From Clinical Perception to Implicit Bias: Understanding Personality Traits in Lymphoma Patients
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Aims and Design of the Study
2.2. Participants/Sample
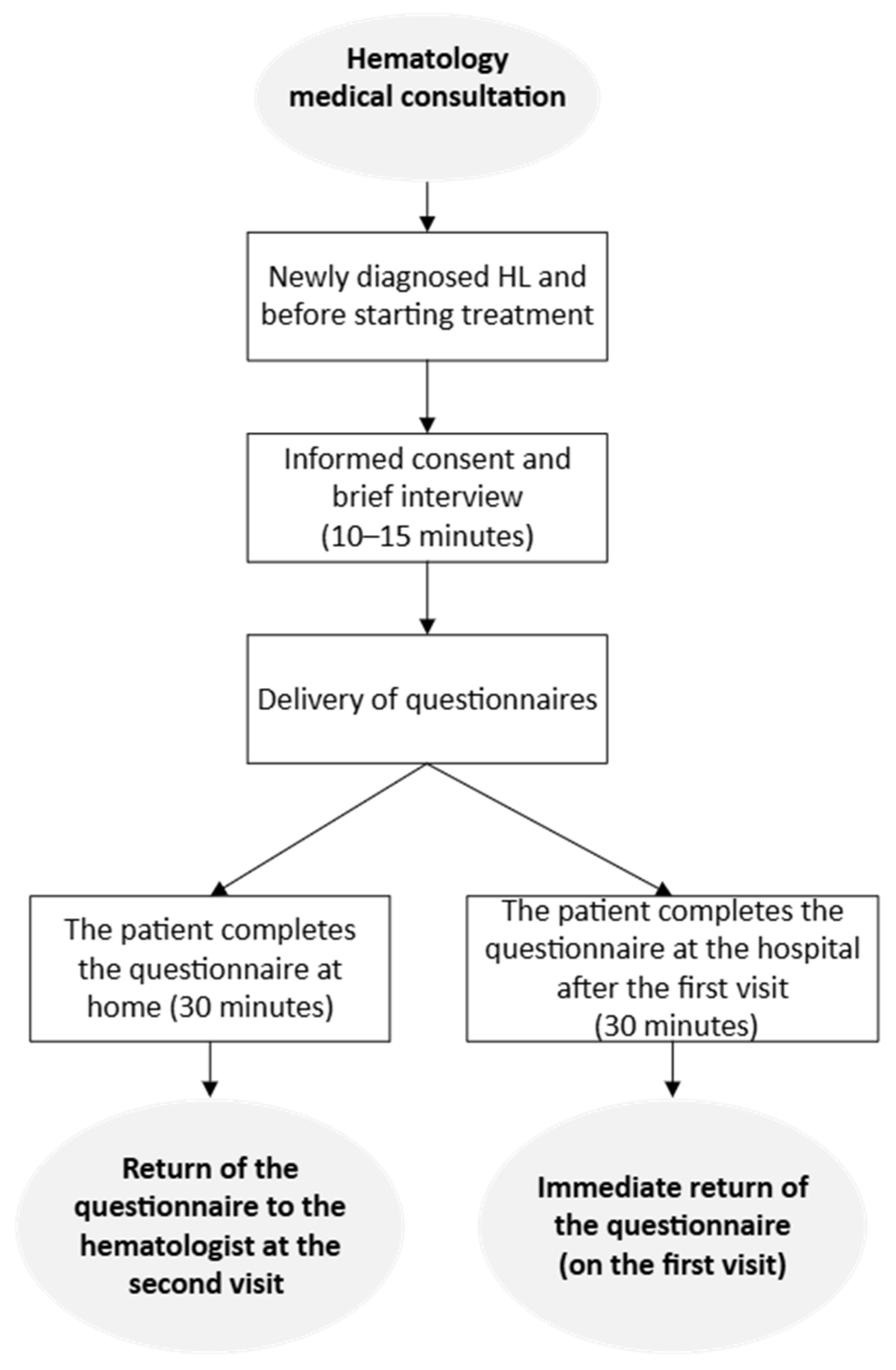
2.3. Procedure
2.4. Measures
NEO Five-Factor Inventory (NEO-FFI)
2.5. Statistical Analysis
3. Results
3.1. Sociodemographic and Psychosocial Data
3.2. Personality Trait Contrasts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HL | Hodgkin’s Lymphoma |
| NHL | Non-Hodgkin’s Lymphoma |
| GELTAMO | Spanish Group of Lymphoma |
| FFM | Five-Factor Model |
| NEO-FFI | NEO Five-Factor Inventory |
| ESS | European Social Survey |
References
- Galli, F.; Scotto, L.; Ravenda, S.; Zampino, M.G.; Pravettoni, G.; Mazzocco, K. Personality Factors in Colorectal Cancer: A Systematic Review. Front. Psychol. 2021, 12, 590320. [Google Scholar] [CrossRef]
- Digman, J.M. Personality Structure: Emergence of the Five-Factor Model. Annu. Rev. Psychol. 1990, 41, 417–440. [Google Scholar] [CrossRef]
- McCrae, R.R.; Costa, P.T. Personality trait structure as a human universal (NEO-PI-R). Am. Psychol. 1997, 52, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.T.; McCrae, R.R. The Revised NEO Personality Inventory (NEO-PI-R). In The SAGE Handbook of Personality Theory and Assessment; Personality Measurement and Testing; Boyle, G.J., Matthews, G., Saklofske, D.H., Eds.; Sage Publications, Inc.: Los Angeles, CA, USA, 2008; Volume 2, pp. 179–198. [Google Scholar] [CrossRef]
- Donnellan, M.B.; Robins, R.W. Resilient, Overcontrolled, and Undercontrolled Personality Types: Issues and Controversies. Soc. Personal. Psychol. Compass 2010, 4, 1070–1083. [Google Scholar] [CrossRef]
- Kinnunen, M.; Metsäpelto, R.; Feldt, T.; Kokko, K.; Tolvanen, A.; Kinnunen, U.; Leppänen, E.; Pulkkinen, L. Personality profiles and health: Longitudinal evidence among Finnish adults. Scand. J. Psychol. 2012, 53, 512–522. [Google Scholar] [CrossRef]
- Rochefort, C.; Hoerger, M.; Turiano, N.A.; Duberstein, P. Big Five personality and health in adults with and without cancer. J. Health Psychol. 2019, 24, 1494–1504. [Google Scholar] [CrossRef] [PubMed]
- Macía, P.; Gorbeña, S.; Gómez, A.; Barranco, M.; Iraurgi, I. Role of neuroticism and extraversion in the emotional health of people with cancer. Heliyon 2020, 6, e04281. [Google Scholar] [CrossRef]
- Rassart, J.; Luyckx, K.; Goossens, E.; Apers, S.; Klimstra, T.A.; Moons, P. Personality traits, quality of life and perceived health in adolescents with congenital heart disease. Psychol. Health 2013, 28, 319–335. [Google Scholar] [CrossRef]
- Sutin, A.R.; Terracciano, A.; Deiana, B.; Naitza, S.; Ferrucci, L.; Uda, M.; Schlessinger, D.; Costa, P.T. High Neuroticism and low Conscientiousness are associated with interleukin-6. Psychol. Med. 2010, 40, 1485–1493. [Google Scholar] [CrossRef]
- Sutin, A.R.; Zonderman, A.B.; Ferrucci, L.; Terracciano, A. Personality Traits and Chronic Disease: Implications for Adult Personality Development. J. Gerontol. Ser. B 2013, 68, 912–920. [Google Scholar] [CrossRef]
- Hengartner, M.P.; Kawohl, W.; Haker, H.; Rössler, W.; Ajdacic-Gross, V. Big Five personality traits may inform public health policy and preventive medicine: Evidence from a cross-sectional and a prospective longitudinal epidemiologic study in a Swiss community. J. Psychosom. Res. 2016, 84, 44–51. [Google Scholar] [CrossRef]
- Lima, M.P.; Moret-Tatay, C.; Irigaray, T.Q. Locus of control, personality and depression symptoms in cancer: Testing a moderated mediation model. Clin. Psychol. Psychother. 2022, 29, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Cerezo, M.V.; Blanca, M.J.; Ferragut, M. Personality Profiles and Psychological Adjustment in Breast Cancer Patients. Int. J. Environ. Res. Public Health 2020, 17, 9452. [Google Scholar] [CrossRef] [PubMed]
- Gempt, J.; Bette, S.; Albertshauser, J.; Cammardella, J.H.; Gradtke, C.; Wiestler, B.; Schirmer, L.; Ryang, Y.-M.; Meyer, B.; Ringel, F. Personality Traits in Patients with Neuroepithelial Tumors—A Prospective Study. Sci. Rep. 2018, 8, 17055. [Google Scholar] [CrossRef]
- Knauer, K.; Bach, A.; Schäffeler, N.; Stengel, A.; Graf, J. Personality Traits and Coping Strategies Relevant to Posttraumatic Growth in Patients with Cancer and Survivors: A Systematic Literature Review. Curr. Oncol. 2022, 29, 9593–9612. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, M.A.; Dawson, A.A.; Walker, L.G. Eysenck Personality Inventory L-scores in patients wtih Hodgkin’s disease and non-Hodgkin’s lymphoma. Psychooncology 1995, 4, 39–45. [Google Scholar] [CrossRef]
- Arts, L.P.; Oerlemans, S.; Schoormans, D.; Sanders, A.L.; Stevens, W.B.; Posthuma, E.F.; Tick, L.W.; van de Poll-Franse, L.V. Psychological distress among patients with lymphoma: The association with personality and coping strategies. J. Psychosoc. Oncol. Res. Pract. 2021, 3, e041. [Google Scholar] [CrossRef]
- Roso-Bas, F.; Alonso-Llobregat, M.D.; Bento, L.; Sanchez-Gonzalez, B.; Herraez, I.; Garcia-Dilla, P.; Vallespir, C.; Rado, F.; Rodriguez, R.; Garcia-Pallarols, F.; et al. Analysis of Personality Traits in Patients with Hodgkin Lymphoma. J. Clin. Med. 2021, 10, 1631. [Google Scholar] [CrossRef]
- Lima, M.P.; Machado, W.d.L.; Irigaray, T.Q. Predictive factors of treatment adherence in cancer outpatients. Psychooncology 2018, 27, 2823–2828. [Google Scholar] [CrossRef]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.d.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef]
- Thewes, B.; Rietjens, J.A.; Berg, S.W.v.D.; Compen, F.R.; Abrahams, H.; Poort, H.; van de Wal, M.; Schellekens, M.P.; Peters, M.E.; Speckens, A.E.; et al. One way or another: The opportunities and pitfalls of self-referral and consecutive sampling as recruitment strategies for psycho-oncology intervention trials. Psycho-Oncology 2018, 27, 2056–2059. [Google Scholar] [CrossRef] [PubMed]
- Cordero, P.A.; Pamos, A.; Seisdedos, C.N.; Avia, A.M.D.; Costa, P.T.; McCrae, R.R. Inventario de Personalidad Neo Revisado (NEO PI-R), Inventario Neo Reducido de Cinco Factores (NEO-FFI): Manual Profesional; TEA: Madrid, Spain, 2008. [Google Scholar]
- Langford, D.J.; Morgan, S.; Cooper, B.; Paul, S.; Kober, K.; Wright, F.; Hammer, M.J.; Conley, Y.P.; Levine, J.D.; Miaskowski, C.; et al. Association of personality profiles with coping and adjustment to cancer among patients undergoing chemotherapy. Psycho-Oncology 2020, 29, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.; Cooper, B.; Paul, S.; Hammer, M.J.; Conley, Y.P.; Levine, J.D.; Miaskowski, C.; Dunn, L.B. Association of personality profiles with depressive, anxiety, and cancer-related symptoms in patients undergoing chemotherapy. Pers. Individ. Dif. 2017, 15, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Bucher, M.A.; Suzuki, T.; Samuel, D.B. A meta-analytic review of personality traits and their associations with mental health treatment outcomes. Clin. Psychol. Rev. 2019, 70, 51–63. [Google Scholar] [CrossRef]
- Kokko, K.; Tolvanen, A.; Pulkkinen, L. Associations between personality traits and psychological well-being across time in middle adulthood. J. Res. Pers. 2013, 47, 748–756. [Google Scholar] [CrossRef]
- Härtl, K.; Engel, J.; Herschbach, P.; Reinecker, H.; Sommer, H.; Friese, K. Personality traits and psychosocial stress: Quality of life over 2 years following breast cancer diagnosis and psychological impact factors. Psychooncology 2010, 19, 160–169. [Google Scholar] [CrossRef]
- Boals, A.; Southard-Dobbs, S.; Blumenthal, H. Adverse Events in Emerging Adulthood Are Associated with Increases in Neuroticism. J. Pers. 2015, 83, 202–211. [Google Scholar] [CrossRef]
- Ogle, C.M.; Rubin, D.C.; Siegler, I.C. Changes in Neuroticism Following Trauma Exposure. J. Pers. 2014, 82, 93–102. [Google Scholar] [CrossRef]
- Costa, P.T.; McCrae, R.R.; Löckenhoff, C.E. Personality Across the Life Span. Annu. Rev. Psychol. 2019, 70, 423–448. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, P.; Maggioncalda, A.; Malik, N.; Flowers, C.R. Incidence Patterns and Outcomes for Hodgkin Lymphoma Patients in the United States. Adv. Hematol. 2011, 2011, 725219. [Google Scholar] [CrossRef]
- Butler, R.N. Why Survive? Being Old in America; Harper & Row: Oxford, UK, 1975; p. 496. [Google Scholar]
- Fernandez-Ballesteros, R.; Huici, C. El edadismo: Una amenaza frente a las personas mayores [Ageism: A threat to the elderly]. Tiempo Paz 2022, 145, 26–39. Available online: www.mpdl.org (accessed on 10 July 2024). (In Spanish).
- Palmore, E. Ageism: Negative and Positive, 2nd ed.; Springer Publishing Company: New York, NY, USA, 1999. [Google Scholar]
- Levy, B.R.; Banaji, M.R. Implicit ageism. In Ageism: Stereotyping and Prejudice Against Older Persons; Nelson, T.D., Ed.; The MIT Press: Cambridge, MA, USA, 2002; pp. 49–75. [Google Scholar] [CrossRef]
- Banaji, M.R.; Greenwald, A.G. Implicit stereotyping and prejudice. In The Psychology of Prejudice, 1st ed.; Zanna, M.P., Olson, J.M., Eds.; Psychology Press: New York, NY, USA, 1994; pp. 55–76. [Google Scholar]
- Bratt, C.; Abrams, D.; Swift, H.J.; Vauclair, C.-M.; Marques, S. Perceived Age Discrimination Across Age in Europe: From an Ageing Society to a Society for All Ages. Dev. Psychol. 2018, 54, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Bratt, C.; Abrams, D.; Swift, H.J. Supporting the Old but Neglecting the Young? The Two Faces of Ageism. Dev. Psychol. 2020, 56, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Francioli, S.P.; North, M.S. Youngism: The Content, Causes, and Consequences of Prejudices Toward Younger Adults. J. Exp. Psychol. Gen. 2021, 150, 2591–2612. [Google Scholar] [CrossRef]
- de la Fuente-Núñez, V.; Cohn-Schwartz, E.; Roy, S.; Ayalon, L. Scoping review on ageism against younger populations. 2021; 18, 3988. [Google Scholar] [CrossRef]
- Karis Allen, T.; Mayo, P.; Koshman, S.; Gray, M.; Babar, A.; Sadowski, C.A. Clinical Pharmacists’ Knowledge of and Attitudes toward Older Adults. Pharmacy 2021, 9, 172. [Google Scholar] [CrossRef]
- López-Hernández, L.; Martínez-Arnau, F.M.; Castellano-Rioja, E.; Botella-Navas, M.; Pérez-Ros, P. Factors Affecting Attitudes towards Older People in Undergraduate Nursing Students. Healthcare 2021, 9, 1231. [Google Scholar] [CrossRef]
- Liu, Y.; Norman, I.J.; While, A.E. Nurses’ attitudes towards older people: A systematic review. Int. J. Nurs. Stud. 2013, 50, 1271–1282. [Google Scholar] [CrossRef]
- Parmač Kovačić, M.; Galić, Z.; Jerneić, Ž. Social desirability scales as indicators of self-enhancement and impression management. J. Pers. Assess. 2014, 96, 532–543. [Google Scholar] [CrossRef]
- The Joint Commission. Implicit Bias in Health Care. Quick Safety. 2016. Available online: https://virginiainterfaithcenter.org/wp-content/uploads/2021/11/Quick-Safety-Issue-23-Apr-2016-FINAL-Rev-implicit-bias.pdf (accessed on 15 August 2024).
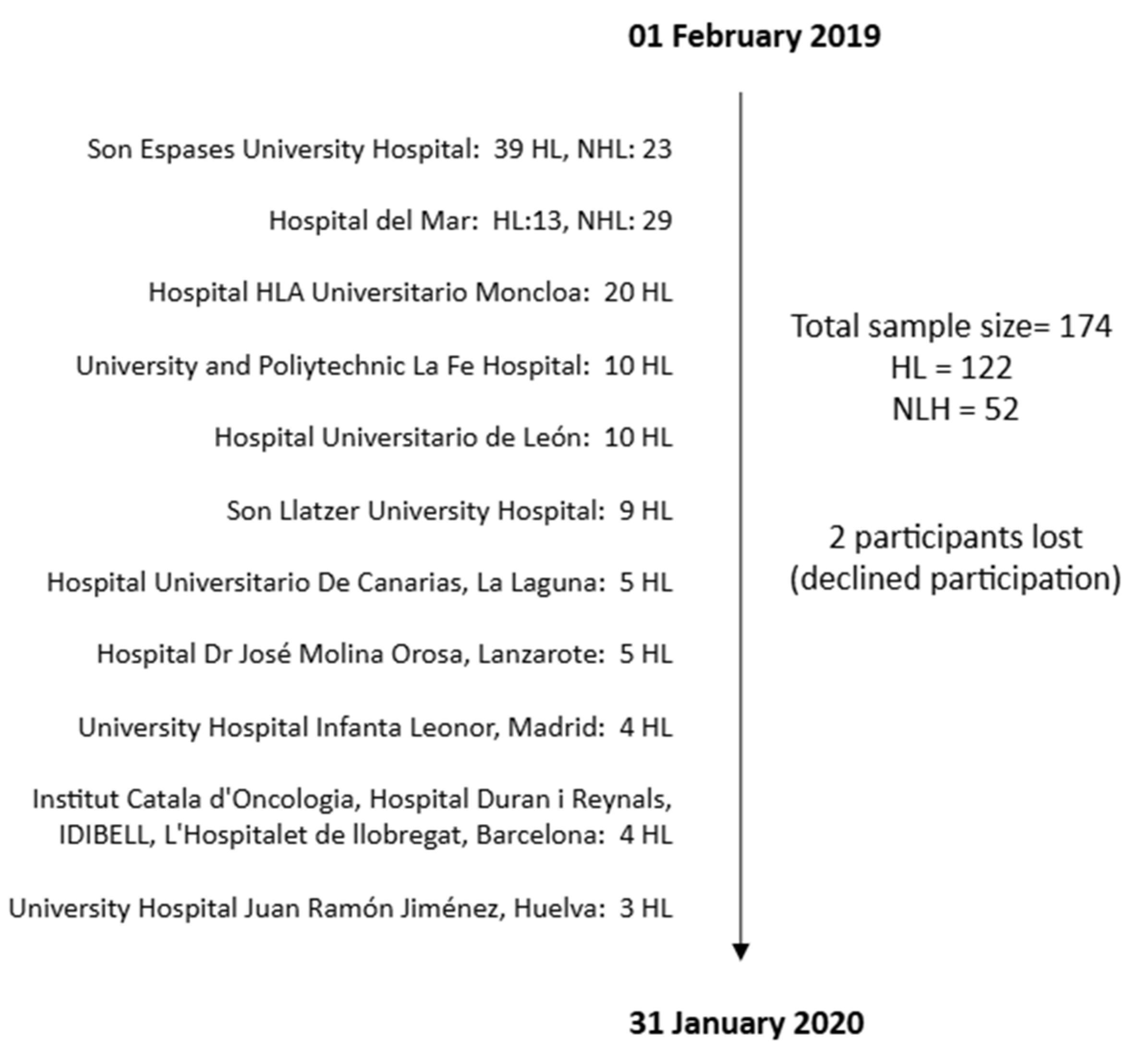
| HL | NHL | ||
|---|---|---|---|
| Age | Med (Rank) | Med (Rank) | p |
| 39 (70) | 51 (58) | 0.003 *a | |
| Gender | n (%) | n (%) | p |
| Male | 55 (45.1) | 31 (59.6) | 0.098 b |
| Female | 67 (54.9) | 21 (40.4) | |
| Civil Status | |||
| Single | 59 (48.8) | 16 (30.8) | 0.066 c |
| Married | 48 (39.7) | 31 (59.6) | |
| Widowed | 3 (2.5) | - | |
| Separated/Divorced | 11 (9.1) | 5 (9.6) | |
| Educational levels | |||
| Uneducated | 1 (0.8) | - | 0.197 c |
| Primary | 25 (20.7) | 12 (23.1) | |
| Secondary | 39 (32.2) | 24 (46.2) | |
| High School | 56 (46.3) | 16 (30.8) | |
| Cohabitation | |||
| Alone | 14 (11.7) | 8 (15.4) | 0.841 c |
| With relatives | 103 (85.8) | 43 (82.7) | |
| Other | 3 (2.5) | 1 (1.9) |
| HL | NHL | ||
|---|---|---|---|
| n (%) | n (%) | p | |
| Perception of social support | |||
| No social support | 1 (1) | 1 (2.6) | 0.296 a |
| Moderate social support | 13 (13) | 2 (5.3) | |
| High social support | 86 (86) | 35 (92.1) | |
| Stressful life events | |||
| No life stressor | 42 (35) | 14 (26.9) | 0.654 a |
| Mild life stressor | 14 (11.7) | 9 (17.3) | |
| Moderate life stressor | 21 (17.5) | 10 (19.2) | |
| Intense life stressor | 43 (35.8) | 19 (36.5) | |
| Impact of family cancer history | |||
| No | 30 (25) | 10 (19.2) | 0.217 a |
| Mild impact | 28 (23.3) | 17 (32.7) | |
| Moderate impact | 32 (36.7) | 8 (15.4) | |
| Intense impact | 30 (25) | 17 (32.7) | |
| Need for psychological support | |||
| No | 87 (79.1) | 33 (64.7) | 0.368 b |
| Yes | 34 (28.1) | 18 (35.3) | |
| Psychiatric diagnosis | |||
| No | 105 (86.8) | 46 (88.5) | 1.0 b |
| Yes | 16 (13.2) | 6 (11.5) | |
| History of autolytic thoughts | |||
| No | 97 (84.3) | 45 (86.5) | 0.817 b |
| Yes | 18 (17.7) | 7 (13.5) | |
| History of suicide attempts | |||
| No | 112 (98.2) | 39 (95.1) | 0.285 b |
| Yes | 2 (1.8) | 2 (4.9) |
| NEO-FFI | α | HL (n = 122) | NHL (n = 52) | ||||
|---|---|---|---|---|---|---|---|
| M (SD) | Med (Rank) | M (SD) | Med (Rank) | U | p | ||
| Neuroticism | 0.827 | 19.89 (8.39) | 19 (37) | 19.28 (8.08) | 17 (39) | 2934.5 | 0.651 |
| Extraversion | 0.837 | 30 (7.34) | 30 (34) | 30.33 (8.52) | 31 (40) | 2834.5 | 0.496 |
| Openness | 0.807 | 26.64 (7.72) | 26 (39) | 27.53 (8.37) | 26.5 (37) | 2787.0 | 0.341 |
| Agreeableness | 0.722 | 31.47 (5.80) | 31 (26) | 30.33 (7.53) | 31 (32) | 2918.5 | 0.693 |
| Conscientiousness | 0.817 | 32.57 (7.13) | 33 (37) | 34.07 (7.30) | 34.5 (33) | 2627.5 | 0.117 |
| NEO-FFI | Reference Population | HL Sample (n = 122) | NHL Sample (n = 52) | |||
|---|---|---|---|---|---|---|
| Mean (SD) | Med | Mean (SD) | Med (Rank) | Mean (SD) | Med (Rank) | |
| Neuroticism | 15.35 (7.40) | 14 | 19.89 (8.39) | 19 (37) | 19.28 (8.08) | 17 (39) |
| p = 0.000 * | p = 0.000 * | |||||
| High cut score = 18 | 55.1% | 46.2% | ||||
| X2 = 0.82; p = 0.364 a | ||||||
| Extraversion | 32.59 (6.35) | 33 | 30 (7.34) | 30 (34) | 30.33 (8.52) | 31 (40) |
| p = 0.000 * | p = 0.057 | |||||
| Low cut score = 28 | 44.5% | 31.4% | ||||
| X2 = 2.04; p = 0.152 a | ||||||
| Openness | 28.64 (6.56) | 29 | 26.64 (7.72) | 26 (39) | 27.53 (8.37) | 26.5 (37) |
| p = 0.001 * | p = 0.293 | |||||
| Low cut score = 24 | 40.7% | 32.7% | ||||
| X2 = 0.66; p = 0.415 a | ||||||
| Agreeableness | 32.79 (5.67) | 33 | 31.47 (5.80) | 31 (26) | 30.33 (7.53) | 31 (32) |
| p = 0.009 * | p = 0.034 * | |||||
| Low cut score = 29 | 37.8% | 41.2% | ||||
| X2 = 0.05; p = 0.810 a | ||||||
| Conscientiousness | 36.01 (6.02) | 36 | 32.57 (7.13) | 33 (37) | 34.07 (7.30) | 34.5 (33) |
| p = 0.000 * | p = 0.176 | |||||
| Low cut score = 32 | 48.7% | 32.7% | ||||
| X2 = 3.16; p = 0.075 a | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roso-Bas, F.; Alonso-Llobregat, M.D.; Bento, L.; Sánchez-González, B.; Lebrahimi, L.A.; Balanzat, I.H.; García-Dilla, P.; García-Pallarols, F.; Gil, S.N.; Romero, S.; et al. From Clinical Perception to Implicit Bias: Understanding Personality Traits in Lymphoma Patients. Cancers 2025, 17, 1743. https://doi.org/10.3390/cancers17111743
Roso-Bas F, Alonso-Llobregat MD, Bento L, Sánchez-González B, Lebrahimi LA, Balanzat IH, García-Dilla P, García-Pallarols F, Gil SN, Romero S, et al. From Clinical Perception to Implicit Bias: Understanding Personality Traits in Lymphoma Patients. Cancers. 2025; 17(11):1743. https://doi.org/10.3390/cancers17111743
Chicago/Turabian StyleRoso-Bas, Fátima, María Dolores Alonso-Llobregat, Leyre Bento, Blanca Sánchez-González, Layla Aoukhiyad Lebrahimi, Inés Herráez Balanzat, Pilar García-Dilla, Francesc García-Pallarols, Sara Nistal Gil, Samuel Romero, and et al. 2025. "From Clinical Perception to Implicit Bias: Understanding Personality Traits in Lymphoma Patients" Cancers 17, no. 11: 1743. https://doi.org/10.3390/cancers17111743
APA StyleRoso-Bas, F., Alonso-Llobregat, M. D., Bento, L., Sánchez-González, B., Lebrahimi, L. A., Balanzat, I. H., García-Dilla, P., García-Pallarols, F., Gil, S. N., Romero, S., Vidal, M.-J., Bonis-Braun, C. D., León, Y. R. d., Infante, M. S., Domingo-Domenech, E., Ramírez, S., Bargay, J., Sampol, A., Salar, A., & Gutiérrez, A. (2025). From Clinical Perception to Implicit Bias: Understanding Personality Traits in Lymphoma Patients. Cancers, 17(11), 1743. https://doi.org/10.3390/cancers17111743







