Effect of Preoperative Single-Inhaler Triple Therapy on Pulmonary Function in Lung Cancer Patients with Chronic Obstructive Pulmonary Disease and FEV1 < 1.5 L
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Study Design and Patients
2.3. COPD Treatments
2.4. Preoperative Tests
2.5. Surgical Strategy
2.6. Surgical Procedure
2.7. Variables and Assessments
2.8. Data Management and Statistical Analysis
3. Results
3.1. Analysis of All Patients Undergoing Lung Resection
3.2. Analysis of Patients Undergoing Anatomical Lung Resection
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| COPD | Chronic obstructive pulmonary disease |
| ACO | Asthma and COPD over lap |
| TIO | Tiotropium |
| FF | Fluticasone furoate |
| UMEC/VI | Umeclidinium and vilanterol |
| FEV1 | Forced expiratory volume in 1 s |
| VATS | Video-assisted thoracoscopic surgery |
| LAMAs | Long-acting muscarinic antagonists |
| LABAS | Long-acting beta agonists |
| ICSs | Inhaled corticosteroids |
| HOT | Home oxygen therappy |
References
- British Thoracic Society; Society of Cardiothoracic Surgeons of Great Britain and Ireland Working Party. Guidelines on the selection of patients with lung cancer for surgery. Thorax 2001, 56, 89–108. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Wang, D.; Chung, C.; Tian, D.; Rimner, A.; Huang, J.; Jones, D.R. A systematic review and meta-analysis of stereotactic body radiation therapy versus surgery for patients with non–small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2019, 157, 362–373.e8. [Google Scholar] [CrossRef] [PubMed]
- Nicastri, D.G.; Alpert, N.; Liu, B.; Wolf, A.; Taioli, E.; Tran, B.V.; Flores, R. Oxygen use after lung cancer surgery. Ann. Thorac. Surg. 2018, 106, 1548–1555. [Google Scholar] [CrossRef]
- Saji, H.; Miyazawa, T.; Sakai, H.; Kimura, Y.; Tsuda, M.; Wakiyama, Y.; Marushima, H.; Kojima, K.; Nakamura, H. Survival significance of coexisting chronic obstructive pulmonary disease in patients with early lung cancer after curative surgery. Thorac. Cancer 2018, 9, 19–24. [Google Scholar] [CrossRef]
- Suzuki, H.; Sekine, Y.; Yoshida, S.; Suzuki, M.; Shibuya, K.; Takiguchi, Y.; Tatsumi, K. Efficacy of perioperative administration of long-acting bronchodilator on postoperative pulmonary function and quality of life in lung cancer patients with chronic obstructive pul-monary disease. Preliminary results of a randomized control study. Surg. Today 2010, 40, 923–930. [Google Scholar] [CrossRef]
- Ueda, K.; Tanaka, T.; Hayashi, M.; Hamano, K. Role of inhaled tiotropium on the perioperative outcomes of patients with lung cancer and chronic obstructive pulmonary disease. Thorac. Cardiovasc. Surg. 2010, 58, 38–42. [Google Scholar] [CrossRef]
- Kobayashi, S.; Suzuki, S.; Niikawa, H.; Sugawara, T.; Yanai, M. Preoperative use of inhaled tiotropium in lung cancer patients with untreated COPD. Respirology 2009, 14, 675–679. [Google Scholar] [CrossRef]
- Bölükbas, S.; Eberlein, M.; Eckhoff, J.; Schirren, J. Short-term effects of inhalative tiotropium/formoterol/budenoside versus tiotropium/formoterol in patients with newly diagnosed chronic obstructive pulmonary disease requiring surgery for lung cancer: A prospective randomized trial. Eur. J. Cardio-Thoracic Surg. 2011, 39, 995–1000. [Google Scholar] [CrossRef]
- Homma, T. Preoperative umeclidinium/vilanterol or tiotropium improves postoperative FEV1 in lung cancer patients with comorbid untreated chronic obstructive pulmonary disease. J. Thorac. Dis. 2023, 15, 1584–1594. [Google Scholar] [CrossRef]
- Lipson, D.A.; Barnhart, F.; Brealey, N.; Brooks, J.; Criner, G.J.; Day, N.C.; Dransfield, M.T.; Halpin, D.M.; Han, M.K.; Jones, C.E.; et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N. Engl. J. Med. 2018, 378, 1671–1680. [Google Scholar] [CrossRef]
- Singh, D.; Papi, A.; Corradi, M.; Pavilisova, I.; Montagna, I.; Francisco, C.; Cohuet, G.; Vezzoli, S.; Scuri, M.; Vestbo, J. Single inhaler triple therapy versus inhaled corticosteroid plus long-acting β2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): A double-blind, parallel group, randomized controlled trial. Lancet 2016, 388, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Vestbo, J.; Fabbri, L.; Corradi, M.; Prunier, H.; Cohuet, G.; Guasconi, A.; Montagna, I.; Vezzoli, S.; Petruzzelli, S.; et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): A double-blind, parallel group, randomised controlled trial. Lancet 2018, 391, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- ALee, L.; Bailes, Z.; Barnes, N.; Boulet, L.-P.; Edwards, D.; Fowler, A.; AHanania, N.; Kerstjens, H.A.M.; Kerwin, E.; Nathan, R.; et al. Efficacy and safety of once-daily single-inhaler triple therapy (FF/UMEC/VI) versus FF/VI in patients with inadequately controlled asthma (CAPTAIN): A double-blind, randomised, phase 3A trial. Lancet Respir. Med. 2021, 9, 69–84. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Guang, J.; Xie, Y.; Shi, Z.; Gu, H.; Zheng, Y. Improving the estimation of PM2.5 concentration in the North China Area by introducing an attention mechanism into random forest. Atmosphere 2024, 15, 384. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, H.; Tang, N.; Zhou, S.; Yu, J.; Ding, B. Spider-Web-Inspired PM0.3 filters based on self-sustained electrostatic nanostructured networks. Adv. Mater. 2020, 32, e2002361. [Google Scholar] [CrossRef]
- Li, J.; Deng, Z.; Soerensen, S.J.C.; Kachuri, L.; Cardenas, A.; Graff, R.E.; Leppert, J.T.; Langston, M.E.; Chung, B.I. Ambient air pollution and urological cancer risk: A systematic review and meta-analysis of epidemiological evidence. Nat. Commun. 2024, 15, 5116. [Google Scholar] [CrossRef]
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2022 Report); Lulu Press: Morrisville, NC, USA, 2021. [Google Scholar]
- A Joint Project of GINA and GOLD. Diagnosis and Initial Treatment of Asthma, COPD and Asthma—COPD Overlap. April 2017. Available online: https://ginasthma.org/wp-content/uploads/2019/11/GINA-GOLD-2017-overlap-pocket-guide-wms-2017-ACO.pdf (accessed on 28 December 2024).
- Saji, H.; Okada, M.; Tsuboi, M.; Nakajima, R.; Suzuki, K.; Aokage, K.; Aoki, T.; Okami, J.; Yoshino, I.; Ito, H.; et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): A multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022, 399, 1607–1617. [Google Scholar] [CrossRef]
- Homma, T.; Shimada, Y.; Tanabe, K. Decreased postoperative complications, neuropathic pain and epidural anesthesia-free effect of uniportal video-assisted thoracoscopic anatomical lung resection: A single-center initial experience of 100 cases. J. Thorac. Dis. 2022, 14, 3154–3166. [Google Scholar] [CrossRef]
- Ali, M.K.; Mountain, C.F.; Ewer, M.S.; Johnston, D.; Haynie, T.P. Predicting loss of pulmonary function after pulmonary resection for bronchogenic carcinoma. Chest 1980, 77, 337–342. [Google Scholar] [CrossRef]
- Cingoz, F.; Oz, B.S.; Arslan, G.; Guler, A.; Sahin, M.A.; Gunay, C.; Arslan, M. Is chronic obstructive pulmonary disease a risk factor for epistaxis after coronary artery bypass graft surgery? Cardiovasc. J. Afr. 2014, 25, 279–281. [Google Scholar] [CrossRef]
- Martin, C.T.; Gao, Y.; Pugely, A.J.; Wolf, B.R. 30-day morbidity and mortality after elective shoulder arthroscopy: A review of 9410 cases. J. Shoulder Elb. Surg. 2013, 22, 1667–1675.e1. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Tsugawa, Y.; Faridi, M.K.; Camargo, C.A.; Hasegawa, K. Reduced risk of acute exacerbation of COPD after bariatric surgery: A self-controlled case series study. Chest 2018, 153, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.; Sousa, I.; Shah, P.L.; Diggle, P.; Goldstraw, P. Lung volume reduction surgery: Reinterpreted with longitudinal data analyses methodology. Ann. Thorac. Surg. 2020, 109, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- Bugge, A.; Lund, M.B.; Brunborg, C.; Solberg, S.; Kongerud, J. Survival After Surgical Resection for Lung Cancer in Patients With Chronic Obstructive Pulmonary Disease. Ann. Thorac. Surg. 2016, 101, 2125–2131. [Google Scholar] [CrossRef]
- Licker, M.J.; Widikker, I.; Robert, J.; Frey, J.-G.; Spiliopoulos, A.; Ellenberger, C.; Schweizer, A.; Tschopp, J.-M. Operative mortality and respiratory complications after lung resection for cancer: Impact of chronic obstructive pulmonary disease and time trends. Ann. Thorac. Surg. 2006, 81, 1830–1837. [Google Scholar] [CrossRef]
- Sekine, Y.; Behnia, M.; Fujisawa, T. Impact of COPD on pulmonary complications and on long-term survival of patients undergoing surgery for NSCLC. Lung Cancer 2002, 37, 95–101. [Google Scholar] [CrossRef]
- Suissa, S.; Coulombe, J.; Ernst, P. Discontinuation of inhaled corticosteroids in COPD and the risk reduction of pneumonia. Chest 2015, 148, 1177–1183. [Google Scholar] [CrossRef]
- AYang, I.; Ferry, O.R.; Clarke, M.S.; Sim, E.H.; Fong, K.M. Inhaled corticosteroids versus placebo for stable chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2023, 2023, CD002991. [Google Scholar] [CrossRef]
- Ernst, P.; Gonzalez, A.V.; Brassard, P.; Suissa, S. Inhaled corticosteroid use in chronic obstructive pulomonary disease and the risk of hospitalization for pneumonia. Am. J. Respir. Crit. Care Med. 2007, 176, 162–166. [Google Scholar] [CrossRef]
- Komase, Y.; Asako, A.; Kobayashi, A.; Sharma, R. Ease-of-use preference for the ELLIPTA® dry powder inhaler over a commonly used single-dose capsule dry powder inhaler by inhalation device-naïve Japanese volunteers aged 40 years or older. Int. J. Chronic Obstr. Pulm. Dis. 2014, 9, 1365–1375. [Google Scholar] [CrossRef]
- Homma, T.; Saji, H.; Shimada, Y.; Tanabe, K.; Kojima, K.; Marushima, H.; Miyazawa, T.; Kimura, H.; Sakai, H.; Otsubo, K.; et al. Surgical outcomes and learning curve of complex versus simple segmentectomy for uniportal video-assisted thoracoscopic surgery: An initial experience of 100 cases of a single experienced surgeon. J. Thorac. Dis. 2023, 16, 7361–7371. [Google Scholar] [CrossRef] [PubMed]
- Kajiwara, A.; Kita, A.; Saruwatari, J.; Morita, K.; Oniki, K.; Yamamura, M.; Murase, M.; Koda, H.; Hirota, S.; Ishizuka, T.; et al. Absence of gargling affects topical adverse symptoms caused by inhaled corticosteroids in females. J. Asthma 2014, 51, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Sano, Y.; Shigematsu, H.; Okazaki, M.; Sakao, N.; Mori, Y.; Yukumi, S.; Izutani, H. Hoarseness after radical surgery with systematic lymph node dissection for primary lung cancer. Eur. J. Cardio-Thoracic Surg. 2019, 55, 280–285. [Google Scholar] [CrossRef]
- Gibson, P.G.; McDonald, V.M. Asthma-COPD overlap 2015: Now we are six. Thorax 2015, 70, 683–691. [Google Scholar] [CrossRef]
- Byun, J.-Y.; Lee, J.-E.; Shim, Y.-B.; Kim, J.; Lee, S.Y.; Shin, B.R.; Yoon, N.R.; Park, M.-H.; Lee, E.-K. Economic burden of recurrence in completely resected stage IB-IIIA non-small cell lung cancer: A retrospective study using nationwide claims data of South Korea. Adv. Ther. 2023, 40, 550–567. [Google Scholar] [CrossRef]
- Svedsater, H.; Stynes, G.; Wex, J.; Frith, L.; Leather, D.; Castelnuovo, E.; Detry, M.; Berry, S. Once-daily fluticasone furoate/vilanterol versus twice daily combination therapies in asthma–mixed treatment comparisons of clinical efficacy. Asthma Res. Pract. 2016, 2, 4. [Google Scholar] [CrossRef][Green Version]
- Lamprecht, B.; McBurnie, M.A.; Vollmer, W.M.; Gudmundsson, G.; Welte, T.; Nizanlowsla-Moglinicka, E.; Studnicka, M.; Bateman, E.; Anto, J.M.; Burney, P.; et al. COPD in never smokers. Results from the population-based burden of obstructive lung disease study. Chest 2011, 139, 752–763. [Google Scholar] [CrossRef]
- Feldman, W.B.; Suissa, S.; Kesselheim, A.S.; Avorn, J.; Russo, M.; Schneeweiss, S.; Wang, S.V. Comparative effectiveness and safety of single inhaler triple therapies for chronic obstructive pulmonary disease: New user cohort study. BMJ 2024, 387, e080409. [Google Scholar] [CrossRef]

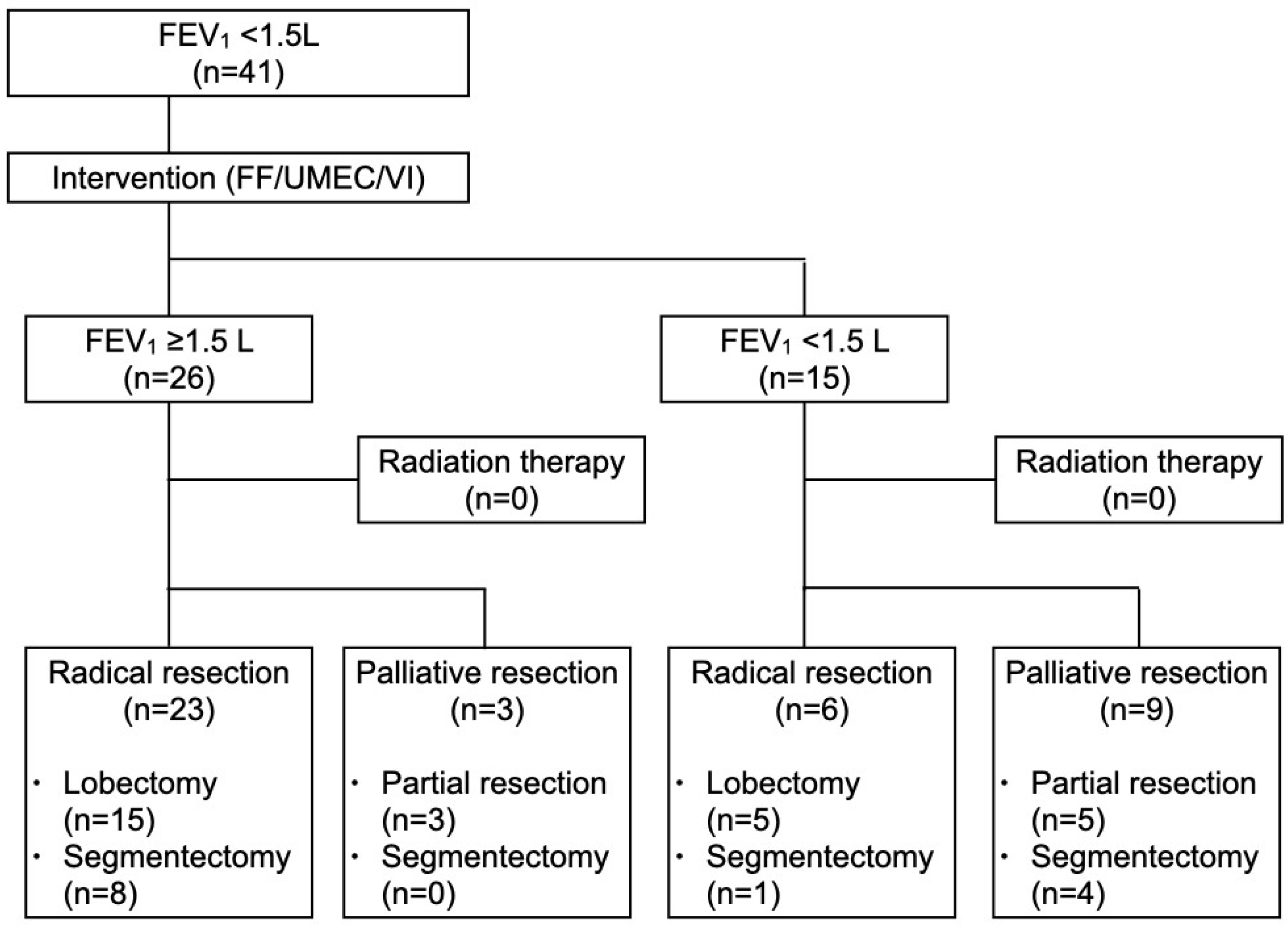


| Characteristic | Post-Interventional FEV1 ≥ 1.5 L (n = 26) | Post-Interventional FEV1 < 1.5 L (n = 15) | p-Value |
|---|---|---|---|
| Age, median [IQR] | 77 [73–81] | 76 [71–81] | 0.49 |
| Sex, male (%) | 12 (46.1) | 7 (46.7) | 0.64 |
| BMI, median [IQR] | 22.4 [19.6–25.4] | 24.8 [21.5–29.1] | 0.091 |
| Hypertension (%) | 15 (57.7) | 10 (66.7) | 0.74 |
| Hyperlipidemia (%) | 10 (38.5) | 5 (33.3) | 0.51 |
| Hyperuricemia (%) | 6 (23.1) | 3 (20.0) | 0.57 |
| Diabetes (%) | 11 (42.3) | 8 (53.3) | 0.53 |
| Smoking history (%) | 24 (92.3) | 14 (93.3) | 0.76 |
| Brinkman index, median [IQR] | 840 [435–1010] | 1120 [1040–1500] | 0.0058 |
| Comorbidities | |||
| Osteoporosis | 2 (7.7) | 1 (6.7) | 0.70 |
| Gastroesophageal reflux | 4 (15.4) | 2 (13.3) | 0.62 |
| Cardiovascular diseases | 7 (26.9) | 6 (40.0) | 0.49 |
| Anxiety and depression | 6 (23.1) | 3 (20.0) | 0.57 |
| Interstitial pneumonia (%) | 5 (19.2) | 0 (0) | 0.14 |
| GOLD classification | |||
| 2 (%) | 20 (76.9) | 10 (66.7) | 0.49 |
| 3 (%) | 6 (23.1) | 5 (33.3) | |
| Spirometry | |||
| FVC (L), median [IQR] | 2.29 [2.11–2.42] | 2.00 [1.88–2.37] | 0.13 |
| %FVC (%), median [IQR] | 94.0 [80.8–105.7] | 86.7 [74.2–96.3] | 0.21 |
| Preint FEV1/FVC (%), median [IQR] | 61.6 [52.5–66.9] | 62.9 [44.3–67.5] | 0.77 |
| Postint FEV1/FVC (%), median [IQR] | 64.9 [52.9–69.2] | 65.3 [45.9–69.8] | 0.54 |
| Preint FEV1 (L), median [IQR] | 1.37 [1.26–1.45] | 1.14 [1.05–1.32] | 0.0050 |
| Postint FEV1 (L), median [IQR] | 1.63 [1.54–1.76] | 1.34 [1.23–1.39] | <0.0001 |
| Postint—Preint FEV1(L), median [IQR] | 0.29 [0.13–0.52] | 0.14 [0.03–0.28] | 0.028 |
| Diseased side, right (%) | 13 (50.0) | 5 (33.3) | 0.35 |
| Tumor size (mm), median [IQR] | 25 [15–32] | 18 [14–23] | 0.24 |
| Clinical N1/2 (%) | 2 (7.7) | 2 (13.3) | 0.61 |
| Location, upper segments (%) | 7 (26.9) | 7 (46.7) | 0.31 |
| Radical surgery (%) | 23 (88.5) | 6 (40.0) | 0.0030 |
| Surgical approach | |||
| Uniportal VATS (%) | 9 (34.6) | 6 (40.0) | 0.75 |
| Multiportal VATS (%) | 15 (57.7) | 9 (60.0) | 0.68 |
| Thoracotomy (%) | 2 (7.7) | 0 (0) | 0.52 |
| Procedure | |||
| Partial resection (%) | 3 (11.5) | 5 (33.3) | 0.12 |
| Segmentectomy (%) | 8 (30.8) | 5 (33.3) | 0.70 |
| Lobectomy (%) | 15 (57.7) | 5 (33.3) | 0.19 |
| Number of resected segments (n), median [IQR] | 3.0 [2.0–4.0] | 2.5 [1.8–4.0] | 0.64 |
| Intraoperative bleeding (mL), median [IQR] | 20 [1–50] | 30 [1–50] | 0.86 |
| Operative time (min), median [IQR] | 157 [131–226] | 158 [82–170] | 0.21 |
| Chest tube duration (days), median [IQR] | 1 [1–1] | 1 [1–2] | 0.71 |
| Complications (%) | |||
| Total | 3 (11.5) | 5 (33.3) | 0.12 |
| Prolonged air leak | 1 (3.9) | 1 (6.7) | 0.87 |
| Pneumonia | 0 (0) | 2 (13.3) | 0.13 |
| Arrhythmia | 1 (3.9) | 2 (13.3) | 0.54 |
| Atelectasis | 0 (0) | 0 (0) | N/A |
| Delirium | 0 (0) | 0 (0) | N/A |
| Others | 2 (7.7) | 2 (13.3) | 0.61 |
| Postoperative hospitalization (days), median [IQR] | 6 [5–8] | 8 [5–10] | 0.28 |
| Postoperative FEV1, median [IQR] | 1.62 [1.31–1.80] | 1.25 [1.11–1.26] | 0.0014 |
| Characteristic | Post-Interventional FEV1 ≥ 1.5 L (n = 23) | Post-Interventional FEV1 < 1.5 L (n = 10) | p-Value |
|---|---|---|---|
| Age, median [IQR] | 77 [73–81] | 76 [71–81] | 0.49 |
| Sex, male (%) | 12 (46.1) | 7 (46.7) | 0.64 |
| BMI, median [IQR] | 22.4 [19.6–25.0] | 24.4 [22.2–30.5] | 0.057 |
| Hypertension (%) | 14 (60.8) | 8 (80.0) | 0.43 |
| Hyperlipidemia (%) | 10 (43.5) | 3 (30.0) | 0.70 |
| Hyperuricemia (%) | 6 (26.1) | 2 (20.0) | 0.54 |
| Diabetes (%) | 10 (43.5) | 5 (50.0) | 0.53 |
| Smoking history (%) | 21 (91.3) | 9 (90.0) | 0.68 |
| Brinkman index, median [IQR] | 840 [305–1000] | 1100 [990–1550] | 0.013 |
| Comorbidities | |||
| Osteoporosis | 2 (8.7) | 1 (10.0) | 0.79 |
| Gastroesophageal reflux | 4 (17.4) | 2 (20.0) | 0.75 |
| Cardiovascular diseases | 7 (30.4) | 5 (50.0) | 0.43 |
| Anxiety and depression | 6 (26.1) | 1 (10.0) | 0.39 |
| Interstitial pneumonia (%) | 4 (17.4) | 0 (0) | 0.29 |
| GOLD classification | |||
| 2 (%) | 18 (78.3) | 7 (70.0) | 0.67 |
| 3 (%) | 5 (21.7) | 3 (30.0) | |
| Spirometry | |||
| FVC (L), median [IQR] | 2.32 [2.10–2.50] | 1.99 [1.86–2.43] | 0.075 |
| %FVC (%), median [IQR] | 93.7 [81.6–104.5] | 87.4 [73.9–94.8] | 0.26 |
| FEV1/FVC (%), median [IQR] | 61.5 [53.4–67.3] | 59.3 [43.3–67.9] | 0.62 |
| Postint FEV1/FVC (%), median [IQR] | 64.8 [52.2–70.3] | 66.5 [45.8–72.2] | 0.93 |
| FEV1 (L), median [IQR] | 1.37 [1.26–1.44] | 1.13 [1.04–1.27] | 0.0013 |
| Postint FEV1 (L), median [IQR] | 1.55 [1.47–1.74] | 1.33 [1.24–1.37] | <0.0001 |
| Postint—Preint FEV1(L), median [IQR] | 0.30 [0.14–0.53] | 0.18 [0.08–0.31] | 0.11 |
| ppo-Preint FEV1 (L), median [IQR] | 1.08 [0.95–1.16] | 0.96 [0.83–1.05] | 0.016 |
| ppo-Postint FEV1 (L), median [IQR] | 1.22 [1.14–1.35] | 1.03 [0.98–1.11] | < 0.0001 |
| Diseased side, right (%) | 11 (47.8) | 3 (30.0) | 0.46 |
| Tumor size (mm), median [IQR] | 25 [16–32] | 21 [15–31] | 0.57 |
| Clinical N1/2 (%) | 2 (8.7) | 2 (20.0) | 0.61 |
| Location, upper segments (%) | 6 (26.1) | 4 (40.0) | 0.44 |
| Radical surgery (%) | 23 (100.0) | 6 (60.0) | 0.0051 |
| Surgical approach | |||
| Uniportal VATS (%) | 8 (34.8) | 3 (30.0) | 0.56 |
| Multiportal VATS (%) | 14 (60.9) | 7 (70.0) | 0.71 |
| Thoracotomy (%) | 1 (4.4) | 0 (0) | 0.69 |
| Procedure | |||
| Segmentectomy (%) | 8 (34.8) | 5 (50.0) | 0.46 |
| Lobectomy (%) | 15 (65.2) | 5 (50.0) | |
| Number of resected segments (n), median [IQR] | 3.0 [2.0–4.0] | 2.5 [1.8–4.0] | 0.64 |
| Intraoperative bleeding (mL), median [IQR] | 20 [1–50] | 50 [28–64] | 0.089 |
| Operative time (min), median [IQR] | 158 [142–225] | 167 [157–189] | 0.69 |
| Chest tube duration (days), median [IQR] | 1 [1–1] | 1 [1–3] | 0.11 |
| Complications (%) | |||
| Total | 3 (13.0) | 5 (50.0) | 0.036 |
| Prolonged air leak | 1 (4.4) | 1 (10.0) | 0.52 |
| Pneumonia | 0 (0) | 2 (20.0) | 0.085 |
| Arrhythmia | 1 (4.4) | 2 (20.0) | 0.21 |
| Atelectasis | 0 (0) | 0 (0) | N/A |
| Delirium | 0 (0) | 0 (0) | N/A |
| Others | 2 (8.7) | 2 (20.0) | 0.57 |
| Postoperative hospitalization (days), median [IQR] | 6 [5–8] | 10 [5–11] | 0.13 |
| Postoperative FEV1, median [IQR] | 1.61 [1.30–1.78] | 1.26 [1.12–1.30] | 0.0040 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Homma, T.; Saji, H.; Shimada, Y.; Tanabe, K.; Kojima, K.; Marushima, H.; Miyazawa, T.; Kimura, H.; Sakai, H.; Otsubo, K.; et al. Effect of Preoperative Single-Inhaler Triple Therapy on Pulmonary Function in Lung Cancer Patients with Chronic Obstructive Pulmonary Disease and FEV1 < 1.5 L. Cancers 2025, 17, 1803. https://doi.org/10.3390/cancers17111803
Homma T, Saji H, Shimada Y, Tanabe K, Kojima K, Marushima H, Miyazawa T, Kimura H, Sakai H, Otsubo K, et al. Effect of Preoperative Single-Inhaler Triple Therapy on Pulmonary Function in Lung Cancer Patients with Chronic Obstructive Pulmonary Disease and FEV1 < 1.5 L. Cancers. 2025; 17(11):1803. https://doi.org/10.3390/cancers17111803
Chicago/Turabian StyleHomma, Takahiro, Hisashi Saji, Yoshifumi Shimada, Keitaro Tanabe, Koji Kojima, Hideki Marushima, Tomoyuki Miyazawa, Hiroyuki Kimura, Hiroki Sakai, Kanji Otsubo, and et al. 2025. "Effect of Preoperative Single-Inhaler Triple Therapy on Pulmonary Function in Lung Cancer Patients with Chronic Obstructive Pulmonary Disease and FEV1 < 1.5 L" Cancers 17, no. 11: 1803. https://doi.org/10.3390/cancers17111803
APA StyleHomma, T., Saji, H., Shimada, Y., Tanabe, K., Kojima, K., Marushima, H., Miyazawa, T., Kimura, H., Sakai, H., Otsubo, K., Hatakeyama, T., Kakizaki, N., Tsuchiya, T., Morikawa, K., & Mineshita, M. (2025). Effect of Preoperative Single-Inhaler Triple Therapy on Pulmonary Function in Lung Cancer Patients with Chronic Obstructive Pulmonary Disease and FEV1 < 1.5 L. Cancers, 17(11), 1803. https://doi.org/10.3390/cancers17111803






