Impact of Perioperative Fluid Strategies on Outcomes in Radical Cystectomy: A Systematic Review
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
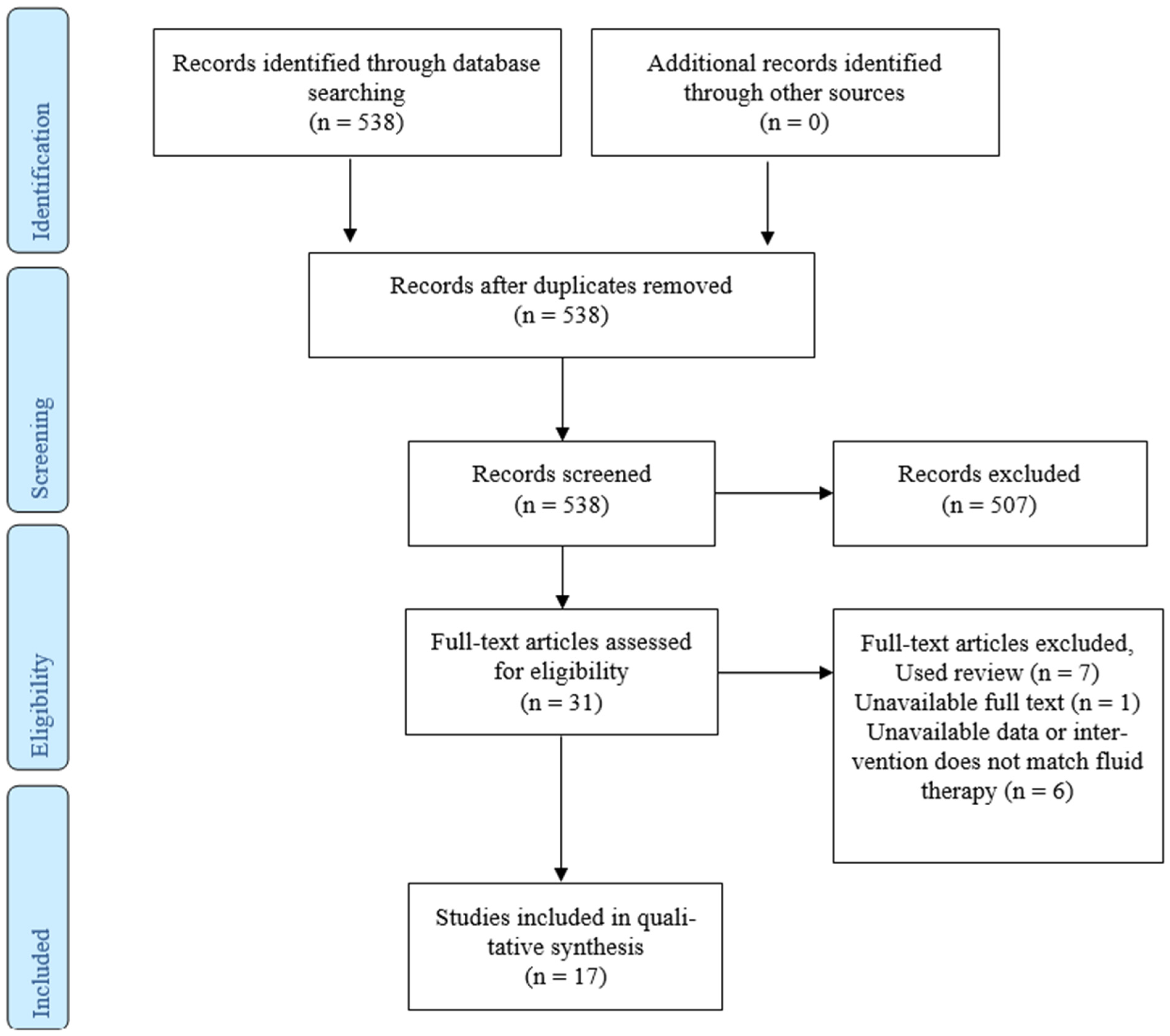
3.1. Study Selection and Characteristics
3.2. Restrictive Fluid Therapy with Norepinephrine Use
3.3. Goal-Directed Fluid Therapy (GDFT)
3.4. Warming Fluid Transfusion
3.5. Vascular Bed Filling Index
3.6. Anesthetist Impact
3.7. Impact on Specific Complications
3.7.1. Acute Kidney Injury
3.7.2. Blood Loss and Transfusions
3.7.3. Postoperative Ileus
3.8. Length of Stay
3.9. Chronic Kidney Disease
3.10. Certainty of Evidence (GRADE Assessment)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maibom, S.L.; Joensen, U.N.; Poulsen, A.M.; Kehlet, H.; Brasso, K.; Røder, M.A. Short-term morbidity and mortality following radical cystectomy: A systematic review. BMJ Open 2021, 11, e043266. [Google Scholar] [CrossRef] [PubMed]
- Duquesne, I.; Benamran, D.; Masson-Lecomte, A.; De La Taille, A.; Peyromaure, M.; Rouprêt, M.; Delongchamps, N.B. Intraoperative complication of radical cystectomy for muscle-invasive bladder cancer: Does the surgical approach matter? A retrospective multicenter study using the EAUiaiC classification. World J. Urol. 2023, 41, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- van Ginkel, N.; Meijer, D.; Boormans, J.L.; Mertens, L.S.; van Beek, S.C.; Vis, A.N. The Dutch Cystectomy Snapshot Research Group Predictors of Major Complications and the Association with Oncological Outcomes After Radical Cystectomy for Bladder Cancer: A Nationwide Registry Study. Med. Res. Arch. 2023, 11. [Google Scholar] [CrossRef]
- Katsimperis, S.; Tzelves, L.; Tandogdu, Z.; Ta, A.; Geraghty, R.; Bellos, T.; Manolitsis, I.; Pyrgidis, N.; Schulz, G.B.; Sridhar, A.; et al. Complications After Radical Cystectomy: A Systematic Review and Meta-analysis of Randomized Controlled Trials with a Meta-regression Analysis. Eur. Urol. Focus 2023, 9, 920–929. [Google Scholar] [CrossRef]
- Ślusarczyk, A.; Pustuła, P.; Garbas, K.; Zapała, Ł.; Radziszewski, P. Mean platelet volume to lymphocyte ratio as an inflammatory marker associated with high-grade recurrence and progression of non-muscle-invasive bladder cancer treated with Bacillus Calmette-Guérin. Cent. Eur. J. Urol. 2024, 77, 599–611. [Google Scholar]
- Gómez-Izquierdo, J.C.; Feldman, L.S.; Carli, F.; Baldini, G. Meta-analysis of the effect of goal-directed therapy on bowel function after abdominal surgery. Br. J. Surg. 2015, 102, 577–589. [Google Scholar] [CrossRef]
- Schwenk, W.; Flemming, S.; Girona-Johannkämper, M.; Wendt, W.; Darwich, I.; Strey, C. Structured implementation of fast-track pathways to enhance recovery after elective colorectal resection: First results from five German hospitals. Chirurgie 2024, 95, 148–156. [Google Scholar] [CrossRef]
- Daneshmand, S.; Ahmadi, H.; Schuckman, A.K.; Mitra, A.P.; Cai, J.; Miranda, G.; Djaladat, H. Enhanced recovery protocol after radical cystectomy for bladder cancer. J. Urol. 2014, 192, 50–56. [Google Scholar] [CrossRef]
- Frees, S.K.; Aning, J.; Black, P.; Struss, W.; Bell, R.; Chavez-Munoz, C.; Gleave, M.; So, A.I. A prospective randomized pilot study evaluating an ERAS protocol versus a standard protocol for patients treated with radical cystectomy and urinary diversion for bladder cancer. World J. Urol. 2018, 36, 215–220. [Google Scholar] [CrossRef]
- Persson, B.; Carringer, M.; Andrén, O.; Andersson, S.-O.; Carlsson, J.; Ljungqvist, O. Initial experiences with the enhanced recovery after surgery (ERAS®) protocol in open radical cystectomy. Scand. J. Urol. 2015, 49, 302–307. [Google Scholar] [CrossRef]
- Tyson, M.D.; Chang, S.S. Enhanced Recovery Pathways Versus Standard Care After Cystectomy: A Meta-analysis of the Effect on Perioperative Outcomes. Eur. Urol. 2016, 70, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhou, L.; Lin, S.; Yan, W.; Huang, L.; Liang, S. Beneficial effect of fluid warming in elderly patients with bladder cancer undergoing Da Vinci robotic-assisted laparoscopic radical cystectomy. Clinics 2020, 75, e1639. [Google Scholar] [CrossRef]
- Kong, Y.-G.; Kim, J.Y.; Yu, J.; Lim, J.; Hwang, J.-H.; Kim, Y.-K. Efficacy and safety of stroke volume variation-guided fluid therapy for reducing blood loss and transfusion requirements during radical cystectomy: A randomized clinical trial. Medicine 2016, 95, e3685. [Google Scholar] [CrossRef]
- Arslan-Carlon, V.; Tan, K.S.; Dalbagni, G.; Pedoto, A.C.; Herr, H.W.; Bochner, B.H.; Cha, E.K.; Donahue, T.F.; Fischer, M.; Donat, S.M. Goal-directed versus standard fluid therapy to decrease ileus after open radical cystectomy: A prospective randomized controlled trial. Anesthesiology 2020, 133, 293–303. [Google Scholar] [CrossRef]
- Pillai, P.; McEleavy, I.; Gaughan, M.; Snowden, C.; Nesbitt, I.; Durkan, G.; Johnson, M.; Cosgrove, J.; Thorpe, A. A double-blind randomized controlled clinical trial to assess the effect of doppler optimized intraoperative fluid management on outcome following radical cystectomy. J. Urol. 2011, 186, 2201–2206. [Google Scholar] [CrossRef]
- Liu, T.-J.; Zhang, J.-C.; Gao, X.-Z.; Tan, Z.-B.; Wang, J.-J.; Zhang, P.-P.; Cheng, A.-B.; Zhang, S.-B. Clinical research of goal-directed fluid therapy in elderly patients with radical resection of bladder cancer. J. Cancer Res. Ther. 2018, 14, S173–S179. [Google Scholar] [CrossRef]
- Ghoreifi, A.; Basin, M.F.; Ghodoussipour, S.; Bazargani, S.T.; Amini, E.; Aslzare, M.; Cai, J.; Miranda, G.; Sugeir, S.; Bhanvadia, S.; et al. Perioperative outcomes of goal-directed versus conventional fluid therapy in radical cystectomy with enhanced recovery protocol. Int. Urol. Nephrol. 2021, 53, 1827–1833. [Google Scholar] [CrossRef]
- Wei, C.; Wan, F.; Zhao, H.; Ma, J.; Gao, Z.; Lin, C. Application of enhanced recovery after surgery in patients undergoing radical cystectomy. J. Int. Med. Res. 2018, 46, 5011–5018. [Google Scholar] [CrossRef]
- Bazargani, S.T.; Ghodoussipour, S.; Tse, B.; Miranda, G.; Cai, J.; Schuckman, A.; Daneshmand, S.; Djaladat, H. The association between intraoperative fluid intake and postoperative complications in patients undergoing radical cystectomy with an enhanced recovery protocol. World J. Urol. 2018, 36, 401–407. [Google Scholar] [CrossRef]
- Dobé, T.-R.; Belhadj, Y.; Michel, C.; Djouadou, M.; Bouchardi, A.; Liron, C.; Bento, C.; Aregui, A.; Meria, P.; Thevenot, A.; et al. Perioperative results of radical cystectomy after neoadjuvant chemotherapy according to the implementation of ERAS pathway. Progres Urol. 2022, 32, 401–409. [Google Scholar] [CrossRef]
- Marques, M.; Tezier, M.; Tourret, M.; Cazenave, L.; Brun, C.; Duong, L.N.; Cambon, S.; Pouliquen, C.; Ettori, F.; Sannini, A.; et al. Risk factors for postoperative acute kidney injury after radical cystectomy for bladder cancer in the era of ERAS protocols: A retrospective observational study. PLoS ONE 2024, 19, e0309549. [Google Scholar] [CrossRef] [PubMed]
- Furrer, M.A.; Schneider, M.P.; Löffel, L.M.; Burkhard, F.C.; Wuethrich, P.Y. Impact of intra-operative fluid and noradrenaline administration on early postoperative renal function after cystectomy and urinary diversion. Eur. J. Anaesthesiol. 2018, 35, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Lipowski, P.; Ostrowski, A.; Adamowicz, J.; Jasiewicz, P.; Kowalski, F.; Drewa, T.; Juszczak, K. Does the Administration of Intravenous Fluid Matter in the Context of the Incidence of Postoperative Complications After Radical Cystectomy? Cancers 2024, 17, 102. [Google Scholar] [CrossRef]
- Wüthrich, P.Y.; Burkhard, F.C.; Thalmann, G.; Stueber, F.; Studer, U. Restrictive Deferred Hydration Combined with Preemptive Norepinephrine Infusion During Radical Cystectomy Reduces Postoperative Complications and Hospitalization Time: A Randomized Clinical Trial. Anesthesiology 2014, 120, 365–377. [Google Scholar] [CrossRef]
- Wuethrich, P.Y.; Studer, U.E.; Thalmann, G.N.; Burkhard, F.C. intraoperative continuous norepinephrine infusion combined with restrictive deferred hydration significantly reduces the need for blood transfusion in patients undergoing open radical cystectomy: Results of a prospective randomised trial. Eur. Urol. 2014, 66, 352–360. [Google Scholar] [CrossRef]
- Wu, F.M.W.; Burkhard, F.; Turri, F.; Furrer, M.; Loeffel, L.; Thalmann, G.; Wuethrich, P. Renal outcome after radical cystectomy and urinary diversion performed with restrictive hydration and vasopressor administration in the frame of an enhanced recovery program: A follow-up study of a randomized clinical trial. Urol. Oncol. Semin. Orig. Investig. 2017, 35, 602.e11–602.e17. [Google Scholar] [CrossRef]
- Jubber, I.; Pang, K.H.; Groves, R.; Reed, O.; Noon, A.P.; Catto, J.W.; Cumberbatch, M.G. Impact of Anaesthetist Volume on Radical Cystectomy Outcomes. Eur. Urol. Focus 2021, 7, 117–123. [Google Scholar] [CrossRef]
- Patel, S.Y.; Getting, R.E.G.; Alford, B.; Hussein, K.; Schaible, B.J.; Boulware, D.; Lee, J.K.; Gilbert, S.M.; Powsang, J.M.; Sexton, W.J.; et al. Improved Outcomes of Enhanced Recovery After Surgery (ERAS) Protocol for Radical Cystectomy with Addition of a Multidisciplinary Care Process in a US Comprehensive Cancer Care Center. World J. Surg. 2018, 42, 2701–2707. [Google Scholar] [CrossRef]
- Tappero, S.; Dell’Oglio, P.; Cerruto, M.A.; Salas, R.S.; Rueda, O.B.; Simone, G.; Hendricksen, K.; Soria, F.; Umari, P.; Antonelli, A.; et al. Ileal Conduit Versus Orthotopic Neobladder Urinary Diversion in Robot-assisted Radical Cystectomy: Results from a Multi-institutional Series. Eur. Urol. Open Sci. 2023, 50, 47–56. [Google Scholar] [CrossRef]
- Crettenand, F.; M’baya, O.; Grilo, N.; Valerio, M.; Dartiguenave, F.B.; Cerantola, Y.; Roth, B.; Rouvé, J.-D.; Blanc, C.; Lucca, I. ERAS® protocol improves survival after radical cystectomy: A single-center cohort study. Medicine 2022, 101, e30258. [Google Scholar] [CrossRef]
- Coeckelenbergh, S.; Soucy-Proulx, M.; Van der Linden, P.; Roullet, S.; Moussa, M.; Kato, H.; Toubal, L.; Naili, S.; Rinehart, J.; Grogan, T.; et al. Restrictive versus Decision Support Guided Fluid Therapy during Major Hepatic Resection Surgery: A Randomized Controlled Trial. Anesthesiology 2024, 141, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Ji, D.; Fang, Q.; Li, Y.; Wang, K.; Liu, J.; Wang, L.; Gu, E.; Zhang, L.; Chen, L. Effect of low-dose norepinephrine combined with goal-directed fluid therapy on postoperative pulmonary complications in lung surgery: A prospective randomized controlled trial. J. Clin. Anesthesia 2024, 99, 111645. [Google Scholar] [CrossRef] [PubMed]
- Al Saied, G.; Almutairi, H.M.; Alharbi, Y.; Almohanna, M.; Almutairi, A. Comparison Between the Impact of Vasopressors and Goal-Directed Fluid Therapy on the Management of Free Flap Reconstruction of Head and Neck and Monitoring in ICU. Cureus 2020, 12, e12108. [Google Scholar] [CrossRef]
- Simegn, G.D.; Bayable, S.D.; Fetene, M.B. Prevention and management of perioperative hypothermia in adult elective surgical patients: A systematic review. Ann. Med. Surg. 2021, 72, 103059. [Google Scholar] [CrossRef]
- Roshan, M.B.A.; Jafarpoor, H.; Shamsalinia, A.; Fotokian, Z.; Hamidi, S.H. Effects of a forced-air warming system and warmed intravenous fluids on hemodynamic parameters, shivering, and time to awakening in elderly patients undergoing open cardiac surgery. Ann. Card. Anaesth. 2023, 26, 386–392. [Google Scholar] [CrossRef]
- Camus, Y.; Delva, E.; Cohen, S.; Lienhart, A. The effects of warming intravenous fluids on intraoperative hypothermia and postoperative shivering during prolonged abdominal surgery. Acta Anaesthesiol. Scand. 1996, 40, 779–782. [Google Scholar] [CrossRef]
- Cella, L.; Basile, G.; Moretto, S.; Paciotti, M.; Hurle, R.; Lughezzani, G.; Avolio, P.P.; Piccolini, A.; Mancon, S.; Lazzeri, M.; et al. Robotic assisted vs open radical cystectomy: An updated systematic review and meta-analysis. J. Robot. Surg. 2024, 18, 277. [Google Scholar] [CrossRef]
- Mastroianni, R.; Tuderti, G.; Ferriero, M.; Anceschi, U.; Bove, A.M.; Brassetti, A.; D’Annunzio, S.; Misuraca, L.; Torregiani, G.; Covotta, M.; et al. Robot-assisted Radical Cystectomy with Totally Intracorporeal Urinary Diversion Versus Open Radical Cystectomy: 3-Year Outcomes from a Randomised Controlled Trial. Eur. Urol. 2024, 85, 422–430. [Google Scholar] [CrossRef]
| No. | Author (Year) | Study Design | Participants (Study Group vs. Control Group) | Intervention | Outcomes |
|---|---|---|---|---|---|
| 1 | Luo J. (2020) [12] | RCT | 53 vs. 55 | Pre-warm fluid infusion use | ↓ transfusion, ↓ LOS |
| 2 | Kong Y. (2016) [13] | RCT | 23 vs. 23 | Fluid infusion based on stroke volume variation (SVV) 10–20% vs. <10% | ↓ transfusion, ↓ blood loss. ↔ complications, ↔ AKI, ↔ LOS |
| 3 | Arslan-Carlon V. (2020) [14] | RCT | 142 vs. 141 | Goal-directed stroke volume (SV) vs. standard fluid therapy | ↑ 30-day complications, ↑ acute kidney injury ↔ ileus, ↔LOS |
| 4 | Pillai P. (2011) [15] | RCT | 32 vs. 34 | Doppler-guided vs. standard fluid therapy | ↓ ileus, ↓ nausea, ↓ vomiting. ↓wound infection. |
| 5 | Liu T. (2016) [16] | RCT | 38 vs. 38 | Goal-directed SVV vs. routine fluid therapy | ↑ cardiac output index, ↑ MAP, ↑ central venous pressure. Better metabolic index. ↓ nausea, ↓ vomiting, ↓ hypotension. |
| 6 | Ghoreifi A. (2021) [17] | Retrospective cohort | 119 vs. 192 | Goal-directed SVV vs. convectional fluid therapy | ↔ kidney function, ↔ LOS, ↔ blood loss, ↔ transfusions, and readmissions. |
| 7 | Wei C. (2018) [18] | Retrospective cohort | 91 vs. 101 | ERAS (less fluids) vs. no ERAS | ↓ blood loss, ↓ transfusions, ↓ readmissions, and ↓ complications. ↓ bowel complications. |
| 8 | Bazargani T. (2018) [19] | Prospective cohort | 180 | Total intraoperative fluid volume, type of fluid impact | ↔ 30 -, 90-day complications, ↔ in LOS. |
| 9 | Dobe T. (2022) [20] | Prospective vs. retrospective control group | 29 vs. 50 | ERAS (less fluids + vasopressive drugs) vs. no ERAS | ↔ blood loss, ↔ transfusions, ↔ ileus, ↔ complications rate. ↓ LOS. |
| 10 | Marques M. (2024) [21] | Retrospective cohort | 51 vs. 71 | No AKI vs. AKI | ↑ AKI if restrictive intraoperative vascular filling, female sex, postoperative sepsis, day 1 SOFA score, creatinin D1. |
| 11 | Furrer M. (2018) [22] | Retrospective cohort | 100 vs. 812 | AKI vs. no AKI | ↑ AKI if surgery time >400 min, male, obesity, high blood loss, blood transfusion, more crystalloids. |
| 12 | Lipowski P. (2024) [23] | Retrospective cohort | 48 vs. 240 | Ileal conduit (IC) vs. ureterocutaneostomy (UCS), Vascular Bed Filling Index (VBFI), adjusted Vascular Bed Filling Index (aVBFI) | VBFI, aVBFI: < 8–UCS ↓ complications; = 8–IC = UCS; >8–IC ↑ complications. |
| 13 | Wuethrich P. (2013) [24] | RCT | 83 vs. 84 | Low volume + noradrenaline vs. balanced Ringer’s solution | ↓ hospital complications, ↓ gastrointestinal, ↓ cardiac, ↓ 90 days complications. ↓ LOS. |
| 14 | Wuethrich P. (2013) [25] | RCT | 83 vs. 84 | Low volume + noradrenaline vs. balanced Ringer’s solution | ↓ blood loss, ↓ transfusions. |
| 15 | Mei Wen Wu F. (2013) [26] | RCT | 83 vs. 84 | Low volume + noradrenaline vs. balanced Ringer’s solution | ↔ in renal function 7 days, 3, 6, and 12 months after surgery. |
| 16 | Jubber I. (2019) [27] | Retrospective cohort | 430 | High-volume anesthetist vs. low-volume anesthetist | ↓ LOS, ↓ blood loss, ↓ transfusion rate. |
| 17 | Patel S. (2018) [28] | Retrospective cohort | 116 vs. 143 | Multidisciplinary ERAS (goal-directed fluid therapy) vs. surgical ERAS | ↓ intraoperative transfusions, ↓ nausea. ↔ in bowel function, ↔ LOS, ↔ 30 and 90 days complications, ↔ readmissions. |
| No. | Author (Year) | Random Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Blinding of Outcome Assessment | Incomplete Data | Selective Reporting | Main Limitations/Quality Considerations |
|---|---|---|---|---|---|---|---|---|
| 1 | Luo J. (2020) [12] | Unclear | Unclear | Unclear | Unclear | Low | Low | Randomization and blinding procedures poorly described, potential risk of bias. |
| 2 | Kong Y. (2016) [13] | Low | Low | Low | Low | Low | Low | Good methodology but small sample size (risk of imprecise estimates). |
| 3 | Arslan-Carlon V. (2020) [14] | Low | Low | Low | Low | Low | Low | Good methodology, large sample size, low risk of bias. |
| 4 | Pillai P. (2011) [15] | High | Unclear | Unclear | Unclear | Low | Low | Small sample size, unclear, randomization and blinding procedures, high risk of bias. |
| 5 | Liu T. (2016) [16] | High | Unclear | Unclear | Unclear | Low | Low | Small sample size, unclear randomization details, moderate risk of bias. |
| 6 | Ghoreifi A. (2021) [17] | High | High | High | High | Low | Low | Retrospective observational design, possible selection and confounding biases. |
| 7 | Wei C. (2018) [18] | High | High | High | High | Low | Unclear | Retrospective, observational study design, risk of selection and confounding biases. |
| 8 | Bazargani T. (2018) [19] | High | High | High | High | Low | Low | Observational design without clear control group, potential confounding bias. |
| 9 | Dobe T. (2022) [20] | High | High | High | High | Low | Low | Small sample size, mixed prospective-retrospective groups, risk of confounding and selection bias. |
| 10 | Marques M. (2024) [21] | High | High | High | High | Low | Low | Retrospective design, limited to AKI risk factors, potential confounding bias. |
| 11 | Furrer M. (2018) [22] | High | High | High | High | Low | Low | Retrospective design, large population but significant confounding risks. |
| 12 | Lipowski P. (2024) [23] | High | High | High | High | Low | Low | Single-center, retrospective, exploratory and hypothesis generating. |
| 13 | Wuethrich P. (2013) [24] | Low | Low | Low | Low | Low | Low | Strong methodology, single population, limited external validity. |
| 14 | Wuethrich P. (2013) [25] | Low | Low | Low | Low | Low | Low | Strong methodology, single population, limited external validity |
| 15 | Mei Wen Wu F. (2013) [26] | Low | Low | Low | Low | Low | Low | Strong methodology, single population, limited external validity |
| 16 | Jubber I. (2019) [27] | High | High | High | High | Unclear | Low | Retrospective design, high-volume anesthetist group limited to few providers, risk of bias. |
| 17 | Patel S. (2018) [28] | High | High | High | High | Low | Low | Retrospective design, historical control group, significant selection and confounding biases. |
| Outcome | No. of Studies | Consistency | Precision | Risk of Bias | Certainty (GRADE) |
|---|---|---|---|---|---|
| Acute Kidney Injury (AKI) | 4 (2 RCTs, 2 obs.) | Inconsistent | Imprecise (small N, some p > 0.05) | Moderate to high (obs. studies, limited blinding) | Low |
| Blood Loss and Transfusions | 4 RCTs | Partially consistent | Moderate (some large effects) | Low to moderate (RCTs well reported) | Moderate |
| Postoperative Ileus | 4 studies (mixed) | Mixed results | Low (small N in key studies) | High in some studies | Low |
| Length of Hospital Stay | 4 RCTs + obs. | Inconsistent | Low (heterogeneity of LOS reporting) | Moderate | Low |
| Chronic Kidney Disease | 1 RCT | Consistent | Moderate | Low | Moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lipowski, P.; Ostrowski, A.; Adamowicz, J.; Kowalski, F.; Drewa, T.; Juszczak, K. Impact of Perioperative Fluid Strategies on Outcomes in Radical Cystectomy: A Systematic Review. Cancers 2025, 17, 1746. https://doi.org/10.3390/cancers17111746
Lipowski P, Ostrowski A, Adamowicz J, Kowalski F, Drewa T, Juszczak K. Impact of Perioperative Fluid Strategies on Outcomes in Radical Cystectomy: A Systematic Review. Cancers. 2025; 17(11):1746. https://doi.org/10.3390/cancers17111746
Chicago/Turabian StyleLipowski, Paweł, Adam Ostrowski, Jan Adamowicz, Filip Kowalski, Tomasz Drewa, and Kajetan Juszczak. 2025. "Impact of Perioperative Fluid Strategies on Outcomes in Radical Cystectomy: A Systematic Review" Cancers 17, no. 11: 1746. https://doi.org/10.3390/cancers17111746
APA StyleLipowski, P., Ostrowski, A., Adamowicz, J., Kowalski, F., Drewa, T., & Juszczak, K. (2025). Impact of Perioperative Fluid Strategies on Outcomes in Radical Cystectomy: A Systematic Review. Cancers, 17(11), 1746. https://doi.org/10.3390/cancers17111746






