Overall Survival and Prognostic Factors in De Novo Metastatic Human Epidermal Growth Factor Receptor (HER)-2-Positive Breast Cancer: A National Cancer Database Analysis
Simple Summary
Abstract
1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast Cancer-Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies-An Updated Review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef] [PubMed]
- Kohler, B.A.; Sherman, R.L.; Howlader, N.; Jemal, A.; Ryerson, A.B.; Henry, K.A.; Boscoe, F.P.; Cronin, K.A.; Lake, A.; Noone, A.M.; et al. Annual Report to the Nation on the Status of Cancer, 1975-2011, Featuring Incidence of Breast Cancer Subtypes by Race/Ethnicity, Poverty, and State. J. Natl. Cancer Inst. 2015, 107, djv048. [Google Scholar] [CrossRef] [PubMed]
- Noone, A.M.; Cronin, K.A.; Altekruse, S.F.; Howlader, N.; Lewis, D.R.; Petkov, V.I.; Penberthy, L. Cancer Incidence and Survival Trends by Subtype Using Data from the Surveillance Epidemiology and End Results Program, 1992–2013. Cancer Epidemiol. Biomark. Prev. 2017, 26, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Gianni, L. HER2-positive breast cancer. Lancet 2017, 389, 2415–2429. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, Y.; Ye, F.; Luo, D.; Jin, Y.; Li, Y.; Zhao, W.; Chen, B.; Wang, L.; Yang, Q. Histologic heterogeneity predicts patient prognosis of HER2-positive metastatic breast cancer: A retrospective study based on SEER database. Cancer Med. 2023, 12, 18597–18610. [Google Scholar] [CrossRef]
- Sánchez-Lorenzo, L.; Bachiller, A.; Gea, C.; Espinós, J. Current Management and Future Perspectives in Metastatic HER2-Positive Breast Cancer. Semin. Oncol. Nurs. 2024, 40, 151554. [Google Scholar] [CrossRef]
- Swain, S.M.; Miles, D.; Kim, S.B.; Im, Y.H.; Im, S.A.; Semiglazov, V.; Ciruelos, E.; Schneeweiss, A.; Loi, S.; Monturus, E.; et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): End-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 519–530. [Google Scholar] [CrossRef]
- Tarantino, P.; Curigliano, G.; Parsons, H.A.; Lin, N.U.; Krop, I.; Mittendorf, E.A.; Waks, A.; Winer, E.P.; Tolaney, S.M. Aiming at a Tailored Cure for ERBB2-Positive Metastatic Breast Cancer: A Review. JAMA Oncol. 2022, 8, 629–635. [Google Scholar] [CrossRef]
- Gong, I.Y.; Yan, A.T.; Earle, C.C.; Trudeau, M.E.; Eisen, A.; Chan, K.K.W. Comparison of outcomes in a population-based cohort of metastatic breast cancer patients receiving anti-HER2 therapy with clinical trial outcomes. Breast Cancer Res. Treat. 2020, 181, 155–165. [Google Scholar] [CrossRef]
- Kesireddy, M.; Elsayed, L.; Shostrom, V.K.; Agarwal, P.; Asif, S.; Yellala, A.; Krishnamurthy, J. Overall Survival and Prognostic Factors in Metastatic Triple-Negative Breast Cancer: A National Cancer Database Analysis. Cancers 2024, 16, 1791. [Google Scholar] [CrossRef]
- Vasista, A.; Stockler, M.R.; West, T.; Wilcken, N.; Kiely, B.E. More than just the median: Calculating survival times for patients with HER2 positive, metastatic breast cancer using data from recent randomised trials. Breast 2017, 31, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, D.; Brufsky, A.; Cobleigh, M.; Jahanzeb, M.; Kaufman, P.A.; Mason, G.; O’Shaughnessy, J.; Rugo, H.S.; Swain, S.M.; Yardley, D.A.; et al. De Novo Versus Recurrent HER2-Positive Metastatic Breast Cancer: Patient Characteristics, Treatment, and Survival from the SystHERs Registry. Oncologist 2020, 25, e214–e222. [Google Scholar] [CrossRef] [PubMed]
- Lobbezoo, D.J.; van Kampen, R.J.; Voogd, A.C.; Dercksen, M.W.; van den Berkmortel, F.; Smilde, T.J.; van de Wouw, A.J.; Peters, F.P.; van Riel, J.M.; Peters, N.A.; et al. Prognosis of metastatic breast cancer: Are there differences between patients with de novo and recurrent metastatic breast cancer? Br. J. Cancer 2015, 112, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Valachis, A.; Carlqvist, P.; Ma, Y.; Szilcz, M.; Freilich, J.; Vertuani, S.; Holm, B.; Lindman, H. Overall survival of patients with metastatic breast cancer in Sweden: A nationwide study. Br. J. Cancer 2022, 127, 720–725. [Google Scholar] [CrossRef]
- Frank, S.; Carton, M.; Dubot, C.; Campone, M.; Pistilli, B.; Dalenc, F.; Mailliez, A.; Levy, C.; D’Hondt, V.; Debled, M.; et al. Impact of age at diagnosis of metastatic breast cancer on overall survival in the real-life ESME metastatic breast cancer cohort. Breast 2020, 52, 50–57. [Google Scholar] [CrossRef]
- Murthy, P.; Kidwell, K.M.; Schott, A.F.; Merajver, S.D.; Griggs, J.J.; Smerage, J.D.; Van Poznak, C.H.; Wicha, M.S.; Hayes, D.F.; Henry, N.L. Clinical predictors of long-term survival in HER2-positive metastatic breast cancer. Breast Cancer Res. Treat. 2016, 155, 589–595. [Google Scholar] [CrossRef]
- Salas, M.; Henderson, M.; Sundararajan, M.; Tu, N.; Islam, Z.; Ebeid, M.; Horne, L. Use of comorbidity indices in patients with any cancer, breast cancer, and human epidermal growth factor receptor-2-positive breast cancer: A systematic review. PLoS ONE 2021, 16, e0252925. [Google Scholar] [CrossRef]
- Tsai, Y.F.; Tseng, L.M.; Lien, P.J.; Hsu, C.Y.; Lin, Y.S.; King, K.L.; Wang, Y.L.; Chao, T.C.; Liu, C.Y.; Chiu, J.H.; et al. HER2 immunohistochemical scores provide prognostic information for patients with HER2-type invasive breast cancer. Histopathology 2019, 74, 578–586. [Google Scholar] [CrossRef]
- Lee, H.J.; Seo, A.N.; Kim, E.J.; Jang, M.H.; Suh, K.J.; Ryu, H.S.; Kim, Y.J.; Kim, J.H.; Im, S.A.; Gong, G.; et al. HER2 heterogeneity affects trastuzumab responses and survival in patients with HER2-positive metastatic breast cancer. Am. J. Clin. Pathol. 2014, 142, 755–766. [Google Scholar] [CrossRef]
- Rivenbark, A.G.; O’Connor, S.M.; Coleman, W.B. Molecular and cellular heterogeneity in breast cancer: Challenges for personalized medicine. Am. J. Pathol. 2013, 183, 1113–1124. [Google Scholar] [CrossRef]
- Arpino, G.; de la Haba Rodríguez, J.; Ferrero, J.M.; De Placido, S.; Osborne, C.K.; Klingbiel, D.; Revelant, V.; Wohlfarth, C.; Poppe, R.; Rimawi, M.F.; et al. Pertuzumab, Trastuzumab, and an Aromatase Inhibitor for HER2-Positive and Hormone Receptor-Positive Metastatic or Locally Advanced Breast Cancer: PERTAIN Final Analysis. Clin. Cancer Res. 2023, 29, 1468–1476. [Google Scholar] [CrossRef] [PubMed]
- Lobbezoo, D.J.; van Kampen, R.J.; Voogd, A.C.; Dercksen, M.W.; van den Berkmortel, F.; Smilde, T.J.; van de Wouw, A.J.; Peters, F.P.; van Riel, J.M.; Peters, N.A.; et al. Prognosis of metastatic breast cancer subtypes: The hormone receptor/HER2-positive subtype is associated with the most favorable outcome. Breast Cancer Res. Treat. 2013, 141, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Gobbini, E.; Ezzalfani, M.; Dieras, V.; Bachelot, T.; Brain, E.; Debled, M.; Jacot, W.; Mouret-Reynier, M.A.; Goncalves, A.; Dalenc, F.; et al. Time trends of overall survival among metastatic breast cancer patients in the real-life ESME cohort. Eur. J. Cancer 2018, 96, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhu, Y.; Liu, X.; Liao, X.; He, J.; Niu, L. The Clinicopathological features and survival outcomes of patients with different metastatic sites in stage IV breast cancer. BMC Cancer 2019, 19, 1091. [Google Scholar] [CrossRef]
- Raghavendra, A.S.; Ibrahim, N.K. Breast Cancer Brain Metastasis: A Comprehensive Review. JCO Oncol. Pract. 2024, 20, 1348–1359. [Google Scholar] [CrossRef]
- Mendes, D.; Alves, C.; Afonso, N.; Cardoso, F.; Passos-Coelho, J.L.; Costa, L.; Andrade, S.; Batel-Marques, F. The benefit of HER2-targeted therapies on overall survival of patients with metastatic HER2-positive breast cancer--a systematic review. Breast Cancer Res. 2015, 17, 140. [Google Scholar] [CrossRef]
- Cardoso, M.J.; Mokbel, K. Locoregional therapy in de novo metastatic breast cancer. The unanswered question. Breast 2021, 58, 170–172. [Google Scholar] [CrossRef]
- Rugo, H.S.; Brufsky, A.M.; Ulcickas Yood, M.; Tripathy, D.; Kaufman, P.A.; Mayer, M.; Yoo, B.; Abidoye, O.O.; Yardley, D.A. Racial disparities in treatment patterns and clinical outcomes in patients with HER2-positive metastatic breast cancer. Breast Cancer Res. Treat. 2013, 141, 461–470. [Google Scholar] [CrossRef]
- Burgaleta, A.M.; Burguete, A.B.; Gutiérrez, L.R.; Nuín, E.B.; Felipe, G.A.; de la Vega, F.A. Local treatment in oligometastasis from breast cancer: An overview. Clin. Transl. Oncol. 2023, 25, 2861–2867. [Google Scholar] [CrossRef]
- Blumenthal, G.M.; Scher, N.S.; Cortazar, P.; Chattopadhyay, S.; Tang, S.; Song, P.; Liu, Q.; Ringgold, K.; Pilaro, A.M.; Tilley, A.; et al. First FDA approval of dual anti-HER2 regimen: Pertuzumab in combination with trastuzumab and docetaxel for HER2-positive metastatic breast cancer. Clin. Cancer Res. 2013, 19, 4911–4916. [Google Scholar] [CrossRef]
- Swain, S.M.; Baselga, J.; Kim, S.B.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.M.; Schneeweiss, A.; Heeson, S.; et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N. Engl. J. Med. 2015, 372, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N. Engl. J. Med. 2020, 382, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Murthy, R.K.; Loi, S.; Okines, A.; Paplomata, E.; Hamilton, E.; Hurvitz, S.A.; Lin, N.U.; Borges, V.; Abramson, V.; Anders, C.; et al. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2020, 382, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Saura, C.; Oliveira, M.; Feng, Y.H.; Dai, M.S.; Chen, S.W.; Hurvitz, S.A.; Kim, S.B.; Moy, B.; Delaloge, S.; Gradishar, W.; et al. Neratinib Plus Capecitabine Versus Lapatinib Plus Capecitabine in HER2-Positive Metastatic Breast Cancer Previously Treated With ≥ 2 HER2-Directed Regimens: Phase III NALA Trial. J. Clin. Oncol. 2020, 38, 3138–3149. [Google Scholar] [CrossRef]
- Rugo, H.S.; Im, S.A.; Cardoso, F.; Cortes, J.; Curigliano, G.; Musolino, A.; Pegram, M.D.; Bachelot, T.; Wright, G.S.; Saura, C.; et al. Margetuximab Versus Trastuzumab in Patients With Previously Treated HER2-Positive Advanced Breast Cancer (SOPHIA): Final Overall Survival Results From a Randomized Phase 3 Trial. J. Clin. Oncol. 2023, 41, 198–205. [Google Scholar] [CrossRef]
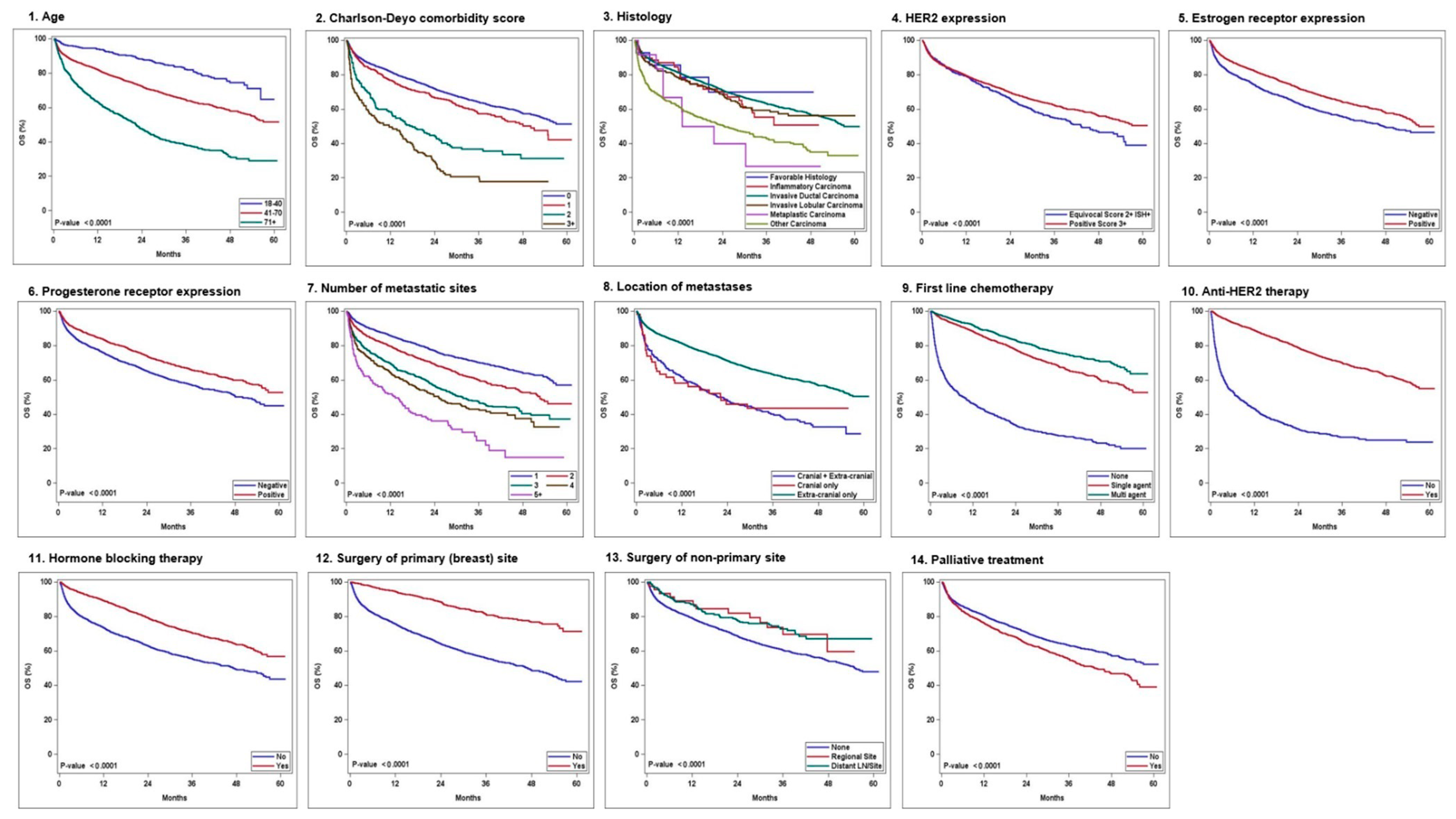
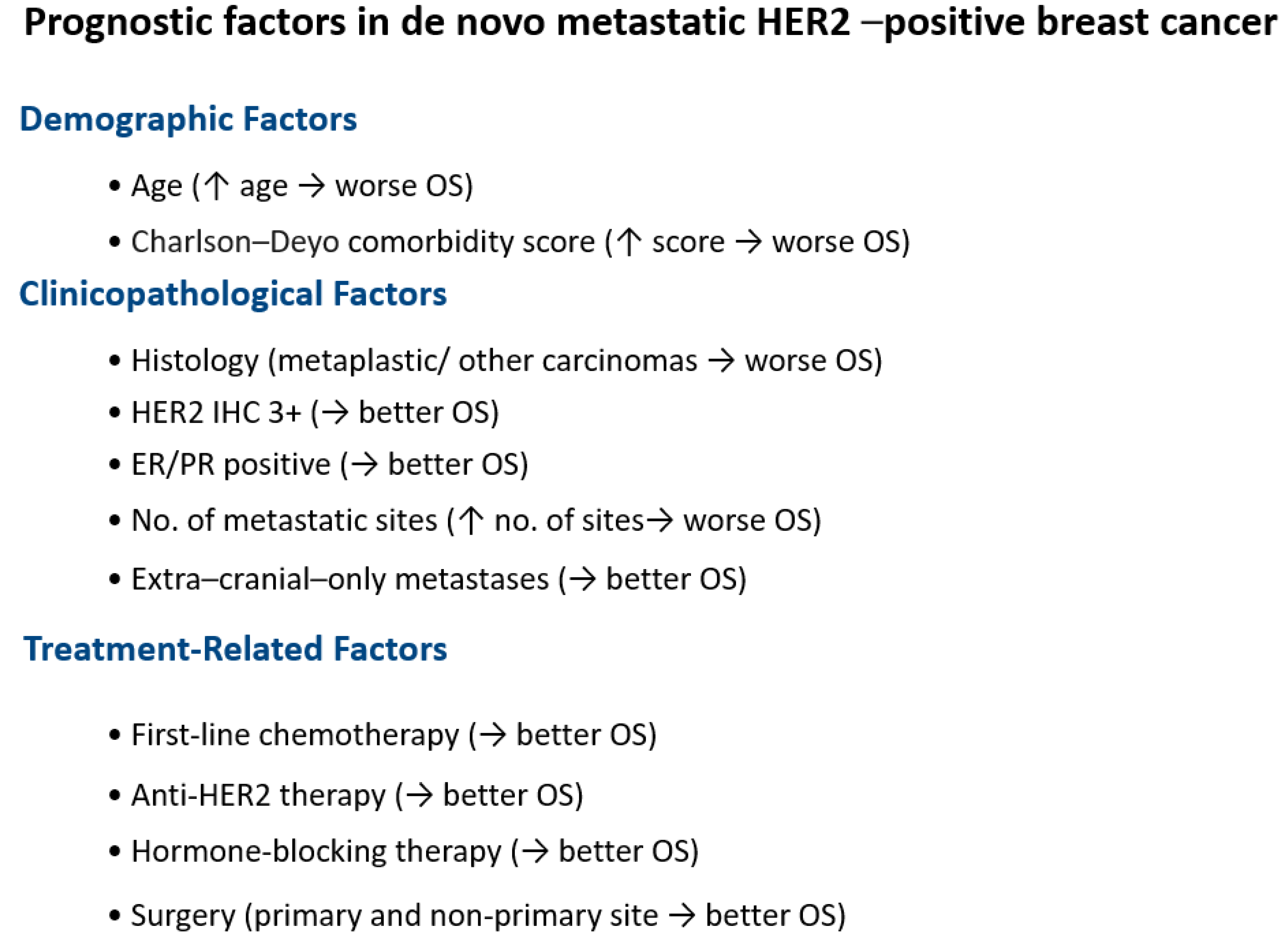
| No. of Patients, n (%) | No. of Events/n | |
|---|---|---|
| Demographic characteristics | ||
| Age | ||
| 19–40 | 633 (11.8%) | 115/633 |
| 41–70 | 3611 (67.2%) | 1239/3611 |
| 71+ | 1132 (21.1%) | 651/1132 |
| Race | ||
| Caucasian | 3976 (74%) | 1472/3976 |
| Black | 951 (17.7%) | 396/951 |
| Other | 395 (7.3%) | 120/395 |
| Unknown | 54 (1%) | 17/54 |
| Charlson–Deyo score | ||
| 0 | 4432 (82.4%) | 1533/4432 |
| 1 | 594 (11%) | 238/594 |
| 2 | 170 (3.2%) | 103/170 |
| 3 or more | 180 (3.3%) | 131/180 |
| Clinicopathological/cancer characteristics | ||
| Histology | ||
| Invasive ductal carcinoma | 4565 (84.9%) | 1618/4565 |
| Invasive lobular carcinoma | 242 (4.5%) | 88/242 |
| Favorable histology | 14 (0.3%) | 4/14 |
| Other carcinoma | 465 (8.6%) | 257/465 |
| Inflammatory carcinoma | 78 (1.5%) | 30/78 |
| Metaplastic carcinoma | 12 (0.2%) | 8/12 |
| HER2 IHC expression | ||
| 2+ with FISH+ | 814 (15.1%) | 351/814 |
| 3+ | 4562 (84.9%) | 1654/4562 |
| Estrogen receptor | ||
| Negative | 2063 (38.4%) | 871/2063 |
| Positive | 3313 (61.6%) | 1134/3313 |
| Progesterone receptor | ||
| Negative | 3011 (56%) | 1232/3011 |
| Positive | 2365 (44%) | 773/2365 |
| No. of metastatic sites | ||
| 1 | 2615 (48.6%) | 754/2615 |
| 2 | 1492 (27.8%) | 578/1492 |
| 3 | 779 (14.5%) | 386/779 |
| 4 | 351 (6.5%) | 191/351 |
| 5+ | 139 (2.6%) | 96/139 |
| Location of metastases | ||
| Intra-cranial only | 58 (1.1%) | 31/58 |
| Extra-cranial only | 4880 (90.8%) | 1717/4880 |
| Intra-cranial + extra-cranial | 438 (8.1%) | 257/438 |
| Treatment characteristics | ||
| First-line chemotherapy | ||
| None | 1290 (24%) | 869/1290 |
| Single-agent | 2502 (46.5%) | 765/2502 |
| Multi-agent | 1584 (29.5%) | 371/1584 |
| Anti-HER2 therapy | ||
| No | 1141 (21.2%) | 769/1141 |
| Yes | 4235 (78.8%) | 1236/4235 |
| Hormone-blocking therapy | ||
| No | 3351 (62.3%) | 1419/3351 |
| Yes | 2025 (37.7%) | 586/2025 |
| Surgery of primary site (breast) | ||
| No | 4305 (80.1%) | 1806/4305 |
| Yes | 1071 (19.9%) | 199/1071 |
| Surgery of non-primary site | ||
| No | 5087 (94.6%) | 1927/5087 |
| Regional nodes | 46 (0.9%) | 13/46 |
| Distant lymph nodes orsites | 243 (4.5%)54 (1%) | 65/243 |
| Radiation | ||
| No | 3675 (68.4%) | 1437/3675 |
| Primary site (breast) | 292 (5.4%) | 75/292 |
| Local lymph nodes | 34 (0.6%) | 5/34 |
| Distant lymph nodes or site | 1375 (25.6%) | 488/1375 |
| Palliative treatment (to alleviate symptoms) | ||
| No | 4010 (74.6%) | 1414/4010 |
| Yes | 1366 (25.4%) | 591/1366 |
| 12-Month Survival Estimate (95% CI) | 36-Month Survival Estimate (95% CI) | 60-Month Survival Estimate (95% CI) | Hazard Ratio (95% CI) | Log-Rank p-Value | |
|---|---|---|---|---|---|
| Demographic Characteristics | |||||
| Age | <0.0001 | ||||
| 19–40 | 0.94 (0.92–0.96) | 0.82 (0.79–0.86) | 0.65 (0.53–0.80) | Reference | |
| 41–70 | 0.82 (0.81–0.83) | 0.64 (0.63–0.66) | 0.52 (0.48–0.55) | 2.18 (1.80–2.64) | |
| 71+ | 0.63 (0.6–0.66) | 0.38 (0.35–0.41) | 0.29 (0.25–0.34) | 4.76 (3.90–5.80) | |
| Race | 0.8 (0.78–0.81) | 0.61 (0.60–0.63) | 0.5 (0.47–0.53) | Reference | 0.0004 |
| Caucasian | 0.77 (0.74–0.8) | 0.57 (0.54–0.61) | 0.41 (0.34–0.5) | 1.19 (1.06–1.33) | |
| Black | |||||
| Other | 0.85 (0.82–0.89) | 0.67 (0.62–0.72) | 0.48 (0.35–0.67) | 0.79 (0.66–0.95) | |
| Unknown | 0.81 (0.71–0.92) | 0.62 (0.48–0.79) | 0.62 (0.48–0.79) | 0.89 (0.55–1.43) | |
| Charlson | <0.0001 | ||||
| –Deyo score | |||||
| 0 | 0.82 (0.81–0.83) | 0.64 (0.63–0.66) | 0.51 (0.48–0.55) | Reference | |
| 1 | 0.77 (0.74–0.80) | 0.57 (0.53–0.62) | 0.42 (0.34–0.52) | 1.23 (1.07–1.41) | |
| 2 | 0.59 (0.51–0.67) | 0.37 (0.30–0.45) | NE (NE–NE) | 2.36 (1.93–2.88) | |
| 3 or more | 0.50 (0.43–0.58) | 0.21 (0.15–0.29) | NE (NE–NE) | 3.72 (3.11–4.45) | |
| Clinicopathologic/Cancer Characteristics | |||||
| Histology | <0.0001 | ||||
| Invasive ductal carcinoma | 0.81 (0.80–0.82) | 0.63 (0.62–0.65) | 0.50 (0.47–0.53) | Reference | |
| Invasive lobular carcinoma | 0.79 (0.74–0.84) | 0.59 (0.53–0.67) | 0.56 (0.49–0.64) | 1.11 (0.89–1.37) | |
| Favorable histology | 0.86 (0.69–1.00) | 0.70 (0.49–1.00) | NE (NE–NE) | 0.87 (0.33–2.33) | |
| Other carcinoma | 0.62 (0.58–0.67) | 0.43 (0.38–0.48) | 0.33 (0.27–0.40) | 1.99 (1.75–2.27) | |
| Inflammatory carcinoma | 0.83 (0.75–0.92) | 0.55 (0.44–0.70) | NE (NE–NE) | 1.17 (0.82–1.68) | |
| Metaplastic carcinoma | 0.67 (0.45–0.99) | 0.27 (0.09–0.78) | NE (NE–NE) | 2.39 (1.19–4.78) | |
| HER2 IHC expression | 0.0007 | ||||
| 3+ | 0.80 (0.78–0.81) | 0.62 (0.61–0.64) | 0.50 (0.47–0.54) | Reference | |
| 2+ with FISH+ | 0.79 (0.77–0.82) | 0.55 (0.51–0.59) | 0.39 (0.32–0.47) | 1.22 (1.09–1.37) | |
| Estrogen receptor | <0.0001 | ||||
| Negative | 0.74 (0.73–0.76) | 0.56 (0.54–0.58) | 0.46 (0.43–0.50) | Reference | |
| Positive | 0.83 (0.81–0.84) | 0.64 (0.63–0.66) | 0.50 (0.46–0.54) | 0.73 (0.67–0.80) | |
| Progesterone receptor | <0.0001 | ||||
| Negative | 0.76 (0.75–0.78) | 0.57 (0.55–0.59) | 0.45 (0.42–0.49) | Reference | |
| Positive | 0.84 (0.82–0.85) | 0.66 (0.64–0.68) | 0.53 (0.48–0.58) | 0.72 (0.66–0.79) | |
| No. of metastatic sites | <0.0001 | ||||
| 1 | 0.86 (0.84–0.87) | 0.70 (0.68–0.72) | 0.57 (0.52–0.62) | Reference | |
| 2 | 0.80 (0.78–0.82) | 0.60 (0.57–0.63) | 0.46 (0.41–0.52) | 1.44 (1.30–1.61) | |
| 3 | 0.70 (0.66–0.73) | 0.47 (0.43–0.51) | 0.37 (0.31–0.44) | 2.14 (1.89–2.42) | |
| 4 | 0.65 (0.60–0.70) | 0.43 (0.38–0.49) | NE (NE–NE) | 2.52 (2.15–2.96) | |
| 5+ | 0.52 (0.44–0.61) | 0.25 (0.17–0.36) | NE (NE–NE) | 4.02 (3.25–4.97) | |
| Site of metastatic involvement (subgroup analysis in those with single metastatic site) | <0.0001 | ||||
| Brain only | 0.58 (0.47–0.72) | 0.44 (0.32–0.59) | NE (NE–NE) | Reference | |
| LN only | 0.94 (0.91–0.96) | 0.76 (0.71–0.81) | 0.61 (0.46–0.80) | 0.30 (0.20–0.46) | |
| Bone only | 0.88 (0.86–0.90) | 0.73 (0.70–0.76) | 0.57 (0.50–0.65) | 0.37 (0.25–0.53) | |
| Liver only | 0.83 (0.80–0.86) | 0.71 (0.67–0.75) | 0.56 (0.48–0.66) | 0.42 (0.29–0.62) | |
| Lung only | 0.85 (0.82–0.89) | 0.66 (0.61–0.72) | 0.61 (0.55–0.67) | 0.45 (0.30–0.67) | |
| Other only | 0.71 (0.63–0.81) | 0.48 (0.38–0.6) | 0.40 (0.29–0.57) | 0.84 (0.53–1.32) | |
| Location of metastases | <0.0001 | ||||
| Intra-cranial only | 0.58 (0.47–0.72) | 0.44 (0.32–0.59) | NE (NE–NE) | Reference | |
| Extra-cranial only | 0.81 (0.80–0.82) | 0.63 (0.62–0.65) | 0.50 (0.47–0.54) | 0.53 (0.37–0.76) | |
| Intra-cranial + extra-cranial | 0.62 (0.58–0.67) | 0.40 (0.36–0.45) | NE (NE–NE) | 1.11 (0.76–1.61) | |
| Treatment characteristics | |||||
| First-line chemotherapy | <0.0001 | ||||
| None | 0.46 (0.44–0.49) | 0.28 (0.25–0.30) | 0.20 (0.16–0.25) | Reference | |
| Single-agent | 0.88 (0.87–0.90) | 0.68 (0.66–0.70) | 0.53 (0.48–0.58) | 0.25 (0.22–0.27) | |
| Multi-agent | 0.92 (0.91–0.93) | 0.76 (0.74–0.78) | 0.64 (0.58–0.69) | 0.18 (0.16–0.20) | |
| Anti-HER2 therapy | <0.0001 | ||||
| No | 0.43 (0.41–0.46) | 0.27 (0.24–0.30) | 0.24 (0.20–0.28) | Reference | |
| Yes | 0.89 (0.88–0.90) | 0.70 (0.68–0.71) | 0.55 (0.52–0.59) | 0.22 (0.20–0.24) | |
| Hormone-blocking therapy | <0.0001 | ||||
| No | 0.74 (0.72–0.75) | 0.55 (0.54–0.57) | 0.44 (0.40–0.48) | Reference | |
| Yes | 0.89 (0.88–0.91) | 0.70 (0.68–0.73) | 0.57 (0.52–0.61) | 0.56 (0.51–0.61) | |
| Surgery of primary site (breast) | <0.0001 | ||||
| No | 0.76 (0.74–0.77) | 0.56 (0.54–0.58) | 0.42 (0.39–0.46) | Reference | |
| Yes | 0.95 (0.93–0.96) | 0.81 (0.78–0.84) | 0.71 (0.66–0.77) | 0.33 (0.29–0.39) | |
| Surgery of non-primary site | 0.0005 | ||||
| No | 0.79 (0.78–0.80) | 0.60 (0.59–0.62) | 0.48 (0.45–0.51) | Reference | |
| Regional nodes | 0.89 (0.81–0.99) | 0.70 (0.56–0.86) | NE (NE–NE) | 0.65 (0.37–1.12) | |
| Distant lymph nodes or site | 0.87 (0.83–0.91) | 0.73 (0.67–0.79) | NE (NE–NE) | 0.64 (0.50–0.82) | |
| Radiation | <0.0001 | ||||
| No | 0.77 (0.76–0.79) | 0.59 (0.57–0.61) | 0.45 (0.42–0.49) | Reference | |
| Primary site (breast) | 0.94 (0.91–0.97) | 0.72 (0.66–0.78) | 0.65 (0.58–0.74) | 0.54 (0.43–0.68) | |
| Local lymph nodes | 0.97 (0.92–1.00) | 0.85 (0.74–0.98) | NE (NE–NE) | 0.29 (0.12–0.69) | |
| Distant lymph nodes or site | 0.82 (0.80–0.84) | 0.64 (0.61–0.66) | 0.52 (0.47–0.58) | 0.83 (0.75–0.92) | |
| Palliative treatment (to alleviate symptoms) | <0.0001 | ||||
| No | 0.81 (0.79–0.82) | 0.63 (0.61–0.65) | 0.52 (0.49–0.56) | Reference | |
| Yes | 0.76 (0.74–0.78) | 0.55 (0.53–0.58) | 0.39 (0.34–0.45) | 1.29 (1.18–1.42) | |
| First-Line Chemotherapy (No. of Patients, n) | 12-Month Survival Estimate (95% CI) | 36-Month Survival Estimate (95% CI) | 60-Month Survival Estimate (95% CI) | Hazard Ratio (95% CI) | Log-Rank p-Value |
| Charlson–Deyo comorbidity score 0; No. of patients = 4432 (82.4%) | |||||
| None (n = 961) | 0.49 (0.46–0.52) | 0.30 (0.27–0.34) | 0.22 (0.18–0.27) | Reference | <0.0001 |
| Single-agent (n = 2123) | 0.89 (0.88–0.91) | 0.70 (0.68–0.72) | 0.54 (0.49–0.60) | 0.25 (0.23–0.28) | |
| Multi-agent (n = 1348) | 0.93 (0.91–0.94) | 0.78 (0.75–0.80) | 0.66 (0.61–0.72) | 0.18 (0.15–0.20) | |
| Charlson–Deyo comorbidity score 1; No. of patients = 594 (11%) | |||||
| None (n = 157) | 0.45 (0.38–0.54) | 0.28 (0.21–0.37) | NE (NE–NE) | Reference | <0.0001 |
| Single-agent (n = 253) | 0.85 (0.81–0.90) | 0.65 (0.59–0.72) | 0.49 (0.38–0.64) | 0.26 (0.20–0.35) | |
| Multi-agent (n = 184) | 0.92 (0.88–0.96) | 0.72 (0.65–0.79) | 0.48 (0.33–0.71) | 0.21 (0.15–0.29) | |
| Charlson–Deyo comorbidity score 2; No. of patients = 170 (3.2%) | |||||
| None (n = 76) | 0.32 (0.23–0.45) | 0.14 (0.08–0.26) | NE (NE–NE) | Reference | <0.0001 |
| Single-agent (n = 63) | 0.81 (0.71–0.91) | 0.58 (0.46–0.73) | NE (NE–NE) | 0.25 (0.16–0.40) | |
| Multi-agent (n = 31) | 0.77 (0.64–0.94) | 0.48 (0.32–0.71) | NE (NE–NE) | 0.29 (0.17–0.52) | |
| Charlson–Deyo comorbidity score = 3 or more; No. of patients = 180 (3.3%) | |||||
| None (n = 96) | 0.32 (0.24–0.43) | 0.10 (0.05–0.20) | NE (NE–NE) | Reference | <0.0001 |
| Single-agent (n = 63) | 0.72 (0.62–0.84) | 0.29 (0.9–0.45) | NE (NE–NE) | 0.44 (0.30–0.64) | |
| Multi-agent (n = 21) | 0.61 (0.43–0.86) | 0.49 (0.31–0.78) | NE (NE–NE) | 0.34 (0.18–0.66) | |
| Anti-HER2 Therapy (No. of Patients, n) | 12-Month Survival Estimate (95% CI) | 36-Month Survival Estimate (95% CI) | 60-Month Survival Estimate (95% CI) | Hazard Ratio (95% CI) | Log-Rank p-Value |
| Charlson–Deyo comorbidity score 0; No. of patients = 4432 (82.4%) | |||||
| No (n = 859) | 0.47 (0.44–0.51) | 0.30 (0.27–0.34) | 0.27 (0.23–0.31) | Reference | <0.0001 |
| Yes (n = 3573) | 0.90 (0.89–0.91) | 0.72 (0.70–0.74) | 0.57 (0.53–0.61) | 0.22 (0.20–0.25) | |
| Charlson–Deyo comorbidity score 1; No. of patients = 594 (11%) | |||||
| No (n = 139) | 0.41 (0.33–0.50) | 0.24 (0.17–0.34) | NE (NE–NE) | Reference | <0.0001 |
| Yes (n = 455) | 0.88 (0.85–0.91) | 0.67 (0.63–0.72) | 0.48 (0.39–0.60) | 0.22 (0.17–0.29) | |
| Charlson–Deyo comorbidity score 2; No. of patients= 170 (3.2%) | |||||
| No (n = 58) | 0.26 (0.16–0.40) | 0.11 (0.04–0.26) | NE (NE–NE) | Reference | <0.0001 |
| Yes (n = 112) | 0.75 (0.68–0.84) | 0.50 (0.41–0.61) | NE (NE–NE) | 0.23 (0.15–0.34) | |
| Charlson–Deyo comorbidity score = 3 or more; No. of patients = 180 (3.3%) | |||||
| No (n = 85) | 0.23 (0.15–0.34) | 0.10 (0.05–0.20) | NE (NE–NE) | Reference | <0.0001 |
| Yes (n = 95) | 0.73 (0.64–0.83) | 0.30 (0.21–0.43) | NE (NE–NE) | 0.31 (0.21–0.44) | |
| Hazard Ratio (95% CI) | Overall p-Value | |
|---|---|---|
| Age | <0.0001 | |
| 19–40 | Reference | |
| 41–70 | 1.56 (1.28–1.89) | |
| 71+ | 2.19 (1.78–2.71) | |
| Charlson–Deyo score | <0.0001 | |
| 0 | Reference | |
| 1 | 1.12 (0.98–1.28) | |
| 2 | 1.67 (1.36–2.04) | |
| 3 or more | 2.15 (1.79–2.58) | |
| Histology | 0.0008 | |
| Invasive ductal carcinoma | Reference | |
| Invasive lobular carcinoma | 0.99 (0.81–1.24) | |
| Favorable histology | 1.61 (0.60–4.32) | |
| Other carcinoma | 1.31 (1.15–1.50) | |
| Inflammatory carcinoma | 1.46 (1.01–2.09) | |
| Metaplastic carcinoma | 1.51 (0.75–3.06) | |
| HER2 IHC expression | <0.0001 | |
| 2+ with FISH+ | Reference | |
| 3+ | 0.78 (0.69–0.88) | |
| Estrogen receptor | 0.0046 | |
| Negative | Reference | |
| Positive | 0.84 (0.75–0.95) | |
| Progesterone receptor | 0.005 | |
| Negative | Reference | |
| Positive | 0.85 (0.76–0.95) | |
| No. of metastatic sites | <0.0001 | |
| 1 | Reference | |
| 2 | 1.36 (1.21–1.52) | |
| 3 | 1.73 (1.52–1.97) | |
| 4 | 2.17 (1.83–2.57) | |
| 5+ | 3.21 (2.52–4.08) | |
| Location of metastases | 0.0093 | |
| Intra-cranial only | Reference | |
| Intra-cranial +extra-cranial | 0.78 (0.53–1.16) | |
| Extra-cranial only | 0.66 (0.46–0.96) | |
| First-line chemotherapy | <0.0001 | |
| None | Reference | |
| Single-agent | 0.41 (0.36–0.47) | |
| Multi-agent | 0.34 (0.29–0.39) | |
| Anti-HER2 therapy | <0.0001 | |
| No | Reference | |
| Yes | 0.42 (0.38–0.48) | |
| Hormone-blocking therapy | <0.0001 | |
| No | Reference | |
| Yes | 0.57 (0.50–0.64) | |
| Surgery of primary site (breast) | <0.0001 | |
| No | Reference | |
| Yes | 0.59 (0.51–0.69) | |
| Surgery of non-primary site | 0.0047 | |
| No | Reference | |
| Regional node | 1.09 (0.63–1.89) | |
| Distant lymph node or site | 0.66 (0.51–0.85) | |
| Palliative treatment (to alleviate symptoms) | 0.0031 | |
| No | Reference | |
| Yes | 1.16 (1.05–1.28) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kesireddy, M.; Masih, D.; Shostrom, V.K.; Yellala, A.; Asif, S.; Krishnamurthy, J. Overall Survival and Prognostic Factors in De Novo Metastatic Human Epidermal Growth Factor Receptor (HER)-2-Positive Breast Cancer: A National Cancer Database Analysis. Cancers 2025, 17, 1823. https://doi.org/10.3390/cancers17111823
Kesireddy M, Masih D, Shostrom VK, Yellala A, Asif S, Krishnamurthy J. Overall Survival and Prognostic Factors in De Novo Metastatic Human Epidermal Growth Factor Receptor (HER)-2-Positive Breast Cancer: A National Cancer Database Analysis. Cancers. 2025; 17(11):1823. https://doi.org/10.3390/cancers17111823
Chicago/Turabian StyleKesireddy, Meghana, Durva Masih, Valerie K. Shostrom, Amulya Yellala, Samia Asif, and Jairam Krishnamurthy. 2025. "Overall Survival and Prognostic Factors in De Novo Metastatic Human Epidermal Growth Factor Receptor (HER)-2-Positive Breast Cancer: A National Cancer Database Analysis" Cancers 17, no. 11: 1823. https://doi.org/10.3390/cancers17111823
APA StyleKesireddy, M., Masih, D., Shostrom, V. K., Yellala, A., Asif, S., & Krishnamurthy, J. (2025). Overall Survival and Prognostic Factors in De Novo Metastatic Human Epidermal Growth Factor Receptor (HER)-2-Positive Breast Cancer: A National Cancer Database Analysis. Cancers, 17(11), 1823. https://doi.org/10.3390/cancers17111823






