Comparison of Efficacy and Safety of Cryotherapy in Chemotherapy-Induced Peripheral Neuropathy Between Upper and Lower Extremities: Results from the Randomized CROPSI Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
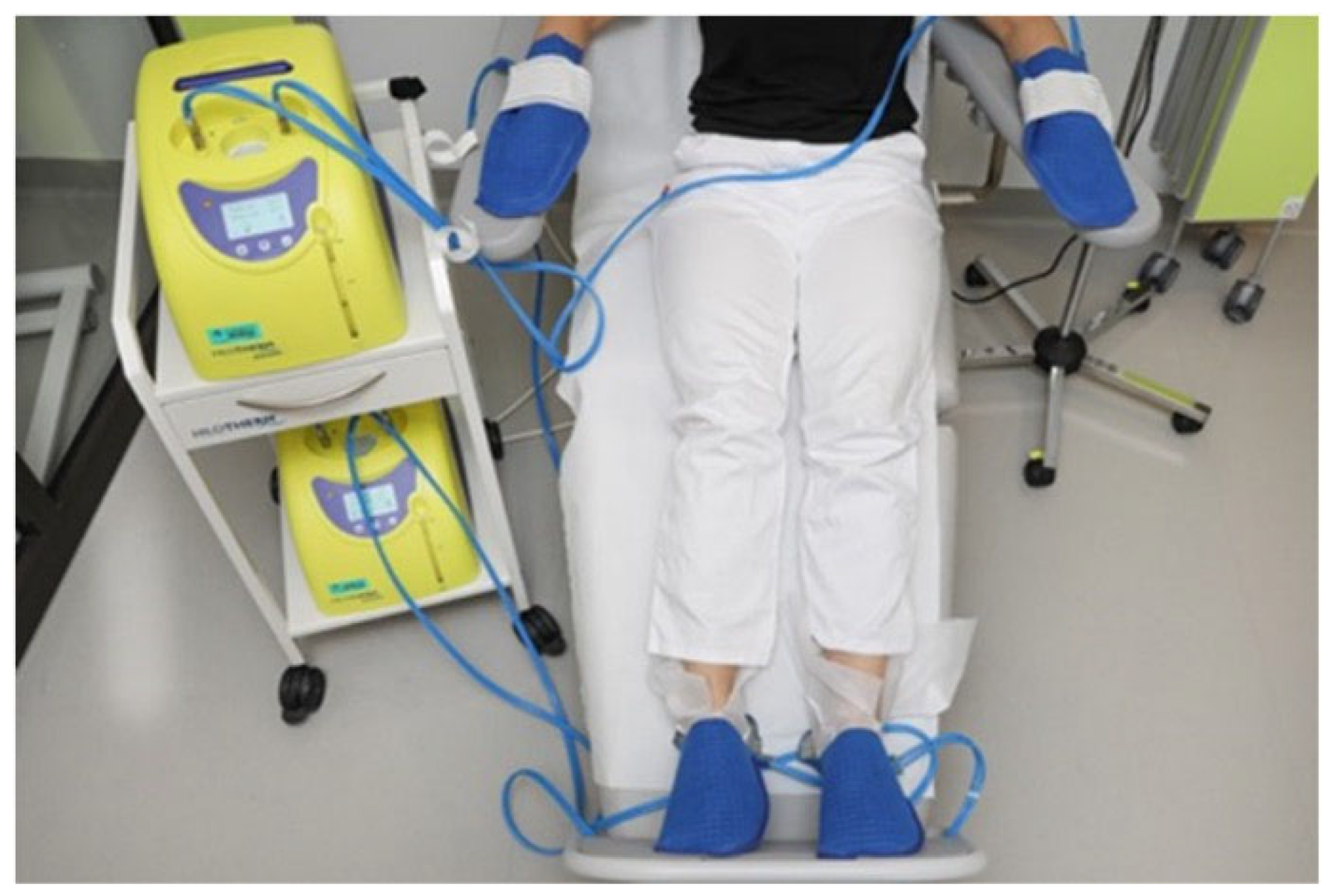
2.3. Cooling Procedures
2.4. Study Endpoints
2.5. Statistical Analysis
3. Results
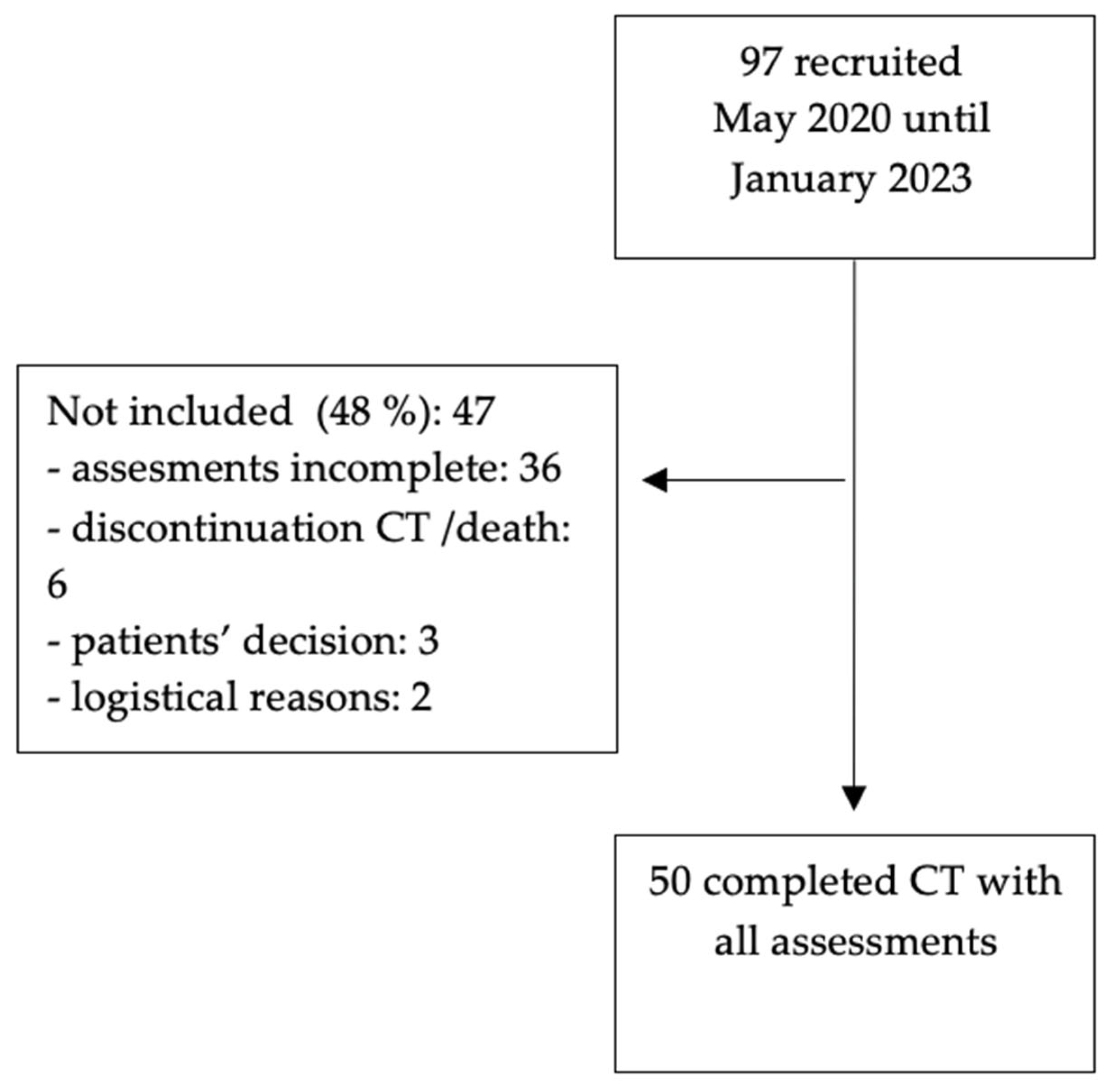
3.1. Study Population
3.2. Comparison of Efficacy of the Two Locations
3.3. Sensory Tests
3.4. Nail Changes
3.5. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| C | Cryotherapy |
| CC | cryocompression |
| CIPN | chemotherapy-induced peripheral neuropathy |
| CT | chemotherapy |
| LEX | Lower extremity |
| RS | Rydell–Seifer vibration test |
| SW | Semmes–Weinstein monofilament test |
| QoL | Quality of life |
| UEX | Upper extremity Directory of open access journals |
References
- Zhu, B.; Gu, H.; Mao, Z.; Beeraka, N.M.; Zhao, X.; Anand, M.P.; Zheng, Y.; Zhao, R.; Li, S.; Manogaran, P.; et al. Global burden of gynaecological cancers in 2022 and projections to 2050. J. Glob. Health 2024, 14, 04155. [Google Scholar] [CrossRef] [PubMed]
- Park, S.B.; Goldstein, D.; Krishnan, A.V.; Lin, C.S.; Friedlander, M.L.; Cassidy, J.; Koltzenburg, M.; Kiernan, M.C. Chemotherapy-induced peripheral neurotoxicity: A critical analysis. CA Cancer J. Clin. 2013, 63, 419–437. [Google Scholar] [CrossRef] [PubMed]
- Korde, L.A.; Somerfield, M.R.; Carey, L.A.; Crews, J.R.; Denduluri, N.; Hwang, E.S.; Khan, S.A.; Loibl, S.; Morris, E.A.; Perez, A.; et al. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. Hershman J. Clin. Oncol. 2021, 39, 1485–1505. [Google Scholar] [CrossRef]
- Vanderpuye, V.D.; Clemenceau, J.R.; Temin, S.; Aziz, Z.; Burke, W.M.; Cevallos, N.L.; Chuang, L.T.; Colgan, T.J.; Del Carmen, M.G.; Fujiwara, K.; et al. Assessment of Adult Women With Ovarian Masses and Treatment of Epithelial Ovarian Cancer: ASCO Resource-Stratified Guideline. JCO Glob. Oncol. 2021, 7, 1032–1066. [Google Scholar] [CrossRef] [PubMed]
- Oneda, E.; Abeni, C.; Zanotti, L.; Zaina, E.; Bighè, S.; Zaniboni, A. Chemotherapy-induced neurotoxicity in the treatment of gynecological cancers: State of art and an innovative approach for prevention. World J. Clin. Oncol. 2021, 12, 458–467. [Google Scholar] [CrossRef]
- Hershman, D.L.; Lacchetti, C.; Dworkin, R.H.; Lavoie Smith, E.M.; Bleeker, J.; Cavaletti, G.; Chauhan, C.; Gavin, P.; Lavino, A.; Lustberg, M.B.; et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 2014, 32, 1941–1967. [Google Scholar] [CrossRef]
- Kanbayashi, Y.; Sakaguchi, K.; Ishikawa, T.; Ouchi, Y.; Nakatsukasa, K.; Tabuchi, Y.; Kanehisa, F.; Hiramatsu, M.; Takagi, R.; Yokota, I.; et al. Comparison of the efficacy of cryotherapy and compression therapy for preventing nanoparticle albumin-bound paclitaxel-induced peripheral neuropathy: A prospective self-controlled trial. Breast 2020, 49, 219–224. [Google Scholar] [CrossRef]
- Tai, H.Y.; Lin, L.Y.; Huang, T.W.; Gautama, M.S.N. Efficacy of cryotherapy in the prevention of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Support. Care Cancer 2024, 32, 482. [Google Scholar] [CrossRef]
- Cavaletti, G.; Cornblath, D.R.; Merkies, I.S.; Postma, T.J.; Rossi, E.; Frigeni, B.; Alberti, P.; Bruna, J.; Velasco, R.; Argyriou, A.A.; et al. The chemotherapy-induced peripheral neuropathy outcome measures standardization study: From consensus to the first validity and reliability findings. Ann. Oncol. 2013, 24, 454–462. [Google Scholar] [CrossRef]
- Stubblefield, M.D.; Burstein, H.J.; Burton, A.W.; Custodio, C.M.; Deng, G.E.; Ho, M.; Junck, L.; Morris, G.S.; Paice, J.A.; Tummala, S.; et al. NCCN task force report: Management of neuropathy in cancer. J. Natl. Compr. Canc Netw. 2009, 7 (Suppl. 5), S1–S26, quiz S7–S8. [Google Scholar] [CrossRef]
- Glare, P.A.; Davies, P.S.; Finlay, E.; Gulati, A.; Lemanne, D.; Moryl, N.; Oeffinger, K.C.; Paice, J.A.; Stubblefield, M.D.; Syrjala, K.L. Pain in cancer survivors. J. Clin. Oncol. 2014, 32, 1739–1747. [Google Scholar] [CrossRef] [PubMed]
- Jordan, B.; Jahn, F.; Sauer, S.; Jordan, K. Prevention and Management of Chemotherapy-Induced Polyneuropathy. Breast Care 2019, 14, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Hertz, D.L.; Tofthagen, C.; Faithfull, S. Predisposing factors for the development of chemotherapy-induced peripheral neuropathy (CIPN). In Diagnosis, Management and Emerging Strategies for Chemotherapy-Induced Neuropathy: A MASCC Book; Springer: Cham, Switzerland, 2021; p. 20. [Google Scholar] [CrossRef]
- Shigematsu, H.; Hirata, T.; Nishina, M.; Yasui, D.; Ozaki, S. Cryotherapy for the prevention of weekly paclitaxel-induced peripheral adverse events in breast cancer patients. Support. Care Cancer 2020, 28, 5005–5011. [Google Scholar] [CrossRef]
- Chitkumarn, P.; Rahong, T.; Achariyapota, V. Efficacy of Siriraj, in-house-developed, frozen gloves for cold therapy reduction of chemotherapy-induced peripheral neuropathy in gynecological cancer patients: Randomized controlled trial. Support. Care Cancer 2022, 30, 4835–4843. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Guo, Y.; Sundar, R.; Bandla, A.; Hao, Z. Cryotherapy for Prevention of Taxane-Induced Peripheral Neuropathy: A Meta-Analysis. Front Oncol. 2021, 11, 781812. [Google Scholar] [CrossRef]
- Hanai, A.; Ishiguro, H.; Sozu, T.; Tsuda, M.; Yano, I.; Nakagawa, T.; Imai, S.; Hamabe, Y.; Toi, M.; Arai, H.; et al. Effects of Cryotherapy on Objective and Subjective Symptoms of Paclitaxel-Induced Neuropathy: Prospective Self-Controlled Trial. J. Natl. Cancer Inst. 2018, 110, 141–148. [Google Scholar] [CrossRef]
- Tandon, M.; Yacur, M.; Brenin, C.; Dillon, P. Cryotherapy for prevention of chemotherapy induced peripheral neuropathy in breast cancer. Crit. Rev. Oncol. Hematol. 2024, 194, 104244. [Google Scholar] [CrossRef]
- Beijers, A.J.M.; Bonhof, C.S.; Mols, F.; Ophorst, J.; de Vos-Geelen, J.; Jacobs, E.M.G.; van de Poll-Franse, L.V.; Vreugdenhil, G. Multicenter randomized controlled trial to evaluate the efficacy and tolerability of frozen gloves for the prevention of chemotherapy-induced peripheral neuropathy. Ann. Oncol. 2020, 31, 131–136. [Google Scholar] [CrossRef]
- Hong, J.S.; Tian, J.; Wu, L.H. The influence of chemotherapy-induced neurotoxicity on psychological distress and sleep disturbance in cancer patients. Curr. Oncol. 2014, 21, 174–180. [Google Scholar] [CrossRef]
- Bao, T.; Basal, C.; Seluzicki, C.; Li, S.Q.; Seidman, A.D.; Mao, J.J. Long-term chemotherapy-induced peripheral neuropathy among breast cancer survivors: Prevalence, risk factors, and fall risk. Breast Cancer Res. Treat. 2016, 159, 327–333. [Google Scholar] [CrossRef]
- Visovsky, C.; Wodzinski, P.T.; Haladay, D.; Ji, M.; Coury, J. Fall Risk Associated with Taxanes: Focus on Chemotherapy-Induced Peripheral Neuropathy. Semin. Oncol. Nurs. 2024, 40, 151687. [Google Scholar] [CrossRef] [PubMed]
- Winters-Stone, K.M.; Horak, F.; Jacobs, P.G.; Trubowitz, P.; Dieckmann, N.F.; Stoyles, S.; Faithfull, S. Falls, Functioning, and Disability Among Women With Persistent Symptoms of Chemotherapy-Induced Peripheral Neuropathy. J. Clin. Oncol. 2017, 35, 2604–2612. [Google Scholar] [CrossRef] [PubMed]
- Brunner, C.; Emmelheinz, M.; Egle, D.; Ritter, M.; Leitner, K.; Wieser, V.; Albertini, C.; Azim, S.A.; Mutz-Dehbalaie, I.; Kögl, J.; et al. Cropsi study: Efficacy and safety of cryotherapy and cryocompression in the prevention of chemotherapy-induced peripheral neuropathy in patients with breast and gynecological cancer-A prospective, randomized trial. Breast 2024, 76, 103763. [Google Scholar] [CrossRef]
- Oneda, E.; Meriggi, F.; Zanotti, L.; Zaina, E.; Bighè, S.; Andreis, F.; Rueda, S.; Zaniboni, A. Innovative Approach for the Prevention of Chemotherapy-Induced Peripheral Neuropathy in Cancer Patients: A Pilot Study With the Hilotherm Device, the Poliambulanza Hospital Experience. Integr. Cancer Ther. 2020, 19, 1534735420943287. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE) v5.0 Publish Date: November 27. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf (accessed on 20 April 2025).
- Ran, L.; Dongxue, G.; Zirui, Z.; Jiwei, H.; Aijun, D.; Yuchen, H.; Lizhi, Z. Effects of intermittent hand-foot hypothermia therapy on chemotherapy-induced peripheral neurotoxicity. Support. Care Cancer 2024, 33, 43. [Google Scholar] [CrossRef]
- Stoner, H.B.; Barker, P.; Riding, G.S.; Hazlehurst, D.E.; Taylor, L.; Marcuson, R.W. Relationships between skin temperature and perfusion in the arm and leg. Clin. Physiol. 1991, 11, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.Y.; Ehrlich, B.E. Prevention of chemotherapy-induced peripheral neuropathy: A review of recent findings. Crit. Rev. Oncol. Hematol. 2020, 145, 102831. [Google Scholar] [CrossRef]
- Michel, A.; Lee, R.T.; Salehi, E.; Accordino, M.K. Improving Quality of Life During Chemotherapy: Cannabinoids, Cryotherapy, and Scalp Cooling. Am. Soc. Clin. Oncol. Educ. Book 2023, 43, e390428. [Google Scholar] [CrossRef]
- Flatters, S.J.L.; Dougherty, P.M.; Colvin, L.A. Clinical and preclinical perspectives on Chemotherapy-Induced Peripheral Neuropathy (CIPN): A narrative review. Br. J. Anaesth. 2017, 119, 737–749. [Google Scholar] [CrossRef]
- Dougherty, P.M.; Cata, J.P.; Cordella, J.V.; Burton, A.; Weng, H.R. Taxol-induced sensory disturbance is characterized by preferential impairment of myelinated fiber function in cancer patients. Pain 2004, 109, 132–142. [Google Scholar] [CrossRef]
- Laforgia, M.; Laface, C.; Calabro, C.; Ferraiuolo, S.; Ungaro, V.; Tricarico, D.; Gadaleta, C.D.; Nardulli, P.; Ranieri, G. Peripheral Neuropathy under Oncologic Therapies: A Literature Review on Pathogenetic Mechanisms. Int. J. Mol. Sci. 2021, 22, 1980. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, C.L.; Lacchetti, C.; Bleeker, J.; Cavaletti, G.; Chauhan, C.; Hertz, D.L.; Kelley, M.R.; Lavino, A.; Lustberg, M.B.; Paice, J.A.; et al. Prevention and Management of Chemotherapy-Induced Peripheral Neuropathy in Survivors of Adult Cancers: ASCO Guideline Update. J. Clin. Oncol. 2020, 38, 3325–3348. [Google Scholar] [CrossRef] [PubMed]
- Jordan, B.; Margulies, A.; Cardoso, F.; Cavaletti, G.; Haugnes, H.S.; Jahn, P.; Le Rhun, E.; Preusser, M.; Scotté, F.; Taphoorn, M.J.; et al. Systemic anticancer therapy-induced peripheral and central neurotoxicity: ESMO-EONS-EANO Clinical Practice Guidelines for diagnosis, prevention, treatment and follow-up. Ann. Oncol. 2020, 31, 1306–1319. [Google Scholar] [CrossRef]
- Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1419 (accessed on 20 April 2025).
- Accordino, M.K.; Lee, S.; Leu, C.S.; Levin, B.; Trivedi, M.S.; Crew, K.D.; Kalinsky, K.; Raghunathan, R.; Faheem, K.; Harden, E.; et al. Randomized adaptive selection trial of cryotherapy, compression therapy, and placebo to prevent taxane-induced peripheral neuropathy in patients with breast cancer. Breast Cancer Res. Treat. 2024, 204, 49–59. [Google Scholar] [CrossRef]
- Kotani, H.; Terada, M.; Mori, M.; Horisawa, N.; Sugino, K.; Kataoka, A.; Adachi, Y.; Gondou, N.; Yoshimura, A.; Hattori, M.; et al. Compression therapy using surgical gloves does not prevent paclitaxel-induced peripheral neuropathy: Results from a double-blind phase 2 trial. BMC Cancer 2021, 21, 548. [Google Scholar] [CrossRef] [PubMed]
- Michel, L.L.; Schwarz, D.; Romar, P.; Feisst, M.; Hamberger, D.; Priester, A.; Kurre, E.; Klein, E.; Müller, J.; Schinköthe, T.; et al. Efficacy of Hand Cooling and Compression in Preventing Taxane-Induced Neuropathy: The POLAR Randomized Clinical Trial. JAMA Oncol. 2025, 11, 408–415. [Google Scholar] [CrossRef]
- Bandla, A.; Tan, S.; Kumarakulasinghe, N.B.; Huang, Y.; Ang, S.; Magarajah, G.; Hairom, Z.; Lim, J.S.; Wong, A.; Chan, G.; et al. Safety and tolerability of cryocompression as a method of enhanced limb hypothermia to reduce taxane-induced peripheral neuropathy. Support. Care Cancer 2020, 28, 3691–3699. [Google Scholar] [CrossRef]
- Molassiotis, A.; Cheng, H.L.; Lopez, V.; Au, J.S.; Chan, A.; Bandla, A.; Leung, K.T.; Li, Y.C.; Wong, K.H.; Suen, L.K.; et al. Are we mis-estimating chemotherapy-induced peripheral neuropathy? Analysis of assessment methodologies from a prospective, multinational, longitudinal cohort study of patients receiving neurotoxic chemotherapy. BMC Cancer 2019, 19, 132. [Google Scholar] [CrossRef]
| n = 50 | |
|---|---|
| Age (years): median (range) | 49.5 (26–78) |
| Menopausal status n (%) | |
| premenopausal | 27 (54) |
| postmenopausal | 22 (44) |
| unknown | 1 (2) |
| BMI: median (range) | 23.7 (18.4–38.4) |
| Tumor features n (%) | |
| breast cancer | 40 (80) |
| ovarian cancer | 8 (16) |
| other | 2 (4) |
| Chemotherapy regimen n (%) | |
| Taxane mono | 6 (12) |
| Taxane + anthracycline | 32 (64) |
| Taxane + platinum | 12 (24) |
| UEX (n = 50) | LEX (n = 50) | p-Value | |
|---|---|---|---|
| Median Cooling time in minutes (range) | 136 (90–285) | 136 (90–285) | |
| Median temperature index finger compared to big toe (°C) | |||
| Median before cooling (range) | 36 (27.9–37.3) | 29 (24–36.3) | |
| Median after cooling (range) | 23 (16.6–35.9) | 21 (15.9–35) | |
| Median differences (range) | −12.5 (−18.7–(−2.7)) | −9.6 (−20.3–(−2)) | <0.001 |
| RS UEX | RS LEX | Ratio UEX/LEX | SW UEX | SW LEX | Ratio UEX/LEX | |
|---|---|---|---|---|---|---|
| T0 (range) | 8 (5–8) | 7 (2–8) | 1.1 | 3 (3–8) | 8 (3–15) | 0.4 |
| T1 (range) | 7 (3–8) | 5 (0–8) | 1.4 | 3 (3–13) | 9 (3–19) | 0.3 |
| T2 (range) | 8 (2–8) | 6 (0–8) | 1.3 | 4 (3–10) | 8 (3–19) | 0.5 |
| T3 (range) | 7.75 (0–8) | 6.5 (0–8) | 1.2 | 4 (3–11) | 8 (3–17) | 0.5 |
| Grade of Nail Change n = 21 | UEX | LEX |
|---|---|---|
| T0 (range) | 0 (0–1) | 0 (0–2) |
| T1 (range) | 0 (0–1) | 0 (0–2) |
| T2 (range) | 0 (0–1) | 1 (0–2) |
| T3 (range) | 0 (0–1) | 1 (0–2) |
| UEX n = 50 (%) | LEX n = 50 (%) | ||
|---|---|---|---|
| Redness hand never | 31 (62) | Redness foot never | 29 (58) |
| Redness hand 1–2x | 17 (34) | Redness foot 1–2x | 20 (40) |
| Redness hand 3–4x | 2 (4) | Redness foot 3x | 1 (2) |
| Blistering hand never | 49 (98) | Blistering foot never | 49 (98) |
| Blistering hand once | 1 (2) | Blistering foot once | 1 (2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emmelheinz, M.; Marth, C.; Egle, D.; Leitner, K.; Albertini, C.; Abdel Azim, S.; Feroz, B.; Strobel, L.; Brunner, C. Comparison of Efficacy and Safety of Cryotherapy in Chemotherapy-Induced Peripheral Neuropathy Between Upper and Lower Extremities: Results from the Randomized CROPSI Study. Cancers 2025, 17, 1748. https://doi.org/10.3390/cancers17111748
Emmelheinz M, Marth C, Egle D, Leitner K, Albertini C, Abdel Azim S, Feroz B, Strobel L, Brunner C. Comparison of Efficacy and Safety of Cryotherapy in Chemotherapy-Induced Peripheral Neuropathy Between Upper and Lower Extremities: Results from the Randomized CROPSI Study. Cancers. 2025; 17(11):1748. https://doi.org/10.3390/cancers17111748
Chicago/Turabian StyleEmmelheinz, Miriam, Christian Marth, Daniel Egle, Katharina Leitner, Carmen Albertini, Samira Abdel Azim, Barin Feroz, Laura Strobel, and Christine Brunner. 2025. "Comparison of Efficacy and Safety of Cryotherapy in Chemotherapy-Induced Peripheral Neuropathy Between Upper and Lower Extremities: Results from the Randomized CROPSI Study" Cancers 17, no. 11: 1748. https://doi.org/10.3390/cancers17111748
APA StyleEmmelheinz, M., Marth, C., Egle, D., Leitner, K., Albertini, C., Abdel Azim, S., Feroz, B., Strobel, L., & Brunner, C. (2025). Comparison of Efficacy and Safety of Cryotherapy in Chemotherapy-Induced Peripheral Neuropathy Between Upper and Lower Extremities: Results from the Randomized CROPSI Study. Cancers, 17(11), 1748. https://doi.org/10.3390/cancers17111748






