Microvascular Complications and Cancer Risk in Type 2 Diabetes: A Population-Based Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
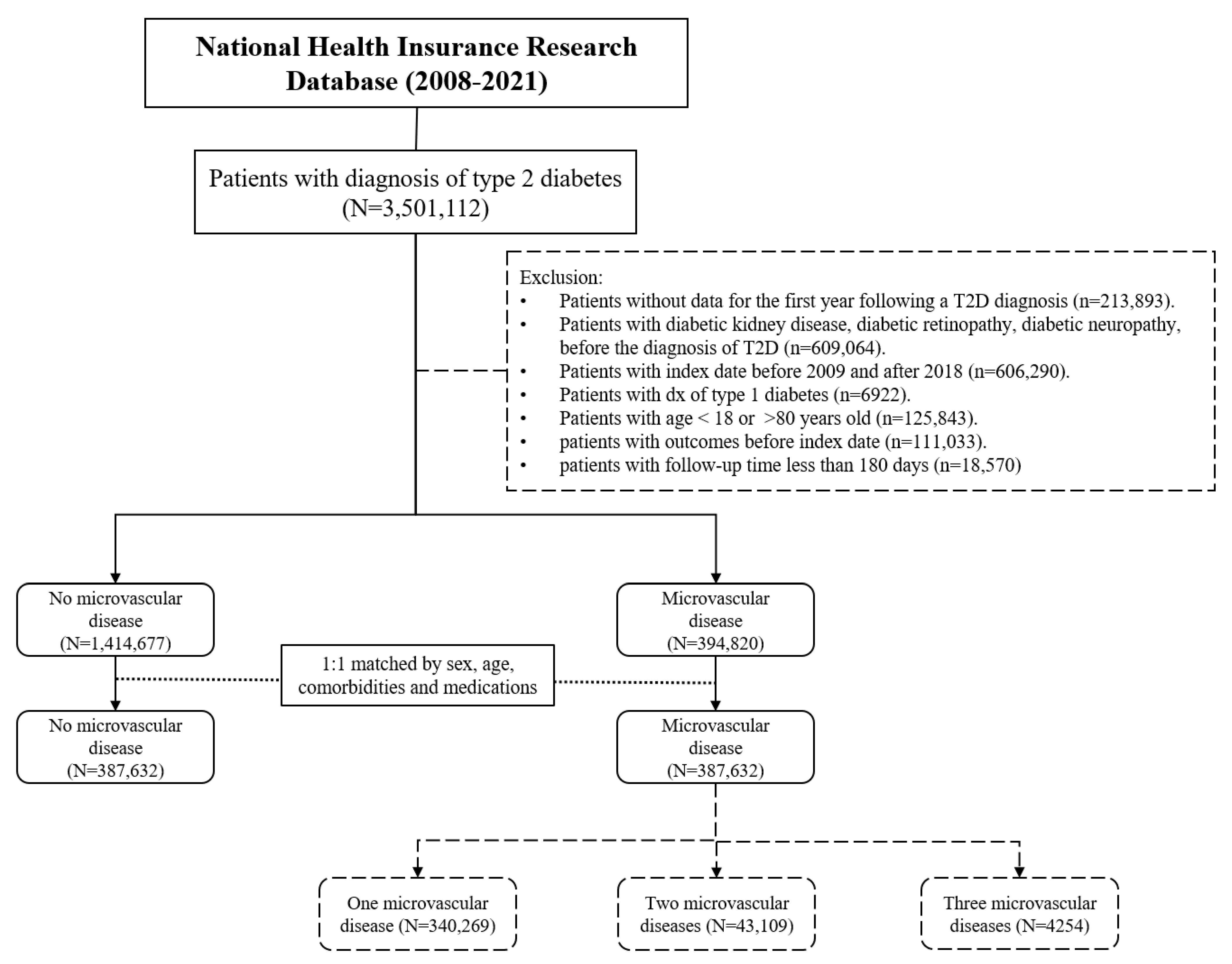
2.1. Study Population and Data Source
2.2. Study Procedures
2.3. Baseline Characteristics and Medications
2.4. Main Outcomes of Interest
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| T2D | Type 2 diabetes |
| DM | Diabetes mellitus |
| CKD | Chronic kidney disease |
| DR | Diabetic retinopathy |
| DN | Diabetic neuropathy |
References
- Yen, F.S.; Wei, J.C.; Chiu, L.T.; Hsu, C.C.; Hwu, C.M. Diabetes, hypertension, and cardiovascular disease development. J. Transl. Med. 2022, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- Yen, F.S.; Wei, J.C.; Yip, H.T.; Hwu, C.M.; Hsu, C.C. Diabetes, Hypertension, and the Risk of Dementia. J. Alzheimer’s Dis. 2022, 89, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Yen, F.S.; Wei, J.C.; Shih, Y.H.; Hsu, C.C.; Hwu, C.M. The Risk of Nephropathy, Retinopathy, and Leg Amputation in Patients with Diabetes and Hypertension: A Nationwide, Population-Based Retrospective Cohort Study. Front. Endocrinol. 2021, 12, 756189. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Yen, F.S.; Lin, K.D.; Shin, S.J.; Hsu, Y.H.; Hsu, C.C. Diabetes Kidney Disease Research Committee of the Diabetes Association of the Republic of China (Taiwan). Epidemiological characteristics of diabetic kidney disease in Taiwan. J. Diabetes Investig. 2021, 12, 2112–2123. [Google Scholar] [CrossRef]
- Yen, F.S.; Wei, J.C.; Shih, Y.H.; Hsu, C.C.; Hwu, C.M. Impact of individual microvascular disease on the risks of macrovascular complications in type 2 diabetes: A nationwide population-based cohort study. Cardiovasc. Diabetol. 2023, 22, 109. [Google Scholar] [CrossRef]
- Yen, Y.H.; Yen, F.S.; Ko, F.S.; Wei, J.C.; Huang, Y.; Yu, T.; Hwu, C.; Hsu, C. Microvascular disease and its association with dementia in patients with type 2 diabetes: A nationwide cohort study in Taiwan. Diabetes Obes. Metab. 2024, 26, 5399–5407. [Google Scholar] [CrossRef]
- Faselis, C.; Katsimardou, A.; Imprialos, K.; Deligkaris, P.; Kallistratos, M.; Dimitriadis, K. Microvascular Complications of Type 2 Diabetes Mellitus. Curr. Vasc. Pharmacol. 2020, 18, 117–124. [Google Scholar] [CrossRef]
- Chang, W.C.; Hsieh, T.C.; Hsu, W.L.; Chang, F.L.; Tsai, H.R.; He, M.S. Diabetes and further risk of cancer: A nationwide population-based study. BMC Med. 2024, 22, 214. [Google Scholar] [CrossRef]
- Bonagiri, P.R.; Shubrook, J.H. Review of Associations Between Type 2 Diabetes and Cancer. Clin. Diabetes 2020, 38, 256–265. [Google Scholar] [CrossRef]
- Noto, H.; Tsujimoto, T.; Sasazuki, T.; Noda, M. Significantly increased risk of cancer in patients with diabetes mellitus: A systematic review and meta-analysis. Endocr. Pract. 2011, 17, 616–628. [Google Scholar] [CrossRef]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Ryu, T.Y.; Park, J.; Scherer, P.E. Hyperglycemia as a Risk Factor for Cancer Progression. Diabetes Metab. J. 2014, 38, 330–336. [Google Scholar] [CrossRef]
- Arcidiacono, B.; Iiritano, S.; Nocera, A.; Possidente, K.; Nevolo, M.T.; Ventura, V.; Foti, D.; Chiefari, E.; Brunetti, A. Insulin Resistance and Cancer Risk: An Overview of the Pathogenetic Mechanisms. Exp. Diabetes Res. 2012, 2012, 789174. [Google Scholar] [CrossRef]
- Fowler, M.J. Microvascular and Macrovascular Complications of Diabetes. Clin. Diabetes 2008, 26, 77–82. [Google Scholar] [CrossRef]
- Tabassum, D.P.; Polyak, K. Tumorigenesis: It takes a village. Nat. Rev. Cancer 2015, 15, 473–483. [Google Scholar] [CrossRef]
- Zhang, S.; Xiao, X.; Yi, Y.; Wang, X.; Zhu, L.; Shen, Y.; Lin, D.; Wu, C. Tumor initiation and early tumorigenesis: Molecular mechanisms and interventional targets. Signal Transduct. Target. Ther. 2024, 9, 149. [Google Scholar] [CrossRef]
- Tseng, Y.; Tsan, Y.; Chen, P. Association between severity of diabetic complications and risk of cancer in middle-aged patients with type 2 diabetes. J. Diabetes Investig. 2024, 16, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel, P. Inflammation in end-stage renal disease: The hidden enemy. Nephrology 2006, 11, 36–41. [Google Scholar] [CrossRef]
- Antonetti, D.A.; Barber, A.J.; Bronson, S.K.; Freeman, W.M.; Gardner, T.W.; Jefferson, L.S.; Kester, M.; Kimball, S.R.; Krady, J.K.; LaNoue, K.F.; et al. JDRF Diabetic Retinopathy Center Group. Diabetic retinopathy: Seeing beyond glucose-induced microvascular disease. Diabetes 2006, 55, 2401–2411. [Google Scholar] [CrossRef]
- Yen, F.S.; Wei, J.C.; Wang, C.; Hou, M.C.; Yu, T.S.; Huang, Y.; Hwu, C.M.; Hsu, C.C. Sodium-Glucose Cotransporter2 inhibitors and associated Liver-Related outcomes in diabetes patients. Diabetes Res. Clin. Pract. 2025, 223, 112174. [Google Scholar] [CrossRef]
- Sung, S.F.; Hsieh, C.Y.; Lin, H.J.; Chen, Y.W.; Yang, Y.H.K.; Li, C.Y. Validation of algorithms to identify stroke risk factors in patients with acute ischemic stroke, transient ischemic attack, or intracerebral hemorrhage in an administrative claims database. Int. J. Cardiol. 2016, 215, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Meduru, P.; Helmer, D.; Rajan, M.; Tseng, C.L.; Pogach, L.; Sambamoorthi, U. Chronic illness with complexity: Implications for performance measurement of optimal glycemic control. J. Gen. Intern. Med. 2007, 22 (Suppl. S3), 408–418. [Google Scholar] [CrossRef]
- Yap, J.; Anbalakan, K.; Tay, W.T.; Ting, D.; Cheung, C.Y.; Sabanayagam, C.; Cheng, C.-Y.; Wong, T.-Y.; Yeo, K.K. Impact of type 2 diabetes and microvascular complications on mortality and cardiovascular outcomes in a multiethnic Asian population. BMJ Open Diabetes Res. Care 2021, 9, e001413. [Google Scholar] [CrossRef]
- Fadini, G.P.; Albiero, M.; Bonora, B.M.; Avogaro, A. Angiogenic Abnormalities in Diabetes Mellitus: Mechanistic and Clinical Aspects. J. Clin. Endocrinol. Metab. 2019, 104, 5431–5444. [Google Scholar] [CrossRef]
- Cheng, R.; Ma, J.-X. Angiogenesis in Diabetes and Obesity. Rev. Endocr. Metab. Disord. 2015, 16, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Moriya, J.; Minamino, T. Angiogenesis, Cancer, and Vascular Aging. Front. Cardiovasc. Med. 2017, 4, 65. [Google Scholar] [CrossRef]
- Wei, X.; Chen, Y.; Jiang, X.; Peng, M.; Liu, Y.; Mo, Y.; Ren, D.; Hua, Y.; Yu, B.; Zhou, Y.; et al. Mechanisms of vasculogenic mimicry in hypoxic tumor microenvironments. Mol. Cancer 2021, 20, 7. [Google Scholar] [CrossRef]
- Sivakumar, B.; Harry, L.E.; Paleolog, E.M. Modulating Angiogenesis: More vs Less. JAMA 2004, 292, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Martín-Timón, I.; Sevillano-Collantes, C.; Segura-Galindo, A.; del Cañizo-Gómez, F.J. Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength? World J. Diabetes 2014, 5, 444–470. [Google Scholar] [CrossRef]
- Risk Factors: Age—NCI. Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk/age (accessed on 23 February 2025).
- Godsland, I.F. Insulin resistance and hyperinsulinaemia in the development and progression of cancer. Clin. Sci. 2009, 118, 315–332. [Google Scholar] [CrossRef]
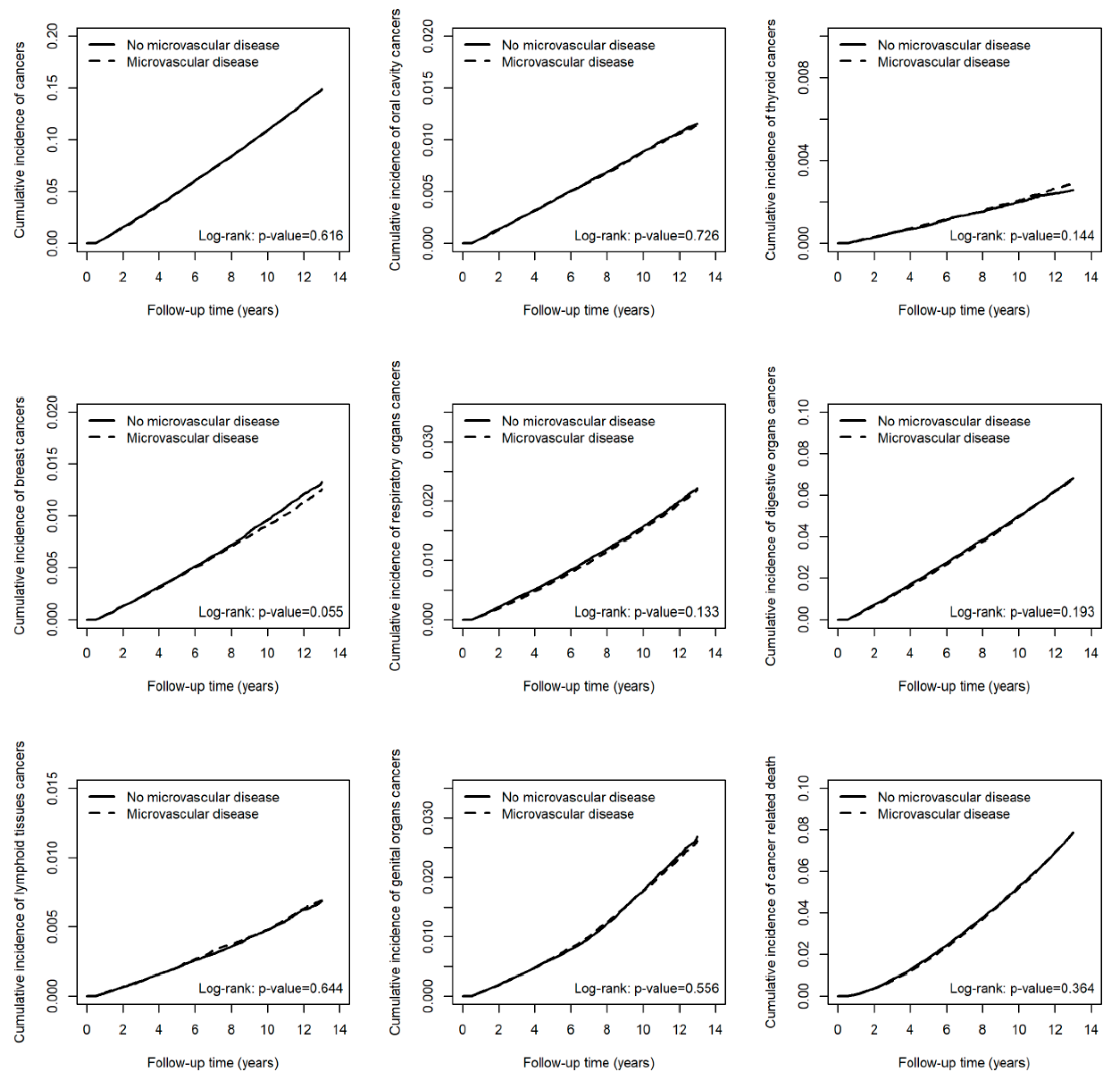
| Exposure | All Cancers | cHR | (95% CI) | p-Value | aHR † | (95% CI) | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|
| n | PY | IR | |||||||
| No microvascular disease | 38,335 | 3,316,979.77 | 11.56 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Microvascular disease | 37,380 | 3,255,697.38 | 11.48 | 1.00 | (0.98, 1.01) | 0.616 | 1.00 | (0.98, 1.01) | 0.695 |
| Exposure | Oral cavity cancers | cHR | (95% CI) | p-value | aHR † | (95% CI) | p-value | ||
| n | PY | IR | |||||||
| No microvascular disease | 2999 | 3,418,211.78 | 0.88 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Microvascular disease | 2913 | 3,355,570.42 | 0.87 | 0.99 | (0.94, 1.04) | 0.726 | 0.99 | (0.94, 1.05) | 0.807 |
| Exposure | Thyroid cancers | cHR | (95% CI) | p-value | aHR † | (95% CI) | p-value | ||
| n | PY | IR | |||||||
| No microvascular disease | 667 | 3,424,998.77 | 0.19 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Microvascular disease | 707 | 3,362,022.27 | 0.21 | 1.08 | (0.97, 1.20) | 0.144 | 1.07 | (0.96, 1.19) | 0.204 |
| Exposure | Breast cancers | cHR | (95% CI) | p-value | aHR † | (95% CI) | p-value | ||
| n | PY | IR | |||||||
| No microvascular disease | 3279 | 3,413,642.23 | 0.96 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Microvascular disease | 3060 | 3,351,524.49 | 0.91 | 0.95 | (0.91, 1.00) | 0.055 | 0.95 | (0.91, 1.00) | 0.058 |
| Exposure | Respiratory organ cancer | cHR | (95% CI) | p-value | aHR † | (95% CI) | p-value | ||
| n | PY | IR | |||||||
| No microvascular disease | 5462 | 3,418,239.84 | 1.60 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Microvascular disease | 5190 | 3,355,484.97 | 1.55 | 0.97 | (0.94, 1.01) | 0.133 | 0.97 | (0.93, 1.01) | 0.112 |
| Exposure | Digestive organ cancers | cHR | (95% CI) | p-value | aHR † | (95% CI) | p-value | ||
| n | PY | IR | |||||||
| No microvascular disease | 17,305 | 3,384,166.79 | 5.11 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Microvascular disease | 16,709 | 3,323,144.91 | 5.03 | 0.99 | (0.97, 1.01) | 0.194 | 0.99 | (0.97, 1.01) | 0.286 |
| Exposure | Lymphoid tissue cancers | cHR | (95% CI) | p-value | aHR † | (95% CI) | p-value | ||
| n | PY | IR | |||||||
| No microvascular disease | 1672 | 3,423,921.91 | 0.49 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Microvascular disease | 1662 | 3,361,202.11 | 0.49 | 1.02 | (0.95, 1.09) | 0.644 | 1.02 | (0.95, 1.09) | 0.651 |
| Exposure | Genital organ cancers | cHR | (95% CI) | p-value | aHR † | (95% CI) | p-value | ||
| n | PY | IR | |||||||
| No microvascular disease | 6169 | 3,407,260.84 | 1.81 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Microvascular disease | 5958 | 3,344,958.26 | 1.78 | 0.99 | (0.95, 1.03) | 0.556 | 0.99 | (0.96, 1.03) | 0.597 |
| Exposure | Cancer-related death | cHR | (95% CI) | p-value | aHR † | (95% CI) | p-value | ||
| n | PY | IR | |||||||
| No microvascular disease | 18,694 | 3,427,998.00 | 5.45 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Microvascular disease | 18,066 | 3,365,206.49 | 5.37 | 0.99 | (0.97, 1.01) | 0.364 | 1.00 | (0.98, 1.02) | 0.695 |
| Exposure | All-cause mortality | cHR | (95% CI) | p-value | aHR † | (95% CI) | p-value | ||
| n | PY | IR | |||||||
| No microvascular disease | 88,676 | 3,427,998.00 | 25.87 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Microvascular disease | 103,547 | 3,365,206.49 | 30.77 | 1.20 | (1.18, 1.21) | <0.001 | 1.21 | (1.20, 1.22) | <0.001 |
| Exposure | All Cancers | cHR | (95% CI) | p-Value | aHR † | (95% CI) | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|
| n | PY | IR | |||||||
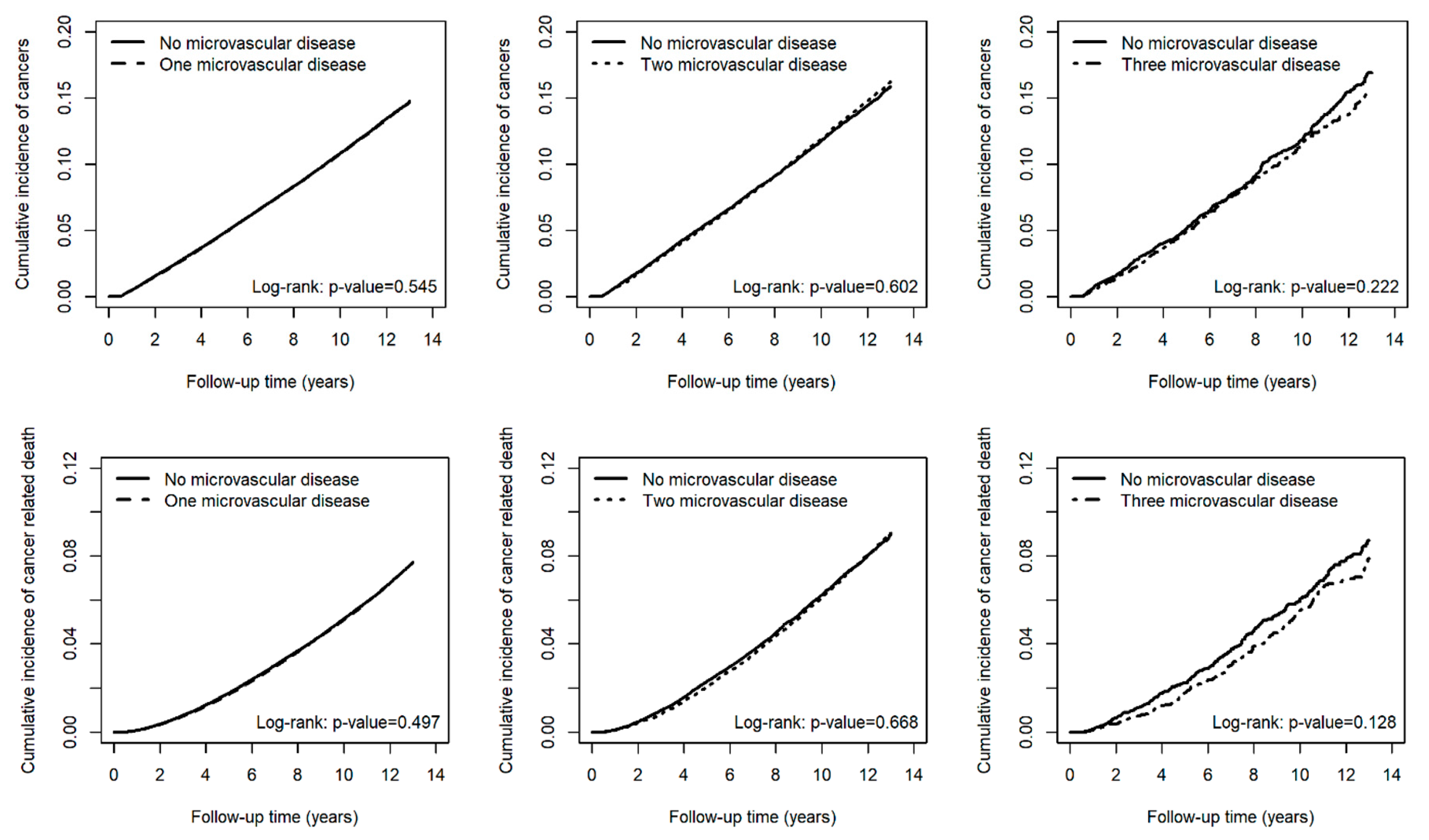
| No microvascular disease (N = 340,269) | 33,405 | 2,923,248.64 | 11.43 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| One microvascular disease (N = 340,269) | 32,494 | 2,865,864.22 | 11.34 | 1.00 | (0.98, 1.01) | 0.545 | 0.99 | (0.98, 1.01) | 0.39 |
| No microvascular disease (N = 43,109) | 4492 | 359,819.98 | 12.48 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Two microvascular diseases (N = 43,109) | 4490 | 356,397.57 | 12.60 | 1.01 | (0.97, 1.05) | 0.602 | 1.02 | (0.98, 1.07) | 0.274 |
| No microvascular disease (N = 4254) | 438 | 33,911.15 | 12.92 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Three microvascular diseases (N = 4254) | 396 | 33,435.58 | 11.84 | 0.92 | (0.80, 1.05) | 0.223 | 0.99 | (0.86, 1.14) | 0.862 |
| Exposure | Oral cavity cancers | cHR | (95% CI) | p-value | aHR † | (95% CI) | p-value | ||
| n | PY | IR | |||||||
| No microvascular disease (N = 340,269) | 2607 | 3,012,368.23 | 0.87 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| One microvascular disease (N = 340,269) | 2547 | 2,952,872.32 | 0.86 | 1.00 | (0.95, 1.05) | 0.946 | 1.00 | (0.95, 1.06) | 0.984 |
| No microvascular disease (N = 43,109) | 359 | 370,937.52 | 0.97 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Two microvascular diseases (N = 43,109) | 329 | 368,167.73 | 0.89 | 0.92 | (0.80, 1.07) | 0.303 | 0.93 | (0.80, 1.08) | 0.359 |
| No microvascular disease (N = 4254) | 33 | 34,906.02 | 0.95 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Three microvascular diseases (N = 4254) | 37 | 34,530.38 | 1.07 | 1.13 | (0.71, 1.81) | 0.605 | 1.15 | (0.71, 1.87) | 0.574 |
| Exposure | Thyroid cancers | cHR | (95% CI) | p-value | aHR † | (95% CI) | p-value | ||
| n | PY | IR | |||||||
| No microvascular disease (N = 340,269) | 611 | 3,018,303.70 | 0.20 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| One microvascular disease (N = 340,269) | 629 | 2,958,586.78 | 0.21 | 1.05 | (0.94, 1.18) | 0.367 | 1.04 | (0.93, 1.16) | 0.477 |
| No microvascular disease (N = 43,109) | 51 | 371,709.52 | 0.14 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Two microvascular diseases (N = 43,109) | 69 | 368,836.10 | 0.19 | 1.37 | (0.95, 1.96) | 0.092 | 1.38 | (0.96, 1.99) | 0.080 |
| No microvascular disease (N = 4254) | 5 | 34,985.55 | 0.14 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Three microvascular diseases (N = 4254) | 9 | 34,599.39 | 0.26 | 1.83 | (0.61, 5.46) | 0.279 | 1.76 | (0.55, 5.64) | 0.34 |
| Exposure | Breast cancers | cHR | (95% CI) | p-value | aHR † | (95% CI) | p-value | ||
| n | PY | IR | |||||||
| No microvascular disease (N = 340,269) | 2870 | 3,008,467.58 | 0.95 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| One microvascular disease (N = 340,269) | 2688 | 2,949,312.20 | 0.91 | 0.96 | (0.91, 1.01) | 0.11 | 0.96 | (0.91, 1.01) | 0.107 |
| No microvascular disease (N = 43,109) | 374 | 370,330.20 | 1.01 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Two microvascular diseases (N = 43,109) | 340 | 367,735.82 | 0.92 | 0.92 | (0.79, 1.06) | 0.242 | 0.91 | (0.78, 1.05) | 0.205 |
| No microvascular disease (N = 4254) | 35 | 34,844.45 | 1.00 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Three microvascular diseases (N = 4254) | 32 | 34,476.47 | 0.93 | 0.93 | (0.57, 1.50) | 0.757 | 0.99 | (0.61, 1.61) | 0.964 |
| Exposure | Respiratory organ cancer | cHR | (95% CI) | p-value | aHR † | (95% CI) | p-value | ||
| n | PY | IR | |||||||
| No microvascular disease (N = 340,269) | 4772 | 3,012,458.33 | 1.58 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| One microvascular disease (N = 340,269) | 4530 | 2,952,831.52 | 1.53 | 0.97 | (0.93, 1.01) | 0.17 | 0.97 | (0.93, 1.01) | 0.087 |
| No microvascular disease (N = 43,109) | 623 | 370,872.46 | 1.68 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Two microvascular diseases (N = 43,109) | 613 | 368,098.22 | 1.67 | 0.99 | (0.89, 1.11) | 0.912 | 1.02 | (0.91, 1.14) | 0.712 |
| No microvascular disease (N = 4254) | 67 | 34,909.05 | 1.92 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Three microvascular diseases (N = 4254) | 47 | 34,555.22 | 1.36 | 0.71 | (0.49, 1.04) | 0.078 | 0.89 | (0.61, 1.30) | 0.552 |
| Exposure | Digestive organ cancers | cHR | (95% CI) | p-value | aHR † | (95% CI) | p-value | ||
| n | PY | IR | |||||||
| No microvascular disease (N = 340,269) | 15,022 | 2,982,745.40 | 5.04 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| One microvascular disease (N = 340,269) | 14,448 | 2,925,036.74 | 4.94 | 0.98 | (0.96, 1.01) | 0.159 | 0.98 | (0.96, 1.01) | 0.142 |
| No microvascular disease (N = 43,109) | 2081 | 366,869.63 | 5.67 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Two microvascular diseases (N = 43,109) | 2080 | 363,960.89 | 5.71 | 1.01 | (0.95, 1.07) | 0.775 | 1.02 | (0.96, 1.09) | 0.452 |
| No microvascular disease (N = 4254) | 202 | 34,551.76 | 5.85 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Three microvascular diseases (N = 4254) | 181 | 34,147.28 | 5.30 | 0.91 | (0.74, 1.11) | 0.337 | 0.94 | (0.76, 1.16) | 0.552 |
| Exposure | Lymphoid tissue cancers | cHR | (95% CI) | p-value | aHR † | (95% CI) | p-value | ||
| n | PY | IR | |||||||
| No microvascular disease (N = 340,269) | 1474 | 3,017,412.92 | 0.49 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| One microvascular disease (N = 340,269) | 1431 | 2,957,979.29 | 0.48 | 0.99 | (0.92, 1.07) | 0.868 | 0.99 | (0.92, 1.07) | 0.811 |
| No microvascular disease (N = 43,109) | 178 | 371,546.45 | 0.48 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Two microvascular diseases (N = 43,109) | 214 | 368,630.99 | 0.58 | 1.21 | (0.99, 1.48) | 0.056 | 1.24 | (1.02, 1.52) | 0.033 |
| No microvascular disease (N = 4254) | 20 | 34,962.54 | 0.57 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Three microvascular diseases (N = 4254) | 17 | 34,591.82 | 0.49 | 0.88 | (0.46, 1.69) | 0.707 | 1.01 | (0.52, 1.96) | 0.968 |
| Exposure | Genital organ cancers | cHR | (95% CI) | p-value | aHR † | (95% CI) | p-value | ||
| n | PY | IR | |||||||
| No microvascular disease (N = 340,269) | 5401 | 3,002,916.16 | 1.80 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| One microvascular disease (N = 340,269) | 5290 | 2,943,288.52 | 1.80 | 1.01 | (0.97, 1.04) | 0.782 | 1.00 | (0.97, 1.04) | 0.856 |
| No microvascular disease (N = 43,109) | 704 | 369,563.22 | 1.90 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Two microvascular diseases (N = 43,109) | 625 | 367,172.57 | 1.70 | 0.90 | (0.81, 1.00) * | 0.048 | 0.91 | (0.82, 1.02) | 0.101 |
| No microvascular disease (N = 4254) | 64 | 34,781.46 | 1.84 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Three microvascular diseases (N = 4254) | 43 | 34,497.18 | 1.25 | 0.68 | (0.46, 1.00) * | 0.048 | 0.74 | (0.49, 1.10) | 0.131 |
| Exposure | Cancer-related death | cHR | (95% CI) | p-value | aHR † | (95% CI) | p-value | ||
| n | PY | IR | |||||||
| No microvascular disease (N = 340,269) | 16,105 | 3,021,083.08 | 5.33 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| One microvascular disease (N = 340,269) | 15,561 | 2,961,395.35 | 5.25 | 0.99 | (0.97, 1.01) | 0.497 | 0.99 | (0.97, 1.02) | 0.558 |
| No microvascular disease (N = 43,109) | 2372 | 371,912.77 | 6.38 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Two microvascular diseases (N = 43,109) | 2320 | 369,175.82 | 6.28 | 0.99 | (0.93, 1.05) | 0.668 | 1.01 | (0.95, 1.07) | 0.814 |
| No microvascular disease (N = 4254) | 217 | 35,002.15 | 6.20 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Three microvascular diseases (N = 4254) | 185 | 34,635.31 | 5.34 | 0.86 | (0.71, 1.05) | 0.129 | 0.99 | (0.81, 1.22) | 0.943 |
| Exposure | All-cause mortality | cHR | (95% CI) | p-value | aHR † | (95% CI) | p-value | ||
| n | PY | IR | |||||||
| No microvascular disease (N = 340,269) | 74,983 | 3,021,083.08 | 24.82 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| One microvascular disease (N = 340,269) | 84,043 | 2,961,395.35 | 28.38 | 1.15 | (1.14, 1.16) | <0.001 | 1.16 | (1.15, 1.17) | <0.001 |
| No microvascular disease (N = 43,109) | 12,300 | 371,912.77 | 33.07 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Two microvascular diseases (N = 43,109) | 17,214 | 369,175.82 | 46.63 | 1.41 | (1.38, 1.45) | <0.001 | 1.42 | (1.38, 1.45) | <0.001 |
| No microvascular disease (N = 4254) | 1393 | 35,002.15 | 39.80 | 1.00 | (reference) | - | 1.00 | (reference) | - |
| Three microvascular diseases (N = 4254) | 2290 | 34,635.31 | 66.12 | 1.66 | (1.56, 1.78) | <0.001 | 1.71 | (1.60, 1.83) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yen, Y.-H.; Wei, J.C.-C.; Yen, F.-S.; Kao, Y.-S.; Lin, H.-J.; Cho, D.-Y.; Hwu, C.-M.; Hsu, C.-C. Microvascular Complications and Cancer Risk in Type 2 Diabetes: A Population-Based Study. Cancers 2025, 17, 1760. https://doi.org/10.3390/cancers17111760
Yen Y-H, Wei JC-C, Yen F-S, Kao Y-S, Lin H-J, Cho D-Y, Hwu C-M, Hsu C-C. Microvascular Complications and Cancer Risk in Type 2 Diabetes: A Population-Based Study. Cancers. 2025; 17(11):1760. https://doi.org/10.3390/cancers17111760
Chicago/Turabian StyleYen, Yu-Hsin, James Cheng-Chung Wei, Fu-Shun Yen, Yung-Shuo Kao, Heng-Jun Lin, Der-Yang Cho, Chii-Min Hwu, and Chih-Cheng Hsu. 2025. "Microvascular Complications and Cancer Risk in Type 2 Diabetes: A Population-Based Study" Cancers 17, no. 11: 1760. https://doi.org/10.3390/cancers17111760
APA StyleYen, Y.-H., Wei, J. C.-C., Yen, F.-S., Kao, Y.-S., Lin, H.-J., Cho, D.-Y., Hwu, C.-M., & Hsu, C.-C. (2025). Microvascular Complications and Cancer Risk in Type 2 Diabetes: A Population-Based Study. Cancers, 17(11), 1760. https://doi.org/10.3390/cancers17111760







