Systemic Therapeutic Options in Radioiodine-Refractory Differentiated Thyroid Cancer: Current Indications and Optimal Timing
Simple Summary
Abstract
1. Introduction
2. Pathophysiology of RAI-R TC
2.1. Alteration of Signaling Pathways
- -
- MAPK pathway:
- -
- PI3K/AKT pathway:
- -
- Transforming growth factor-β (TGF-β)/Smad pathway:
- -
- Wnt/β-catetin pathway:
- -
- Notch pathway:
- -
- Thyroid-stimulating hormone receptor (TSHR) pathway:
2.2. Tumor Genetic Profiling
- -
- BRAF mutation and rearrangement:
- -
- RAS mutation:
- -
- RET rearrangement:
- -
- Telomerase reverse transcriptase (TERT) promoter mutation:
- -
- NTRK fusion:
- -
- Anaplastic lymphoma kinase (ALK) mutation and rearrangement:
- -
- SWI/SNF (SWItch/Sucrose Non-Fermentable) complex mutation:
2.3. Tumor Microenvironment
3. Current Systemic Therapeutic Options
3.1. TKIs
- -
- Lenvatinib:
- -
- Sorafenib:
- -
- Cabozantinib:
- -
- Vandetanib
- -
- TKIs with evidence from phase II clinical trials:
3.2. Immune Checkpoint Inhibitors (ICIs)
- -
- Pembrolizumab:
3.3. Chemotherapy
3.4. Other Novel Therapies
- -
- RET Inhibitors:
- -
- BRAF Inhibitors:
4. Clinical Indications for Systemic Therapy
4.1. Criteria for Determining Refractoriness (RAI-R) [106]
- -
- Tumors that show no RAI uptake in all local or distant lesions on a post-treatment whole-body scan, despite being DTC.
- -
- Partial loss of RAI uptake, with some lesions failing to concentrate RAI.
- -
- Progressive disease within 6–12 months, despite receiving adequate doses of RAI, as defined by RECIST criteria.
- -
- Patients who have received a cumulative RAI dose (typically >600 mCi) without a significant response.
- -
- Locally advanced thyroid tumors in which surgical resection is not feasible, precluding proper assessment of RAI uptake.
4.2. Criteria for Determining Advanced/Aggressive Forms
- Structural/surgical category:
- -
- Invasive or inoperable locoregional disease.
- -
- Recurrence of the disease.
- -
- Presence of distant metastases.
- -
- Rapid progression detectable on imaging studies.
- Biochemical category:
- -
- Tumors resistant to RAI treatment.
- -
- Tumors unresponsive to TSH suppression therapy.
- -
- Rapid doubling time of specific biomarkers (e.g., TG).
- Histologic/molecular category:
- -
- Aggressive histologic variants (e.g., poorly DTC or ATC).
- -
- High Ki67 proliferation index.
- -
- Elevated mitotic count.
- Clinical judgment:
4.3. Patient Selection
5. Timing of Systemic Therapy
5.1. Early vs. Late Intervention
- -
- Early intervention:
- -
- Late intervention:
5.2. Active Surveillance
5.3. Integration with Local Treatments
- -
- Surgery
- -
- Radiation therapy
6. AEs and Toxicity Management
6.1. Common AEs
6.2. Toxicity Management
- -
- Fatigue/asthenia
- -
- Hypertension
- -
- Proteinuria
- -
- Gastrointestinal AEs
- -
- Stomatitis/mucositis
- -
- Palmar–plantar erythrodysesthesia syndrome (PPES):
- -
- Hemorrhagic events
- -
- Hepatotoxicity
7. Challenges, Practical Implications, and Future Directions
7.1. Challenges and Limitations of Emerging Therapies
7.2. Practical Recommendations for Clinicians and Researchers
- -
- Routine molecular profiling: All patients with progressive RAI-R TC should undergo comprehensive molecular testing early in the disease course to identify potential targets for therapy.
- -
- Multidisciplinary evaluation: Treatment decisions should involve tumor boards including endocrinologists, oncologists, nuclear medicine specialists, and molecular pathologists to ensure individualized care.
- -
- Cost-effectiveness analysis: Institutions should prioritize the implementation of cost-effectiveness assessments when introducing new targeted therapies, especially in resource-constrained settings.
- -
- Proactive toxicity management: Early recognition and intervention for treatment-related AEs can prolong therapy duration and maintain QoL.
- -
- Participation in clinical trials: Clinicians should encourage eligible patients to enroll in ongoing studies evaluating novel agents or treatment sequences to help build robust evidence.
7.3. Integration of Artificial Intelligence (AI) in Clinical Practice
- -
- Molecular profiling: AI tools can rapidly analyze NGS data, identifying actionable mutations and predicting potential resistance mechanisms.
- -
- Treatment selection: Predictive models based on real-world data could support clinicians in selecting the most appropriate systemic therapy based on patient and tumor characteristics.
- -
- Monitoring and toxicity prediction: AI-driven digital platforms, including mobile health apps and wearable devices, can enable real-time monitoring of symptoms and early detection of AEs.
- -
- Clinical trial matching: Automated systems can help match patients to appropriate clinical trials based on their molecular profiles and clinical parameters, facilitating access to emerging therapies.
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization International Agency for Research on Cancer (IARC). GLOBOCAN: Estimated Cancer Incidence, Mortality and Prevalence Worlwide. Available online: http://gco.iarc.fr/en (accessed on 8 February 2024).
- Cabanillas, M.E.; McFadden, D.G.; Durante, C. Thyroid cancer. Lancet 2016, 388, 2783–2795. [Google Scholar] [CrossRef] [PubMed]
- La Vecchia, C.; Negri, E. Thyroid cancer: The thyroid cancer epidemic—Overdiagnosis or a real increase? Nat. Rev. Endocrinol. 2017, 13, 318–319. [Google Scholar] [CrossRef] [PubMed]
- Sciuto, R.; Romano, L.; Rea, S.; Marandino, F.; Sperduti, I.; Maini, C.L. Natural history and clinical outcome of differentiated thyroid carcinoma: A retrospective analysis of 1503 patients treated at a single institution. Ann. Oncol. 2009, 20, 1728–1735. [Google Scholar] [CrossRef] [PubMed]
- Durante, C.; Haddy, N.; Baudin, E.; Leboulleux, S.; Hartl, D.; Travagli, J.P.; Caillou, B.; Ricard, M.; Lumbroso, J.D.; De Vathaire, F.; et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: Benefits and limits of radioiodine therapy. J. Clin. Endocrinol. Metab. 2006, 91, 2892–2899. [Google Scholar] [CrossRef]
- Lirov, R.; Worden, F.P.; Cohen, M.S. The Treatment of Advanced Thyroid Cancer in the Age of Novel Targeted Therapies. Drugs 2017, 77, 733–745. [Google Scholar] [CrossRef]
- Schlumberger, M.; Tahara, M.; Wirth, L.J.; Robinson, B.; Brose, M.S.; Elisei, R.; Habra, M.A.; Newbold, K.; Shah, M.H.; Hoff, A.O.; et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N. Engl. J. Med. 2015, 372, 621–630. [Google Scholar] [CrossRef]
- Brose, M.S.; Nutting, C.M.; Jarzab, B.; Elisei, R.; Siena, S.; Bastholt, L.; de la Fouchardiere, C.; Pacini, F.; Paschke, R.; Shong, Y.K.; et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: A randomised, double-blind, phase 3 trial. Lancet 2014, 384, 319–328. [Google Scholar] [CrossRef]
- Brose, M.S.; Robinson, B.; Sherman, S.I.; Krajewska, J.; Lin, C.C.; Vaisman, F.; Hoff, A.O.; Hitre, E.; Bowles, D.W.; Hernando, J.; et al. Cabozantinib for radioiodine-refractory differentiated thyroid cancer (COSMIC-311): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021, 22, 1126–1138. [Google Scholar] [CrossRef]
- Gild, M.L.; Tsang, V.H.M.; Clifton-Bligh, R.J.; Robinson, B.G. Multikinase inhibitors in thyroid cancer: Timing of targeted therapy. Nat. Rev. Endocrinol. 2021, 17, 225–234. [Google Scholar] [CrossRef]
- Wirth, L.J.; Sherman, E.; Robinson, B.; Solomon, B.; Kang, H.; Lorch, J.; Worden, F.; Brose, M.; Patel, J.; Leboulleux, S.; et al. Efficacy of Selpercatinib in RET-Altered Thyroid Cancers. N. Engl. J. Med. 2020, 383, 825–835. [Google Scholar] [CrossRef]
- Subbiah, V.; Hu, M.I.; Wirth, L.J.; Schuler, M.; Mansfield, A.S.; Curigliano, G.; Brose, M.S.; Zhu, V.W.; Leboulleux, S.; Bowles, D.W.; et al. Pralsetinib for patients with advanced or metastatic RET-altered thyroid cancer (ARROW): A multi-cohort, open-label, registrational, phase 1/2 study. Lancet Diabetes Endocrinol. 2021, 9, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.W.; Feng, F.Y. A Tumor-Agnostic NTRK (TRK) Inhibitor. Cell 2019, 177, 8. [Google Scholar] [CrossRef] [PubMed]
- Cocco, E.; Scaltriti, M.; Drilon, A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat. Rev. Clin. Oncol. 2018, 15, 731–747. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.L.; Ward, L.S. Translating the immune microenvironment of thyroid cancer into clinical practice. Endocr. Relat. Cancer 2022, 29, R67–R83. [Google Scholar] [CrossRef]
- Oh, D.Y.; Algazi, A.; Capdevila, J.; Longo, F.; Miller WJr Chun Bing, J.T.; Bonilla, C.E.; Chung, H.C.; Guren, T.K.; Lin, C.C.; Motola-Kuba, D.; et al. Efficacy and safety of pembrolizumab monotherapy in patients with advanced thyroid cancer in the phase 2 KEYNOTE-158 study. Cancer 2023, 129, 1195–1204. [Google Scholar] [CrossRef]
- Dierks, C.; Seufert, J.; Aumann, K.; Ruf, J.; Klein, C.; Kiefer, S.; Rassner, M.; Boerries, M.; Zielke, A.; la Rosee, P.; et al. Combination of Lenvatinib and Pembrolizumab Is an Effective Treatment Option for Anaplastic and Poorly Differentiated Thyroid Carcinoma. Thyroid 2021, 31, 1076–1085. [Google Scholar] [CrossRef]
- Reed, N.; Glen, H.; Gerrard, G.; Good, J.; Lei, M.; Lyon, A.R.; Strachan, M.; Wadsley, J.; Newbold, K. Expert Consensus on the Management of Adverse Events During Treatment with Lenvatinib for Thyroid Cancer. Clin. Oncol. (R. Coll. Radiol.) 2020, 32, e145–e153. [Google Scholar] [CrossRef]
- Haddad, R.I.; Schlumberger, M.; Wirth, L.J.; Sherman, E.J.; Shah, M.H.; Robinson, B.; Dutcus, C.E.; Teng, A.; Gianoukakis, A.G.; Sherman, S.I. Incidence and timing of common adverse events in Lenvatinib-treated patients from the SELECT trial and their association with survival outcomes. Endocrine 2017, 56, 121–128. [Google Scholar] [CrossRef]
- Xing, M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat. Rev. Cancer 2013, 13, 184–199. [Google Scholar] [CrossRef]
- Castro, M.R.; Bergert, E.R.; Goellner, J.R.; Hay, I.D.; Morris, J.C. Immunohistochemical analysis of sodium iodide symporter expression in metastatic differentiated thyroid cancer: Correlation with radioiodine uptake. J. Clin. Endocrinol. Metab. 2001, 86, 5627–5632. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, D.; Murugan, A.K.; Liu, Z.; Xing, M. Histone deacetylation of NIS promoter underlies BRAF V600E-promoted NIS silencing in thyroid cancer. Endocr. Relat. Cancer 2014, 21, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Romitti, M.; Ceolin, L.; Siqueira, D.R.; Ferreira, C.V.; Wajner, S.M.; Maia, A.L. Signaling pathways in follicular cell-derived thyroid carcinomas (review). Int. J. Oncol. 2013, 42, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Nagarajah, J.; Le, M.; Knauf, J.A.; Ferrandino, G.; Montero-Conde, C.; Pillarsetty, N.; Bolaender, A.; Irwin, C.; Krishnamoorthy, G.P.; Saqcena, M.; et al. Sustained ERK inhibition maximizes responses of BrafV600E thyroid cancers to radioiodine. J. Clin. Investig. 2016, 126, 4119–4124. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, N.; Panebianco, F.; Condello, V.; Barletta, J.A.; Kaya, C.; Yip, L.; Nikiforova, M.N.; Nikiforov, Y.E. Characterization of Activating Mutations of the MEK1 Gene in Papillary Thyroid Carcinomas. Thyroid 2019, 29, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Zhu, R.; Liu, Y.; Hong, Y.; Ge, J.; Xuan, J.; Niu, W.; Yu, X.; Qin, J.J.; Li, Q. Radioiodine-refractory differentiated thyroid cancer: Molecular mechanisms and therapeutic strategies for radioiodine resistance. Drug Resist. Updat. 2024, 72, 101013. [Google Scholar] [CrossRef]
- Zhu, S.; Guo, Y.; Zhang, X.; Liu, H.; Yin, M.; Chen, X.; Peng, C. Pyruvate kinase M2 (PKM2) in cancer and cancer therapeutics. Cancer Lett. 2021, 503, 240–248. [Google Scholar] [CrossRef]
- Azouzi, N.; Cailloux, J.; Cazarin, J.M.; Knauf, J.A.; Cracchiolo, J.; Al Ghuzlan, A.; Hartl, D.; Polak, M.; Carré, A.; El Mzibri, M.; et al. NADPH Oxidase NOX4 Is a Critical Mediator of BRAFV600E-Induced Downregulation of the Sodium/Iodide Symporter in Papillary Thyroid Carcinomas. Antioxid. Redox Signal 2017, 26, 864–877. [Google Scholar] [CrossRef]
- Zhang, W.; Ruan, X.; Li, Y.; Zhi, J.; Hu, L.; Hou, X.; Shi, X.; Wang, X.; Wang, J.; Ma, W.; et al. KDM1A promotes thyroid cancer progression and maintains stemness through the Wnt/β-catenin signaling pathway. Theranostics 2022, 12, 1500–1517. [Google Scholar] [CrossRef]
- Lan, L.; Basourakos, S.; Cui, D.; Zuo, X.; Deng, W.; Huo, L.; Chen, L.; Zhang, G.; Deng, L.; Shi, B.; et al. Inhibiting β-catenin expression promotes efficiency of radioiodine treatment in aggressive follicular thyroid cancer cells probably through mediating NIS localization. Oncol. Rep. 2017, 37, 426–434. [Google Scholar] [CrossRef]
- Somnay, Y.R.; Yu, X.M.; Lloyd, R.V.; Leverson, G.; Aburjania, Z.; Jang, S.; Jaskula-Sztul, R.; Chen, H. Notch3 expression correlates with thyroid cancer differentiation, induces apoptosis, and predicts disease prognosis. Cancer 2017, 123, 769–782. [Google Scholar] [CrossRef]
- Szkudlinski, M.W.; Fremont, V.; Ronin, C.; Weintraub, B.D. Thyroid-stimulating hormone and thyroid-stimulating hormone receptor structure-function relationships. Physiol. Rev. 2002, 82, 473–502. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Xi, Z.; Xiao, Y.; Zhao, X.; Li, J.; Feng, N.; Hu, L.; Zheng, R.; Zhang, N.; Wang, S.; et al. TSH-TSHR axis promotes tumor immune evasion. J. Immunother. Cancer 2022, 10, e004049. [Google Scholar] [CrossRef] [PubMed]
- Fiskus, W.; Mitsiades, N. B-Raf Inhibition in the Clinic: Present and Future. Annu. Rev. Med. 2016, 67, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Efanov, A.A.; Brenner, A.V.; Bogdanova, T.I.; Kelly, L.M.; Liu, P.; Little, M.P.; Wald, A.I.; Hatch, M.; Zurnadzy, L.Y.; Nikiforova, M.N.; et al. Investigation of the Relationship Between Radiation Dose and Gene Mutations and Fusions in Post-Chernobyl Thyroid Cancer. J. Natl. Cancer Inst. 2018, 110, 371–378. [Google Scholar] [CrossRef]
- Zou, M.; Baitei, E.Y.; Alzahrani, A.S.; BinHumaid, F.S.; Alkhafaji, D.; Al-Rijjal, R.A.; Meyer, B.F.; Shi, Y. Concomitant RAS, RET/PTC, or BRAF mutations in advanced stage of papillary thyroid carcinoma. Thyroid 2014, 24, 1256–1266. [Google Scholar] [CrossRef]
- Romei, C.; Ciampi, R.; Elisei, R. A comprehensive overview of the role of the RET proto-oncogene in thyroid carcinoma. Nat. Rev. Endocrinol. 2016, 12, 192–202. [Google Scholar] [CrossRef]
- Greider, C.W. Telomere length regulation. Annu. Rev. Biochem. 1996, 65, 337–365. [Google Scholar] [CrossRef]
- Cao, J.; Zhu, X.; Sun, Y.; Li, X.; Yun, C.; Zhang, W. The genetic duet of BRAF V600E and TERT promoter mutations predicts the poor curative effect of radioiodine therapy in papillary thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3470–3481. [Google Scholar] [CrossRef]
- Prasad, M.L.; Vyas, M.; Horne, M.J.; Virk, R.K.; Morotti, R.; Liu, Z.; Tallini, G.; Nikiforova, M.N.; Christison-Lagay, E.R.; Udelsman, R.; et al. NTRK fusion oncogenes in pediatric papillary thyroid carcinoma in northeast United States. Cancer 2016, 122, 1097–1107. [Google Scholar] [CrossRef]
- Kelly, L.M.; Barila, G.; Liu, P.; Evdokimova, V.N.; Trivedi, S.; Panebianco, F.; Gandhi, M.; Carty, S.E.; Hodak, S.P.; Luo, J.; et al. Identification of the transforming STRN-ALK fusion as a potential therapeutic target in the aggressive forms of thyroid cancer. Proc. Natl. Acad. Sci. USA 2014, 111, 4233–4238. [Google Scholar] [CrossRef]
- Saqcena, M.; Leandro-Garcia, L.J.; Maag, J.L.V.; Tchekmedyian, V.; Krishnamoorthy, G.P.; Tamarapu, P.P.; Tiedje, V.; Reuter, V.; Knauf, J.A.; de Stanchina, E.; et al. SWI/SNF Complex Mutations Promote Thyroid Tumor Progression and Insensitivity to Redifferentiation Therapies. Cancer Discov. 2021, 11, 1158–1175. [Google Scholar] [CrossRef] [PubMed]
- Kiyota, N.; Tahara, M.; Robinson, B.; Schlumberger, M.; Sherman, S.I.; Leboulleux, S.; Lee, E.K.; Suzuki, T.; Ren, M.; Fushimi, K.; et al. Impact of baseline tumor burden on overall survival in patients with radioiodine-refractory differentiated thyroid cancer treated with lenvatinib in the SELECT global phase 3 trial. Cancer 2022, 128, 2281–2287. [Google Scholar] [CrossRef] [PubMed]
- Tahara, M.; Brose, M.S.; Wirth, L.J.; Suzuki, T.; Miyagishi, H.; Fujino, K.; Dutcus, C.E.; Gianoukakis, A. Impact of dose interruption on the efficacy of lenvatinib in a phase 3 study in patients with radioiodine-refractory differentiated thyroid cancer. Eur. J. Cancer 2019, 106, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Viola, D.; Cappagli, V.; Elisei, R. Cabozantinib (XL184) for the treatment of locally advanced or metastatic progressive medullary thyroid cancer. Future Oncol. 2013, 9, 1083–1092. [Google Scholar] [CrossRef]
- Grüllich, C. Cabozantinib: Multi-kinase Inhibitor of MET, AXL, RET, and VEGFR2. Recent Results Cancer Res. 2018, 211, 67–75. [Google Scholar]
- Brose, M.S.; Robinson, B.G.; Sherman, S.I.; Jarzab, B.; Lin, C.C.; Vaisman, F.; Hoff, A.O.; Hitre, E.; Bowles, D.W.; Sen, S.; et al. Cabozantinib for previously treated radioiodine-refractory differentiated thyroid cancer: Updated results from the phase 3 COSMIC-311 trial. Cancer 2022, 128, 4203–4212. [Google Scholar] [CrossRef]
- Cochin, V.; Gross-Goupil, M.; Ravaud, A.; Godbert, Y.; Le Moulec, S. Cabozantinib: Modalités d’action, efficacité et indications [Cabozantinib: Mechanism of action, efficacy and indications]. Bull Cancer 2017, 104, 393–401. [Google Scholar] [CrossRef]
- Krajewska, J.; Olczyk, T.; Jarzab, B. Cabozantinib for the treatment of progressive metastatic medullary thyroid cancer. Expert Rev. Clin. Pharmacol. 2016, 9, 69–79. [Google Scholar] [CrossRef]
- Chau, N.G.; Haddad, R.I. Vandetanib for the treatment of medullary thyroid cancer. Clin. Cancer Res. 2013, 19, 524–529. [Google Scholar] [CrossRef]
- Deshpande, H.; Roman, S.; Thumar, J.; Sosa, J.A. Vandetanib (ZD6474) in the Treatment of Medullary Thyroid Cancer. Clin. Med. Insights Oncol. 2011, 5, 213–221. [Google Scholar] [CrossRef]
- Sim, M.W.; Cohen, M.S. The discovery and development of vandetanib for the treatment of thyroid cancer. Expert Opin. Drug Discov. 2014, 9, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.J.; Seib, C.D.; Gosnell, J. Vandetanib and the management of advanced medullary thyroid cancer. Curr. Opin. Oncol. 2013, 25, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Karras, S.; Anagnostis, P.; Krassas, G.E. Vandetanib for the treatment of thyroid cancer: An update. Expert Opin. Drug Metab. Toxicol. 2014, 10, 469–481. [Google Scholar] [CrossRef]
- Fallahi, P.; Di Bari, F.; Ferrari, S.M.; Spisni, R.; Materazzi, G.; Miccoli, P.; Benvenga, S.; Antonelli, A. Selective use of vandetanib in the treatment of thyroid cancer. Drug Des. Devel Ther. 2015, 9, 3459–3470. [Google Scholar]
- Liu, D.; Offin, M.; Harnicar, S.; Li, B.T.; Drilon, A. Entrectinib: An orally available, selective tyrosine kinase inhibitor for the treatment of NTRK, ROS1, and ALK fusion-positive solid tumors. Ther. Clin. Risk Manag. 2018, 14, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Delgado, J.; Pean, E.; Melchiorri, D.; Migali, C.; Josephson, F.; Enzmann, H.; Pignatti, F. The European Medicines Agency review of entrectinib for the treatment of adult or paediatric patients with solid tumours who have a neurotrophic tyrosine receptor kinase gene fusions and adult patients with non-small-cell lung cancer harbouring ROS1 rearrangements. ESMO Open 2021, 6, 100087. [Google Scholar]
- Marcus, L.; Donoghue, M.; Aungst, S.; Myers, C.E.; Helms, W.S.; Shen, G.; Zhao, H.; Stephens, O.; Keegan, P.; Pazdur, R. FDA Approval Summary: Entrectinib for the Treatment of NTRK gene Fusion Solid Tumors. Clin. Cancer Res. 2021, 27, 928–932. [Google Scholar] [CrossRef]
- Chu, Y.H.; Dias-Santagata, D.; Farahani, A.A.; Boyraz, B.; Faquin, W.C.; Nosé, V.; Sadow, P.M. Clinicopathologic and molecular characterization of NTRK-rearranged thyroid carcinoma (NRTC). Mod Pathol 2020, 33, 2186–2197. [Google Scholar] [CrossRef]
- Liu, S.V.; Macke, L.A.; Colton, B.S.; Imran, S.S.; Christiansen, J.; Chow-Maneval, E.; Hornby, Z.; Multani, P.S. Response to Entrectinib in Differentiated Thyroid Cancer With a ROS1 Fusion. JCO Precis. Oncol. 2017, 1, PO.17.00105. [Google Scholar] [CrossRef]
- Drilon, A.; Siena, S.; Ou, S.I.; Patel, M.; Ahn, M.J.; Lee, J.; Bauer, T.M.; Farago, A.F.; Wheler, J.J.; Liu, S.V.; et al. Safety and Antitumor Activity of the Multitargeted Pan-TRK, ROS1, and ALK Inhibitor Entrectinib: Combined Results from Two Phase I Trials (ALKA-372-001 and STARTRK-1). Cancer Discov. 2017, 7, 400–409. [Google Scholar] [CrossRef]
- Ardini, E.; Menichincheri, M.; Banfi, P.; Bosotti, R.; De Ponti, C.; Pulci, R.; Ballinari, D.; Ciomei, M.; Texido, G.; Degrassi, A.; et al. Entrectinib, a Pan-TRK, ROS1, and ALK Inhibitor with Activity in Multiple Molecularly Defined Cancer Indications. Mol. Cancer Ther. 2016, 15, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, M.E.; Ryder, M.; Jimenez, C. Targeted Therapy for Advanced Thyroid Cancer: Kinase Inhibitors and Beyond. Endocr. Rev. 2019, 40, 1573–1604. [Google Scholar] [CrossRef] [PubMed]
- Waguespack, S.G.; Drilon, A.; Lin, J.J.; Brose, M.S.; McDermott, R.; Almubarak, M.; Bauman, J.; Casanova, M.; Krishnamurthy, A.; Kummar, S.; et al. Efficacy and safety of larotrectinib in patients with TRK fusion-positive thyroid carcinoma. Eur. J. Endocrinol. 2022, 186, 631–643. [Google Scholar] [CrossRef]
- Pitoia, F. Complete response to larotrectinib treatment in a patient with papillary thyroid cancer harboring an ETV6-NTRK3 gene fusion. Clin. Case Rep. 2021, 9, 1905–1912. [Google Scholar] [CrossRef]
- Hong, D.S.; Bauer, T.M.; Lee, J.J.; Dowlati, A.; Brose, M.S.; Farago, A.F.; Taylor, M.; Shaw, A.T.; Montez, S.; Meric-Bernstam, F.; et al. Larotrectinib in adult patients with solid tumours: A multi-centre, open-label, phase I dose-escalation study. Ann. Oncol. 2019, 30, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Suh, K.; Carlson, J.J.; Xia, F.; Williamson, T.; Sullivan, S.D. The potential long-term comparative effectiveness of larotrectinib vs standard of care for treatment of metastatic TRK fusion thyroid cancer, colorectal cancer, and soft tissue sarcoma. J. Manag. Care Spec. Pharm. 2022, 28, 622–630. [Google Scholar] [CrossRef]
- Groussin, L.; Theodon, H.; Bessiene, L.; Bricaire, L.; Bonnet-Serrano, F.; Cochand-Priollet, B.; Leroy, K.; Garinet, S.; Pasmant, E.; Zerbit, J.; et al. Redifferentiating Effect of Larotrectinib in NTRK-Rearranged Advanced Radioactive-Iodine Refractory Thyroid Cancer. Thyroid 2022, 32, 594–598. [Google Scholar] [CrossRef]
- Cohen, E.E.; Tortorici, M.; Kim, S.; Ingrosso, A.; Pithavala, Y.K.; Bycott, P. A Phase II trial of axitinib in patients with various histologic subtypes of advanced thyroid cancer: Long-term outcomes and pharmacokinetic/pharmacodynamic analyses. Cancer Chemother. Pharmacol. 2014, 74, 1261–1270. [Google Scholar] [CrossRef][Green Version]
- Capdevila, J.; Trigo, J.M.; Aller, J.; Manzano, J.L.; Adrián, S.G.; Llopis, C.Z.; Reig, Ò.; Bohn, U.; Cajal, T.R.Y.; Duran-Poveda, M.; et al. Axitinib treatment in advanced RAI-resistant differentiated thyroid cancer (DTC) and refractory medullary thyroid cancer (MTC). Eur. J. Endocrinol. 2017, 177, 309–317. [Google Scholar] [CrossRef]
- Ferrari, S.M.; Centanni, M.; Virili, C.; Miccoli, M.; Ferrari, P.; Ruffilli, I.; Ragusa, F.; Antonelli, A.; Fallahi, P. Sunitinib in the Treatment of Thyroid Cancer. Curr. Med. Chem. 2019, 26, 963–972. [Google Scholar] [CrossRef]
- Gómez-Sáez, J.M. Sunitinib for the treatment of thyroid cancer. Expert Opin. Investig. Drugs 2016, 25, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.; Rosen, L.S.; Mulay, M.; Vanvugt, A.; Dinolfo, M.; Tomoda, C.; Sugawara, M.; Hershman, J.M. Sunitinib induces hypothyroidism in advanced cancer patients and may inhibit thyroid peroxidase activity. Thyroid 2007, 17, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Bible, K.C.; Suman, V.J.; Menefee, M.E.; Smallridge, R.C.; Molina, J.R.; Maples, W.J.; Karlin, N.J.; Traynor, A.M.; Kumar, P.; Goh, B.C.; et al. A multiinstitutional phase 2 trial of pazopanib monotherapy in advanced anaplastic thyroid cancer. J. Clin. Endocrinol. Metab. 2012, 97, 3179–3184. [Google Scholar] [CrossRef] [PubMed]
- Wells SAJr Robinson, B.G.; Gagel, R.F.; Dralle, H.; Fagin, J.A.; Santoro, M.; Baudin, E.; Elisei, R.; Jarzab, B.; Vasselli, J.R.; Read, J.; et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: A randomized, double-blind phase III trial. J. Clin. Oncol. 2012, 30, 134–141. [Google Scholar] [CrossRef]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef]
- Hong, D.S.; DuBois, S.G.; Kummar, S.; Farago, A.F.; Albert, C.M.; Rohrberg, K.S.; van Tilburg, C.M.; Nagasubramanian, R.; Berlin, J.D.; Federman, N.; et al. Larotrectinib in patients with TRK fusion-positive solid tumours: A pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020, 21, 531–540. [Google Scholar] [CrossRef]
- Carr, L.L.; Mankoff, D.A.; Goulart, B.H.; Eaton, K.D.; Capell, P.T.; Kell, E.M.; Bauman, J.E.; Martins, R.G. Phase II study of daily sunitinib in FDG-PET-positive, iodine-refractory differentiated thyroid cancer and metastatic medullary carcinoma of the thyroid with functional imaging correlation. Clin. Cancer Res. 2010, 16, 5260–5268. [Google Scholar] [CrossRef]
- Bible, K.C.; Menefee, M.E.; Lin, C.J.; Millward, M.J.; Maples, W.J.; Goh, B.C.; Karlin, N.J.; Kane, M.A.; Adkins, D.R.; Molina, J.R.; et al. An International Phase 2 Study of Pazopanib in Progressive and Metastatic Thyroglobulin Antibody Negative Radioactive Iodine Refractory Differentiated Thyroid Cancer. Thyroid 2020, 30, 1254–1262. [Google Scholar] [CrossRef]
- Hadoux, J.; Elisei, R.; Brose, M.S.; Hoff, A.O.; Robinson, B.G.; Gao, M.; Jarzab, B.; Isaev, P.; Kopeckova, K.; Wadsley, J.; et al. Phase 3 Trial of Selpercatinib in Advanced RET-Mutant Medullary Thyroid Cancer. N. Engl. J. Med. 2023, 389, 1851–1861. [Google Scholar] [CrossRef]
- Subbiah, V.; Hu, M.I.; Mansfield, A.S.; Taylor, M.H.; Schuler, M.; Zhu, V.W.; Hadoux, J.; Curigliano, G.; Wirth, L.; Gainor, J.F.; et al. Pralsetinib in Patients with Advanced/Metastatic Rearranged During Transfection (RET)-Altered Thyroid Cancer: Updated Efficacy and Safety Data from the ARROW Study. Thyroid 2024, 34, 26–40. [Google Scholar] [CrossRef]
- Mehnert, J.M.; Varga, A.; Brose, M.S.; Aggarwal, R.R.; Lin, C.C.; Prawira, A.; de Braud, F.; Tamura, K.; Doi, T.; Piha-Paul, S.A.; et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced, PD-L1-positive papillary or follicular thyroid cancer. BMC Cancer 2019, 19, 196. [Google Scholar] [CrossRef] [PubMed]
- Chintakuntlawar, A.V.; Yin, J.; Foote, R.L.; Kasperbauer, J.L.; Rivera, M.; Asmus, E.; Garces, N.I.; Janus, J.R.; Liu, M.; Ma, D.J.; et al. A Phase 2 Study of Pembrolizumab Combined with Chemoradiotherapy as Initial Treatment for Anaplastic Thyroid Cancer. Thyroid 2019, 29, 1615–1622. [Google Scholar] [CrossRef] [PubMed]
- Iyer, P.C.; Dadu, R.; Gule-Monroe, M.; Busaidy, N.L.; Ferrarotto, R.; Habra, M.A.; Zafereo, M.; Williams, M.D.; Gunn, G.B.; Grosu, H.; et al. Salvage pembrolizumab added to kinase inhibitor therapy for the treatment of anaplastic thyroid carcinoma. J. Immunother. Cancer 2018, 6, 68. [Google Scholar] [CrossRef]
- Boudin, L.; Morvan, J.B.; Thariat, J.; Métivier, D.; Marcy, P.Y.; Delarbre, D. Rationale Efficacy and Safety Evidence of Lenvatinib and Pembrolizumab Association in Anaplastic Thyroid Carcinoma. Curr. Oncol. 2022, 29, 7718–7731. [Google Scholar] [CrossRef]
- Haugen, B.; French, J.; Worden, F.; Konda, B.; Sherman, E.; Dadu, R.; Gianoukakis, A.; Mccue, S.; Foster, N.; Bowles, D.; et al. 1917P Pembrolizumab salvage add-on therapy in patients with radioiodine-refractory (RAIR), progressive differentiated thyroid cancer (DTC) progressing on lenvatinib: Results of a multicenter phase II International Thyroid Oncology Group Trial. Ann. Oncol. 2020, 31, S1086–S1087. [Google Scholar] [CrossRef]
- Wirth, L.; Subbiah, V.; Worden, F.; Solomon, B.; Robinson, B.; Hadoux, J.; Tomasini, P.; Weiler, D.; Deschler-Baier, B.; Tan, D.; et al. 2229P Updated safety and efficacy of selpercatinib in patients (pts) with RET-activated thyroid cancer: Data from LIBRETTO-001. Ann. Oncol. 2023, 34, S1147–S1148. [Google Scholar] [CrossRef]
- Kroiss, M.; Sherman, E.; Wirth, L.; Cabanillas, M.; Robinson, B.; Subbiah, V.; Drilon, A.; Godbert, Y.; Fasnacht, N.; Soldatenkova, V.; et al. 1656P Durable efficacy of selpercatinib in patients (pts) with medullary thyroid cancer (MTC): Update of the LIBRETTO-001 trial. Ann. Oncol. 2022, 33, S1299–S1300. [Google Scholar] [CrossRef]
- Subbiah, V.; Wolf, J.; Konda, B.; Kang, H.; Spira, A.; Weiss, J.; Takeda, M.; Ohe, Y.; Khan, S.; Ohashi, K.; et al. Tumour-agnostic efficacy and safety of selpercatinib in patients with RET fusion-positive solid tumours other than lung or thyroid tumours (LIBRETTO-001): A phase 1/2, open-label, basket trial. Lancet Oncol. 2022, 23, 1261–1273. [Google Scholar] [CrossRef]
- Markham, A. Selpercatinib: First Approval. Drugs 2020, 80, 1119–1124. [Google Scholar] [CrossRef]
- Della Corte, C.M.; Morgillo, F. Rethinking treatment for RET-altered lung and thyroid cancers: Selpercatinib approval by the EMA. ESMO Open 2021, 6, 100041. [Google Scholar] [CrossRef]
- Zheng, X.; Fang, M.; Fan, Y.; Sun, Y.; Sun, M.; Yang, A.; Zhang, B.; Liu, Q.; Liu, H.; Zhou, X.; et al. Efficacy and safety of pralsetinib in Chinese advanced RET-mutant medullary thyroid cancer patients. Endocr. Relat. Cancer 2024, 31, e230134. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Bradford, D.; Larkins, E.; Pai-Scherf, L.H.; Chatterjee, S.; Mishra-Kalyani, P.S.; Wearne, E.; Helms, W.S.; Ayyoub, A.; Bi, Y.; et al. FDA Approval Summary: Pralsetinib for the Treatment of Lung and Thyroid Cancers With RET Gene Mutations or Fusions. Clin. Cancer Res. 2021, 27, 5452–5456. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Kreitman, R.J.; Wainberg, Z.A.; Cho, J.Y.; Schellens, J.H.M.; Soria, J.C.; Wen, P.Y.; Zielinski, C.; Cabanillas, M.E.; Urbanowitz, G.; et al. Dabrafenib and Trametinib Treatment in Patients With Locally Advanced or Metastatic BRAF V600-Mutant Anaplastic Thyroid Cancer. J. Clin. Oncol. 2018, 36, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Kreitman, R.J.; Wainberg, Z.A.; Cho, J.Y.; Schellens, J.H.M.; Soria, J.C.; Wen, P.Y.; Zielinski, C.C.; Cabanillas, M.E.; Boran, A.; et al. Dabrafenib plus trametinib in patients with BRAF V600E-mutant anaplastic thyroid cancer: Updated analysis from the phase II ROAR basket study. Ann. Oncol. 2022, 33, 406–415. [Google Scholar] [CrossRef]
- Jeon, Y.; Park, S.; Lee, S.H.; Kim, T.H.; Kim, S.W.; Ahn, M.J.; Jung, H.A.; Chung, J.H. Combination of Dabrafenib and Trametinib in Patients with Metastatic BRAFV600E-Mutated Thyroid Cancer. Cancer Res. Treat. 2024, 56, 1270–1276. [Google Scholar] [CrossRef]
- Lorimer, C.; Cheng, L.; Chandler, R.; Garcez, K.; Gill, V.; Graham, K.; Grant, W.; Sardo Infirri, S.; Wadsley, J.; Wall, L.; et al. Dabrafenib and Trametinib Therapy for Advanced Anaplastic Thyroid Cancer—Real-World Outcomes From UK Centres. Clin. Oncol. (R. Coll. Radiol.) 2023, 35, e60–e66. [Google Scholar] [CrossRef]
- Leboulleux, S.; Dupuy, C.; Lacroix, L.; Attard, M.; Grimaldi, S.; Corre, R.; Ricard, M.; Nasr, S.; Berdelou, A.; Hadoux, J.; et al. Redifferentiation of a BRAFK601E-Mutated Poorly Differentiated Thyroid Cancer Patient with Dabrafenib and Trametinib Treatment. Thyroid 2019, 29, 735–742. [Google Scholar] [CrossRef]
- Busaidy, N.L.; Konda, B.; Wei, L.; Wirth, L.J.; Devine, C.; Daniels, G.A.; DeSouza, J.A.; Poi, M.; Seligson, N.D.; Cabanillas, M.E.; et al. Dabrafenib Versus Dabrafenib + Trametinib in BRAF-Mutated Radioactive Iodine Refractory Differentiated Thyroid Cancer: Results of a Randomized, Phase 2, Open-Label Multicenter Trial. Thyroid 2022, 32, 1184–1192. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, J.R.; Dadu, R.; Busaidy, N.L.; Xu, L.; Learned, K.O.; Chasen, N.N.; Vu, T.; Maniakas, A.; Eguia, A.A.; et al. Surgery After BRAF-Directed Therapy Is Associated with Improved Survival in BRAFV600E Mutant Anaplastic Thyroid Cancer: A Single-Center Retrospective Cohort Study. Thyroid 2023, 33, 484–491. [Google Scholar] [CrossRef]
- Ball, D.W.; Jin, N.; Xue, P.; Bhan, S.; Ahmed, S.R.; Rosen, D.M.; Schayowitz, A.; Clark, D.P.; Nelkin, B.D. Trametinib with and without pazopanib has potent preclinical activity in thyroid cancer. Oncol. Rep. 2015, 34, 2319–2324. [Google Scholar] [CrossRef]
- Kurzrock, R.; Ball, D.W.; Zahurak, M.L.; Nelkin, B.D.; Subbiah, V.; Ahmed, S.; O’Connor, A.; Karunsena, E.; Parkinson, R.M.; Bishop, J.A.; et al. A Phase I Trial of the VEGF Receptor Tyrosine Kinase Inhibitor Pazopanib in Combination with the MEK Inhibitor Trametinib in Advanced Solid Tumors and Differentiated Thyroid Cancers. Clin. Cancer Res. 2019, 25, 5475–5484. [Google Scholar] [CrossRef] [PubMed]
- White, P.S.; Pudusseri, A.; Lee, S.L.; Eton, O. Intermittent Dosing of Dabrafenib and Trametinib in Metastatic BRAFV600E Mutated Papillary Thyroid Cancer: Two Case Reports. Thyroid 2017, 27, 1201–1205. [Google Scholar] [CrossRef]
- Bible, K.C.; Suman, V.J.; Molina, J.R.; Smallridge, R.C.; Maples, W.J.; Menefee, M.E.; Rubin, J.; Karlin, N.; Sideras, K.; Morris JC3rd McIver, B.; et al. A multicenter phase 2 trial of pazopanib in metastatic and progressive medullary thyroid carcinoma: MC057H. J. Clin. Endocrinol. Metab. 2014, 99, 1687–1693. [Google Scholar] [CrossRef]
- Hamidi, S.; Iyer, P.C.; Dadu, R.; Gule-Monroe, M.K.; Maniakas, A.; Zafereo, M.E.; Wang, J.R.; Busaidy, N.L.; Cabanillas, M.E. Checkpoint Inhibition in Addition to Dabrafenib/Trametinib for BRAFV600E-Mutated Anaplastic Thyroid Carcinoma. Thyroid 2024, 34, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, M.; Brose, M.; Elisei, R.; Leboulleux, S.; Luster, M.; Pitoia, F.; Pacini, F. Definition and management of radioactive iodine-refractory differentiated thyroid cancer. Lancet Diabetes Endocrinol. 2014, 2, 356–358. [Google Scholar] [CrossRef]
- Shonka DCJr Ho, A.; Chintakuntlawar, A.V.; Geiger, J.L.; Park, J.C.; Seetharamu, N.; Jasim, S.; Abdelhamid Ahmed, A.H.; Bible, K.C.; Brose, M.S.; Cabanillas, M.E.; et al. American Head and Neck Society Endocrine Surgery Section and International Thyroid Oncology Group consensus statement on mutational testing in thyroid cancer: Defining advanced thyroid cancer and its targeted treatment. Head Neck 2022, 44, 1277–1300. [Google Scholar] [CrossRef]
- Tuttle, R.M.; Ahuja, S.; Avram, A.M.; Bernet, V.J.; Bourguet, P.; Daniels, G.H.; Dillehay, G.; Draganescu, C.; Flux, G.; Führer, D.; et al. Controversies, Consensus, and Collaboration in the Use of 131I Therapy in Differentiated Thyroid Cancer: A Joint Statement from the American Thyroid Association, the European Association of Nuclear Medicine, the Society of Nuclear Medicine and Molecular Imaging, and the European Thyroid Association. Thyroid 2019, 29, 461–470. [Google Scholar] [PubMed]
- Pitoia, F.; Jerkovich, F.; Trimboli, P.; Smulever, A. New approaches for patients with advanced radioiodine-refractory thyroid cancer. World J. Clin. Oncol. 2022, 13, 9–27. [Google Scholar] [CrossRef]
- Nikiforova, M.N.; Wald, A.I.; Roy, S.; Durso, M.B.; Nikiforov, Y.E. Targeted next-generation sequencing panel (ThyroSeq) for detection of mutations in thyroid cancer. J. Clin. Endocrinol. Metab. 2013, 98, E1852–E1860. [Google Scholar] [CrossRef]
- Giordano, T.J.; Beaudenon-Huibregtse, S.; Shinde, R.; Langfield, L.; Vinco, M.; Laosinchai-Wolf, W.; Labourier, E. Molecular testing for oncogenic gene mutations in thyroid lesions: A case-control validation study in 413 postsurgical specimens. Hum. Pathol. 2014, 45, 1339–1347. [Google Scholar] [CrossRef]
- Vitale, M. Mutational testing and its utility in thyroid cancer management: The need for something more. Biomark. Med. 2013, 7, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Babu, G.; Kainickal, C.T. Update on the systemic management of radioactive iodine refractory differentiated thyroid cancer (Review). Mol. Clin. Oncol. 2021, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Haddad, R.I.; Bischoff, L.; Ball, D.; Bernet, V.; Blomain, E.; Busaidy, N.L.; Campbell, M.; Dickson, P.; Duh, Q.; Ehya, H.; et al. Thyroid Carcinoma, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 925–951. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Filetti, S.; Durante, C.; Hartl, D.; Leboulleux, S.; Locati, L.D.; Newbold, K.; Papotti, M.G.; Berruti, A.; ESMO Guidelines Committee. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1856–1883. [Google Scholar] [CrossRef]
- Fugazzola, L.; Elisei, R.; Fuhrer, D.; Jarzab, B.; Leboulleux, S.; Newbold, K.; Smit, J. 2019 European Thyroid Association Guidelines for the Treatment and Follow-Up of Advanced Radioiodine-Refractory Thyroid Cancer. Eur. Thyroid J. 2019, 8, 227–245. [Google Scholar] [CrossRef]
- Ito, Y.; Onoda, N.; Okamoto, T. The revised clinical practice guidelines on the management of thyroid tumors by the Japan Associations of Endocrine Surgeons: Core questions and recommendations for treatments of thyroid cancer. Endocr. J. 2020, 67, 669–717. [Google Scholar] [CrossRef]
- Bible, K.C.; Ryder, M. Evolving molecularly targeted therapies for advanced-stage thyroid cancers. Nat. Rev. Clin. Oncol. 2016, 13, 403–416. [Google Scholar] [CrossRef]
- Brose, M.S.; Smit, J.W.A.; Lin, C.C.; Tori, M.; Bowles, D.W.; Worden, F.; Shen, D.H.; Huang, S.M.; Tsai, H.J.; Alevizaki, M.; et al. Multikinase Inhibitors for the Treatment of Asymptomatic Radioactive Iodine-Refractory Differentiated Thyroid Cancer: Global Noninterventional Study (RIFTOS MKI). Thyroid 2022, 32, 1059–1068. [Google Scholar] [CrossRef]
- Vaisman, F.; Tala, H.; Grewal, R.; Tuttle, R.M. In differentiated thyroid cancer, an incomplete structural response to therapy is associated with significantly worse clinical outcomes than only an incomplete thyroglobulin response. Thyroid 2011, 21, 1317–1322. [Google Scholar] [CrossRef]
- American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer; Cooper, D.S.; Doherty, G.M.; Haugen, B.R.; Kloos, R.T.; Lee, S.L.; Mandel, S.J.; Mazzaferri, E.L.; McIver, B.; Pacini, F.; et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009, 19, 1167–1214. [Google Scholar]
- Tufano, R.P.; Clayman, G.; Heller, K.S.; Inabnet, W.B.; Kebebew, E.; Shaha, A.; Steward, D.L.; Tuttle, R.M.; American Thyroid Association Surgical Affairs Committee Writing Task Force. Management of recurrent/persistent nodal disease in patients with differentiated thyroid cancer: A critical review of the risks and benefits of surgical intervention versus active surveillance. Thyroid 2015, 25, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Urken, M.L.; Milas, M.; Randolph, G.W.; Tufano, R.; Bergman, D.; Bernet, V.; Brett, E.M.; Brierley, J.D.; Cobin, R.; Doherty, G.; et al. Management of recurrent and persistent metastatic lymph nodes in well-differentiated thyroid cancer: A multifactorial decision-making guide for the Thyroid Cancer Care Collaborative. Head Neck 2015, 37, 605–614. [Google Scholar] [CrossRef]
- Guy, A.; Hirsch, D.; Shohat, T.; Bachar, G.; Tirosh, A.; Robenshtok, E.; Shimon, I.; Benbassat, C.A. Papillary thyroid cancer: Factors involved in restaging N1 disease after total thyroidectomy and radioactive iodine treatment. J. Clin. Endocrinol. Metab. 2014, 99, 4167–4173. [Google Scholar] [CrossRef][Green Version]
- Tuttle, R.M.; Haddad, R.I.; Ball, D.W.; Byrd, D.; Dickson, P.; Duh, Q.Y.; Ehya, H.; Haymart, M.; Hoh, C.; Hunt, J.P.; et al. Thyroid carcinoma, version 2.2014. J. Natl. Compr. Cancer Netw. 2014, 12, 1671–1680; quiz 1680. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Kane, M.A. Approach to the thyroid cancer patient with extracervical metastases. J. Clin. Endocrinol. Metab. 2010, 95, 987–993. [Google Scholar] [CrossRef]
- Brierley, J.D.; Tsang, R.W. External-beam radiation therapy in the treatment of differentiated thyroid cancer. Semin. Surg. Oncol. 1999, 16, 42–49. [Google Scholar] [CrossRef]
- Brierley, J.D.; Tsang, R.W. External beam radiation therapy for thyroid cancer. Endocrinol. Metab. Clin. N. Am. 2008, 37, 497–509. [Google Scholar] [CrossRef]
- Pöllinger, B.; Dühmke, E. External radiotherapy of thyroid cancer. Onkologie 2001, 24, 134–138. [Google Scholar] [CrossRef]
- Mangoni, M.; Gobitti, C.; Autorino, R.; Cerizza, L.; Furlan, C.; Mazzarotto, R.; Monari, F.; Simontacchi, G.; Vianello, F.; Basso, M.; et al. External beam radiotherapy in thyroid carcinoma: Clinical review and recommendations of the AIRO “Radioterapia Metabolica” Group. Tumori 2017, 103, 114–123. [Google Scholar] [CrossRef]
- Brierley, J.D. Update on external beam radiation therapy in thyroid cancer. J. Clin. Endocrinol. Metab. 2011, 96, 2289–2295. [Google Scholar] [CrossRef] [PubMed]
- Roukoz, C.; Gregoire, V. Indications of external beams radiation for thyroid cancer. Curr. Opin. Otolaryngol. Head Neck Surg. 2022, 30, 137–144. [Google Scholar] [CrossRef]
- Giuliani, M.; Brierley, J. Indications for the use of external beam radiation in thyroid cancer. Curr. Opin. Oncol. 2014, 26, 45–50. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Leo, A.; Di Simone, E.; Spano, A.; Puliani, G.; Petrone, F. Nursing Management and Adverse Events in Thyroid Cancer Treatments with Tyrosine Kinase Inhibitors: A Narrative Review. Cancers 2021, 13, 5961. [Google Scholar] [CrossRef] [PubMed]
- De Leo, S.; Trevisan, M.; Moneta, C.; Colombo, C. Endocrine-related adverse conditions induced by tyrosine kinase inhibitors. Ann. Endocrinol. 2023, 84, 374–381. [Google Scholar] [CrossRef]
- Bai, Y.; Li, J.Y.; Li, J.; Zhang, B.; Liu, Y.H.; Zhang, B.Y.; Jing, J. Risk of venous and arterial thromboembolic events associated with tyrosine kinase inhibitors in advanced thyroid cancer: A meta-analysis and systematic review. Oncotarget 2018, 10, 4205–4212. [Google Scholar] [CrossRef]
- Yu, S.T.; Ge, J.N.; Luo, J.Y.; Wei, Z.G.; Sun, B.H.; Lei, S.T. Treatment-related adverse effects with TKIs in patients with advanced or radioiodine refractory differentiated thyroid carcinoma: A systematic review and meta-analysis. Cancer Manag. Res. 2019, 11, 1525–1532. [Google Scholar] [CrossRef]
- Nervo, A.; Ragni, A.; Piovesan, A.; Marica, V.; Migliore, E.; Gallo, M.; Arvat, E. Quality of Life during Treatment with Lenvatinib for Thyroid Cancer: The Patients’ Perspective beyond the Medical Evaluation. Eur. Thyroid J. 2021, 10, 65–71. [Google Scholar] [CrossRef]
- Taylor, M.H.; Leboulleux, S.; Panaseykin, Y.; Konda, B.; de La Fouchardiere, C.; Hughes, B.G.M.; Gianoukakis, A.G.; Park, Y.J.; Romanov, I.; Krzyzanowska, M.K.; et al. Health-related quality-of-life analyses from a multicenter, randomized, double-blind phase 2 study of patients with differentiated thyroid cancer treated with lenvatinib 18 or 24 mg/day. Cancer Med. 2023, 12, 4332–4342. [Google Scholar] [CrossRef]
- Enokida, T.; Tahara, M. Management of VEGFR-Targeted TKI for Thyroid Cancer. Cancers 2021, 13, 5536. [Google Scholar] [CrossRef]
- Krajewska, J.; Paliczka-Cieslik, E.; Jarzab, B. Managing tyrosine kinase inhibitors side effects in thyroid cancer. Expert Rev. Endocrinol. Metab. 2017, 12, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Gabora, K.; Piciu, A.; Bădulescu, I.C.; Larg, M.I.; Stoian, I.A.; Piciu, D. Current evidence on thyroid related adverse events in patients treated with protein tyrosine kinase inhibitors. Drug Metab. Rev. 2019, 51, 562–569. [Google Scholar] [CrossRef]
- Jimenez-Fonseca, P. Use of multikinase inhibitors/lenvatinib in patients with high cardiovascular risk/vasculopathy and radioiodine refractory-differentiated thyroid cancer. Cancer Med. 2022, 11 (Suppl. S1), 17–25. [Google Scholar] [CrossRef] [PubMed]
- Pitoia, F.; Schmidt, A.; Bueno, F.; Abelleira, E.; Jerkovich, F. Rare complications of multikinase inhibitor treatment. Arch. Endocrinol. Metab. 2018, 62, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, M.E.; Takahashi, S. Managing the adverse events associated with lenvatinib therapy in radioiodine-refractory differentiated thyroid cancer. Semin. Oncol. 2019, 46, 57–64. [Google Scholar] [CrossRef]
- Cohen, R.B.; Oudard, S. Antiangiogenic therapy for advanced renal cell carcinoma: Management of treatment-related toxicities. Investig. New Drugs 2012, 30, 2066–2079. [Google Scholar] [CrossRef]
- Lacouture, M.E.; Ciccolini, K.; Kloos, R.T.; Agulnik, M. Overview and management of dermatologic events associated with targeted therapies for medullary thyroid cancer. Thyroid 2014, 24, 1329–1340. [Google Scholar] [CrossRef]
- Teo, Y.L.; Ho, H.K.; Chan, A. Risk of tyrosine kinase inhibitors-induced hepatotoxicity in cancer patients: A meta-analysis. Cancer Treat. Rev. 2013, 39, 199–206. [Google Scholar] [CrossRef]
- Béchade, D.; Chakiba, C.; Desjardin, M.; Bécouarn, Y.; Fonck, M. Toxicité hépatique des inhibiteurs des tyrosines kinases: Mécanismes en cause et conséquences pratiques [Hepatotoxicity of tyrosine kinase inhibitors: Mechanisms involved and practical implications]. Bull. Cancer 2018, 105, 290–298. [Google Scholar] [CrossRef]
| Drug | Clinical Trial | Phase | Indication | Main Molecular Targets | Dosage | PFS Compared to Placebo (Months) |
|---|---|---|---|---|---|---|
| Lenvatinib [7] | NCT01321554 | III | First/second-line DTC | VEGFR1-3, FGFR1-4, PDGFRα, RET, c-KIT | 24 mg orally per day | 18.3 vs. 3.6, HR 0.21 (99% CI 0.14–0.31, p < 0.001) |
| Sorafenib [8] | NCT00984282 | III | First * line DTC | VEGFR1-3, PDGFRβ, Raf, c-KIT, FLT-3. BRAF, CRAF | 400 mg orally twice daily | 10.8 vs. 5.8, HR 0.58 (95% CI 0.45–0.75, p < 0.0001) |
| Cabozantinib [9,45] | NCT01811212/NCT00704730 | III (DTC)/ III (MTC) | Second-line DTC First-line non-RETm MTC Second-line RETm MTC | MET, VEGFR2, RET, c-KIT, FLT-3, AXL | 60 mg orally per day | DTC: NE vs. 1.9, HR 0.22 (96% CI 0.13–0.36; p < 0.0001) MTC: 11.2 vs. 4.0; HR 0.28 (95% CI 0.19–0.40; p < 0.001) |
| Vandetanib [75] | NCT00537095/NCT00410761 | II/III | First-line non-RETm MTC Second-line RETm MTC Subsequent in DTC | VEGFR2/3, EGFR, RET | 300 mg orally per day | DTC: 11.1 (95% CI, 7.7–14.0) MTC: 30.5 (model prediction; NE) vs. 19.3, HR 0.46 (95% CI 0.31–0.69; p < 0.001) |
| Entrectinib [76] | NCT02568267 | II | Any line | NTRK, ROS1, and ALK fusions | 600 mg orally per day | 19.9 (95% CI, 6.5–33.8) |
| Larotrectinib [77] | NCT02122913/ NCT02637687/NCT02576431 | I/II | Any line | NTRK fusions | 100 mg orally twice daily | 44.0 (95% CI, 16.6–NE) at a median follow-up of 38.7 months |
| Axitinib [69] | NCT00094055 | II | Subsequent line if no driver mutation | VEGFR1-3 | 5 mg orally twice daily | 15–18.1 (95% CI) |
| Sunitinib [78] | NCT00381641 | II | Subsequent line if no driver mutation | VEGFR1/2, PDGFR, RET, KIT, FLT3, CSF1R | 37.5 mg orally per day | 12.8 (95% CI, 8.9–NE) |
| Pazopanib [79] | NCT00625846 | II | Subsequent line if no driver mutation | VEGFR, PDGFR | 800 mg orally per day | 11.4 (95% CI) |
| Selpercatinib [80] | NCT04211337 | III | First/second-line DTC (targeted therapy) First-line RETm MTC First-line RETm ATC | RET | 160 mg orally twice daily | NE vs. 16.8, HR 0.28 (95% CI 0.16–0.48; p < 0.001) |
| Pralsetinib [81] | NCT03037385 | II | Targeted therapy | RET | 400 mg orally per day | 25.9, NR, 25.4 (95% CI) ** |
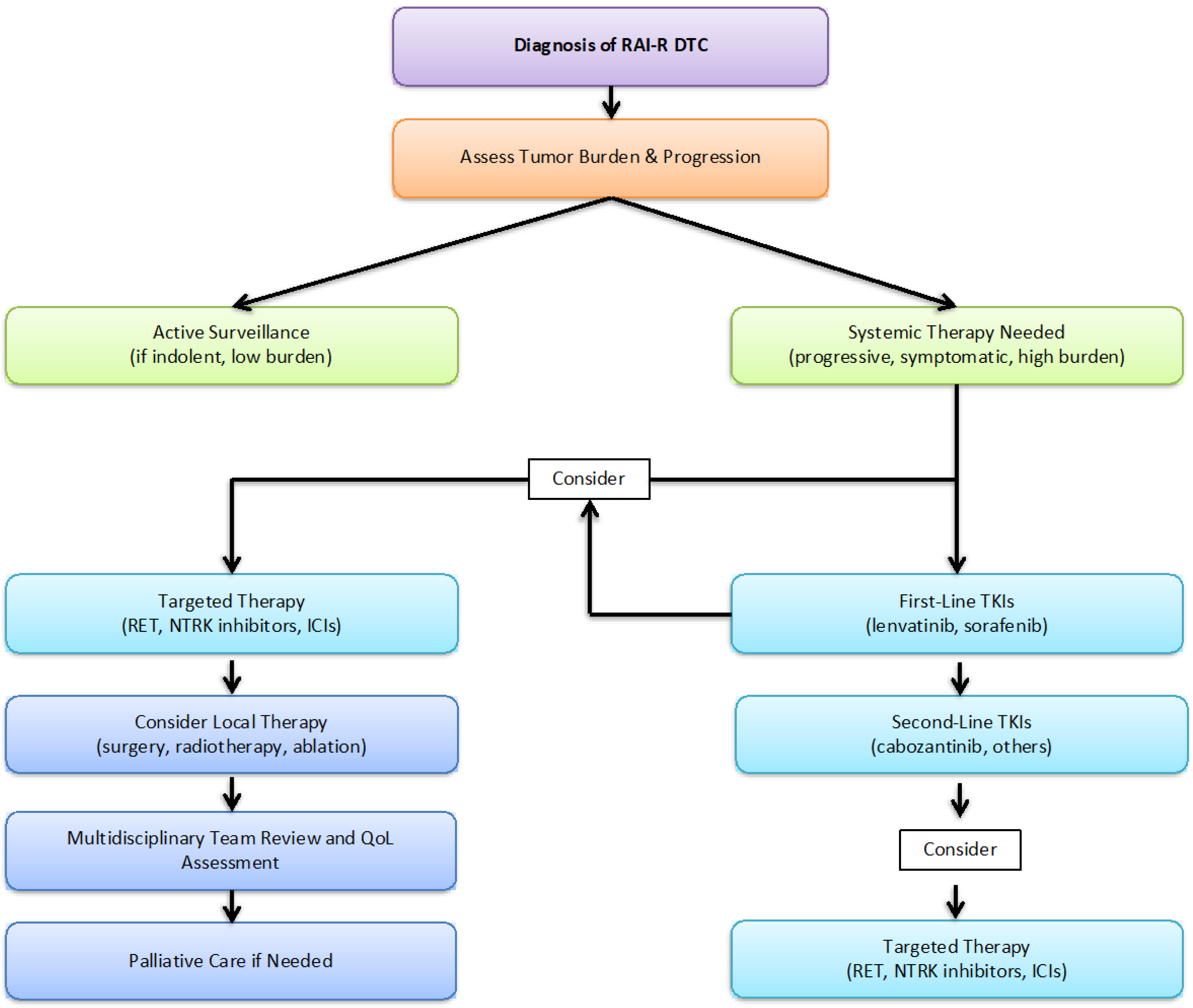
| Clinical Situation | Recommended Approach | |
|---|---|---|
| 1 | Diagnosis of RAI-R DTC | Assess tumor burden and disease progression |
| 2 | Low tumor burden, indolent disease | Active surveillance with periodic monitoring |
| 3 | Progressive or symptomatic disease | Systemic therapy initiation (TKIs or targeted agents) |
| 4 | First-line systemic therapy | Lenvatinib or Sorafenib |
| 5 | Disease progression after first-line therapy | Cabozantinib or alternative systemic agents |
| 6 | Molecularly targeted therapy | RET/NTRK inhibitors, immune checkpoint inhibitors |
| 7 | Consideration of local treatments | Surgery, radiotherapy, or ablative technique |
| 8 | Quality of life assessment | Multidisciplinary team review and supportive care |
| 9 | Palliative care if necessary | End-of-life care planning and symptom management |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz Vico, T.; Martínez-Amores Martínez, B.; Mihic Góngora, L.; Jiménez-Fonseca, P.; Peinado Martín, P.; Grao Torrente, I.; García Muñoz-Nájar, A.; Durán-Poveda, M. Systemic Therapeutic Options in Radioiodine-Refractory Differentiated Thyroid Cancer: Current Indications and Optimal Timing. Cancers 2025, 17, 1800. https://doi.org/10.3390/cancers17111800
Díaz Vico T, Martínez-Amores Martínez B, Mihic Góngora L, Jiménez-Fonseca P, Peinado Martín P, Grao Torrente I, García Muñoz-Nájar A, Durán-Poveda M. Systemic Therapeutic Options in Radioiodine-Refractory Differentiated Thyroid Cancer: Current Indications and Optimal Timing. Cancers. 2025; 17(11):1800. https://doi.org/10.3390/cancers17111800
Chicago/Turabian StyleDíaz Vico, Tamara, Brezo Martínez-Amores Martínez, Luka Mihic Góngora, Paula Jiménez-Fonseca, Paloma Peinado Martín, Irene Grao Torrente, Alejandro García Muñoz-Nájar, and Manuel Durán-Poveda. 2025. "Systemic Therapeutic Options in Radioiodine-Refractory Differentiated Thyroid Cancer: Current Indications and Optimal Timing" Cancers 17, no. 11: 1800. https://doi.org/10.3390/cancers17111800
APA StyleDíaz Vico, T., Martínez-Amores Martínez, B., Mihic Góngora, L., Jiménez-Fonseca, P., Peinado Martín, P., Grao Torrente, I., García Muñoz-Nájar, A., & Durán-Poveda, M. (2025). Systemic Therapeutic Options in Radioiodine-Refractory Differentiated Thyroid Cancer: Current Indications and Optimal Timing. Cancers, 17(11), 1800. https://doi.org/10.3390/cancers17111800






