Rethinking the Prognostic Role of Necrosis in Soft-Tissue Sarcoma: Multidisciplinary Insights from the Sarcoma Academy
Simple Summary
Abstract
1. Introduction
2. Pathology Overview
2.1. Defining Necrosis in STS
2.2. Heterogeneity of Necrosis
3. Critical Analysis of Existing Evidence
3.1. Summaries of the Four Presented Studies
3.1.1. Bridging Perspective
3.1.2. Dian Wang, Rush University—RTOG 9415 and 0630 Trials [11,21]
3.1.3. Arash Naghavi, Moffitt Cancer Center, HEAT Trial, and Precision Radiation
3.1.4. Julien Montreuil, University of Miami—Diverse Cohort Analysis
3.2. Challenges in Prognostication
3.3. Insights and Integrations
4. Consensus Statements
4.1. Therapy-Induced Necrosis as a Standalone Marker
4.2. Incorporating Other Factors
4.3. Standardization and Protocols
4.4. Future Research Directions (Innovative Methodologies)
4.5. Target Trial Emulation and Hybrid Designs
5. Discussion
5.1. Expert Opinions and Points of Agreement
5.2. Clinical and Research Implications
5.2.1. Clinical Context
5.2.2. Research Perspectives
5.2.3. Radiologist’s Point of View in Necrosis Assessment
5.3. Considering the Consequences of Escalating Preoperative Therapy to Increase Necrosis
6. Conclusions
- 1.
- Therapy-induced necrosis alone is not a definitive prognostic indicator in STS.High levels of necrosis may reflect either inherent tumor aggressiveness or effective therapy, complicating interpretation—especially when differentiating spontaneous necrosis (as used in the FNCLCC system) from treatment-related cell death. A uniform pathologic protocol that measures % viable tumor cells may provide an alternative or complimentary approach, but consistency is critical. However like necrosis, the potential prognostic value of % viable tumor cells is also likely to depend on factors such as tumor subtype, timing of resection, and surgical margins.
- 2.
- Percent of viable tumor cells (rather than necrosis alone) offers a more reliable measure of therapeutic effect.Reporting the percentage of viable tumor cells (%VTC) provides a uniform endpoint—the amount of living tissue that remains post-therapy. All other treatment-related changes (necrosis, fibrosis, hemorrhage, and inflammation) are classified as non-viable, a simplification that reduces inter-observer variation and enables more reliable cross-study comparison.
- 3.
- Complete pathologic response is rare but may correlate with better outcomes in selected settings.In certain prospective trials (e.g., RTOG 9514, 0630), 19–27% of patients achieved complete absence of viable tumor cells, often linked to improved survival (and retrospective series [26]). However, due to STS heterogeneity, potential toxicities, and the possibility of selecting resistant clones by delaying surgery, universal escalation of therapy to maximize necrosis remains neither routinely advisable nor consistently beneficial.
- 4.
- Surgical resection margins trump necrosis and subtype for local control.Securing negative surgical margins remains the cornerstone of local control in STS and outweighs both necrosis extent and histological subtype. Although tumor biology and other factors contribute, margin status consistently exerts the strongest influence on recurrence risk.
- 5.
- Pathologic and radiologic measures of response are complementary but distinct.Pathologists assess histological changes, including necrosis, viable cells, and fibrosis, whereas radiologists evaluate imaging-based criteria (e.g., tumor shrinkage and metabolic shifts). Integrating both perspectives provides a more comprehensive picture of therapeutic impact.
- 6.
- Jointly agreed pathology-plus-imaging protocols are essential for comparability across centers.Inconsistent thresholds and variable pathological sampling underscore the need for universally accepted protocols (for example, [13]). Harmonizing radiological response criteria (e.g., RECIST or PERCIST) with standardized pathology procedures is similarly essential.
- 7.
- Integrating imaging-based biomarkers, molecular data, and immunologic profiling may enhance prognostic accuracy.Techniques such as radiomics, genomic analysis, and immune microenvironment characterization can provide deeper insights into tumor behavior, enabling more precise risk stratification.
- 8.
- Real-world evidence and pragmatic trials can circumvent some limitations of traditional RCTs, improving clinical relevance.Broader, more inclusive study designs in RWE frameworks capture a wider range of patient and treatment variations, refining our understanding of necrosis, % viable cells, and response in everyday STS care.
- 9.
- Patient-reported outcomes and toxicity data should be standard secondary endpoints, ensuring treatments remain patient-focused.Balancing efficacy with side-effect profiles is imperative, ensuring any push to increase necrosis remains aligned with patient well-being and quality of life.
- 10.
- Target trial emulation and hybrid study designs can yield better causal insights in rare, heterogeneous STSs.These methodologies enable more nuanced exploration of necrosis and % viable cells in smaller or diverse patient populations, approximating randomized conditions while reflecting real-world clinical practice.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antonescu, C.; Blay, J. WHO Classification of Tumours: Soft Tissue and Bone Tumours, 5th ed.; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Gannon, N.P.; Stemm, M.H.; King, D.M.; Bedi, M. Pathologic necrosis following neoadjuvant radiotherapy or chemoradiotherapy is prognostic of poor survival in soft tissue sarcoma. J. Cancer Res. Clin. Oncol. 2019, 145, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Salah, S.; Lewin, J.; Amir, E.; Abdul Razak, A. Tumor necrosis and clinical outcomes following neoadjuvant therapy in soft tissue sarcoma: A systematic review and meta-analysis. Cancer Treat. Rev. 2018, 69, 1–10. [Google Scholar] [CrossRef]
- Patel, N.; Werenski, J.O.; Gonzalez, M.R.; Clunk, M.J.; McCadden, M.R.; Richard, A.; Chebib, I.; Hung, Y.P.; Nielsen, G.P.; Lozano-Calderon, S.A. Tumor Necrosis Drives Prognosis in Osteosarcoma: No Difference in Chemotherapy Response and Survival Between Chondroblastic and Osteoblastic Osteosarcoma. Surg. Oncol. 2024, 57, 102155. [Google Scholar] [CrossRef]
- Lozano-Calderon, S.A.; Albergo, J.I.; Groot, O.Q.; Merchan, N.A.; El Abiad, J.M.; Salinas, V.; Gomez Mier, L.C.; Montoya, C.S.; Ferrone, M.L.; Ready, J.E.; et al. Complete tumor necrosis after neoadjuvant chemotherapy defines good responders in patients with Ewing sarcoma. Cancer 2023, 129, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Wunder, J.S.; Paulian, G.; Huvos, A.G.; Heller, G.; Meyers, P.A.; Healey, J.H. The histological response to chemotherapy as a predictor of the oncological outcome of operative treatment of Ewing sarcoma. J. Bone Jt. Surg. Am. 1998, 80, 1020–1033. [Google Scholar] [CrossRef]
- Crombe, A.; Marcellin, P.J.; Buy, X.; Stoeckle, E.; Brouste, V.; Italiano, A.; Le Loarer, F.; Kind, M. Soft-Tissue Sarcomas: Assessment of MRI Features Correlating with Histologic Grade and Patient Outcome. Radiology 2019, 291, 710–721. [Google Scholar] [CrossRef]
- Nystrom, H.; Jonsson, M.; Nilbert, M.; Carneiro, A. Immune-cell infiltration in high-grade soft tissue sarcomas; prognostic implications of tumor-associated macrophages and B-cells. Acta Oncol. 2023, 62, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Xu, R.; Ren, X.; Zhang, W.; Yang, Z.; Tu, C.; Li, Z. Comprehensive Profiling Reveals Prognostic and Immunogenic Characteristics of Necroptosis in Soft Tissue Sarcomas. Front. Immunol. 2022, 13, 877815. [Google Scholar] [CrossRef]
- Vogin, G.; Lepage, M.; Salleron, J.; Cuenin, M.; Blum, A.; Gondim Teixeira, P.A. Evaluation of the Prognostic Value of Pretherapeutic Magnetic Resonance Imaging in Predicting Soft Tissue Sarcoma Radiation Response: A Retrospective Study from a Large Institutional Sarcoma Imaging Database. Cancers 2024, 16, 878. [Google Scholar] [CrossRef]
- Wang, D.; Harris, J.; Kraybill, W.G.; Eisenberg, B.; Kirsch, D.G.; Ettinger, D.S.; Kane, J.M., 3rd; Barry, P.N.; Naghavi, A.; Freeman, C.R.; et al. Pathologic Complete Response and Clinical Outcomes in Patients With Localized Soft Tissue Sarcoma Treated With Neoadjuvant Chemoradiotherapy or Radiotherapy: The NRG/RTOG 9514 and 0630 Nonrandomized Clinical Trials. JAMA Oncol. 2023, 9, 646–655. [Google Scholar] [CrossRef]
- Boulouta, A.; Kyriazoglou, A.; Kotsantis, I.; Economopoulou, P.; Anastasiou, M.; Pantazopoulos, A.; Kyrkasiadou, M.; Moutafi, M.; Gavrielatou, N.; Zazas, E.; et al. Pathologic complete response in patients with localized soft tissue sarcoma treated with neoadjuvant therapy and its correlation with clinical outcomes: A systematic review. Cancer Treat. Rev. 2024, 130, 102820. [Google Scholar] [CrossRef]
- Smolle, M.A.; Andreou, D.; Tunn, P.U.; Szkandera, J.; Liegl-Atzwanger, B.; Leithner, A. Diagnosis and treatment of soft-tissue sarcomas of the extremities and trunk. EFORT Open Rev. 2017, 2, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Wardelmann, E.; Haas, R.L.; Bovee, J.V.; Terrier, P.; Lazar, A.; Messiou, C.; LePechoux, C.; Hartmann, W.; Collin, F.; Fisher, C.; et al. Evaluation of response after neoadjuvant treatment in soft tissue sarcomas; the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC-STBSG) recommendations for pathological examination and reporting. Eur. J. Cancer 2016, 53, 84–95. [Google Scholar] [CrossRef]
- Sugita, S.; Tanaka, K.; Oda, Y.; Nojima, T.; Konishi, N.; Machida, R.; Kita, R.; Fukuda, H.; Ozaki, T.; Hasegawa, T. Prognostic evaluation of the Ki-67 labeling system in histological grading of non-small round cell sarcoma: A supplementary analysis of a randomized controlled trial, JCOG1306. Jpn. J. Clin. Oncol. 2024, 54, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Stergioula, A.; Kormas, T.; Kokkali, S.; Memos, N.; Pantelis, E.; Pouloudi, D.; Agrogiannis, G. What Is the Prognostic Value of the Pathologic Response after Neoadjuvant Radiotherapy in Soft Tissue Sarcoma? An Institutional Study Using the EORTC–STBSG Response Score. Cancers 2024, 16, 3449. [Google Scholar] [CrossRef] [PubMed]
- Boxberg, M.; Langer, R.; Woertler, K.; Knebel, C.; Rechl, H.; von Eisenhart-Rothe, R.; Weichert, W.; Combs, S.E.; Hadjamu, M.; Roper, B.; et al. Neoadjuvant Radiation in High-Grade Soft-Tissue Sarcomas: Histopathologic Features and Response Evaluation. Am. J. Surg. Pathol. 2022, 46, 1060–1070. [Google Scholar] [CrossRef]
- Gennaro, N.; van der Loo, I.; Reijers, S.J.M.; van Boven, H.; Snaebjornsson, P.; Bekers, E.M.; Bodalal, Z.; Trebeschi, S.; Schrage, Y.M.; van der Graaf, W.T.A.; et al. Heterogeneity in response to neoadjuvant radiotherapy between soft tissue sarcoma histotypes: Associations between radiology and pathology findings. Eur. Radiol. 2024, 35, 1337–1350. [Google Scholar] [CrossRef]
- Hanafi, H.; Freeman, C.R.; Tsui, J.; Ramia, P.; Turcotte, R.; Aoude, A.; Bozzo, A.; Cury, F.L. A retrospective study on the comparision of pathological tumour necrosis of conventional versus ultrahypofractionated preoperative radiotherapy in localised extremity soft tissue sarcoma and its correlation with clinical outcomes: A retrospective study on the comparision of pathological tumour necrosis of CONV-RT versus UHYPO-RT preoperative radiotherapy in localised extremity soft tissue sarcoma and its correlation with clinical outcomes. Pract. Radiat. Oncol. 2024, 15, e189–e197. [Google Scholar] [CrossRef]
- Fromm, J.; Klein, A.; Kirilova, M.; Lindner, L.H.; Nachbichler, S.; Holzapfel, B.M.; Goller, S.S.; Knosel, T.; Durr, H.R. The Effect of chemo- and radiotherapy on tumor necrosis in soft tissue sarcoma- does it influence prognosis? BMC Cancer 2024, 24, 303. [Google Scholar] [CrossRef]
- Wang, D.; Abrams, R.A. Radiotherapy for soft tissue sarcoma: 50 years of change and improvement. Am. Soc. Clin. Oncol. Educ. Book 2014, 34, 244–251. [Google Scholar] [CrossRef]
- Naghavi, A.O.; Bryant, J.M.; Kim, Y.; Weygand, J.; Redler, G.; Sim, A.J.; Miller, J.; Coucoules, K.; Michael, L.T.; Gloria, W.E.; et al. Habitat escalated adaptive therapy (HEAT): A phase 2 trial utilizing radiomic habitat-directed and genomic-adjusted radiation dose (GARD) optimization for high-grade soft tissue sarcoma. BMC Cancer 2024, 24, 437. [Google Scholar] [CrossRef] [PubMed]
- Vanzulli, A.; Vigorito, R.; Buonomenna, C.; Palmerini, E.; Quagliuolo, V.; Broto, J.M.; Lopez Pousa, A.; Grignani, G.; Brunello, A.; Blay, J.Y.; et al. Redefining radiologic responses in high-risk soft-tissue sarcomas treated with neoadjuvant chemotherapy: Final results of ISG-STS 1001, a randomized clinical trial. ESMO Open 2025, 10, 104299. [Google Scholar] [CrossRef]
- White, L.M.; Atinga, A.; Naraghi, A.M.; Lajkosz, K.; Wunder, J.S.; Ferguson, P.; Tsoi, K.; Griffin, A.; Haider, M. T2-weighted MRI radiomics in high-grade intramedullary osteosarcoma: Predictive accuracy in assessing histologic response to chemotherapy, overall survival, and disease-free survival. Skelet. Radiol. 2023, 52, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Isaac, C.; Kavanagh, J.; Griffin, A.M.; Dickie, C.I.; Mohankumar, R.; Chung, P.W.; Catton, C.N.; Shultz, D.; Ferguson, P.C.; Wunder, J.S. Radiological progression of extremity soft tissue sarcoma following pre-operative radiotherapy predicts for poor survival. Br. J. Radiol. 2022, 95, 20210936. [Google Scholar] [CrossRef]
- Bonvalot, S.; Wunder, J.; Gronchi, A.; Broto, J.M.; Turcotte, R.; Rastrelli, M.; Papai, Z.; Radaelli, S.; Lindner, L.H.; Shumelinsky, F.; et al. Complete pathological response to neoadjuvant treatment is associated with better survival outcomes in patients with soft tissue sarcoma: Results of a retrospective multicenter study. Eur. J. Surg. Oncol. 2021, 47, 2166–2172. [Google Scholar] [CrossRef]
- Chodyla, M.; Demircioglu, A.; Schaarschmidt, B.M.; Bertram, S.; Morawitz, J.; Bauer, S.; Podleska, L.; Rischpler, C.; Forsting, M.; Herrmann, K.; et al. Evaluation of the Predictive Potential of 18F-FDG PET and DWI Data Sets for Relevant Prognostic Parameters of Primary Soft-Tissue Sarcomas. Cancers 2021, 13, 2753. [Google Scholar] [CrossRef] [PubMed]
- Crompton, J.G.; Armstrong, W.R.; Eckardt, M.A.; Seyedroudbari, A.; Tap, W.D.; Dry, S.M.; Abt, E.R.; Calais, J.; Herrmann, K.; Czernin, J.; et al. (18)F-FLT PET/CT as a Prognostic Imaging Biomarker of Disease-Specific Survival in Patients with Primary Soft-Tissue Sarcoma. J. Nucl. Med. 2022, 63, 708–712. [Google Scholar] [CrossRef]
- Hernan, M.A.; Wang, W.; Leaf, D.E. Target Trial Emulation: A Framework for Causal Inference From Observational Data. JAMA 2022, 328, 2446–2447. [Google Scholar] [CrossRef]
- Hernan, M.A.; Robins, J.M. Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available. Am. J. Epidemiol. 2016, 183, 758–764. [Google Scholar] [CrossRef]
- Subhawong, T.K.; Jacobs, M.A.; Fayad, L.M. Insights into quantitative diffusion-weighted MRI for musculoskeletal tumor imaging. AJR Am. J. Roentgenol. 2014, 203, 560–572. [Google Scholar] [CrossRef]
- Di Masi, P.; Colangeli, M.; Simonetti, M.; Bianchi, G.; Righi, A.; Bilancia, G.; Palmerini, E.; Crombe, A.; Spinnato, P. Clear Cell Sarcoma of Soft Tissues: Radiological Analysis of 14 Patients-MRI Findings Related to Metastatic Disease. Diagnostics 2025, 15, 1027. [Google Scholar] [CrossRef] [PubMed]
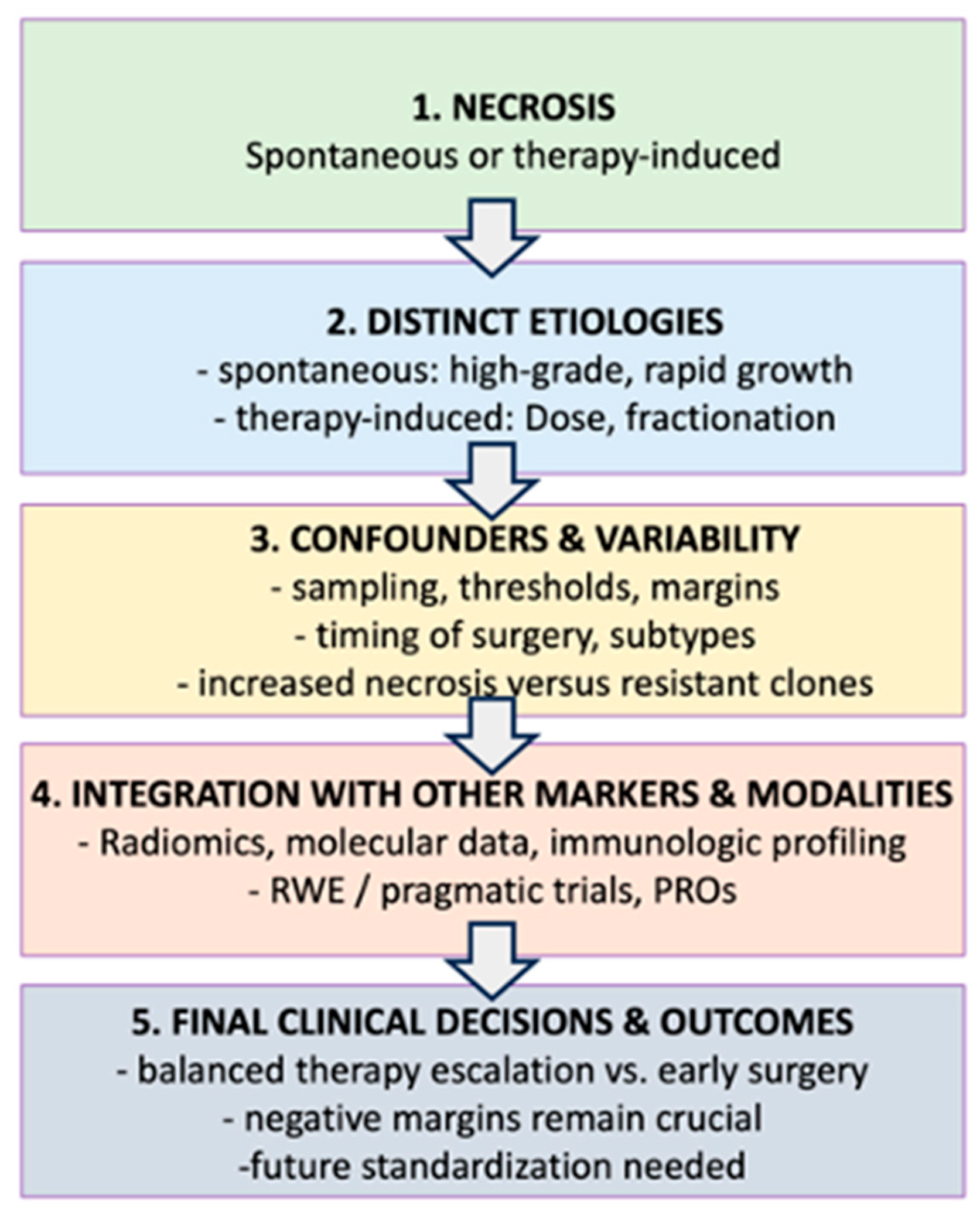
| Label | Topic or Factor | Detail/Key Points | Connection to Flow Chart |
|---|---|---|---|
| A | Spontaneous Necrosis |
| Referenced at node (2) for difference in etiologies. |
| B | Preoperative Therapy induced Necrosis |
| Node (2) to compare with spontaneous necrosis. |
| C | Confounders & Variability |
| Node (3) describes multiple confounders that Influence necrosis interpretation. |
| D | Integration with other markers/Modalities |
| Node (4) where necrosis merges with advanced diagnostics ad trial designs. |
| E | Clinical Decisions/Future Directions |
| Node (5) final step in the flowchart: linking necrosis to actual treatment outcomes. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bode-Lesniewska, B.; Dürr, H.R.; Wang, D.; Naghavi, A.; Montreuil, J.; Fischer, T.; Ghert, M.; Lazarides, A.; Lindner, L.; Martin-Broto, J.; et al. Rethinking the Prognostic Role of Necrosis in Soft-Tissue Sarcoma: Multidisciplinary Insights from the Sarcoma Academy. Cancers 2025, 17, 1779. https://doi.org/10.3390/cancers17111779
Bode-Lesniewska B, Dürr HR, Wang D, Naghavi A, Montreuil J, Fischer T, Ghert M, Lazarides A, Lindner L, Martin-Broto J, et al. Rethinking the Prognostic Role of Necrosis in Soft-Tissue Sarcoma: Multidisciplinary Insights from the Sarcoma Academy. Cancers. 2025; 17(11):1779. https://doi.org/10.3390/cancers17111779
Chicago/Turabian StyleBode-Lesniewska, Beata, Hans Roland Dürr, Dian Wang, Arash Naghavi, Julien Montreuil, Tim Fischer, Michelle Ghert, Alexander Lazarides, Lars Lindner, Javier Martin-Broto, and et al. 2025. "Rethinking the Prognostic Role of Necrosis in Soft-Tissue Sarcoma: Multidisciplinary Insights from the Sarcoma Academy" Cancers 17, no. 11: 1779. https://doi.org/10.3390/cancers17111779
APA StyleBode-Lesniewska, B., Dürr, H. R., Wang, D., Naghavi, A., Montreuil, J., Fischer, T., Ghert, M., Lazarides, A., Lindner, L., Martin-Broto, J., Mazza, M., Scanferla, R., Studer, G., Temple, H. T., Wunder, J., & Fuchs, B. (2025). Rethinking the Prognostic Role of Necrosis in Soft-Tissue Sarcoma: Multidisciplinary Insights from the Sarcoma Academy. Cancers, 17(11), 1779. https://doi.org/10.3390/cancers17111779






