristics | N (%) |
|---|---|
| Male | 8 (53%) |
| Female | 7 (46%) |
| Age (median, range) | 1 year, 7 months–8 years |
| High-risk neuroblastoma | 12 (80%) |
| MYCN amplification | 2 (13.3%) |
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| Age at diagnosis | 8 months | 5 years | 7 years | 3 years | 7 months |
| Sex | Male | Female | Male | Male | Female |
| Tumor location | Right adrenal gland tu. paraaortic lymph node periorbital metastases MYCN+ | Retroperitoneal tu. hepatic, retroperitoneal lymph node MYCN– inoperable | Right-sided paravertebral tu. mpx bone met. MYCN– | Retroperitoneal tu. paravertebral met. mpx bone met. MYCN– inoperable | Retroperitoneal tu. mesenterial mass mpx cranial bone met. MYCN– inoperable |
| Induction treatment | Chemotherapy (2 VP-Carbo, 4 CADO) Primary tu. resection Chemotherapy (Rapid COJEC) auto-HSCT radiotherapy | Chemotherapy (1 CO, Rapid COJEC, 4 TVD) auto-HSCT | Paravertebral tu. resection Chemotherapy (Rapid COJEC, 2 TVD) auto-HSCTradiotherapy | Chemotherapy (Rapid COJEC, 2 TVD) auto-HSCT radiotherapy | Chemotherapy (2 CO, 4 VP-Carbo, 4 CADO, Rapid COJEC, 2 TVD) auto-HSCT |
| Residual disease before immunoth. | Post-HSCT BM: 1–2% Post-RTx MR: CR | Post-HSCT BM: neg. Post-RTx MR: resid.tu Post-RTx mIBG: resid.tu | Post-HSCT BM: 5–10% Post-RTx MR: mpx bone met Post-RTx mIBG: neg. | Post-HSCT BM: neg. Post-RTx MR: resid.tu Post-RTx mIBG: resid.tu | Post-HSCT BM: neg. Post-HSCT MR: resid. tu. Post-HSCT mIBG: resid. tu. |
| Immunotherapy | Dinutuximab beta + Isotretinoin | Dinutuximabbeta + Isotretinoin | Dinutuximab beta + Isotretinoin | Dinutuximab beta + Isotretinoin | Dinutuximab beta + Cyclo-phosphamide + Irinotecan |
| Results after 5 cycles of Dinutuximab beta | CR | PR/SD | CR Relapse (4 months later) | PR/SD | PR |
| Further treatment and results | - | Nivolumab + Etoposide Progression | TEMIRI Alternative treatment Progression | Nivolumab + Etoposide Progression | Nivolumab + Etoposide Progression |
| Further treatment after progression—ongoing treatments | - | TEMIRI + Dinutuximab beta | - | TEMIRI + Dinutuximab beta | Nivolumab + TEMIRI + Dinutuximab beta |
| Latest follow-up results | CR | PR/SD | End-stage, progressive disease | PR/SD | PR/SD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zsigrai, E.; Barna, S.; Gaál, Z.; Macsi, L.; Szegedi, I.; Petrás, M.; Kiss, C. Single-Center Cohort of Pediatric Patients with High-Risk Neuroblastoma Receiving Immunotherapy. Cancers 2025, 17, 1824. https://doi.org/10.3390/cancers17111824
Zsigrai E, Barna S, Gaál Z, Macsi L, Szegedi I, Petrás M, Kiss C. Single-Center Cohort of Pediatric Patients with High-Risk Neuroblastoma Receiving Immunotherapy. Cancers. 2025; 17(11):1824. https://doi.org/10.3390/cancers17111824
Chicago/Turabian StyleZsigrai, Emese, Sándor Barna, Zsuzsanna Gaál, Lilla Macsi, István Szegedi, Miklós Petrás, and Csongor Kiss. 2025. "Single-Center Cohort of Pediatric Patients with High-Risk Neuroblastoma Receiving Immunotherapy" Cancers 17, no. 11: 1824. https://doi.org/10.3390/cancers17111824
APA StyleZsigrai, E., Barna, S., Gaál, Z., Macsi, L., Szegedi, I., Petrás, M., & Kiss, C. (2025). Single-Center Cohort of Pediatric Patients with High-Risk Neuroblastoma Receiving Immunotherapy. Cancers, 17(11), 1824. https://doi.org/10.3390/cancers17111824








