Low CD25 in ALK+ Anaplastic Large Cell Lymphoma Is Associated with Older Age, Thrombocytopenia, and Increased Expression of Surface CD3 and CD8
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection
2.2. Immunophenotypic Analysis
2.3. Statistical Analysis
3. Results
3.1. Clinical Findings
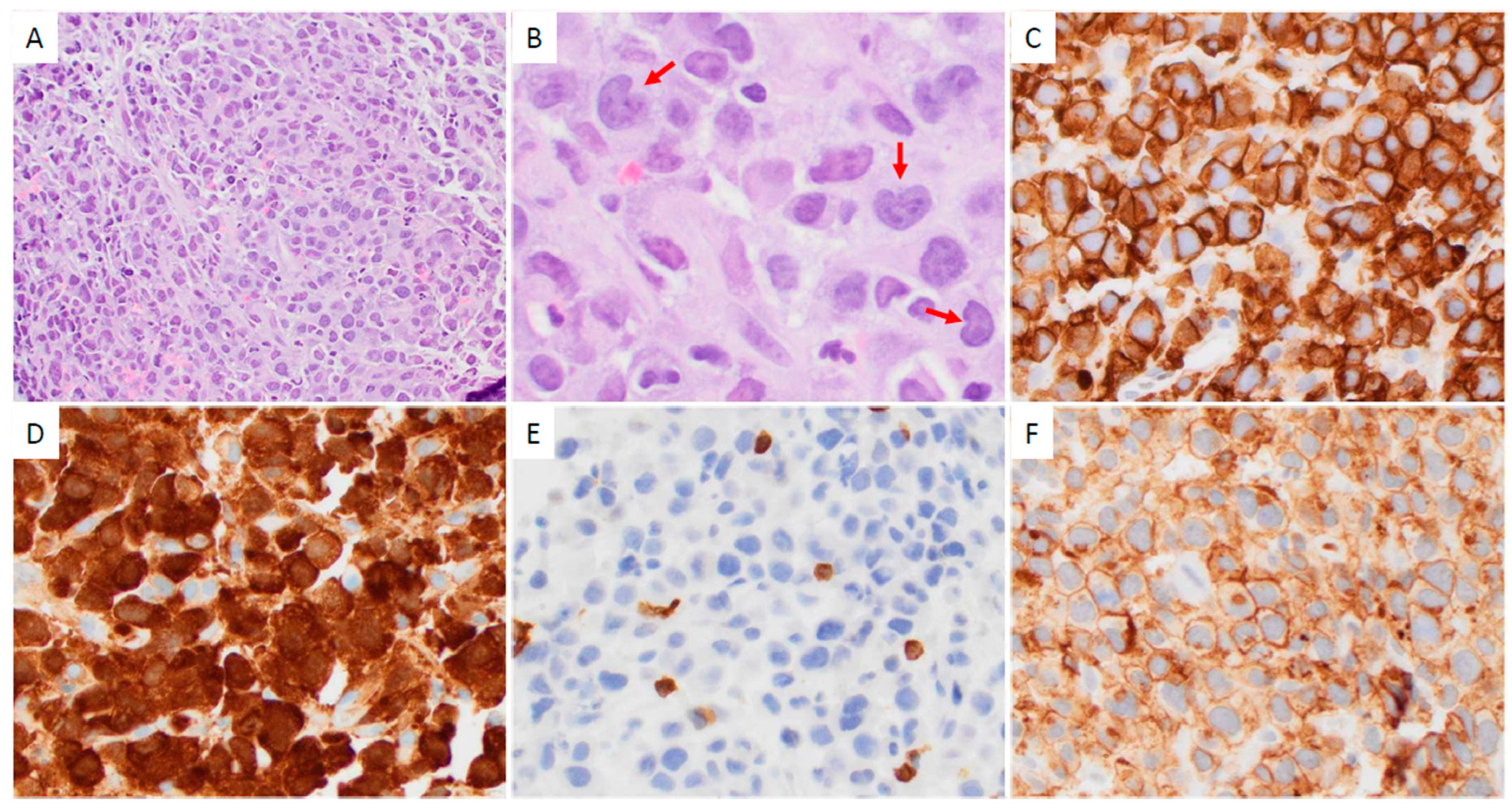
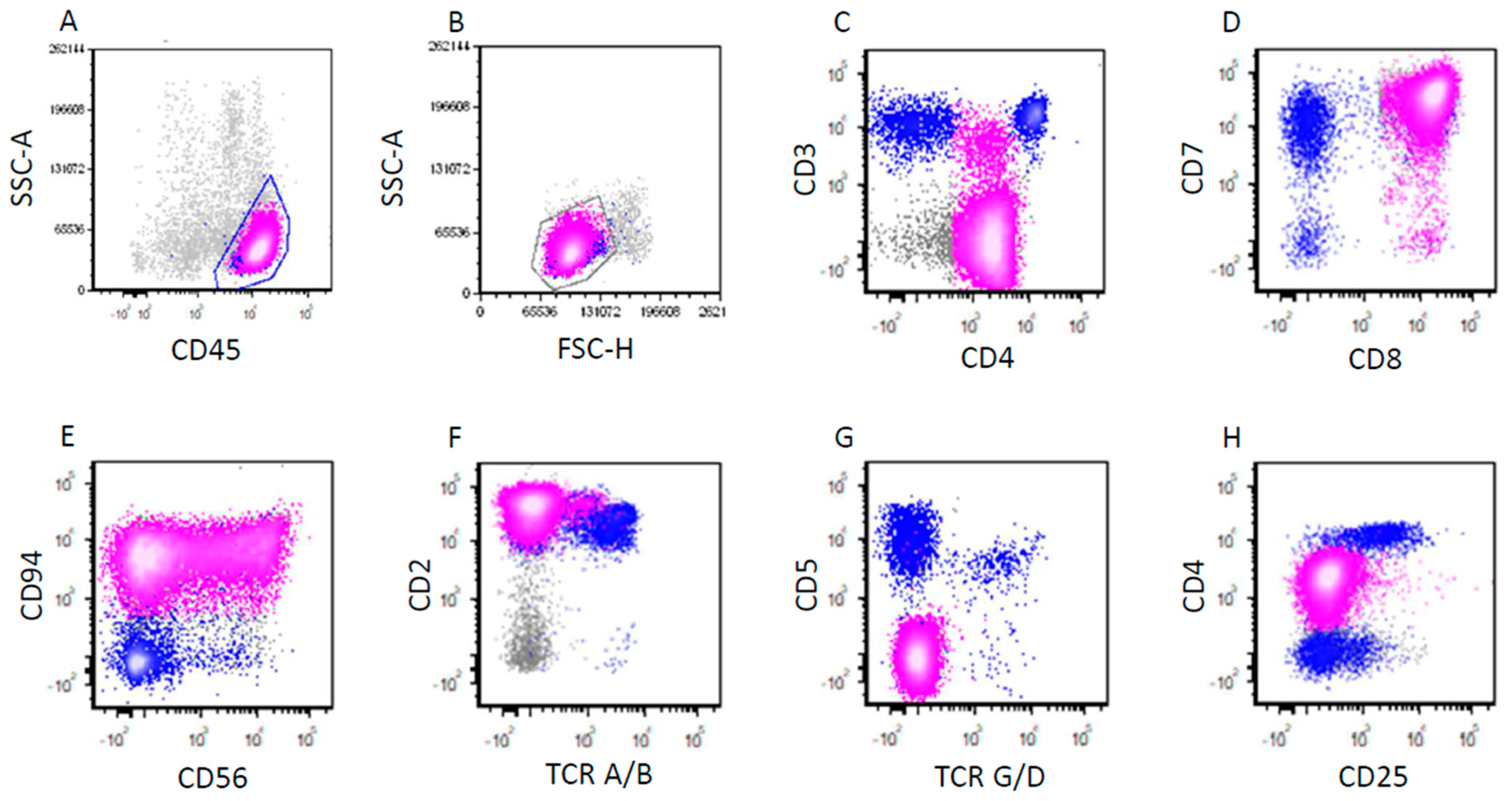
3.2. Pathologic Findings
3.3. Treatment and Response
3.4. Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef] [PubMed]
- Campo, E.; Jaffe, E.S.; Cook, J.R.; Quintanilla-Martinez, L.; Swerdlow, S.H.; Anderson, K.C.; Brousset, P.; Cerroni, L.; de Leval, L.; Dirnhofer, S.; et al. The International Consensus Classification of Mature Lymphoid Neoplasms: A report from the Clinical Advisory Committee. Blood 2022, 140, 1229–1253. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, L.J.; Elenitoba-Johnson, K.S. Anaplastic Large Cell Lymphoma. Am. J. Clin. Pathol. 2007, 127, 707–722. [Google Scholar] [CrossRef]
- Vose, J.; Armitage, J.; Weisenburger, D.; International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: Pathology findings and clinical outcomes. J. Clin. Oncol. 2008, 26, 4124–4130. [Google Scholar] [CrossRef]
- Falini, B.; Pileri, S.; Zinzani, P.L.; Carbone, A.; Zagonel, V.; Wolf-Peeters, C.; Verhoef, G.; Menestrina, F.; Todeschini, G.; Paulli, M.; et al. ALK+ lymphoma: Clinico-pathological findings and outcome. Blood 1999, 93, 2697–2706. [Google Scholar]
- Sibon, D.; Fournier, M.; Briere, J.; Lamant, L.; Haioun, C.; Coiffier, B.; Bologna, S.; Morel, P.; Gabarre, J.; Hermine, O.; et al. Long-term outcome of adults with systemic anaplastic large-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte trials. J. Clin. Oncol. 2012, 30, 3939–3946. [Google Scholar] [CrossRef] [PubMed]
- ten Berge, R.L.; de Bruin, P.C.; Oudejans, J.J.; Ossenkoppele, G.J.; van der Valk, P.; Meijer, C.J. ALK-negative anaplastic large-cell lymphoma demonstrates similar poor prognosis to peripheral T-cell lymphoma, unspecified. Histopathology 2003, 43, 462–469. [Google Scholar] [CrossRef]
- Parrilla Castellar, E.R.; Jaffe, E.S.; Said, J.W.; Swerdlow, S.H.; Ketterling, R.P.; Knudson, R.A.; Sidhu, J.S.; Hsi, E.D.; Karikehalli, S.; Jiang, L.; et al. ALK-negative anaplastic large cell lymphoma is a genetically heterogeneous disease with widely disparate clinical outcomes. Blood 2014, 124, 1473–1480. [Google Scholar] [CrossRef]
- Ellin, F.; Landstrom, J.; Jerkeman, M.; Relander, T. Real-world data on prognostic factors and treatment in peripheral T-cell lymphomas: A study from the Swedish Lymphoma Registry. Blood 2014, 124, 1570–1577. [Google Scholar] [CrossRef]
- Jaffe, E.S. Anaplastic large cell lymphoma: The shifting sands of diagnostic hematopathology. Mod. Pathol. 2001, 14, 219–228. [Google Scholar] [CrossRef]
- Lamant, L.; McCarthy, K.; d'’Amore, E.; Klapper, W.; Nakagawa, A.; Fraga, M.; Maldyk, J.; Simonitsch-Klupp, I.; Oschlies, I.; Delsol, G.; et al. Prognostic impact of morphologic and phenotypic features of childhood ALK-positive anaplastic large-cell lymphoma: Results of the ALCL99 study. J. Clin. Oncol. 2011, 29, 4669–4676. [Google Scholar] [CrossRef] [PubMed]
- Arce Vargas, F.; Furness, A.J.S.; Solomon, I.; Joshi, K.; Mekkaoui, L.; Lesko, M.H.; Miranda Rota, E.; Dahan, R.; Georgiou, A.; Sledzinska, A.; et al. Fc-Optimized Anti-CD25 Depletes Tumor-Infiltrating Regulatory T Cells and Synergizes with PD-1 Blockade to Eradicate Established Tumors. Immunity 2017, 46, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, T.A.; Goldman, C.K.; Robb, R.J.; Depper, J.M.; Leonard, W.J.; Sharrow, S.O.; Bongiovanni, K.F.; Korsmeyer, S.J.; Greene, W.C. Expression of interleukin 2 receptors on activated human B cells. J. Exp. Med. 1984, 160, 1450–1466. [Google Scholar] [CrossRef]
- Maggiano, N.; Colotta, F.; Castellino, F.; Ricci, R.; Valitutti, S.; Larocca, L.M.; Musiani, P. Interleukin-2 receptor expression in human mast cells and basophils. Int. Arch. Allergy Appl. Immunol. 1990, 91, 8–14. [Google Scholar] [CrossRef]
- Herrmann, F.; Cannistra, S.A.; Levine, H.; Griffin, J.D. Expression of interleukin 2 receptors and binding of interleukin 2 by gamma interferon-induced human leukemic and normal monocytic cells. J. Exp. Med. 1985, 162, 1111–1116. [Google Scholar] [CrossRef]
- Holter, W.; Goldman, C.K.; Casabo, L.; Nelson, D.L.; Greene, W.C.; Waldmann, T.A. Expression of functional IL 2 receptors by lipopolysaccharide and interferon-gamma stimulated human monocytes. J. Immunol. 1987, 138, 2917–2922. [Google Scholar] [CrossRef]
- Nakase, K.; Kita, K.; Shirakawa, S.; Tanaka, I.; Tsudo, M. Induction of cell surface interleukin 2 receptor alpha chain expression on non-T lymphoid leukemia cells. Leuk. Res. 1994, 18, 855–859. [Google Scholar] [CrossRef]
- Nakase, K.; Kita, K.; Nasu, K.; Ueda, T.; Tanaka, I.; Shirakawa, S.; Tsudo, M. Differential expression of interleukin-2 receptors (alpha and beta chain) in mature lymphoid neoplasms. Am. J. Hematol. 1994, 46, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Gualco, G.; Weiss, L.M.; Harrington, W.J., Jr.; Bacchi, C.E. BCL6, MUM1, and CD10 expression in mantle cell lymphoma. Appl. Immunohistochem. Mol. Morphol. 2010, 18, 103–108. [Google Scholar] [CrossRef]
- Lim, K.H.; Tefferi, A.; Lasho, T.L.; Finke, C.; Patnaik, M.; Butterfield, J.H.; McClure, R.F.; Li, C.Y.; Pardanani, A. Systemic mastocytosis in 342 consecutive adults: Survival studies and prognostic factors. Blood 2009, 113, 5727–5736. [Google Scholar] [CrossRef]
- Ambrosetti, A.; Nadali, G.; Vinante, F.; Ricetti, M.M.; Todeschini, G.; Morosato, L.; de Sabata, D.; Bergamo Andreis, I.A.; Chilosi, M.; Semenzato, G.; et al. Soluble interleukin-2 receptor in hairy-cell leukemia: A reliable marker of disease. Int. J. Clin. Lab. Res. 1993, 23, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, S.; Koyanagi, Y.; Tanaka, Y.; Waki, M.; Matsumoto, A.; Zhou, Y.W.; Yamamoto, M.; Yamamoto, N. Altered interleukin-2 receptor alpha-chain is expressed in human T-cell leukaemia virus type-I-infected T-cell lines and human peripheral blood mononuclear cells of adult T-cell leukaemia patients through an alternative splicing mechanism. Immunology 1997, 91, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Paietta, E.; Racevskis, J.; Neuberg, D.; Rowe, J.M.; Goldstone, A.H.; Wiernik, P.H. Expression of CD25 (interleukin-2 receptor alpha chain) in adult acute lymphoblastic leukemia predicts for the presence of BCR/ABL fusion transcripts: Results of a preliminary laboratory analysis of ECOG/MRC Intergroup Study E2993. Eastern Cooperative Oncology Group/Medical Research Council. Leukemia 1997, 11, 1887–1890. [Google Scholar] [CrossRef] [PubMed]
- Juco, J.; Holden, J.T.; Mann, K.P.; Kelley, L.G.; Li, S. Immunophenotypic analysis of anaplastic large cell lymphoma by flow cytometry. Am. J. Clin. Pathol. 2003, 119, 205–212. [Google Scholar] [CrossRef]
- Liang, H.C.; Costanza, M.; Prutsch, N.; Zimmerman, M.W.; Gurnhofer, E.; Montes-Mojarro, I.A.; Abraham, B.J.; Prokoph, N.; Stoiber, S.; Tangermann, S.; et al. Super-enhancer-based identification of a BATF3/IL-2R-module reveals vulnerabilities in anaplastic large cell lymphoma. Nat. Commun. 2021, 12, 5577. [Google Scholar] [CrossRef]
- Janik, J.E.; Morris, J.C.; Pittaluga, S.; McDonald, K.; Raffeld, M.; Jaffe, E.S.; Grant, N.; Gutierrez, M.; Waldmann, T.A.; Wilson, W.H. Elevated serum-soluble interleukin-2 receptor levels in patients with anaplastic large cell lymphoma. Blood 2004, 104, 3355–3357. [Google Scholar] [CrossRef]
- Lyapichev, K.A.; Tang, G.; Li, S.; You, M.J.; Cheng, T.J.; Miranda, R.N.; Iyer, S.; Yin, C.C.; Konoplev, S.; Bueso-Ramos, C.; et al. MYC expression is associated with older age, common morphology, increased MYC copy number, and poorer prognosis in patients with ALK+ anaplastic large cell lymphoma. Hum. Pathol. 2021, 108, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Medeiros, L.J.; Li, S.; Wang, S.A.; Lin, P.; Khanlari, M.; Iyer, S.P.; Yin, C.C.; Tang, G.; Jorgensen, J.L.; et al. CD8 expression in anaplastic large cell lymphoma correlates with noncommon morphologic variants and T-cell antigen expression suggesting biological differences with CD8-negative anaplastic large cell lymphoma. Hum. Pathol. 2020, 98, 1–9. [Google Scholar] [CrossRef]
- Muzzafar, T.; Wei, E.X.; Lin, P.; Medeiros, L.J.; Jorgensen, J.L. Flow cytometric immunophenotyping of anaplastic large cell lymphoma. Arch. Pathol. Lab. Med. 2009, 133, 49–56. [Google Scholar] [CrossRef]
- Nakase, K.; Kita, K.; Kyo, T.; Ueda, T.; Tanaka, I.; Katayama, N. Prognostic Relevance of Cytokine Receptor Expression in Acute Myeloid Leukemia: Interleukin-2 Receptor alpha-Chain (CD25) Expression Predicts a Poor Prognosis. PLoS ONE 2015, 10, e0128998. [Google Scholar] [CrossRef]
- Nakase, K.; Kita, K.; Otsuji, A.; Anazawa, H.; Hoshino, K.; Sekine, T.; Shirakawa, S.; Tanaka, I.; Nasu, K.; Tsutani, H.; et al. Diagnostic and clinical importance of interleukin-2 receptor alpha chain expression on non-T-cell acute leukaemia cells. Br. J. Haematol. 1992, 80, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Terwijn, M.; Feller, N.; van Rhenen, A.; Kelder, A.; Westra, G.; Zweegman, S.; Ossenkoppele, G.; Schuurhuis, G.J. Interleukin-2 receptor alpha-chain (CD25) expression on leukaemic blasts is predictive for outcome and level of residual disease in AML. Eur. J. Cancer 2009, 45, 1692–1699. [Google Scholar] [CrossRef] [PubMed]
- Gonen, M.; Sun, Z.; Figueroa, M.E.; Patel, J.P.; Abdel-Wahab, O.; Racevskis, J.; Ketterling, R.P.; Fernandez, H.; Rowe, J.M.; Tallman, M.S.; et al. CD25 expression status improves prognostic risk classification in AML independent of established biomarkers: ECOG phase 3 trial, E1900. Blood 2012, 120, 2297–2306. [Google Scholar] [CrossRef]
- Cerny, J.; Yu, H.; Ramanathan, M.; Raffel, G.D.; Walsh, W.V.; Fortier, N.; Shanahan, L.; O’Rourke, E.; Bednarik, J.; Barton, B.; et al. Expression of CD25 independently predicts early treatment failure of acute myeloid leukaemia (AML). Br. J. Haematol. 2013, 160, 262–266. [Google Scholar] [CrossRef]
- Nakase, K.; Kita, K.; Miwa, H.; Nishii, K.; Shikami, M.; Tanaka, I.; Tsutani, H.; Ueda, T.; Nasu, K.; Kyo, T.; et al. Clinical and prognostic significance of cytokine receptor expression in adult acute lymphoblastic leukemia: Interleukin-2 receptor alpha-chain predicts a poor prognosis. Leukemia 2007, 21, 326–332. [Google Scholar] [CrossRef]
- Hapgood, G.; Savage, K.J. The biology and management of systemic anaplastic large cell lymphoma. Blood 2015, 126, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Richardson, N.C.; Kasamon, Y.L.; Chen, H.; de Claro, R.A.; Ye, J.; Blumenthal, G.M.; Farrell, A.T.; Pazdur, R. FDA Approval Summary: Brentuximab Vedotin in First-Line Treatment of Peripheral T-Cell Lymphoma. Oncologist 2019, 24, e180–e187. [Google Scholar] [CrossRef]
- Merino, M.; Kasamon, Y.; Li, H.; Ma, L.; Leong, R.; Zhou, J.; Reaman, G.; Chambers, W.; Richardson, N.; Theoret, M.; et al. FDA approval summary: Crizotinib for pediatric and young adult patients with relapsed or refractory systemic anaplastic large cell lymphoma. Pediatr. Blood Cancer 2022, 69, e29602. [Google Scholar] [CrossRef]
- Ito, M.; Zhao, N.; Zeng, Z.; Zhou, X.; Chang, C.C.; Zu, Y. Interleukin-2 Functions in Anaplastic Large Cell Lymphoma Cells through Augmentation of Extracellular Signal-Regulated Kinases 1/2 Activation. Int. J. Biomed. Sci. 2011, 7, 181–190. [Google Scholar]
- Peuchmaur, M.; Emilie, D.; Crevon, M.C.; Solal-Celigny, P.; Maillot, M.C.; Lemaigre, G.; Galanaud, P. IL-2 mRNA expression in Tac-positive malignant lymphomas. Am. J. Pathol. 1990, 136, 383–390. [Google Scholar]
- Solomon, I.; Amann, M.; Goubier, A.; Arce Vargas, F.; Zervas, D.; Qing, C.; Henry, J.Y.; Ghorani, E.; Akarca, A.U.; Marafioti, T.; et al. CD25-T(reg)-depleting antibodies preserving IL-2 signaling on effector T cells enhance effector activation and antitumor immunity. Nat. Cancer 2020, 1, 1153–1166. [Google Scholar] [CrossRef] [PubMed]
- Emilie, D.; Peuchmaur, M.; Solal-Celigny, P.; Boue, F.; Defraissy, J.F.; Tertian, G.; Dormont, J.; Galanaud, P. ANTI-IL-2 Receptor Antibody Treatment for Lymphoproliferative Disease: Transient Response in A Case of Ki-1+ Anaplastic Large Cell Lymphoma. Leuk. Lymphoma 1991, 3, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, T.A.; Goldman, C.K.; Bongiovanni, K.F.; Sharrow, S.O.; Davey, M.P.; Cease, K.B.; Greenberg, S.J.; Longo, D.L. Therapy of patients with human T-cell lymphotrophic virus I-induced adult T-cell leukemia with anti-Tac, a monoclonal antibody to the receptor for interleukin-2. Blood 1988, 72, 1805–1816. [Google Scholar] [CrossRef] [PubMed]
- Prince, H.M.; Duvic, M.; Martin, A.; Sterry, W.; Assaf, C.; Sun, Y.; Straus, D.; Acosta, M.; Negro-Vilar, A. Phase III placebo-controlled trial of denileukin diftitox for patients with cutaneous T-cell lymphoma. J. Clin. Oncol. 2010, 28, 1870–1877. [Google Scholar] [CrossRef]
- Linden, O. Remission of a refractory, anaplastic large-cell lymphoma after treatment with daclizumab. N. Engl. J. Med. 2004, 351, 1466–1467. [Google Scholar] [CrossRef]
- Costa, V.; Oliva, T.; Norton, L. Successful treatment with daclizumab of refractory anaplastic lymphoma. Pediatr. Blood Cancer 2009, 53, 1130–1131. [Google Scholar] [CrossRef]
- Suzuki, R.; Kagami, Y.; Takeuchi, K.; Kami, M.; Okamoto, M.; Ichinohasama, R.; Mori, N.; Kojima, M.; Yoshino, T.; Yamabe, H.; et al. Prognostic significance of CD56 expression for ALK-positive and ALK-negative anaplastic large-cell lymphoma of T/null cell phenotype. Blood 2000, 96, 2993–3000. [Google Scholar]
- Abramov, D.; Oschlies, I.; Zimmermann, M.; Konovalov, D.; Damm-Welk, C.; Wossmann, W.; Klapper, W. Expression of CD8 is associated with non-common type morphology and outcome in pediatric anaplastic lymphoma kinase-positive anaplastic large cell lymphoma. Haematologica 2013, 98, 1547–1553. [Google Scholar] [CrossRef]


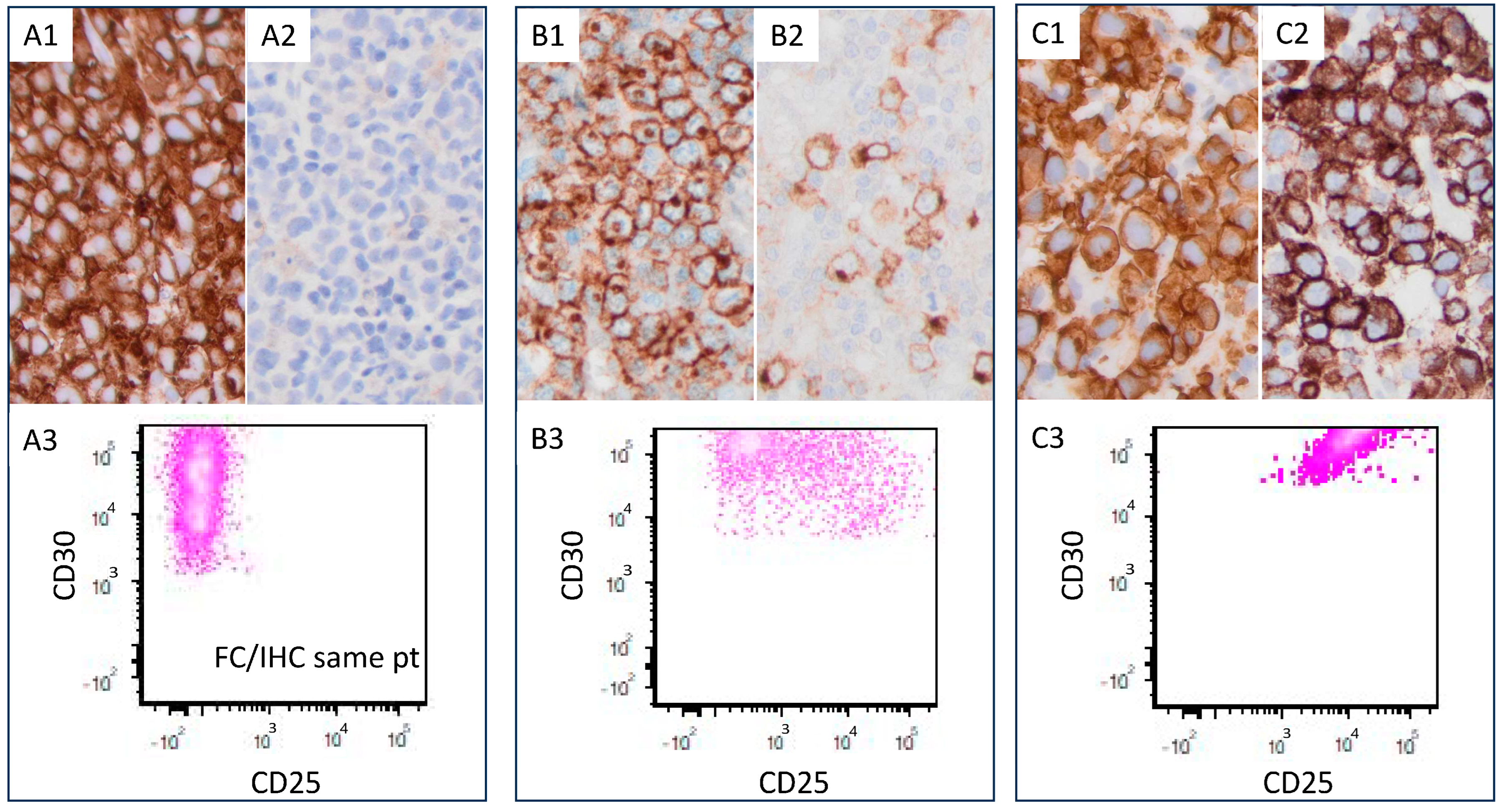

| CD25-High (n = 42) | CD25-Low (n = 12) | p Value | |||||
|---|---|---|---|---|---|---|---|
| Positive | Total Tested | % | Positive | Total Tested | % | ||
| Clinical features (at diagnosis) | |||||||
| Male/female | 25/17 (1.5:1) | 4/8 (0.5:1) | 0.19 | ||||
| Age (median, range) | 29 (5–58) | 40 (9–74) | 0.01 | ||||
| Nodal presentation | 36 | 42 | 86 | 7 | 11 | 64 | 0.19 |
| Extra-nodal involvement | 23 | 38 | 61 | 6 | 8 | 75 | 0.69 |
| BM+ | 8 | 36 | 22 | 3 | 7 | 43 | 0.35 |
| PB+ | 1 | 8 | 13 | 1 | 3 | 33 | 0.49 |
| Stage III–IV | 26 | 36 | 72 | 7 | 8 | 88 | 0.66 |
| B symptoms | 20 | 33 | 61 | 6 | 7 | 86 | 0.39 |
| Laboratory at diagnosis | |||||||
| Elevated WBC (>11.0 × 109/L) | 9 | 26 | 35 | 4 | 5 | 80 | 0.13 |
| Absolute lymphocytosis (>4.8 × 109/L) | 2 | 24 | 8 | 1 | 5 | 20 | 0.45 |
| Absolute lymphopenia (<1.0 × 109/L) | 4 | 24 | 17 | 1 | 5 | 20 | 1 |
| Anemia (M < 14; F < 12 g/dL) | 18 | 26 | 69 | 4 | 5 | 80 | 1 |
| Thrombocytopenia (<140 × 109/L) | 0 | 26 | 0 | 2 | 5 | 40 | 0.02 |
| Elevated LDH * | 11 | 23 | 48 | 3 | 5 | 60 | 1 |
| IPI ≥ 3 | 6 | 28 | 21 | 0 | 3 | 0 | 1 |
| Initial treatment | |||||||
| CHOP or modified CHOP | 26 | 37 | 70 | 6 | 6 | 100 | 0.31 |
| Other treatment (other chemo, or RT) | 11 | 37 | 30 | 0 | 6 | 0 | |
| Initial CR | 31 | 36 | 86 | 4 | 6 | 67 | 0.26 |
| SCT performed | 13 | 31 | 42 | 3 | 7 | 43 | 1 |
| Outcome | |||||||
| Alive | 29 | 38 | 76 | 4 | 9 | 44 | 0.1 |
| Dead | 9 | 38 | 24 | 5 | 9 | 56 | |
| OS (months) (median, range) | 40.0 (0–382.8) | 14.1 (0.3–108.1) | 0.02 | ||||
| CD25-High (n = 42) | CD25-Low (n = 12) | p Value | |||||
|---|---|---|---|---|---|---|---|
| Positive | Total Tested | % | Positive | Total Tested | % | ||
| Morphology | |||||||
| Common pattern | 34 | 42 | 81 | 4 | 8 | 50 | 0.08 |
| SC/LH pattern | 8 | 42 | 19 | 4 | 8 | 50 | |
| Immunophenotype | |||||||
| CD2+ | 16 | 32 | 50 | 7 | 8 | 88 | 0.11 |
| Surface CD3+ | 1 | 33 | 3 | 6 | 6 | 100 | 0.001 |
| CD4+ | 26 | 33 | 79 | 6 | 8 | 75 | 1 |
| CD8+ | 4 | 28 | 14 | 4 | 7 | 57 | 0.03 |
| CD4+CD8+ | 2 | 27 | 7 | 2 | 7 | 29 | 0.18 |
| CD5+ | 9 | 31 | 29 | 6 | 9 | 67 | 0.053 |
| CD7+ | 7 | 20 | 35 | 4 | 7 | 57 | 0.39 |
| CD15+ | 1 | 18 | 6 | 0 | 5 | 0 | 1 |
| CD43+ | 17 | 20 | 85 | 4 | 6 | 67 | 0.56 |
| CD45+ | 23 | 29 | 79 | 7 | 8 | 88 | 1 |
| CD52+ | 5 | 7 | 71 | 1 | 1 | 100 | 1 |
| CD56+ | 4 | 14 | 29 | 1 | 3 | 33 | 1 |
| BCL2+ | 1 | 9 | 11 | 1 | 4 | 25 | 1 |
| EMA+ | 16 | 16 | 100 | 7 | 8 | 88 | 0.33 |
| Granzyme B+ | 10 | 11 | 91 | 3 | 4 | 75 | 0.48 |
| TIA1+ | 7 | 7 | 100 | 2 | 2 | 100 | 1 |
| TCR A/B+ | 6 | 10 | 60 | 3 | 4 | 75 | 1 |
| TCR G/D+ | 0 | 9 | 0 | 0 | 4 | 0 | 1 |
| Mean Ki67 (%) | 85 | 81 | 0.55 | ||||
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| p Value | HR | 95% CI | p Value | HR | 95% CI | |
| Age ≥ 30 years old | 0.09 | 2.67 | 0.90–7.94 | 0.16 | 2.40 | 0.72–8.10 |
| Low CD25 expression | 0.02 | 3.41 | 0.76–15.33 | 0.15 | 2.34 | 0.73–7.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
E, S.; Medeiros, L.J.; Fang, H.; Li, S.; Tang, G.; Wang, S.A.; Wang, W.; Yin, C.C.; You, M.J.; Iyer, S.P.; et al. Low CD25 in ALK+ Anaplastic Large Cell Lymphoma Is Associated with Older Age, Thrombocytopenia, and Increased Expression of Surface CD3 and CD8. Cancers 2025, 17, 1767. https://doi.org/10.3390/cancers17111767
E S, Medeiros LJ, Fang H, Li S, Tang G, Wang SA, Wang W, Yin CC, You MJ, Iyer SP, et al. Low CD25 in ALK+ Anaplastic Large Cell Lymphoma Is Associated with Older Age, Thrombocytopenia, and Increased Expression of Surface CD3 and CD8. Cancers. 2025; 17(11):1767. https://doi.org/10.3390/cancers17111767
Chicago/Turabian StyleE, Shuyu, L. Jeffrey Medeiros, Hong Fang, Shaoying Li, Guilin Tang, Sa A. Wang, Wei Wang, C. Cameron Yin, M. James You, Swaminathan P. Iyer, and et al. 2025. "Low CD25 in ALK+ Anaplastic Large Cell Lymphoma Is Associated with Older Age, Thrombocytopenia, and Increased Expression of Surface CD3 and CD8" Cancers 17, no. 11: 1767. https://doi.org/10.3390/cancers17111767
APA StyleE, S., Medeiros, L. J., Fang, H., Li, S., Tang, G., Wang, S. A., Wang, W., Yin, C. C., You, M. J., Iyer, S. P., Malpica, L., Qiu, L., Tang, Z., Wei, Q., Lin, P., & Xu, J. (2025). Low CD25 in ALK+ Anaplastic Large Cell Lymphoma Is Associated with Older Age, Thrombocytopenia, and Increased Expression of Surface CD3 and CD8. Cancers, 17(11), 1767. https://doi.org/10.3390/cancers17111767








