Bone Marrow Myeloid–Lymphatic Progenitors Expand Tumor Lymphatic Vasculature Through Cell Fusion
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Antibodies
2.3. Animal Ethics Statement
2.4. Human Blood and Tissues
2.5. Isolation and Differentiation of Blood-Circulating CD14+ Human Monocytes
2.6. Isolation and Differentiation of Mouse BM Cells into M-LECPs
2.7. RT-qPCR Analysis
2.8. Flow Cytometry
2.9. Culture of Human and Mouse Breast Carcinoma Cell Lines
2.10. Immunofluorescence
2.11. Quantification of Mouse Tumor Lyve-1+ M-LECP Expressing Fusogenic Markers
2.12. Quantification of Human Tumor LYVE-1+ M-LECP-Expressing Fusogenic Markers
2.13. In Vitro Model for Fusion of Myeloid and Lymphatic Endothelial Cells
2.14. Quantification of Fusion Events In Vitro
2.15. High-Resolution Imaging for Multinucleation
2.16. Generation of Chimera Female Mice with Male Bone Marrow
2.17. Orthotopic Breast Cancer Models and TAK-242 Treatment
2.18. Measurement of Metastatic Burden
2.19. Fluorescent in Situ Hybridization for Detection of Y-Chromosomes
2.20. Quantification of Y-Chromosome-Positive Structures
2.21. Quantification of Dividing Endothelial Cells in Tumor Lymphatic and Blood Vessels
2.22. Statistical Analysis
3. Results
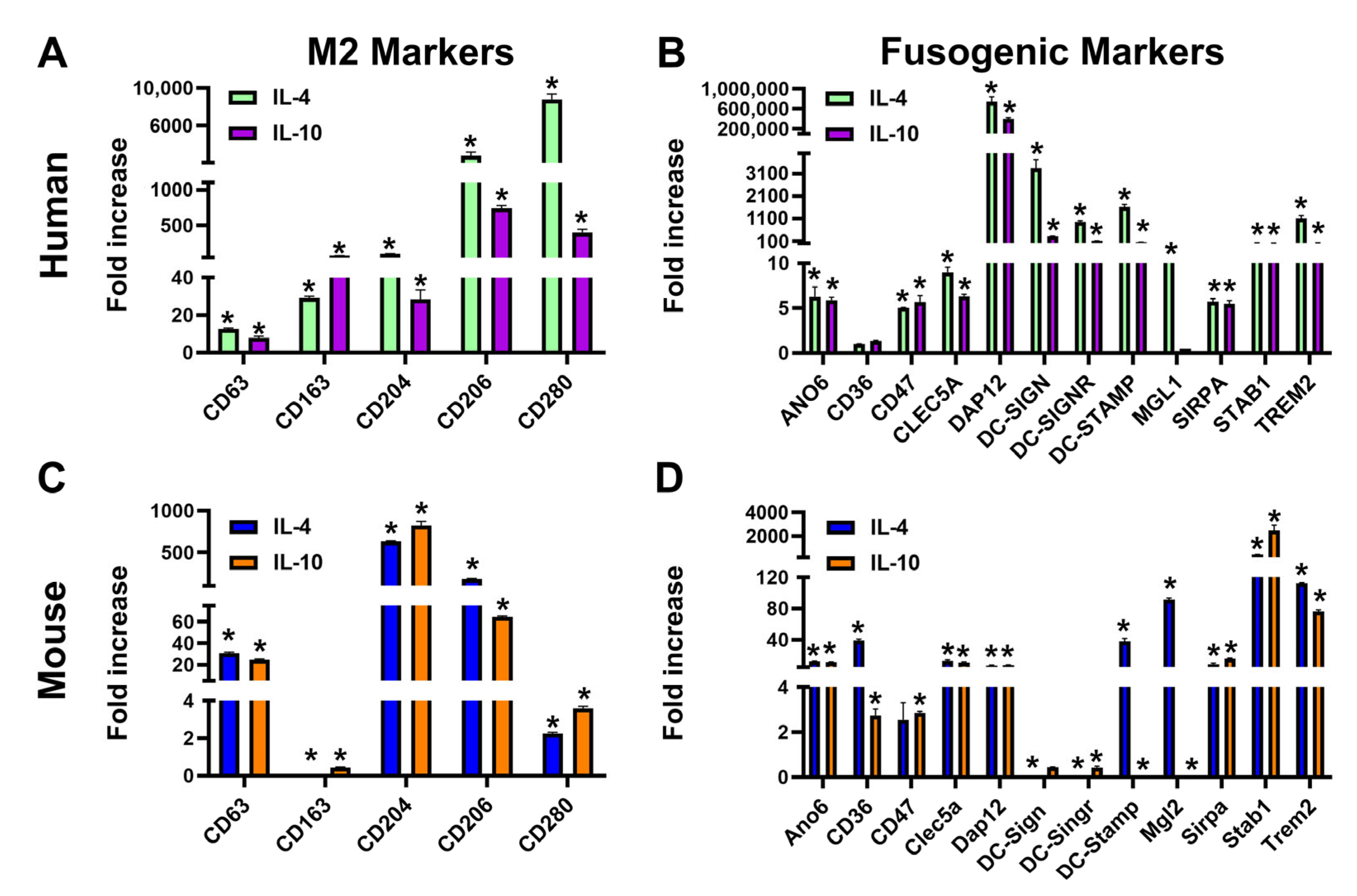
3.1. Th2 Cytokines IL-4 and IL-10 Induce Transcripts of Fusogenic Markers in Mouse M-LECPs and Human Monocytes
3.2. TLR4 and Th2 Factors Upregulate Multiple Fusogenic Proteins During Differentiation of M-LECPs
3.3. Multiple Fusogenic Proteins Are Expressed in M-LECPs Recruited to Mouse and Human Tumors In Vivo
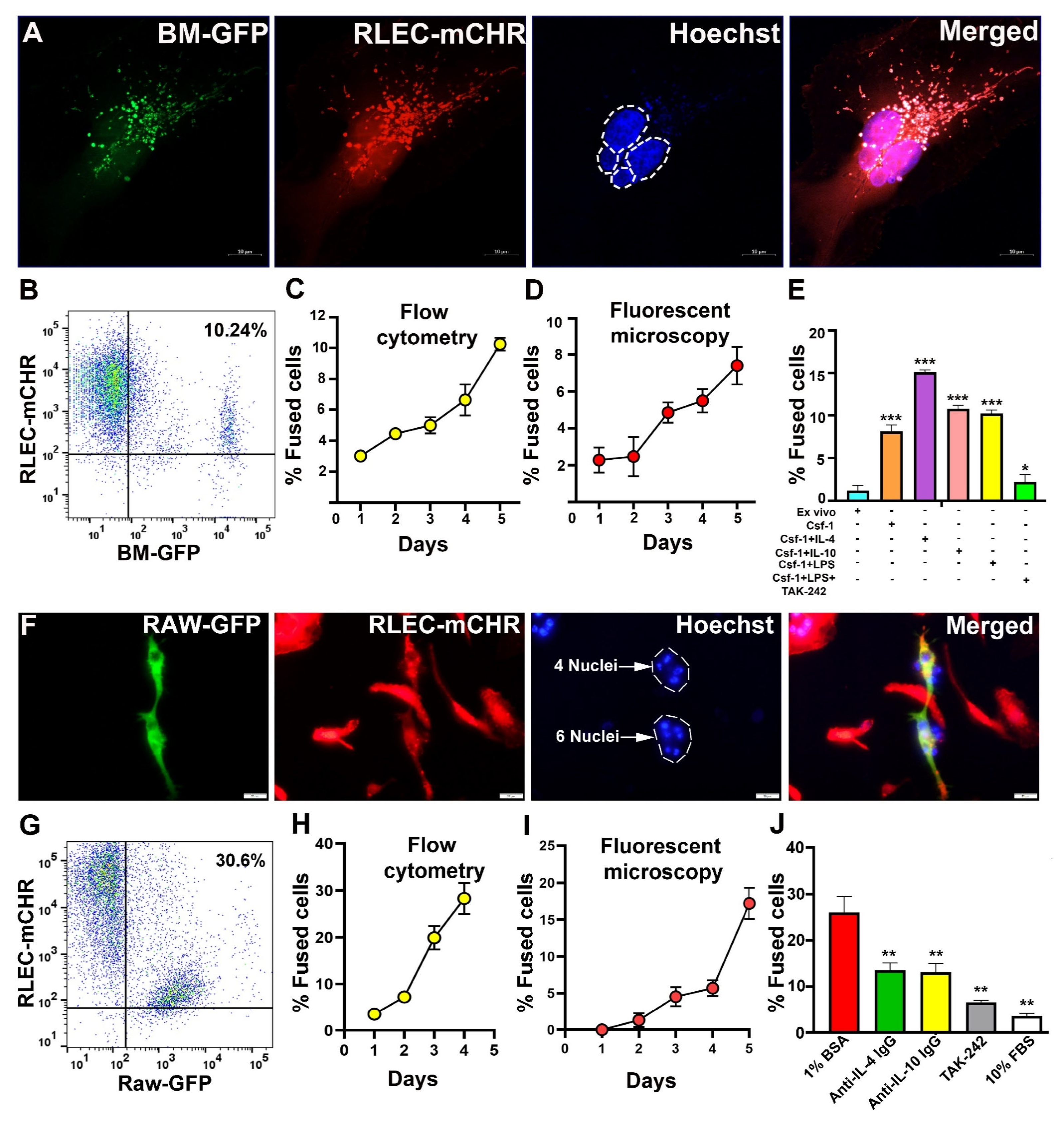
3.4. BM-Derived M-LECPs and RAW264.7 Macrophages Fuse with Lymphatic Endothelial Cells In Vitro
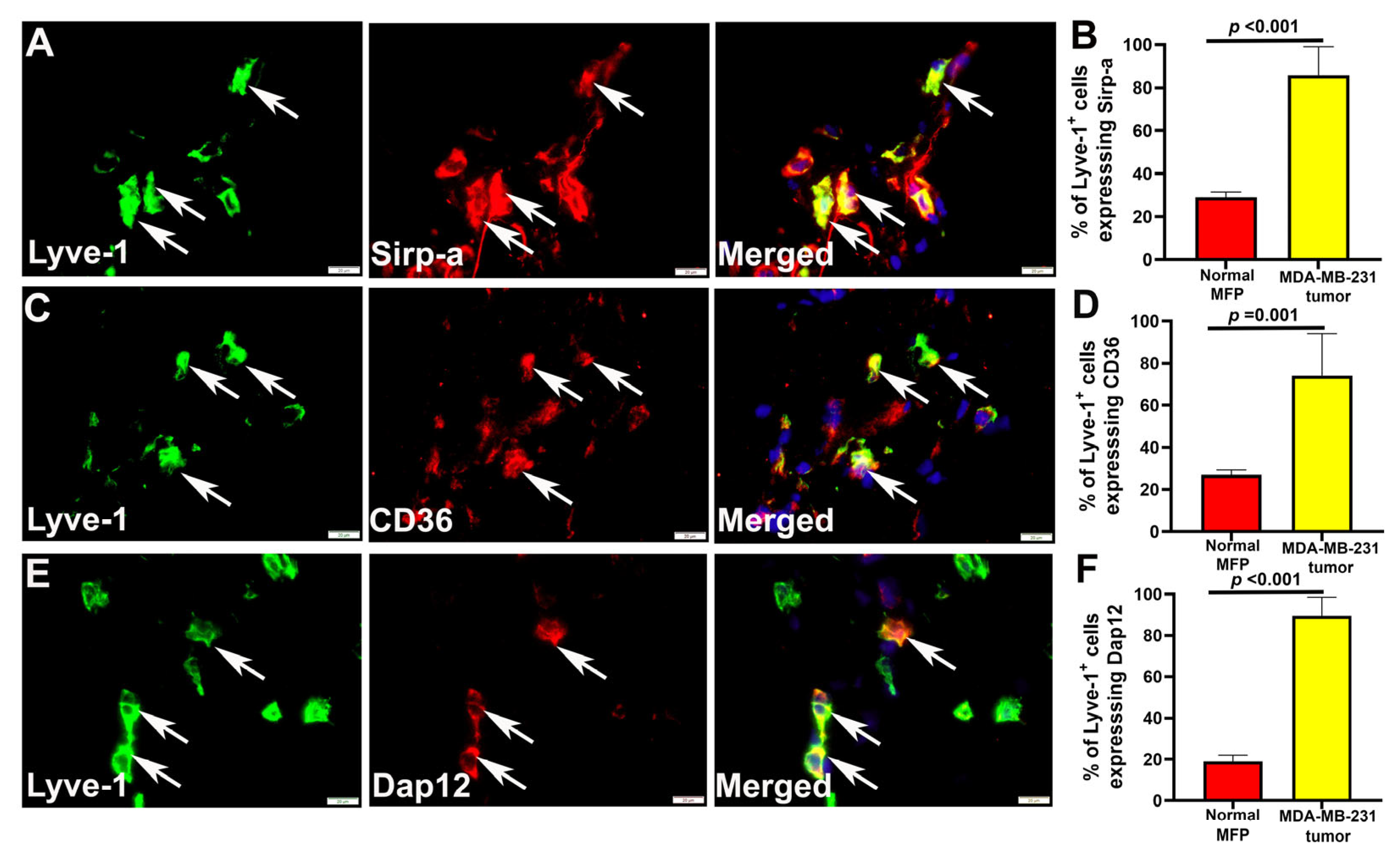
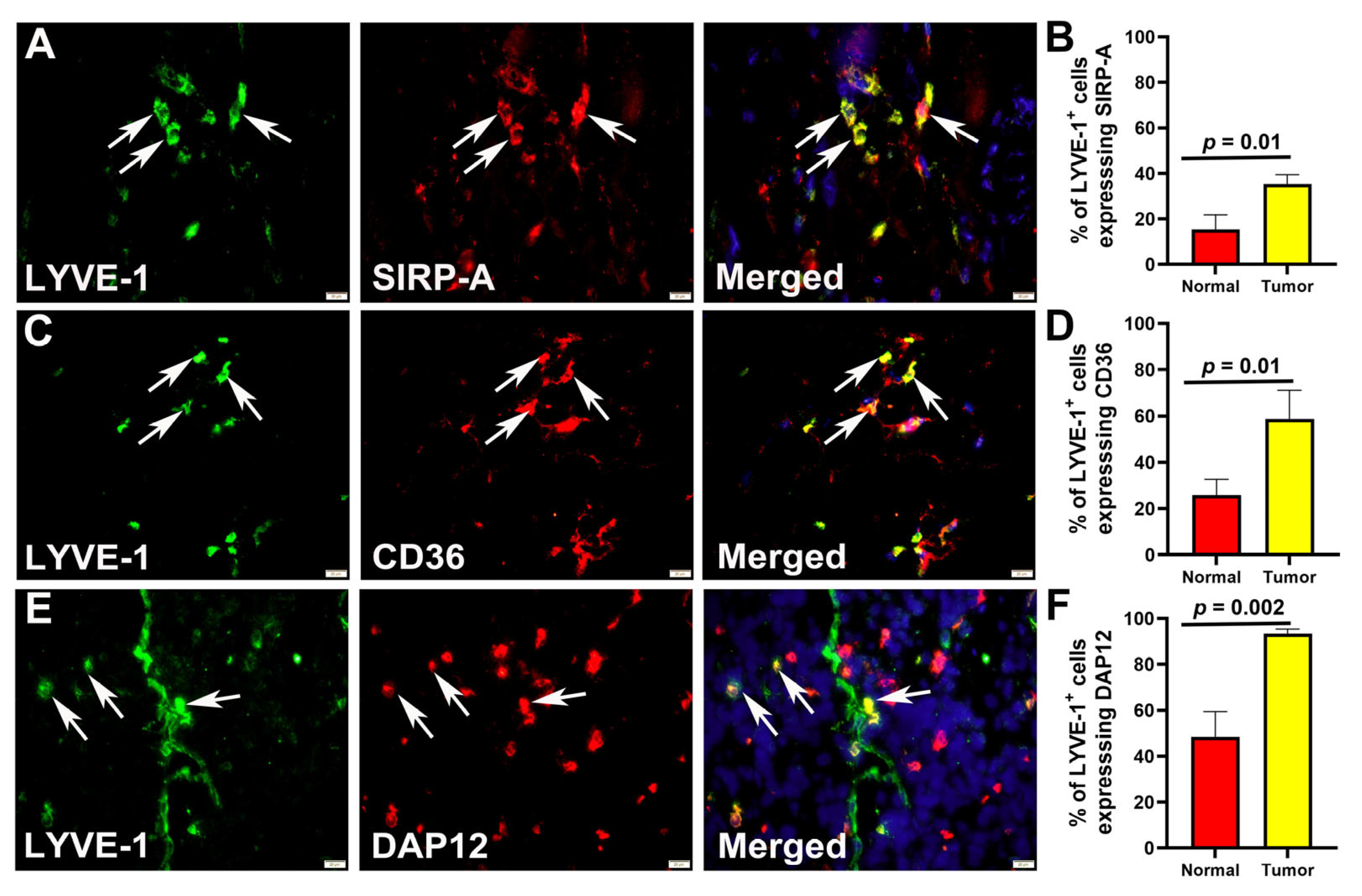
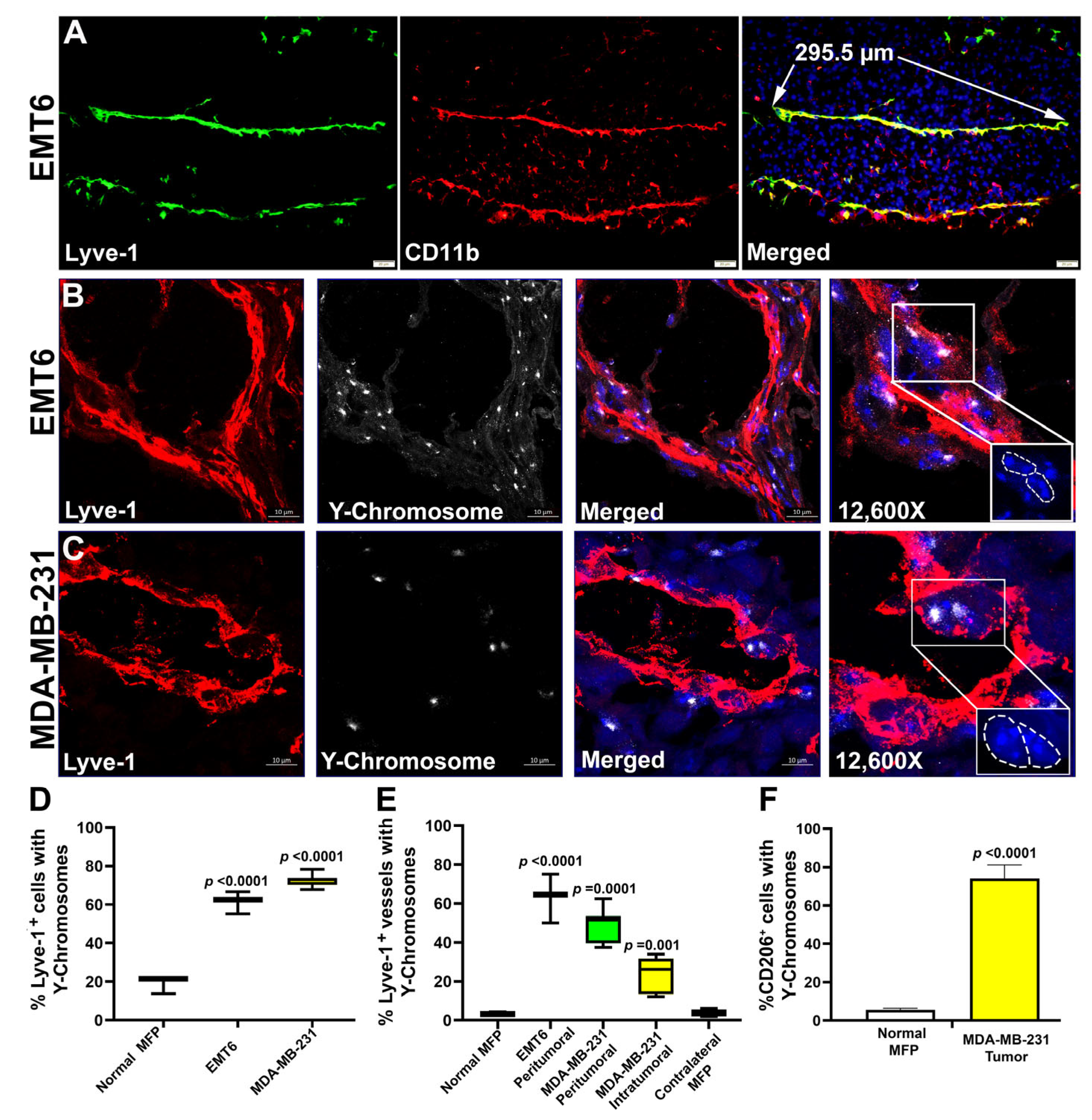
3.5. M-LECPs Recruited to EMT6 and MDA-MB-231 Tumors Fuse with Lymphatic Vessels In Vivo
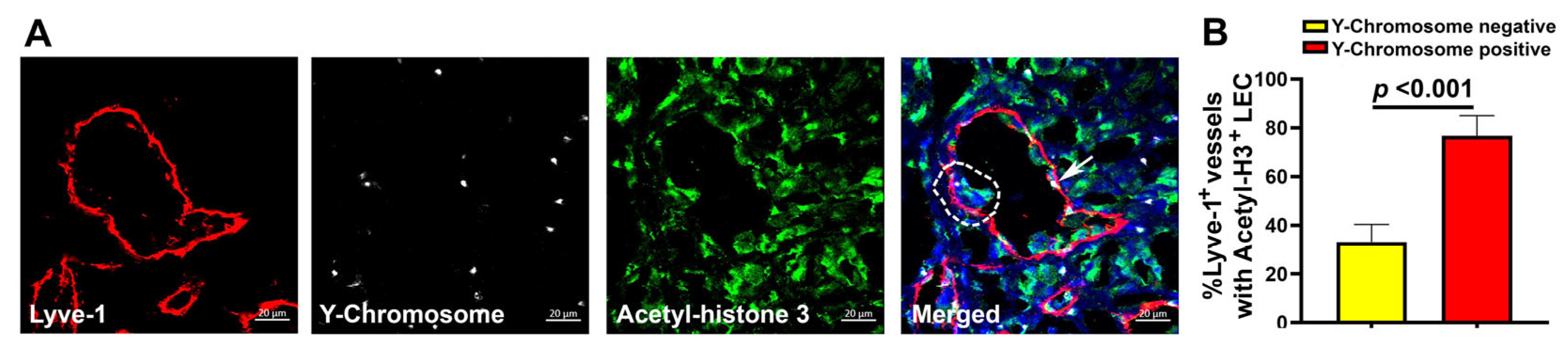
3.6. Fusion of Tumor Lymphatics with BM Progenitors Correlates with Increased LEC Proliferation
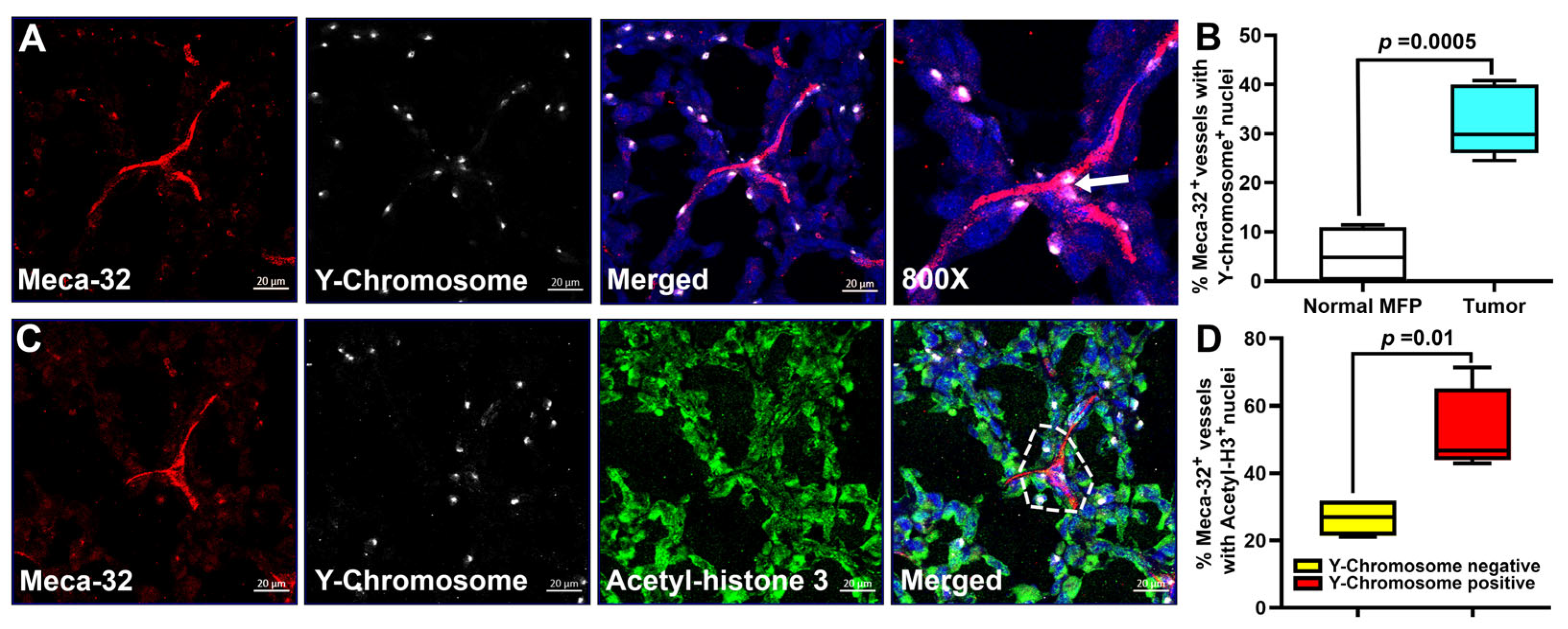
3.7. Fusion of Tumor Blood Vessels with BM Progenitors Also Correlates with Endothelial Cell Division
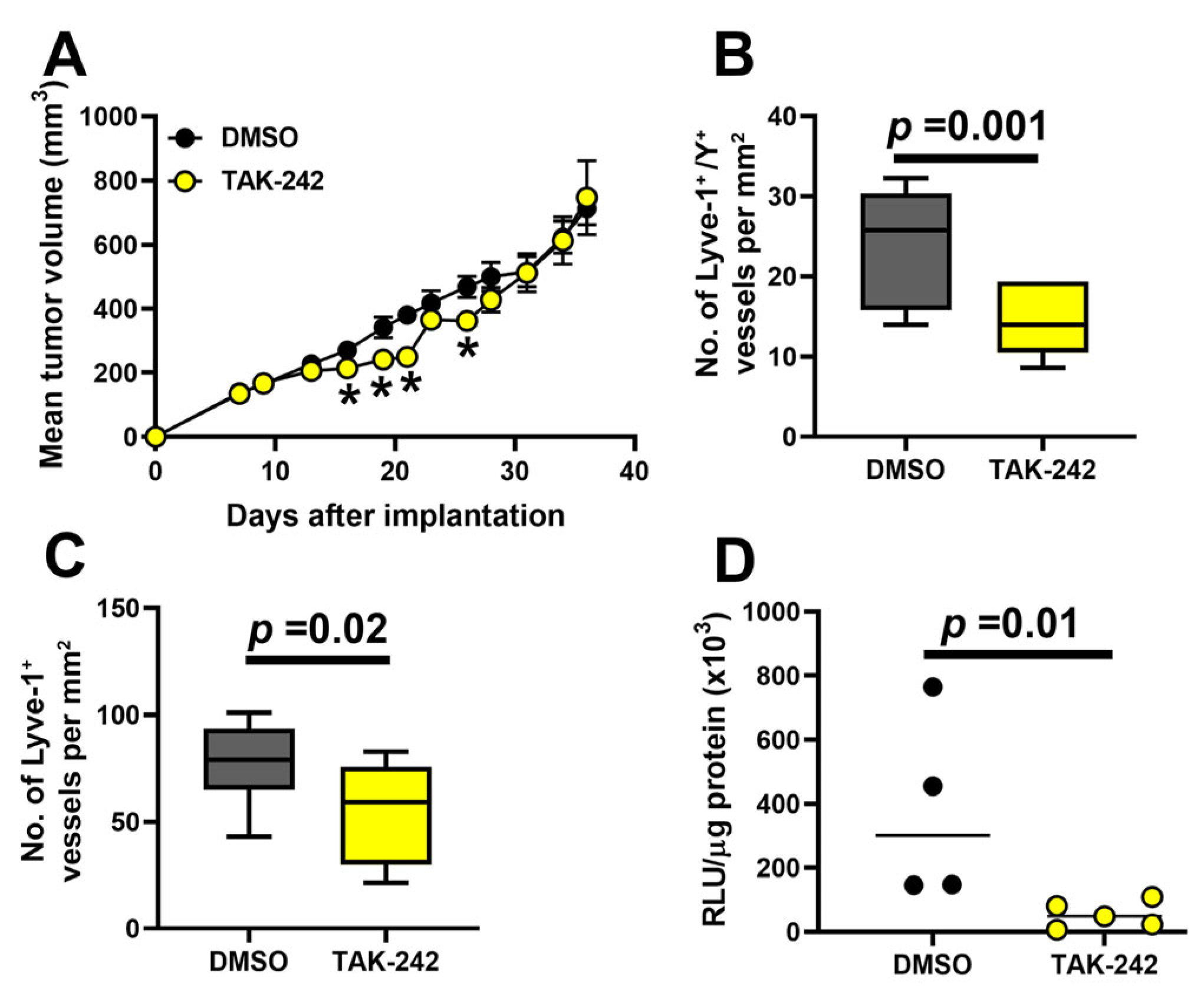
3.8. Inhibition of TLR4 Reduces Fusion of BM Progenitors with Tumor Lymphatics, LVD, and Lymph Node Metastasis
4. Discussion
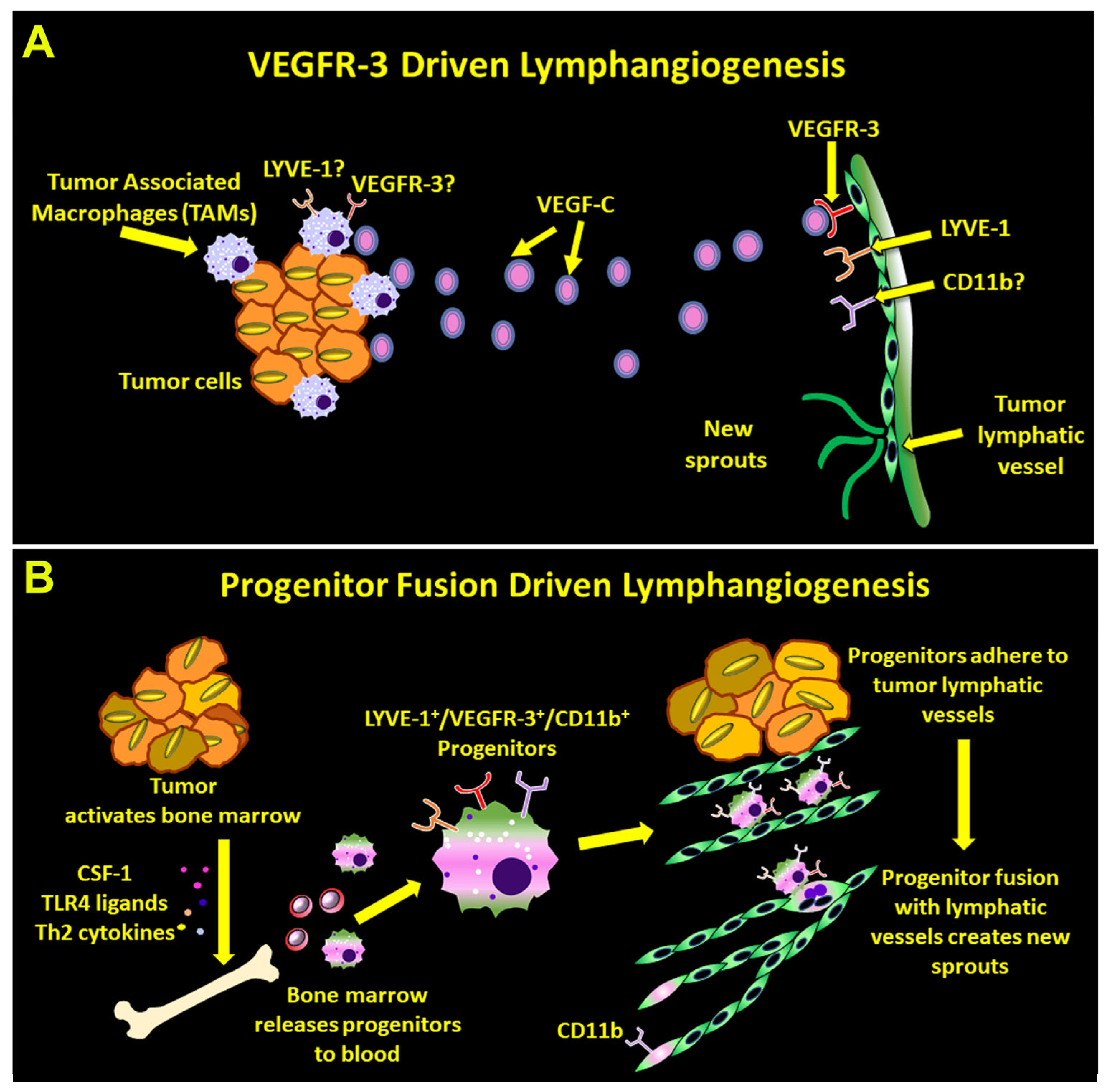
4.1. BM-Derived Myeloid–Lymphatic Progenitors Are Fusogenic and Use Fusion to Induce Vascular Sprouting
4.2. Fusogenicity of Myeloid–Lymphatic Progenitors Is Regulated by CSF1-TLR4-Th2 Pathway Axis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BC | Breast cancer |
| BM | Bone marrow |
| BMT | Bone marrow transplant |
| GFP | Green fluorescent protein |
| LEC | Lymphatic endothelial cells |
| LNs | Lymph nodes |
| LV | Lymphatic vessel |
| LVD | Lymphatic vessel density |
| MDSC | Myeloid-derived suppressor cell |
| M-LECP | Myeloid-lymphatic endothelial cell progenitor |
| TAMs | Tumor-associated macrophages |
| VEGFR-3 | Vascular endothelial growth factor receptor-3 |
References
- Kesler, C.T.; Liao, S.; Munn, L.L.; Padera, T.P. Lymphatic vessels in health and disease. Wiley. Interdiscip. Rev. Syst. Biol. Med. 2013, 5, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Vieira, J.M.; Norman, S.; Villa Del, C.C.; Cahill, T.J.; Barnette, D.N.; Gunadasa-Rohling, M.; Johnson, L.A.; Greaves, D.R.; Carr, C.A.; Jackson, D.G.; et al. The cardiac lymphatic system stimulates resolution of inflammation following myocardial infarction. J. Clin. Invest 2018, 128, 3402–3412. [Google Scholar] [CrossRef]
- Ravaud, C.; Ved, N.; Jackson, D.G.; Vieira, J.M.; Riley, P.R. Lymphatic Clearance of Immune Cells in Cardiovascular Disease. Cells 2021, 10, 2594. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Zhou, Q.; Proulx, S.T.; Wood, R.; Ji, R.C.; Ritchlin, C.T.; Pytowski, B.; Zhu, Z.; Wang, Y.J.; Schwarz, E.M.; et al. Inhibition of lymphangiogenesis and lymphatic drainage via vascular endothelial growth factor receptor 3 blockade increases the severity of inflammation in a mouse model of chronic inflammatory arthritis. Arthritis Rheum. 2009, 60, 2666–2676. [Google Scholar] [CrossRef]
- Ran, S.; Volk, L.; Hall, K.; Flister, M.J. Lymphangiogenesis and lymphatic metastasis in breast cancer. Pathophysiology 2009, 17, 229–251. [Google Scholar] [CrossRef]
- Brown, M.; Assen, F.P.; Leithner, A.; Abe, J.; Schachner, H.; Asfour, G.; Bago-Horvath, Z.; Stein, J.V.; Uhrin, P.; Sixt, M.; et al. Lymph node blood vessels provide exit routes for metastatic tumor cell dissemination in mice. Science 2018, 359, 1408–1411. [Google Scholar] [CrossRef]
- Dong, R.; Wang, Q.; He, X.L.; Chu, Y.K.; Lu, J.G.; Ma, Q.J. Role of nuclear factor kappa B and reactive oxygen species in the tumor necrosis factor-alpha-induced epithelial-mesenchymal transition of MCF-7 cells. Braz. J. Med. Biol. Res. 2007, 40, 1071–1078. [Google Scholar] [CrossRef]
- Al-Rawi, M.A.; Jiang, W.G. Lymphangiogenesis and cancer metastasis. Front Biosci. 2011, 16, 723–739. [Google Scholar] [CrossRef]
- Achen, M.G.; Stacker, S.A. Molecular control of lymphatic metastasis. Ann. N. Y. Acad. Sci. 2008, 1131, 225–234. [Google Scholar] [CrossRef]
- Goldman, J.; Rutkowski, J.M.; Shields, J.D.; Pasquier, M.C.; Cui, Y.; Schmokel, H.G.; Willey, S.; Hicklin, D.J.; Pytowski, B.; Swartz, M.A. Cooperative and redundant roles of VEGFR-2 and VEGFR-3 signaling in adult lymphangiogenesis. FASEB J. 2007, 21, 1003–1012. [Google Scholar] [CrossRef]
- Mattila, M.M.; Ruohola, J.K.; Karpanen, T.; Jackson, D.G.; Alitalo, K.; Harkonen, P.L. VEGF-C induced lymphangiogenesis is associated with lymph node metastasis in orthotopic MCF-7 tumors. Int. J. Cancer 2002, 98, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Blanc, I.; Gueguen-Dorbes, G.; Duclos, O.; Bonnin, J.; Barron, P.; Laplace, M.C.; Morin, G.; Gaujarengues, F.; Dol, F.; et al. SAR131675, a potent and selective VEGFR-3-TK inhibitor with antilymphangiogenic, antitumoral, and antimetastatic activities. Mol. Cancer Ther. 2012, 11, 1637–1649. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Kim, C.; Kim, M.J.; Schwendener, R.A.; Alitalo, K.; Heston, W.; Kim, I.; Kim, W.J.; Koh, G.Y. Soluble vascular endothelial growth factor receptor-3 suppresses lymphangiogenesis and lymphatic metastasis in bladder cancer. Mol. Cancer 2011, 10, 36. [Google Scholar] [CrossRef]
- Zumsteg, A.; Baeriswyl, V.; Imaizumi, N.; Schwendener, R.; Ruegg, C.; Christofori, G. Myeloid cells contribute to tumor lymphangiogenesis. PLoS ONE 2009, 4, e7067. [Google Scholar] [CrossRef]
- Schledzewski, K.; Falkowski, M.; Moldenhauer, G.; Metharom, P.; Kzhyshkowska, J.; Ganss, R.; Demory, A.; Falkowska-Hansen, B.; Kurzen, H.; Ugurel, S.; et al. Lymphatic endothelium-specific hyaluronan receptor LYVE-1 is expressed by stabilin-1+, F4/80+, CD11b+ macrophages in malignant tumours and wound healing tissue in vivo and in bone marrow cultures in vitro: Implications for the assessment of lymphangiogenesis. J. Pathol. 2006, 209, 67–77. [Google Scholar]
- Lee, J.Y.; Park, C.; Cho, Y.P.; Lee, E.; Kim, H.; Kim, P.; Yun, S.H.; Yoon, Y.S. Podoplanin-expressing cells derived from bone marrow play a crucial role in postnatal lymphatic neovascularization. Circulation 2010, 122, 1413–1425. [Google Scholar] [CrossRef]
- Volk-Draper, L.; Patel, R.; Bhattarai, N.; Yang, J.; Wilber, A.; DeNardo, D.; Ran, S. Myeloid-Derived Lymphatic Endothelial Cell Progenitors Significantly Contribute to Lymphatic Metastasis in Clinical Breast Cancer. Am. J. Pathol. 2019, 189, 2269–2292. [Google Scholar] [CrossRef]
- Hall, K.L.; Volk-Draper, L.D.; Flister, M.J.; Ran, S. New model of macrophage acquisition of the lymphatic endothelial phenotype. PLoS ONE 2012, 7, e31794. [Google Scholar] [CrossRef]
- Ran, S.; Wilber, A. Novel role of immature myeloid cells in formation of new lymphatic vessels associated with inflammation and tumors. J. Leukoc. Biol. 2017, 102, 253–263. [Google Scholar] [CrossRef]
- Van’t Hull, E.F.; Bron, S.; Henry, L.; Ifticene-Treboux, A.; Turrini, R.; Coukos, G.; Delaloye, J.F.; Doucey, M.A. Bone marrow-derived cells are implicated as a source of lymphatic endothelial progenitors in human breast cancer. Oncoimmunology 2014, 3, e29080. [Google Scholar] [CrossRef]
- Bogos, K.; Renyi-Vamos, F.; Dobos, J.; Kenessey, I.; Tovari, J.; Timar, J.; Strausz, J.; Ostoros, G.; Klepetko, W.; Ankersmit, H.J.; et al. High VEGFR-3-positive circulating lymphatic/vascular endothelial progenitor cell level is associated with poor prognosis in human small cell lung cancer. Clin. Cancer Res. 2009, 15, 1741–1746. [Google Scholar] [CrossRef] [PubMed]
- Schoppmann, S.F.; Birner, P.; Stockl, J.; Kalt, R.; Ullrich, R.; Caucig, C.; Kriehuber, E.; Nagy, K.; Alitalo, K.; Kerjaschki, D. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am. J. Pathol. 2002, 161, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Cao, L.; Wang, D.; Xu, H.; Liang, Z. High levels of circulating CD34+/VEGFR3+ lymphatic/vascular endothelial progenitor cells is correlated with lymph node metastasis in patients with epithelial ovarian cancer. J. Obstet. Gynaecol. Res. 2013, 39, 1268–1275. [Google Scholar] [CrossRef] [PubMed]
- Religa, P.; Cao, R.; Bjorndahl, M.; Zhou, Z.; Zhu, Z.; Cao, Y. Presence of bone marrow-derived circulating progenitor endothelial cells in the newly formed lymphatic vessels. Blood 2005, 106, 4184–4190. [Google Scholar] [CrossRef]
- Volk-Draper, L.D.; Hall, K.L.; Wilber, A.C.; Ran, S. Lymphatic endothelial progenitors originate from plastic myeloid cells activated by toll-like receptor-4. PLoS ONE 2017, 12, e0179257. [Google Scholar] [CrossRef]
- Tawada, M.; Hayashi, S.; Ikegame, Y.; Nakashima, S.; Yoshida, K. Possible involvement of tumor-producing VEGF-A in the recruitment of lymphatic endothelial progenitor cells from bone marrow. Oncol. Rep. 2014, 32, 2359–2364. [Google Scholar] [CrossRef]
- Kerjaschki, D.; Huttary, N.; Raab, I.; Regele, H.; Bojarski-Nagy, K.; Bartel, G.; Krober, S.M.; Greinix, H.; Rosenmaier, A.; Karlhofer, F.; et al. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat. Med. 2006, 12, 230–234. [Google Scholar] [CrossRef]
- Bron, S.; Henry, L.; Faes-Van’t Hull, E.; Turrini, R.; Vanhecke, D.; Guex, N.; Ifticene-Treboux, A.; Marina, I.E.; Semilietof, A.; Rufer, N.; et al. TIE-2-expressing monocytes are lymphangiogenic and associate specifically with lymphatics of human breast cancer. Oncoimmunology 2016, 5, e1073882. [Google Scholar] [CrossRef]
- Weimann, J.M.; Johansson, C.B.; Trejo, A.; Blau, H.M. Stable reprogrammed heterokaryons form spontaneously in Purkinje neurons after bone marrow transplant. Nat. Cell Biol. 2003, 5, 959–966. [Google Scholar] [CrossRef]
- Bonde, S.; Pedram, M.; Stultz, R.; Zavazava, N. Cell fusion of bone marrow cells and somatic cell reprogramming by embryonic stem cells. FASEB J. 2010, 24, 364–373. [Google Scholar] [CrossRef]
- Camargo, F.D.; Green, R.; Capetanaki, Y.; Jackson, K.A.; Goodell, M.A. Single hematopoietic stem cells generate skeletal muscle through myeloid intermediates. Nat. Med. 2003, 9, 1520–1527. [Google Scholar] [CrossRef] [PubMed]
- Yeh, M.H.; Chang, Y.H.; Tsai, Y.C.; Chen, S.L.; Huang, T.S.; Chiu, J.F.; Ch’ang, H.J. Bone marrow derived macrophages fuse with intestine stromal cells and contribute to chronic fibrosis after radiation. Radiother. Oncol. 2016, 119, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Quintana-Bustamante, O.; Alvarez-Barrientos, A.; Kofman, A.V.; Fabregat, I.; Bueren, J.A.; Theise, N.D.; Segovia, J.C. Hematopoietic mobilization in mice increases the presence of bone marrow-derived hepatocytes via in vivo cell fusion. Hepatology 2006, 43, 108–116. [Google Scholar] [CrossRef]
- Davies, P.S.; Powell, A.E.; Swain, J.R.; Wong, M.H. Inflammation and proliferation act together to mediate intestinal cell fusion. PLoS ONE 2009, 4, e6530. [Google Scholar] [CrossRef]
- Spees, J.L.; Whitney, M.J.; Sullivan, D.E.; Lasky, J.A.; Laboy, M.; Ylostalo, J.; Prockop, D.J. Bone marrow progenitor cells contribute to repair and remodeling of the lung and heart in a rat model of progressive pulmonary hypertension. FASEB J. 2008, 22, 1226–1236. [Google Scholar] [CrossRef]
- Palermo, A.; Doyonnas, R.; Bhutani, N.; Pomerantz, J.; Alkan, O.; Blau, H.M. Nuclear reprogramming in heterokaryons is rapid, extensive, and bidirectional. FASEB J. 2009, 23, 1431–1440. [Google Scholar] [CrossRef]
- Shabo, I.; Svanvik, J.; Lindstrom, A.; Lechertier, T.; Trabulo, S.; Hulit, J.; Sparey, T.; Pawelek, J. Roles of cell fusion, hybridization and polyploid cell formation in cancer metastasis. World J. Clin. Oncol. 2020, 11, 121–135. [Google Scholar] [CrossRef]
- Johansson, C.B.; Youssef, S.; Koleckar, K.; Holbrook, C.; Doyonnas, R.; Corbel, S.Y.; Steinman, L.; Rossi, F.M.; Blau, H.M. Extensive fusion of haematopoietic cells with Purkinje neurons in response to chronic inflammation. Nat. Cell Biol. 2008, 10, 575–583. [Google Scholar] [CrossRef]
- Jiang, E.; Yan, T.; Xu, Z.; Shang, Z. Tumor Microenvironment and Cell Fusion. Biomed. Res. Int. 2019, 2019, 5013592. [Google Scholar] [CrossRef]
- Cortez-Retamozo, V.; Etzrodt, M.; Newton, A.; Rauch, P.J.; Chudnovskiy, A.; Berger, C.; Ryan, R.J.; Iwamoto, Y.; Marinelli, B.; Gorbatov, R.; et al. Origins of tumor-associated macrophages and neutrophils. Proc. Natl. Acad. Sci. USA 2012, 109, 2491–2496. [Google Scholar] [CrossRef]
- Sun, C.; Zhao, D.; Dai, X.; Chen, J.; Rong, X.; Wang, H.; Wang, A.; Li, M.; Dong, J.; Huang, Q.; et al. Fusion of cancer stem cells and mesenchymal stem cells contributes to glioma neovascularization. Oncol. Rep. 2015, 34, 2022–2030. [Google Scholar] [CrossRef] [PubMed]
- Clawson, G.A.; Matters, G.L.; Xin, P.; Imamura-Kawasawa, Y.; Du, Z.; Thiboutot, D.M.; Helm, K.F.; Neves, R.I.; Abraham, T. Macrophage-tumor cell fusions from peripheral blood of melanoma patients. PLoS ONE 2015, 10, e0134320. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, M.; Mathur, S.R.; Mukhopadhyay, A. Fusion-derived epithelial cancer cells express hematopoietic markers and contribute to stem cell and migratory phenotype in ovarian carcinoma. Cancer Res. 2013, 73, 5360–5370. [Google Scholar] [CrossRef]
- Aguirre, L.A.; Montalban-Hernandez, K.; Avendano-Ortiz, J.; Marin, E.; Lozano, R.; Toledano, V.; Sanchez-Maroto, L.; Terron, V.; Valentin, J.; Pulido, E.; et al. Tumor stem cells fuse with monocytes to form highly invasive tumor-hybrid cells. Oncoimmunology 2020, 9, 1773204. [Google Scholar] [CrossRef]
- Powell, A.E.; Anderson, E.C.; Davies, P.S.; Silk, A.D.; Pelz, C.; Impey, S.; Wong, M.H. Fusion between Intestinal epithelial cells and macrophages in a cancer context results in nuclear reprogramming. Cancer Res. 2011, 71, 1497–1505. [Google Scholar] [CrossRef]
- Shabo, I.; Midtbo, K.; Andersson, H.; Akerlund, E.; Olsson, H.; Wegman, P.; Gunnarsson, C.; Lindstrom, A. Macrophage traits in cancer cells are induced by macrophage-cancer cell fusion and cannot be explained by cellular interaction. BMC Cancer 2015, 15, 922. [Google Scholar] [CrossRef]
- Mia, S.; Warnecke, A.; Zhang, X.M.; Malmstrom, V.; Harris, R.A. An optimized protocol for human M2 macrophages using M-CSF and IL-4/IL-10/TGF-beta yields a dominant immunosuppressive phenotype. Scand. J. Immunol. 2014, 79, 305–314. [Google Scholar] [CrossRef]
- Van Dyken, S.J.; Locksley, R.M. Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: Roles in homeostasis and disease. Annu. Rev. Immunol. 2013, 31, 317–343. [Google Scholar] [CrossRef]
- Espinosa Gonzalez, M.; Volk-Draper, L.; Bhattarai, N.; Wilber, A.; Ran, S. Th2 Cytokines IL-4, IL-13, and IL-10 Promote Differentiation of Pro-Lymphatic Progenitors Derived from Bone Marrow Myeloid Precursors. Stem Cells Dev. 2022, 31, 322–333. [Google Scholar] [CrossRef]
- Amarasekara, D.S.; Yun, H.; Kim, S.; Lee, N.; Kim, H.; Rho, J. Regulation of Osteoclast Differentiation by Cytokine Networks. Immune. Netw. 2018, 18, e8. [Google Scholar] [CrossRef]
- Moreno, J.L.; Mikhailenko, I.; Tondravi, M.M.; Keegan, A.D. IL-4 promotes the formation of multinucleated giant cells from macrophage precursors by a STAT6-dependent, homotypic mechanism: Contribution of E-cadherin. J. Leukoc. Biol. 2007, 82, 1542–1553. [Google Scholar] [CrossRef] [PubMed]
- Helming, L.; Gordon, S. Molecular mediators of macrophage fusion. Trends Cell Biol. 2009, 19, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Chen, L.Y.; Papadimos, T.J.; Huang, S.; Zuraw, B.L.; Pan, Z.K. Lipopolysaccharide-driven Th2 cytokine production in macrophages is regulated by both MyD88 and TRAM. J. Biol. Chem. 2009, 284, 29391–29398. [Google Scholar] [CrossRef]
- Volk-Draper, L.; Hall, K.; Griggs, C.; Rajput, S.; Kohio, P.; DeNardo, D.; Ran, S. Paclitaxel therapy promotes breast cancer metastasis in a TLR4-dependent manner. Cancer Res. 2014, 74, 5421–5434. [Google Scholar] [CrossRef]
- Whitehurst, B.; Eversgerd, C.; Flister, M.; Bivens, C.M.; Pickett, B.; Zawieja, D.C.; Ran, S. Molecular profile and proliferative responses of rat lymphatic endothelial cells in culture. Lymphat. Res. Biol. 2006, 4, 119–142. [Google Scholar] [CrossRef]
- Aricthota, S.; Rana, P.P.; Haldar, D. Histone acetylation dynamics in repair of DNA double-strand breaks. Front Genet. 2022, 13, 926577. [Google Scholar] [CrossRef]
- Helming, L.; Winter, J.; Gordon, S. The scavenger receptor CD36 plays a role in cytokine-induced macrophage fusion. J. Cell Sci. 2009, 122, 453–459. [Google Scholar] [CrossRef]
- Ul-Haq, Z.; Naz, S.; Mesaik, M.A. Interleukin-4 receptor signaling and its binding mechanism: A therapeutic insight from inhibitors tool box. Cytokine Growth Factor Rev. 2016, 32, 3–15. [Google Scholar] [CrossRef]
- Kim, G.W.; Park, S.Y.; Kim, I.S. Novel function of stabilin-2 in myoblast fusion: The recognition of extracellular phosphatidylserine as a “fuse-me” signal. BMB. Rep. 2016, 49, 303–304. [Google Scholar] [CrossRef]
- Whitlock, J.M.; Chernomordik, L.V. Flagging fusion: Phosphatidylserine signaling in cell-cell fusion. J. Biol. Chem. 2021, 296, 100411. [Google Scholar] [CrossRef]
- Vignery, A. Osteoclasts and giant cells: Macrophage-macrophage fusion mechanism. Int. J. Exp. Pathol. 2000, 81, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Vignery, A. Macrophage fusion: Are somatic and cancer cells possible partners? Trends Cell Biol. 2005, 15, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Helming, L.; Tomasello, E.; Kyriakides, T.R.; Martinez, F.O.; Takai, T.; Gordon, S.; Vivier, E. Essential role of DAP12 signaling in macrophage programming into a fusion-competent state. Sci. Signal. 2008, 1, ra11. [Google Scholar] [CrossRef]
- Yagi, M.; Ninomiya, K.; Fujita, N.; Suzuki, T.; Iwasaki, R.; Morita, K.; Hosogane, N.; Matsuo, K.; Toyama, Y.; Suda, T.; et al. Induction of DC-STAMP by alternative activation and downstream signaling mechanisms. J. Bone Miner. Res. 2007, 22, 992–1001. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, I.S. Stabilin Receptors: Role as Phosphatidylserine Receptors. Biomolecules 2019, 9, 387. [Google Scholar] [CrossRef]
- Brooks, P.J.; Wang, Y.; Magalhaes, M.A.; Glogauer, M.; McCulloch, C.A. CD301 mediates fusion in IL-4-driven multinucleated giant cell formation. J. Cell Sci. 2020, 133, jcs248864. [Google Scholar] [CrossRef]
- Helming, L.; Gordon, S. Macrophage fusion induced by IL-4 alternative activation is a multistage process involving multiple target molecules. Eur. J. Immunol. 2007, 37, 33–42. [Google Scholar] [CrossRef]
- Whitehurst, B.; Flister, M.J.; Bagaitkar, J.; Volk, L.; Bivens, C.M.; Pickett, B.; Castro-Rivera, E.; Brekken, R.A.; Gerard, R.D.; Ran, S. Anti-VEGF-A therapy reduces lymphatic vessel density and expression of VEGFR-3 in an orthotopic breast tumor model. Int. J. Cancer 2007, 121, 2181–2191. [Google Scholar] [CrossRef]
- Volk, L.D.; Flister, M.J.; Chihade, D.; Desai, N.; Trieu, V.; Ran, S. Synergy of nab-paclitaxel and bevacizumab in eradicating large orthotopic breast tumors and preexisting metastases. Neoplasia 2011, 13, 327–338. [Google Scholar] [CrossRef]
- Dalton, P.; Christian, H.C.; Redman, C.W.; Sargent, I.L.; Boyd, C.A. Membrane trafficking of CD98 and its ligand galectin 3 in BeWo cells—Implication for placental cell fusion. FEBS J. 2007, 274, 2715–2727. [Google Scholar] [CrossRef]
- Ceccaldi, P.E.; Delebecque, F.; Prevost, M.C.; Moris, A.; Abastado, J.P.; Gessain, A.; Schwartz, O.; Ozden, S. DC-SIGN facilitates fusion of dendritic cells with human T-cell leukemia virus type 1-infected cells. J. Virol. 2006, 80, 4771–4780. [Google Scholar] [CrossRef]
- Oursler, M.J. Recent advances in understanding the mechanisms of osteoclast precursor fusion. J. Cell Biochem. 2010, 110, 1058–1062. [Google Scholar] [CrossRef]
- Dietrich, J.; Cella, M.; Seiffert, M.; Buhring, H.J.; Colonna, M. Cutting edge: Signal-regulatory protein beta 1 is a DAP12-associated activating receptor expressed in myeloid cells. J. Immunol. 2000, 164, 9–12. [Google Scholar] [CrossRef]
- Shi, L.; Kidder, K.; Bian, Z.; Chiang, S.K.T.; Ouellette, C.; Liu, Y. SIRPalpha sequesters SHP-2 to promote IL-4 and IL-13 signaling and the alternative activation of macrophages. Sci. Signal. 2021, 14, eabb3966. [Google Scholar] [CrossRef]
- Kawamoto, T.; Ii, M.; Kitazaki, T.; Iizawa, Y.; Kimura, H. TAK-242 selectively suppresses Toll-like receptor 4-signaling mediated by the intracellular domain. Eur. J. Pharmacol. 2008, 584, 40–48. [Google Scholar] [CrossRef]
- Ran, S.; Volk-Draper, L. Lymphatic Endothelial Cell Progenitors in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1234, 87–105. [Google Scholar]
- Freeman, B.T.; Jung, J.P.; Ogle, B.M. Single-cell RNA-seq reveals activation of unique gene groups as a consequence of stem cell-parenchymal cell fusion. Sci. Rep. 2016, 6, 23270. [Google Scholar] [CrossRef]
- Espagnolle, N.; Barron, P.; Mandron, M.; Blanc, I.; Bonnin, J.; Agnel, M.; Kerbelec, E.; Herault, J.P.; Savi, P.; Bono, F.; et al. Specific Inhibition of the VEGFR-3 Tyrosine Kinase by SAR131675 Reduces Peripheral and Tumor Associated Immunosuppressive Myeloid Cells. Cancers 2014, 6, 472–490. [Google Scholar] [CrossRef]
- Okubo, M.; Kioi, M.; Nakashima, H.; Sugiura, K.; Mitsudo, K.; Aoki, I.; Taniguchi, H.; Tohnai, I. M2-polarized macrophages contribute to neovasculogenesis, leading to relapse of oral cancer following radiation. Sci. Rep. 2016, 6, 27548. [Google Scholar] [CrossRef]
- Dittmar, T.; Nagler, C.; Niggemann, B.; Zanker, K.S. The dark side of stem cells: Triggering cancer progression by cell fusion. Curr. Mol. Med. 2013, 13, 735–750. [Google Scholar] [CrossRef]
- Zhang, L.N.; Huang, Y.H.; Zhao, L. Fusion of macrophages promotes breast cancer cell proliferation, migration and invasion through activating epithelial-mesenchymal transition and Wnt/beta-catenin signaling pathway. Arch. Biochem. Biophys. 2019, 676, 108137. [Google Scholar] [CrossRef] [PubMed]
- Lindstrom, A.; Midtbo, K.; Arnesson, L.G.; Garvin, S.; Shabo, I. Fusion between M2-macrophages and cancer cells results in a subpopulation of radioresistant cells with enhanced DNA-repair capacity. Oncotarget 2017, 8, 51370–51386. [Google Scholar] [CrossRef]
- Adams, R.H.; Alitalo, K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 2007, 8, 464–478. [Google Scholar] [CrossRef]
- Kang, S.; Lee, S.P.; Kim, K.E.; Kim, H.Z.; Memet, S.; Koh, G.Y. Toll-like receptor 4 in lymphatic endothelial cells contributes to LPS-induced lymphangiogenesis by chemotactic recruitment of macrophages. Blood 2009, 113, 2605–2613. [Google Scholar] [CrossRef]
- Zampell, J.; Elhadad, S.; Avraham, T.; Weitman, E.; Aschen, S.; Yan, A.; Mehrara, B.J. Toll-Like Receptor Deficiency Worsens Inflammation and Lymphedema after Lymphatic Injury. Am. J. Physiol Cell Physiol 2011, 4, 709–719. [Google Scholar] [CrossRef]
- Volk-Draper, L.; Athaiya, S.; Espinosa, G.M.; Bhattarai, N.; Wilber, A.; Ran, S. Tumor microenvironment restricts IL-10 induced multipotent progenitors to myeloid-lymphatic phenotype. PLoS ONE 2024, 19, e0298465. [Google Scholar] [CrossRef]
- Hernandez, J.M.; Podbilewicz, B. The hallmarks of cell-cell fusion. Development 2017, 144, 4481–4495. [Google Scholar] [CrossRef]
- Frendo, J.L.; Cronier, L.; Bertin, G.; Guibourdenche, J.; Vidaud, M.; Evain-Brion, D.; Malassine, A. Involvement of connexin 43 in human trophoblast cell fusion and differentiation. J. Cell Sci. 2003, 116, 3413–3421. [Google Scholar] [CrossRef]
- Kneppers, A.; Verdijk, L.; de Theije, C.; Corten, M.; Gielen, E.; van Loon, L.; Schols, A.; Langen, R. A novel in vitro model for the assessment of postnatal myonuclear accretion. Skelet. Muscle 2018, 8, 4. [Google Scholar] [CrossRef]
- Pereira, M.; Petretto, E.; Gordon, S.; Bassett, J.H.D.; Williams, G.R.; Behmoaras, J. Common signalling pathways in macrophage and osteoclast multinucleation. J. Cell Sci. 2018, 131, jcs216267. [Google Scholar] [CrossRef]
- Puissegur, M.P.; Lay, G.; Gilleron, M.; Botella, L.; Nigou, J.; Marrakchi, H.; Mari, B.; Duteyrat, J.L.; Guerardel, Y.; Kremer, L.; et al. Mycobacterial lipomannan induces granuloma macrophage fusion via a TLR2-dependent, ADAM9- and beta1 integrin-mediated pathway. J. Immunol. 2007, 178, 3161–3169. [Google Scholar] [CrossRef]
- Dornen, J.; Sieler, M.; Weiler, J.; Keil, S.; Dittmar, T. Cell Fusion-Mediated Tissue Regeneration as an Inducer of Polyploidy and Aneuploidy. Int. J. Mol. Sci. 2020, 21, 1811. [Google Scholar] [CrossRef]
- Shultes, P.V.; Weaver, D.T.; Tadele, D.S.; Barker-Clarke, R.J.; Scott, J.G. Cell-cell fusion in cancer: The next cancer hallmark? Int. J. Biochem. Cell Biol. 2024, 175, 106649. [Google Scholar] [CrossRef]
- Shand, F.H.; Ueha, S.; Otsuji, M.; Koid, S.S.; Shichino, S.; Tsukui, T.; Kosugi-Kanaya, M.; Abe, J.; Tomura, M.; Ziogas, J.; et al. Tracking of intertissue migration reveals the origins of tumor-infiltrating monocytes. Proc. Natl. Acad. Sci. USA 2014, 111, 7771–7776. [Google Scholar] [CrossRef]
- Lee, S.G.; Moon, S.H.; Kim, H.J.; Lee, J.Y.; Park, S.J.; Chung, H.M.; Ha, T.Y.; Song, G.W.; Jung, D.H.; Park, H.; et al. Role of bone marrow-derived progenitor cells in de novo liver regeneration in human liver transplants. Liver Transpl. 2015, 21, 1186–1194. [Google Scholar] [CrossRef]
- Fukata, M.; Ishikawa, F.; Najima, Y.; Yamauchi, T.; Saito, Y.; Takenaka, K.; Miyawaki, K.; Shimazu, H.; Shimoda, K.; Kanemaru, T.; et al. Contribution of bone marrow-derived hematopoietic stem/progenitor cells to the generation of donor-marker+ cardiomyocytes in vivo. PLoS ONE 2013, 8, e62506. [Google Scholar] [CrossRef]
- Ma, S.F.; Chen, Y.J.; Zhang, J.X.; Shen, L.; Wang, R.; Zhou, J.S.; Hu, J.G.; Lu, H.Z. Adoptive transfer of M2 macrophages promotes locomotor recovery in adult rats after spinal cord injury. Brain Behav. Immun. 2015, 45, 157–170. [Google Scholar] [CrossRef]
- Metharom, P.; Liu, C.; Wang, S.; Stalboerger, P.; Chen, G.; Doyle, B.; Ikeda, Y.; Caplice, N.M. Myeloid lineage of high proliferative potential human smooth muscle outgrowth cells circulating in blood and vasculogenic smooth muscle-like cells in vivo. Atherosclerosis 2008, 198, 29–38. [Google Scholar] [CrossRef]
- Maruyama, K.; Ii, M.; Cursiefen, C.; Jackson, D.G.; Keino, H.; Tomita, M.; Van Rooijen, N.; Takenaka, H.; D’Amore, P.A.; Stein-Streilein, J.; et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J. Clin. Invest 2005, 115, 2363–2372. [Google Scholar] [CrossRef]
- Tawada, M.; Hayashi, S.; Osada, S.; Nakashima, S.; Yoshida, K. Human gastric cancer organizes neighboring lymphatic vessels via recruitment of bone marrow-derived lymphatic endothelial progenitor cells. J. Gastroenterol. 2012, 47, 1057–1060. [Google Scholar] [CrossRef]
- Peters, B.A.; Diaz, L.A.; Polyak, K.; Meszler, L.; Romans, K.; Guinan, E.C.; Antin, J.H.; Myerson, D.; Hamilton, S.R.; Vogelstein, B.; et al. Contribution of bone marrow-derived endothelial cells to human tumor vasculature. Nat. Med. 2005, 11, 261–262. [Google Scholar] [CrossRef]
- Balaji, S.; King, A.; Crombleholme, T.M.; Keswani, S.G. The Role of Endothelial Progenitor Cells in Postnatal Vasculogenesis: Implications for Therapeutic Neovascularization and Wound Healing. Adv. Wound. Care 2013, 2, 283–295. [Google Scholar] [CrossRef]
- Vignery, A. Macrophage fusion: The making of osteoclasts and giant cells. J. Exp. Med. 2005, 202, 337–340. [Google Scholar] [CrossRef]
- Kukreja, A.; Radfar, S.; Sun, B.H.; Insogna, K.; Dhodapkar, M.V. Dominant role of CD47-thrombospondin-1 interactions in myeloma-induced fusion of human dendritic cells: Implications for bone disease. Blood 2009, 114, 3413–3421. [Google Scholar] [CrossRef]
- Chiu, Y.G.; Ritchlin, C.T. Characterization of DC-STAMP+ Cells in Human Bone Marrow. J. Bone Marrow Res. 2013, 1, 127. [Google Scholar] [CrossRef]
- Helming, L.; Gordon, S. The molecular basis of macrophage fusion. Immunobiology 2007, 212, 785–793. [Google Scholar] [CrossRef]
- McNally, A.K.; Anderson, J.M. Macrophage fusion and multinucleated giant cells of inflammation. Adv. Exp. Med. Biol. 2011, 713, 97–111. [Google Scholar]
- Li, G.; Kikuchi, K.; Radka, M.; Abraham, J.; Rubin, B.P.; Keller, C. IL-4 receptor blockade abrogates satellite cell: Rhabdomyosarcoma fusion and prevents tumor establishment. Stem Cells 2013, 31, 2304–2312. [Google Scholar] [CrossRef]
- Shrivastava, P.; Bagchi, T. IL-10 modulates in vitro multinucleate giant cell formation in human tuberculosis. PLoS ONE 2013, 8, e77680. [Google Scholar] [CrossRef]
- Dresner-Pollak, R.; Gelb, N.; Rachmilewitz, D.; Karmeli, F.; Weinreb, M. Interleukin 10-deficient mice develop osteopenia, decreased bone formation, and mechanical fragility of long bones. Gastroenterology 2004, 127, 792–801. [Google Scholar] [CrossRef]
- Deng, B.; Wehling-Henricks, M.; Villalta, S.A.; Wang, Y.; Tidball, J.G. IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. J. Immunol. 2012, 189, 3669–3680. [Google Scholar] [CrossRef] [PubMed]
- Irvine, K.M.; Burns, C.J.; Wilks, A.F.; Su, S.; Hume, D.A.; Sweet, M.J. A CSF-1 receptor kinase inhibitor targets effector functions and inhibits pro-inflammatory cytokine production from murine macrophage populations. FASEB J. 2006, 20, 1921–1923. [Google Scholar] [CrossRef] [PubMed]
- Kubota, Y.; Takubo, K.; Shimizu, T.; Ohno, H.; Kishi, K.; Shibuya, M.; Saya, H.; Suda, T. M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis. J. Exp. Med. 2009, 206, 1089–1102. [Google Scholar] [CrossRef]
- Rossol, M.; Heine, H.; Meusch, U.; Quandt, D.; Klein, C.; Sweet, M.J.; Hauschildt, S. LPS-induced cytokine production in human monocytes and macrophages. Crit Rev. Immunol. 2011, 31, 379–446. [Google Scholar] [CrossRef]
- Cao, S.; Liu, J.; Song, L.; Ma, X. The protooncogene c-Maf is an essential transcription factor for IL-10 gene expression in macrophages. J. Immunol. 2005, 174, 3484–3492. [Google Scholar] [CrossRef]
- Daassi, D.; Hamada, M.; Jeon, H.; Imamura, Y.; Nhu Tran, M.T.; Takahashi, S. Differential expression patterns of MafB and c-Maf in macrophages in vivo and in vitro. Biochem. Biophys. Res. Commun. 2016, 473, 118–124. [Google Scholar] [CrossRef]
- Biswas, R.; Datta, S.; Gupta, J.D.; Novotny, M.; Tebo, J.; Hamilton, T.A. Regulation of chemokine mRNA stability by lipopolysaccharide and IL-10. J. Immunol. 2003, 170, 6202–6208. [Google Scholar] [CrossRef]
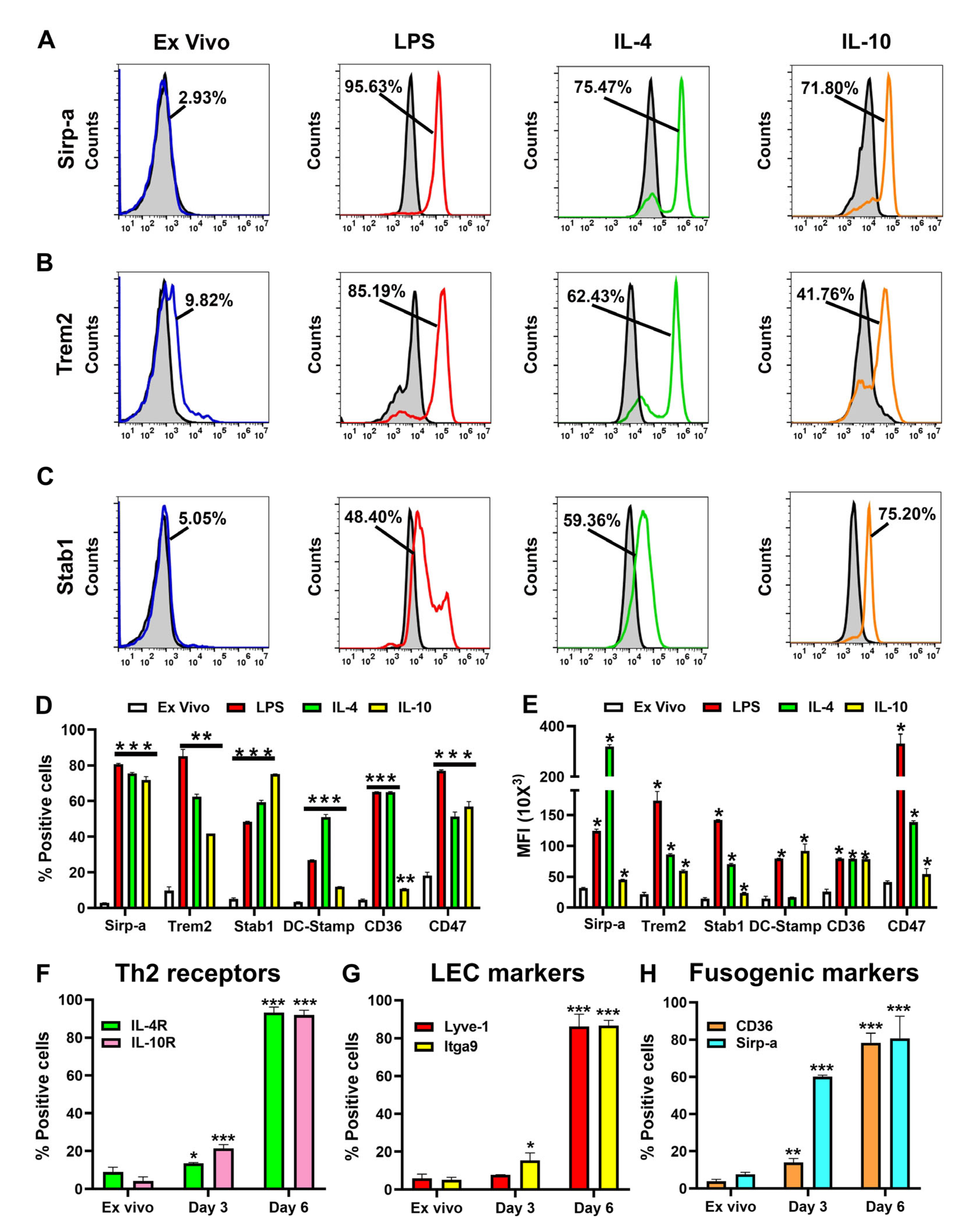
| Percent Positive Cells | |||||||
|---|---|---|---|---|---|---|---|
| Ex Vivo a | LPS b | IL-4 b | IL-10 b | ||||
| Marker | % | % | p-Value | % | p-Value | % | p-Value |
| Sirp-a | 2.93 ± 0.05 | 80.69 ± 0.20 | <0.0001 | 75.47 ± 0.62 | <0.0001 | 71.80 ± 1.89 | 0.0008 |
| Trem2 | 9.82 ± 2.06 | 85.19 ± 3.80 | 0.0020 | 62.43 ± 1.47 | 0.0023 | 41.76 ± 0.01 | 0.0041 |
| Stab1 | 5.05 ± 0.64 | 48.40 ± 0.20 | 0.0001 | 59.36 ± 1.13 | 0.0003 | 75.20 ± 0.10 | <0.0001 |
| DC-Stamp | 3.41 ± 0.08 | 26.88 ± 0.28 | 0.0001 | 51.05 ± 1.46 | 0.0009 | 11.94 ± 0.09 | 0.0002 |
| CD36 | 4.60 ± 0.44 | 65.10 ± 0.20 | <0.0001 | 65.08 ± 0.33 | 0.0001 | 10.78 ± 0.18 | 0.0057 |
| CD47 | 18.29 ± 1.74 | 77.02 ± 0.48 | 0.0001 | 51.40 ± 2.46 | 0.0082 | 57.01 ± 2.67 | 0.0069 |
| Mean Fluorescent Intensity (MFI) | |||||||
| Marker | MFI (×103) | MFI (×103) | p-Value | MFI (×103) | p-Value | MFI (×103) | p-Value |
| Sirp-a | 31.62 ± 1.13 | 124.26 ± 2.66 | 0.0010 | 319.92 ± 8.00 | 0.0004 | 45.25 ± 0.58 | 0.0085 |
| Trem2 | 21.72 ± 2.79 | 172.92 ± 15.32 | 0.0054 | 86.11 ± 1.25 | 0.0023 | 59.48 ± 1.58 | 0.0063 |
| Stab1 | 14.62 ± 1.54 | 141.64 ± 0.87 | 0.0001 | 70.10 ± 1.36 | 0.0007 | 23.90 ± 0.60 | 0.0159 |
| DC-Stamp | 14.59 ± 3.75 | 79.39 ± 0.73 | 0.0034 | 16.95 ± 0.30 | NSc | 92.10 ± 11.03 | 0.0218 |
| CD36 | 25.52 ± 4.21 | 79.55 ± 1.01 | 0.0064 | 79.55 ± 1.01 | 0.0064 | 78.53 ± 1.91 | 0.0057 |
| CD47 | 41.17 ± 2.20 | 331.93 ± 37.63 | 0.0162 | 70.10 ± 1.36 | 0.0007 | 23.90 ± 0.60 | 0.0159 |
| Marker | Normal MFP | MDA-MB-231 | p-Value | Reported Role in Fusion | Reference |
|---|---|---|---|---|---|
| CD36 | 26.89 ± 2.37 a | 74.10 ± 2.03 | 0.001 | Binds to phosphatidylserine to facilitate cell adhesion | [57] |
| CD98 | ND b | 86.03 ± 12.15 | Promotes placental syncytia by binding to galectin-3 | [70] | |
| CD209 | ND | 86.99 ± 12.31 | Promotes fusion of myeloid and virus-infected cells | [71] | |
| Dap12 | 18.95 ± 3.05 | 86.58 ± 9.73 | 0.001 | Mediates Dc-stamp and SIRP-a signaling that confers fusion competence of myeloid cells | [63] |
| DC-Stamp | 25.95 ± 9.46 | 77.66 ± 4.71 | 0.001 | Promotes multinucleation in IL-4-treated macrophages | [63] |
| Sirp alpha | 28.92 ± 2.53 | 85.79 ± 16.21 | 0.001 | Mediates Th2-dependent induction of key M2 and fusogenic traits | [61,74] |
| Sirp beta-1 | ND | 73.74 ± 10.94 | Activates pro-fusogenic Dap12 signaling in myeloid cells | [73] | |
| Stabilin-1 | ND | 98.92 ± 3.42 | Binds to phosphatidylserine to facilitate cell adhesion | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Athaiya, S.; Volk-Draper, L.; Cox, E.; Robinson, K.; Zinkevich, N.; Ran, S. Bone Marrow Myeloid–Lymphatic Progenitors Expand Tumor Lymphatic Vasculature Through Cell Fusion. Cancers 2025, 17, 1804. https://doi.org/10.3390/cancers17111804
Athaiya S, Volk-Draper L, Cox E, Robinson K, Zinkevich N, Ran S. Bone Marrow Myeloid–Lymphatic Progenitors Expand Tumor Lymphatic Vasculature Through Cell Fusion. Cancers. 2025; 17(11):1804. https://doi.org/10.3390/cancers17111804
Chicago/Turabian StyleAthaiya, Shaswati, Lisa Volk-Draper, Emma Cox, Kathy Robinson, Natalya Zinkevich, and Sophia Ran. 2025. "Bone Marrow Myeloid–Lymphatic Progenitors Expand Tumor Lymphatic Vasculature Through Cell Fusion" Cancers 17, no. 11: 1804. https://doi.org/10.3390/cancers17111804
APA StyleAthaiya, S., Volk-Draper, L., Cox, E., Robinson, K., Zinkevich, N., & Ran, S. (2025). Bone Marrow Myeloid–Lymphatic Progenitors Expand Tumor Lymphatic Vasculature Through Cell Fusion. Cancers, 17(11), 1804. https://doi.org/10.3390/cancers17111804






