Where Reflectance Confocal Microscopy Provides the Greatest Benefit for Diagnosing Skin Cancers: The Experience of the National Cancer Institute of Naples
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Workflow
2.2. Lesion Classification at ELM
2.3. Reflectance Confocal Microscopy (RCM) Assessment
2.4. Statistical Methods
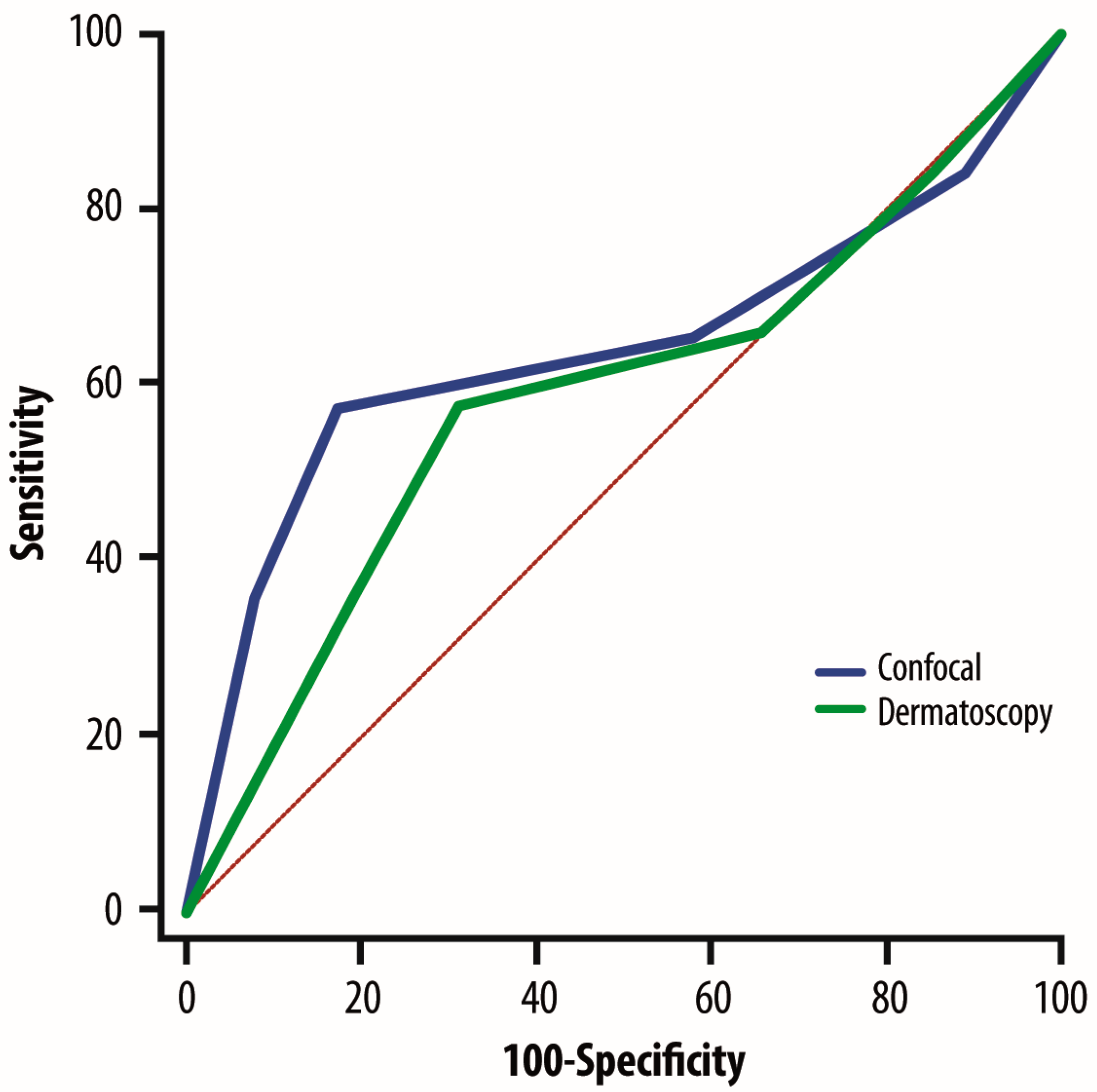
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R.; Sondak, V.K.; Long, G.V.; Ross, M.I.; Lazar, A.J.; Faries, M.B.; Kirkwood, J.M.; McArthur, G.A.; et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 472–492. [Google Scholar] [CrossRef] [PubMed]
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Basset-Seguin, N.; Bastholt, L.; Bataille, V.; Brochez, L.; del Marmol, V.; et al. European consensus-based interdisciplinary guideline for melanoma. Part 1: Diagnostics—Update 2024. Eur. J. Cancer 2025, 215, 115152. [Google Scholar] [CrossRef] [PubMed]
- Dinnes, J.; Deeks, J.J.; Saleh, D.; Chuchu, N.; Bayliss, S.E.; Patel, L.; Davenport, C.; Takwoingi, Y.; Godfrey, K.; Matin, R.N.; et al. Reflectance confocal microscopy for diagnosing cutaneous melanoma in adults. Cochrane Database Syst. Rev. 2018, 12, CD013190. [Google Scholar] [CrossRef]
- Waddell, A.; Star, P.; Guitera, P. Advances in the use of reflectance confocal microscopy in melanoma. Melanoma Manag. 2018, 5, MMT04. [Google Scholar] [CrossRef]
- Pizzichetta, M.A.; Kittler, H.; Stanganelli, I.; Ghigliotti, G.; Corradin, M.T.; Rubegni, P.; Cavicchini, S.; De Giorgi, V.; Bono, R.; Alaibac, M.; et al. Dermoscopic diagnosis of amelanotic/hypomelanotic melanoma. Br. J. Dermatol. 2017, 177, 538–540. [Google Scholar] [CrossRef]
- Guitera, P.; Menzies, S.W.; Longo, C.; Cesinaro, A.M.; Scolyer, R.A.; Pellacani, G. In vivo confocal microscopy for diagnosis of melanoma and basal cell carcinoma using a two-step method: Analysis of 710 consecutive clinically equivocal cases. J. Investig. Dermatol. 2012, 132, 2386–2394. [Google Scholar] [CrossRef]
- Mandal, A.; Priyam, S.; Chan, H.H.; Gouveia, B.M.; Guitera, P.; Song, Y.; Baker, M.A.B.; Vafaee, F. Computer-Aided Diagnosis of Melanoma Subtypes Using Reflectance Confocal Images. Cancers 2023, 15, 1428. [Google Scholar] [CrossRef]
- Levine, A.; Markowitz, O. Introduction to reflectance confocal microscopy and its use in clinical practice. JAAD Case Rep. 2018, 4, 1014–1023. [Google Scholar] [CrossRef]
- Filoni, A.; Alaibac, M. Reflectance Confocal Microscopy in Evaluating Skin Cancer: A Clinicians’s Perspective. Front. Oncol. 2019, 9, 1457. [Google Scholar] [CrossRef]
- Rajadhyaksha, M.; Grossman, M.; Esterowitz, D.; Webb, R.H.; Anderson, R.R. In vivo confocal scanning laser microscopy of human skin: Melanin provides strong contrast. J. Investig. Dermatol. 1995, 104, 946–952. [Google Scholar] [CrossRef]
- Abbasi, N.R.; Shaw, H.M.; Rigel, D.S.; Friedman, R.J.; McCarthy, W.H.; Osman, I.; Kopf, A.W.; Polsky, D. Early diagnosis of cutaneous melanoma: Revisiting the ABCD criteria. JAMA 2004, 292, 2771–2776. [Google Scholar] [CrossRef] [PubMed]
- Farabi, B.; Khan, S.; Jamgochian, M.; Atak, M.F.; Jain, M.; Rao, B.K. The role of reflectance confocal microscopy in the diagnosis and management of pigmentary disorders: A review. J. Cosmet. Dermatol. 2023, 22, 3213–3222. [Google Scholar] [CrossRef]
- Ricci, C. Advances in Diagnosis of Skin and Superficial Tissue Disorders—“Old and Emerging” Diagnostic Tools. Diagnostics 2024, 14, 2414. [Google Scholar] [CrossRef]
- Shahriari, N.; Grant-Kels, J.M.; Rabinovitz, H.; Oliviero, M.; Scope, A. Reflectance confocal microscopy: Principles, basic terminology, clinical indications, limitations, and practical considerations. J. Am. Acad. Dermatol. 2021, 84, 1–14. [Google Scholar] [CrossRef]
- Finnane, A.; Curiel-Lewandrowski, C.; Wimberley, G.; Caffery, L.; Katragadda, C.; Halpern, A.; Marghoob, A.A.; Malvehy, J.; Kittler, H.; Hofmann-Wellenhof, R.; et al. Proposed Technical Guidelines for the Acquisition of Clinical Images of Skin-Related Conditions. JAMA Dermatol. 2017, 153, 453–457. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Palmieri, G.; Celentano, E.; Parasole, R.; Caracò, C.; Daponte, A.; Chiofalo, M.; Melucci, M.; Mozzillo, N.; Satriano, R.; et al. Sensitivity and specificity of epiluminescence microscopy: Evaluation on a sample of 2731 excised cutaneous pigmented lesions. Br. J. Dermatol. 2000, 142, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Scope, A.; Benvenuto-Andrade, C.; Agero, A.L.; Malvehy, J.; Puig, S.; Rajadhyaksha, M.; Busam, K.J.; Marra, D.E.; Torres, A.; Propperova, I.; et al. In vivo reflectance confocal microscopy imaging of melanocytic skin lesions: Consensus terminology glossary and illustrative images. J. Am. Acad. Dermatol. 2007, 57, 644–658. [Google Scholar] [CrossRef]
- Navarrete-Dechent, C.; Liopyris, K.; Monnier, J.; Aleissa, S.; Boyce, L.M.; Longo, C.; Oliviero, M.; Rabinovitz, H.; Marghoob, A.A.; Halpern, A.C.; et al. Reflectance confocal microscopy terminology glossary for melanocytic skin lesions: A systematic review. J. Am. Acad. Dermatol. 2021, 84, 102–119. [Google Scholar] [CrossRef]
- Pellacani, G.; Guitera, P.; Longo, C.; Avramidis, M.; Seidenari, S.; Menzies, S. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J. Investig. Dermatol. 2007, 127, 2759–2765. [Google Scholar] [CrossRef]
- Xiong, Y.Q.; Ma, S.J.; Mo, Y.; Huo, S.T.; Wen, Y.Q.; Chen, Q. Comparison of dermoscopy an reflectance confocal microscopy for the diagnosis of malignant skin tumours: A meta-analysis. J. Cancer Res. Clin. Oncol. 2017, 143, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Wen, J.; Cao, S.; Yin, T.; Jiang, B.; Lou, Y.; Zhu, J.; An, X.; Suo, H.; Li, D.; et al. The diagnostic accuracy of dermoscopy and reflectance confocal microscopy for amelanotic/hypomelanotic melanoma: A systematic review and meta-analysis. Br. J. Dermatol. 2020, 183, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Pellacani, G.; Farnetani, F.; Ciardo, S.; Chester, J.; Kaleci, S.; Mazzoni, L.; Bassoli, S.; Casari, A.; Pampena, R.; Mirra, M.; et al. Effect of Reflectance Confocal Microscopy for Suspect Lesions on Diagnostic Accuracy in Melanoma: A Randomized Clinical Trial. JAMA Dermatol. 2022, 158, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Pellacani, G.; Stanganelli, I.; Longo, C. Use of Reflectance Confocal Microscopy in Equivocal Lesions for the Diagnosis of Melanoma-Reply. JAMA Dermatol. 2023, 159, 115–116. [Google Scholar] [CrossRef] [PubMed]
- Marushchak, O.; Yakubov, R.; Yakubov, R.; Goldenberg, G. New Technologies in Diagnosis and Prognosis of Melanocytic Lesions. J. Clin. Aesthetic Dermatol. 2023, 16, 44–49. [Google Scholar]
- Atak, M.F.; Farabi, B.; Navarrete-Dechent, C.; Rubinstein, G.; Rajadhyaksha, M.; Jain, M. Confocal Microscopy for Diagnosis and Management of Cutaneous Malignancies: Clinical Impacts and Innovation. Diagnostics 2023, 13, 854. [Google Scholar] [CrossRef] [PubMed]
- Harris, U.; Rajadhyaksha, M.; Jain, M. Combining Reflectance Confocal Microscopy with Optical Coherence Tomography for Noninvasive Diagnosis of Skin Cancers via Image Acquisition. J. Vis. Exp. 2022, 186, e63789. [Google Scholar] [CrossRef]
- Malciu, A.M.; Lupu, M.; Voiculescu, V.M. Artificial Intelligence-Based Approaches to Reflectance Confocal Microscopy Image Analysis in Dermatology. J. Clin. Med. 2022, 11, 429. [Google Scholar] [CrossRef]
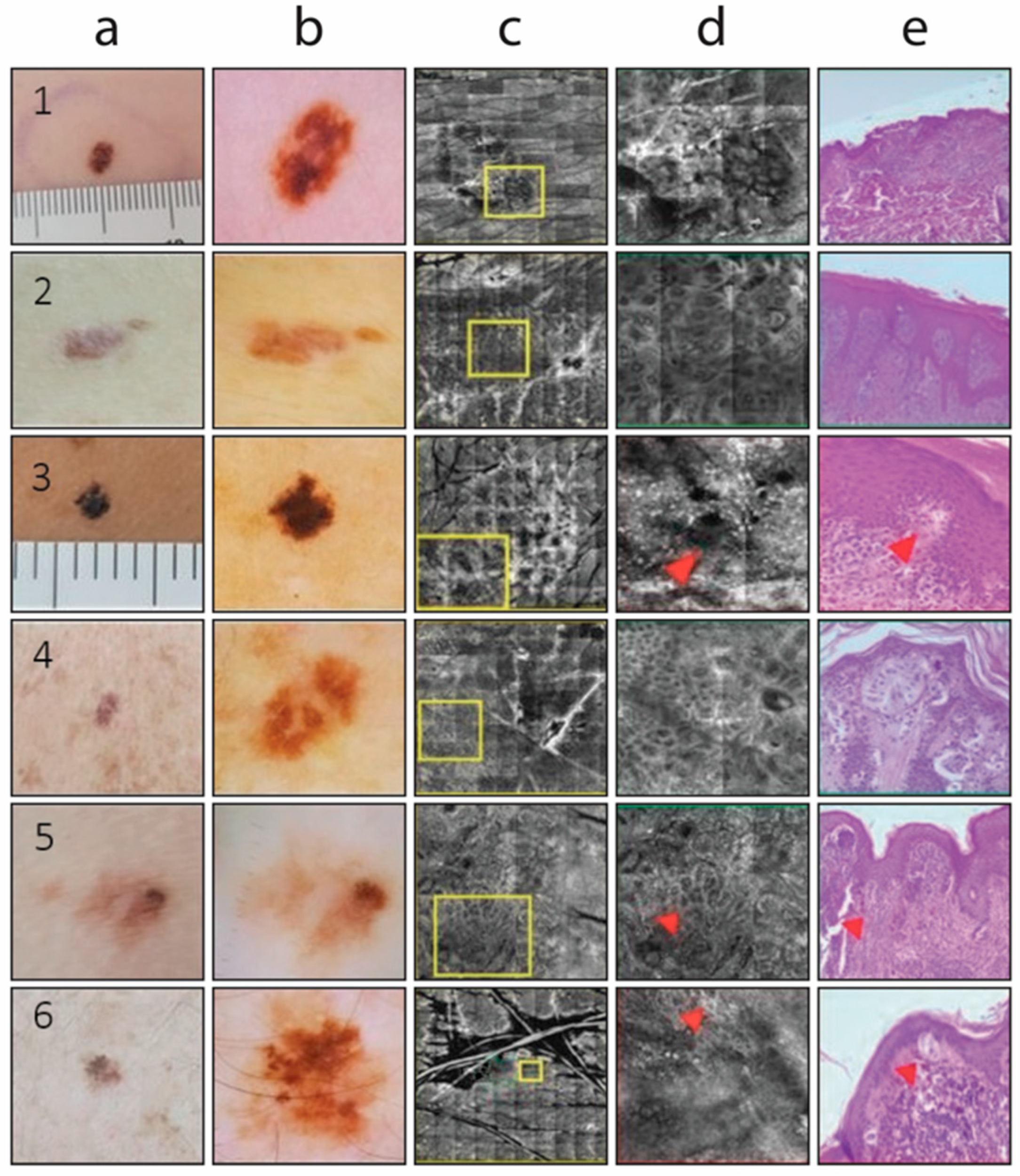
| Melanocytic Lesions | ELM Features |
|---|---|
| Type 1 (very high risk) | Lesion with a pigment network and distinct ELM features strongly associated with melanoma, such as pseudopods, radial streaming, a blue-gray veil, or atypical vessels. |
| Type 2 (high risk) | Lesion with a pigment network and subtle emerging ELM features that may indicate melanoma but are also commonly found in atypical nevi. |
| Type 3 (medium risk) | Lesion with a pigment network showing mild structural irregularities, which can be observed in atypical nevi and melanocytic hyperplasia. |
| Type 4 (low risk) | Lesion with a uniformly benign-appearing pigment network. |
| Type 5 (very low risk) | Lesion with a benign-appearing pigment network, often exhibiting a globular pattern or another characteristic benign ELM structure. |
| Breslow Thickness Categories | Cases (n = 657), n | Median Breslow Thickness (mm) | Range (mm) |
|---|---|---|---|
| Diffuse intraepidermal atypical cells, melanoma in situ, MELTUMP | 93 | No | No |
| <0.8 mm | 257 | 0.5 | 0.3–0.8 |
| <0.8 mm with ulceration or 0.8–1.0 mm | 189 | 0.9 | 0.8–1 |
| >1.1–2.0 mm | 153 | 1.65 | 1.3–2.0 |
| >2.1–4.0 mm | 39 | 2.45 | 2.1–2 |
| >4.0 mm | 19 | 4.65 | >4 |
| ELM Levels of Risk | N (no. of MMs, %) | ELM Agrees with Histology, n (%) | RCM Agrees with Histology, n (%) |
|---|---|---|---|
| Very high | 364 (15.9) | 335 (92.0) | 330 (90.9) |
| High | 468 (20.5) | 414 (88.5) | 394 (84.4) |
| Medium | 235 (10.3) | 156 (66.3) | 219 (93.1) |
| Low | 463 (20.4) | 450 (96.3) | 418 (90.5) |
| Very low | 752 (32.9) | 737 (98.0) | 722 (96.2) |
| Total | 2282 | 2092 | 2083 |
| ELM Levels of Risk | N | ELM Sensitivity | ELM Specificity | RCM Sensitivity | RCM Specificity |
|---|---|---|---|---|---|
| Very high | 364 | 98.4 | 78.2 | 95.6 | 68.2 |
| High | 468 | 93.8 | 70.6 | 88.6 | 67.6 |
| Medium | 235 | 73.5 | 55.7 | 92.4 | 94.0 |
| Low | 463 | 98.5 | 85.1 | 92.9 | 85.3 |
| Very low | 752 | 98.3 | 83.3 | 96.6 | 81.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palla, M.; Ferrara, G.; Caracò, C.; Scarpato, L.; Anniciello, A.M.; Meinardi, P.; Amore, A.; Di Trolio, R.; Marano, G.; Alfano, B.; et al. Where Reflectance Confocal Microscopy Provides the Greatest Benefit for Diagnosing Skin Cancers: The Experience of the National Cancer Institute of Naples. Cancers 2025, 17, 1745. https://doi.org/10.3390/cancers17111745
Palla M, Ferrara G, Caracò C, Scarpato L, Anniciello AM, Meinardi P, Amore A, Di Trolio R, Marano G, Alfano B, et al. Where Reflectance Confocal Microscopy Provides the Greatest Benefit for Diagnosing Skin Cancers: The Experience of the National Cancer Institute of Naples. Cancers. 2025; 17(11):1745. https://doi.org/10.3390/cancers17111745
Chicago/Turabian StylePalla, Marco, Gerardo Ferrara, Corrado Caracò, Luigi Scarpato, Anna Maria Anniciello, Paolo Meinardi, Alfonso Amore, Rossella Di Trolio, Giuseppina Marano, Benedetta Alfano, and et al. 2025. "Where Reflectance Confocal Microscopy Provides the Greatest Benefit for Diagnosing Skin Cancers: The Experience of the National Cancer Institute of Naples" Cancers 17, no. 11: 1745. https://doi.org/10.3390/cancers17111745
APA StylePalla, M., Ferrara, G., Caracò, C., Scarpato, L., Anniciello, A. M., Meinardi, P., Amore, A., Di Trolio, R., Marano, G., Alfano, B., Tuccillo, M., Mallardo, D., Pellacani, G., & Ascierto, P. A. (2025). Where Reflectance Confocal Microscopy Provides the Greatest Benefit for Diagnosing Skin Cancers: The Experience of the National Cancer Institute of Naples. Cancers, 17(11), 1745. https://doi.org/10.3390/cancers17111745






